Abstract
As global plastic production reaches unprecedented levels, the increasing amount of plastic entering our oceans poses serious environmental risks. The Circular Economy (CE) concept plays a key role in this shift, from linear to circular, by promoting waste reduction, improved recycling methods, and innovative technologies that convert waste into value-added products. Among waste polymers, poly (ethylene terephthalate) PET, known for its enhanced properties like chemical resistance and mechanical properties, which is widely used for water bottles and food containers, is a prime candidate for recycling efforts. Taking advantage of 3D printing and recycled PET as a feedstock emerged as one of the potential solutions to reuse waste PET. However, 3D printing recycled PET (r-PET) is challenging due to the deterioration of physical properties after recycling compared to virgin PET, like reduced viscosity and melt strength, as well as chain scission. To address these challenges and direct 3D printing of r-PET, this study, for the first time, modified r-PET using a proprietary additive, to make it compatible with 3D printing and enhance properties. Through analysis of flow behavior and examinations of thermal stability, chemical structures, and mechanical properties, it is found that 0.75% is the optimum amount of additive added to r-PET, which improves the printability of r-PET feedstock. After the addition of 0.75% additive, the complex viscosity of the modified r-PET increased from 200 Pa.s to around 1000 Pa.s, which meets the viscosity requirements for 3D printing. In addition, ultimate tensile strength of modified r-PET reached around 51 MPa which is comparable with commercial filaments like ABS, PETG and PLA. The findings of this study highlight the importance of using modifiers as additives to enhance the properties of recycled waste plastic, rendering them suitable for 3D printing. This facilitates the reprocessing and remanufacturing of new products within the framework of a circular economy with the aid of 3D printing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In modern life, polymers are inseparable parts of many industries, such as aerospace, automotive, packaging, and construction. To meet the huge demands of these industries, the worldwide rate of plastic production increased from 2 million metric tons in 1950 to 391 million metric tons in 2021. As a result of this high rate of production, each year, an estimated range of 4.8 million to 12.7 million tons of plastic waste finds its way into the ocean [1,2,3]. It is projected that without intervention, the weight of plastic in the ocean will exceed that of fish by 2050. More importantly, one of the main objectives in the context of sustainable development goals (SDGs) is responsible consumption and production [4]. To prevent entering more plastic waste into the ocean, inefficient use of raw materials and reach the goal of circular economy in plastic production, it is imperative to use them for recycling and energy recovery [2]. Energy recovery involves extracting energy from plastic waste through processes like incineration or pyrolysis. This approach not only reduces the amount of plastic waste ending up in landfills or oceans but also harnesses the energy potential stored in these materials. The Circular Economy is a production system concept that aims to increase the value and use of materials by promoting their retention and restoration within the economy. This material retention can be achieved by reducing waste, increasing recycling rates, improving collection efficiency, enhancing recycling methods, or developing technologies for upcycling and downcycling [5].
Among industries using plastics, packaging is the primary end-user market for polymers, specifically polyethylene (PE), poly (ethylene terephthalate) (PET), and polypropylene (PP) [6]. PET is the most recycled thermoplastic in Canada and is widely recognized globally. It is commonly used in bottle manufacturing and various industries such as food, cosmetics, and healthcare. This semi-crystalline polyester offers excellent barrier properties, mechanical strength, ductility, and resilience to large deformations. [2, 7]. PET is traditionally manufactured by injection blow molding, which promotes rapid cooling rates and inhibits crystallization. This technology enables the manufacture of flexible and translucent PET packages and films [8,9,10]. PET can also be made using other technologies, including lamination, injection molding, thermoforming, or coextrusion [7].
In addition to the abovementioned traditional methods for manufacturing PET parts, the other method is reprocessing PET flakes or pellets into filaments via extrusion, which can be utilized as a feedstock for Fused Deposition Modeling (FDM) which is one of the additive manufacturing (AM) (also called 3D printing) techniques [11, 12]. A key benefit of AM is its reduced waste production in comparison to traditional manufacturing methods, thereby aligning with sustainability and economic objectives. Stratasys created the FDM method, the process of extruding filament material through a heated nozzle and depositing it layer by layer onto a substrate while it is still semisolid [13]. Among many 3D printing technologies, FDM has grown in popularity due to its low cost, accessibility, and adaptability with a wide range of materials [14, 15]. Over time, the range of 3D printable materials with FDM has been expanded to a variety of plastics, metal powders, ceramics, and composites, for use in the aerospace, medical, mold design, and automotive industries [16, 17].
However, the number of studies for developing filaments from recycled PET (r-PET) and subsequent FDM 3D printing is very limited [18,19,20,21,22,23]. The main challenge for making filaments from r-PET is its low intrinsic viscosity (IV) and melt strength, which makes it unsuitable for the extrusion process. Mechanical recycling methods for PET, such as heat-induced processes like extrusion, cause property degradation due to imposing high shear stress and temperature, leading to the reduction in IV by the formation of low molar mass PET. The intrinsic viscosity of PET serves as a crucial indicator of its printability and final product quality. In addition, parameters such as molecular weight, melting point, crystallinity, and tensile strength are significantly influenced by the IV of PET [24].
In the very first study by Zander et al. [25], they developed r-PET filament for FDM without additives using a twin-screw extruder. The filament was pelletized and re-extruded to achieve a consistent diameter. The study examined the effects of processing conditions, drying, and PET source on mechanical, rheological, and thermal properties. Crystallinity ranged from 12.2% for water-cooled filaments to 24.9% without active cooling. The tensile strength of printed parts was 35.1 ± 8 MPa, comparable to commercial polycarbonate-ABS filament, with an elongation to failure of 3.5%, similar to injection-molded parts. Three-point bending tests showed that parts made from recycled filament had equivalent loads at failure compared to those made from commercial filament." In another study by Voorde et al. [26], they studied how FDM and extrusion parameters affect the crystallinity and mechanical properties of recycled PET using a twin-screw extruder. Polymer degradation depended on extruder residence time, with 270 °C for 5 min being optimal. Extrusion conditions, such as barrel temperature and residence time, influenced filament crystallinity and mechanical properties. Optimal FDM printing temperatures were 250 °C for Ultimaker 3 and 260 °C for Prusa i3 MK3S. Fan cooling and the interaction between printing and build plate temperatures significantly affected the crystallinity and mechanical properties of printed parts. Tensile testing showed significant differences (p < 0.05) in tensile modulus between Ultimaker (2.65 ± 0.14 GPa) and Prusa (2.41 ± 0.04 GPa) printed components.
These two groups that worked on processing r-PET using FDM employed a certain grade to manufacture filament and print it, with no chemical change. Most studies have concentrated on optimizing process parameters rather than finding a technique to improve the properties of the main material feedstock with different grades and discover a practical strategy for employing r-PET for AM on a commercial scale. The primary challenge encountered when processing r-PET for FDM lies in the significant degradation of r-PET properties with different grades, produced from different sources, during their lifespan and recycling process. This degradation results in lowered viscosity and melt strength, rendering direct extrusion into filament forms unfeasible. More importantly, PET undergoes different processing methods for various applications, such as injection molding or blow molding, utilizing different grades. However, during recycling, these grades get mixed, creating a blend of different PET grades. Some of these streams of r-PET can be processable for extrusion and some of them not. Separating these grades proves challenging, prompting the use of chemical modification instead. The proposed approach in this study, by adding a suitable modifier, adjusts the rheological properties of the mixed PET grades and makes it extrudable. By modifying the chemical composition or structure, the mixed PET becomes suitable for processes like FDM. To this end, in this study, r-PET flakes sourced from water bottle waste were procured and subjected to rheological analysis, revealing insufficient shear thinning behavior and viscosity for making filaments and 3D printing, due to chain scission during mechanical recycling. To address this issue, a reactive modification of r-PET was investigated using a proprietary additive (hereafter called PA), via a batch mixer, to modify the rheological property of r-PET and make it printable. Subsequently, the filament-making process was executed using a single-screw extruder (Filabot). Various concentrations of additives (0.5, 0.75, 1, and 1.5 wt %) were employed to modify r-PET viscosity. Following reactive modification, comprehensive investigations into thermal stability, chemical structures, rheology, and mechanical properties were conducted to determine the optimal additive concentration. As the showcase, the parts of a centrifugal pump was 3D printed successfully using the modified r-PET. Through a systematic approach, this study aimed to mitigate the inherent limitations of remanufacturing and reusing recycled PET using a low-cost desktop 3D printer, thereby enhancing reprocessing and remanufacturing waste plastics into new value-added products.
2 Experiment
2.1 Materials
Recycled PET flakes (r-PET), sourced from post-industrial water bottle waste, were purchased from Post Plastics Inc. Canada. The additive PA was supplied by Sigma-Aldrich in the form of white powders with 99% purity.
2.2 Reactive modification, filament making, and 3D printing
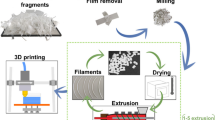
Reactive modification process of r-PET with PA additive was carried out in a Haake Rheomix 3000P (ThermoFisher Scientific; Waltham, MA) at a rotor speed and mixing temperature of 60 rpm and 260 °C, respectively. To prevent a hydrolysis reaction, r-PET flakes, and the PA powders were pre-dried for 12 h at 100 °C in an oven before the melting process. Dried flakes were melted and mixed for 4 min in the batch before the PA addition. PA contents were selected at 0, 0.5, 0.75, 1, 1.5 wt%. After adding the additive, the reaction process was continued for 6 min when the torque became stable. After that, samples were detached from the batch, grinded, and then dried for another 12 h prior to filament-making process. To make filaments, the granules were fed into a single-screw extruder (EX2, Filabot, Barre, USA), with one heating zone and air fans cooling system. The heating zone temperature and the nozzle diameter were adjusted to 245 °C and 1.75 mm, respectively. The screw and spooling speeds were adjusted to make filaments with 1.75 ± 0.05 mm diameter, which is compatible with Prusa FDM 3D printer. After filament making, type V tensile test specimens based on ASTM D638 were printed by using a low-cost desktop Prusa i3 MK3S + 3D printer. The STL files of the specimens were converted to G-code files by using the Cura Slicer software. All printing parameters are represented in Table 1. The show case of centrifugal pump parts was printed with the same parameters in Table 1. In addition, the process schematic is presented in Fig. 1.
2.3 Thermal properties
2.3.1 Differential scanning calorimetry (DSC)
To investigate the thermal properties of r-PET modified with PA, a DSC test (Q20 instrument from TA Instruments in New Castle, DE, USA) was conducted. The experiments were implemented under the 50 mL/min nitrogen flow rate. The average weight of the samples was 6 mg. To eliminate the thermal history of the materials, 3 cycles, including heating, cooling, and reheating were conducted. Samples were heated up until 300 °C, followed by an isothermal hold at this temperature for 3 min. Subsequently, the cooling phase occurred at 10 °C/min down to 30 °C, succeeded by a second heating phase at 10 °C/min up to 300 °C. From the DSC test, melting temperature (\({T}_{m}),\) cold crystallization temperature (\({T}_{cc}),\) glass transition temperature \({(T}_{g})\) and the degree of the crystallinity of the samples \({X}_{C}\) were extracted. The degree of crystallinity is calculated using Eq. (1), where \(\Delta H_f\) is the fusion enthalpy, \(\Delta H_{cc}\) is the cold crystallization enthalpy and \(\Delta H_f^0\) is the heat fusion of 100% crystalline PET (140 J/g) [27]:
2.3.2 Thermal gravimetric analysis (TGA)
TGA test was conducted by utilizing a thermal gravimetric analyzer (Q50, TA Instruments, USA) to extract decomposition temperature as well as additives content in r-PET samples. The sample's average weight was 5 mg. The process was carried out under a nitrogen-controlled atmosphere with a temperature range of 30–600 °C and 10 °C/min heating rate.
2.4 Fourier transform infrared spectroscopy (FTIR)
PerkinElmer Spectrum 100 FT-IR Spectrometer was used to determine the chemical structure of r-PET and the additive and indicate the chemical reactions.
2.5 Rheology
The melt viscosity of the samples at 260 °C was measured by using a parallel plate rheometer (DHR, TA Instruments, USA) with a 25 mm parallel plate and 1500 µm gap. The samples were prepared in the form of disks with 25 mm diameter and 2 mm thickness. The shear frequencies were set in a range between 1 and 100 rad/s with a constant strain rate of 5%, which is in the linear viscoelastic region.
2.6 Mechanical test
The tensile evaluation of the 3D-printed doge-bone-shaped samples (ASTM D638) was carried out utilizing a cutting-edge Universal Testing Machine (LS100 Plus, Lloyd, UK), boasting a remarkable load capacity of up to 100 kN. Each specimen was positioned between upper and lower flat support surfaces, ensuring alignment. In addition, the long axis of the specimen was carefully oriented along the central axis of the testing apparatus for optimal testing conditions. The testing protocol was executed, maintaining a consistent speed of 5 mm/min throughout the evaluation process. This deliberate approach ensured uniformity and accuracy in the assessment of the mechanical properties. For each formulation, 3 samples were printed. Images of the printed samples and dimensions are represented in Fig. 2.
3 Results and discussion
3.1 DSC and TGA analysis
According to DSC results, shown in Fig. 3a, the melting temperature of modified r-PET samples with PA (0–1.5%) showed a decrease of around 3 0C in their melting point compared to neat r-PET. This decrease is attributed to the fact that steric hindrance is increased by adding the additive and changing the side chain from carbon-zero to carbon [25]. Thus, in these cases, a decrease in the melting point was caused by an increase in the amount of additive agents. The addition of PA increases branching in the PET chain, which results in a lower melting temperature due to changes in the size of the crystals and the length of the chain. Because the melting temperature depends on the size of the crystal, it drops as branching rises. The most significant changes were observed in the crystallization temperature by adding PA to r-PET in that \({T}_{c}\) of all modified samples in Fig. 3b shifted to a higher temperature compared to unmodified r-PET. It appears that increasing the amount of additive that serve as uniform nucleation site leads to the formation of branch structures which increase the crystallization temperature [28, 29]. This results in an elevation of both the initial rate of crystallization and the crystallization temperature. In Table 2, the values of Tm, Tc, enthalpies, and degree of crystallization (calculated using Eq. 1 are illustrated. Regarding the degree of crystallinity, adding 0.5% PA led to a decrease, indicating that at this concentration, PA disrupted the crystallization process. However, when the concentration was increased to 0.75% and 1% PA, the degree of crystallinity improved. This suggests that these higher concentrations enhanced chain mobility and facilitated optimal crystallization, resulting in increased crystallinity.
In the TGA analysis shown in Fig. 3c, both decomposition temperature and the residue content of r-PET were investigated. The results indicated that r-PET typically leaves behind approximately 15% residue, which shows the small quantity of impurities or additives added by the producers or recycling center to r-PET. Another key parameter in TGA results is the decomposition temperature which tends to hover around 400 °C for r-PET (Fig. 3c). When additives were added, there was no notable alteration observed in the decomposition temperature compared to the neat r-PET. All sample decomposition temperatures were around 390.
3.2 Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared spectroscopy was done to analyze the chemical modifications that occurred after adding PA additive to r-PET. FTIR analysis provided insight into the chemical structure of pure as well as functionalized r-PET, Fig. 4. The PET spectra showed absorption bands at 725 cm−1, 1015 cm−1 (aromatic bands), 1090 cm−1 (methylene), 1235 cm−1 (ester group), and 1720 cm−1 (ester carbonyl). In the FTIR spectra, double shoulder peaks from 1250 to 1050 cm−1 were associated with the vibration of C–O from carboxyl groups, and absorption bands at 1340 cm−1 and 1370 cm−1 were assigned to the wagging of the ethylene units. Absorption peaks in PA spectra indicate symmetric and asymmetric stretching of carbonyl groups at 1780–1763 cm−1. For comparative analysis, a graph was plotted for r-PET, PA and r-PET/0.75%PA. The intensity of these peaks at 1700 cm−1 and 1770 cm−1 was reduced in the r-PET/0.75%PA sample, indicating that the reactive modification was done completely [30].
3.3 Rheology analysis
In Fig. 5 a, b the storage and loss modulus of all samples represented. Similar trend to the complex viscosity results was observed here for all formulations. For PA-modified samples, the storage modulus significantly exceeded that of pure r-PET. It is attributed to the reason that increasing the number of entanglements enhances the material's capability to transfer external forces, resulting in improved melt elasticity and a consequent increase in the storage modulus [31, 32]. The storage modulus rises as frequency increases because, at low frequencies, there's ample time for chain relaxation, resulting in a lower number of contributing values to the storage modulus. Conversely, at higher frequencies, the chains lack sufficient time for relaxation, leading to an increase in the storage modulus. This increase in storage modulus which is representative of melt elasticity improves filament-making process and makes it stable without any failure during the extrusion process. As for loss modulus, Fig. 5b, a similar trend to storage modulus is observed, overall, it is dominant to storage modulus, which illustrates that the material is more viscous rather than elastic.
The complex viscosity graphs of all samples are illustrated in Fig. 5c. In the neat sample, it is obvious that by increasing the frequency, no significant changes in viscosity were detected in the range of 1 to 100 rad/s. It shows that the neat r-PET flakes do not show shear thinning behavior, which is not favorable for material extrusion (both printing and filament-making process) and the complex viscosity was around 200 Pa.s in the linear region. However, in modified samples with PA, the behavior was different. PA-modified r-PET samples demonstrated higher viscosity compared to r-PET. This increasing trend in viscosity was continued until the PA amount of 0.75% (complex viscosity: around 1000 Pa.s); however, after increasing the PA content to 1% and 1.5%, the complex viscosity of the samples dropped to around 430 Pa.s in the linear region. The decline in complex viscosity could be attributed to the excessive addition of additive, which causes disruption to the reaction process. The other important point is that, by adding 0.75% PA, the shear thinning behavior of the material improved significantly, which is favorable for 3D printing. Shear thinning behavior enables the material to flow more readily under the high shear rates encountered during extrusion through the printer nozzle, promoting smoother extrusion and deposition. In addition, shear-thinning materials typically regain viscosity after deposition, which aids in preserving the shape and structural integrity of the deposited layers, thus promoting suitable interlayer adhesion. The PA additive which is used falls under the category of anhydride additives. This additive functions by connecting polymer chains together, thereby creating longer branches that significantly increase the viscosity of the material.
3.4 Mechanical properties
Table 3 and Figs. 6, 7 represent the mechanical properties of samples containing varying PA concentrations (0.5%, 0.75%, and 1%). The ultimate tensile strength (UTS) values for all modified formulations display no significant difference, averaging 51 MPa. Comparative analysis with existing literature on 3D-printed recycled PET (r-PET), reveals UTS values ranging from 39 to 70 MPa. This disparity can be attributed to various production methodologies, including the use of twin-screw extruders or batch mixers, divergent mixing temperatures, and variations in recycled PET sources [23]. Regarding elastic modulus and elongation at break, samples with 1% and 0.75% PA exhibited diminished values compared to r-PET/0.5%PA, a trend validated by differential scanning calorimetry findings (Fig. 3b). These samples indicated higher degrees of crystallinity (27.3% and 28.27%, respectively), resulting in higher elastic modulus and reduced elongation at break. Conversely, the sample containing 0.5% PA displayed elevated elongation at break, attributable to a lower degree of crystallinity (19%). This underscores PA's role as a nucleating agent, enhancing crystallinity and consequently improving mechanical properties. In addition, in the previous studies by Zader et al., the printed r-PET ultimate tensile strength was 35 MPa, which is 15 MPa lower compared to our modified r-PET [25]. Crucially, when compared with widely used commercial feedstocks such as acrylonitrile butadiene styrene (ABS), polylactic acid (PLA), and polyethylene terephthalate glycol (PETG), which exhibit average tensile strengths of 43 MPa, 59 MPa, and 53 MPa, respectively, it becomes evident that recycled PET modified by PA in this work presents a promising eco-friendly alternative for commercial feedstocks in 3D printing applications. This is noteworthy even though the PET used in this research is a recycled grade and typically would have lower properties compared to the mentioned virgin plastics [33].
As a proof of concept for this work, a centrifugal pump was 3D printed (FDM) using a selected formulation (r-PET/0.75 PA) that provided optimized properties. The successful printing of stable and fairly complex part shapes in this research showcases the effective use of our proprietary additive in recycled PET, making it suitable for 3D printing and enabling the successful reprocessing and remanufacturing of waste plastic.
4 Conclusion
This study aims to address the pressing issue of plastic waste management by exploring sustainable solutions within the framework of the Circular Economy (CE). In this study, the primary objective was to enhance the processability of recycled PET (r-PET), whose properties had deteriorated due to the recycling process, to make it suitable for 3D printing. This was achieved through the utilization of a proprietary additive (PA), to modify the recycled PET for 3D printing. Key findings revealed that:
-
The optimal concentration of 0.75% PA significantly improved the complex viscosity of modified r-PET from 200 Pa·s to 1000 Pa·s, facilitating extrusion in FDM 3D printing.
-
The introduction of PA led to beneficial changes in the thermal, crystallinity, and mechanical properties of r-PET. Differential scanning calorimetry showed a crystallinity of 27.3% and a crystallization temperature of 187.4 °C for samples with 0.75% PA. Fourier Transform Infrared Spectroscopy confirmed successful chemical modification, resulting in a branched structure that enhanced mechanical properties. Rheological assessments revealed a substantial increase in storage modulus, essential for stable filament production.
-
As for mechanical properties, samples with 0.75% PA exhibited an ultimate tensile strength (UTS) of 51 MPa, while elongation at break was reduced by 75%, indicating improved structural integrity. The elastic modulus also increased with PA concentration, reaching 680.26 ± 21.31 MPa. The modified r-PET demonstrated tensile strengths comparable to conventional virgin materials like ABS, PLA, and PETG, despite using recycled grade PET. These findings support the potential of modified r-PET as a viable material for various applications in additive manufacturing, contributing to sustainable plastic waste management.
Future research should focus on optimizing the mechanical properties of r-PET to enhance its strength and uniformity, making it suitable for a broader range of applications. Experiments could explore different additives, compatibilizers, or processing approaches, such as using a twin-screw extruder to prepare feedstock for 3D printing. A detailed analysis of the material's impact, bending, and compressive strengths could reveal additional uses, like as a core for sandwich panels. Modeling efforts could simulate r-PET performance in real-world applications, such as load-bearing structures and stress analysis, guiding the development of new formulations. Based on the investigated properties, r-PET is suitable for producing plastic parts in the automotive and aerospace industries, including brackets, structural support, panel components, and as an affordable material for printing prototypes in research studies. In addition, it can be used for printing container and packages with specific geometry as well.
References
Yuan Z, Nag R, Cummins E (2022) Human health concerns regarding microplastics in the aquatic environment: from marine to food systems. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.153730
Van de Voorde B, Katalagarianakis A, Huysman S, Toncheva A, Raquez JM, Duretek I, Holzer C, Cardon L, Bernaerts KV, Van Hemelrijck D, Pyl L, Van Vlierberghe S (2022) Effect of extrusion and fused filament fabrication processing parameters of recycled poly(ethylene terephthalate) on the crystallinity and mechanical properties. Addit Manuf. https://doi.org/10.1016/j.addma.2021.102518
Boschi A, Scieuzo C, Salvia R, Arias CF, Perez RP, Bertocchini F, Falabella P (2023) Beyond microbial biodegradation: plastic degradation by galleria mellonella. J Polym Environ. https://doi.org/10.1007/s10924-023-03084-6
Hák T, Janoušková S, Moldan B (2016) Sustainable development goals: a need for relevant indicators. Ecol Indic 60:565–573. https://doi.org/10.1016/j.ecolind.2015.08.003
Gracida-Alvarez UR, Xu H, Benavides PT, Wang M, Hawkins TR (2023) Circular economy sustainability analysis framework for plastics: application for poly(ethylene Terephthalate) (PET). ACS Sustain Chem Eng 11:514–524. https://doi.org/10.1021/acssuschemeng.2c04626
Tariq A, Afzal A, Rashid IA, Shakir MF (2020) Study of thermal, morphological, barrier and viscoelastic properties of PP grafted with maleic anhydride (PP-g-MAH) and PET blends. J Polym Res 27:309. https://doi.org/10.1007/s10965-020-02291-2
Burgos Pintos P, Sanz de León A, Molina SI (2024) Large format additive manufacturing of polyethylene terephthalate (PET) by material extrusion. Addit Manuf. https://doi.org/10.1016/j.addma.2023.103908
A Subramaniam, S Sethuraman (2014) Biomedical applications of nondegradable polymers, in: Natural and Synthetic Biomedical Polymers, Elsevier pp. 301–308
TE Long, J Scheirs (2005), Modern polyesters: chemistry and technology of polyesters and copolyesters, John Wiley & Sons
Dahmen KG, Maurin N, Richter HA, Mittermayer CH (1997) Screening of biomedical polymer biocompatibility in NMRI-mice peritoneal cavity: a comparison between ultra-high-molecular-weight polyethylene (UHMW-PE) and polyethyleneterephthalate (PET). J Mater Sci Mater Med 8:239–245
A Kaushik, U Punia, S Gahletia, RK Garg, D Chhabra (2023) Identification and Overcoming Key Challenges in the 3D Printing Revolution, in: 3D Printing and Sustainable Product Development, CRC Press pp. 87–104
Kaushik A, Garg RK (2023) Tapping the potential of rapid prototyping techniques in creating a paradigm shift in the fabrication of occlusal splints. Rapid Prototyp J 29:2176–2192. https://doi.org/10.1108/RPJ-12-2022-0412
Penumakala PK, Santo J, Thomas A (2020) A critical review on the fused deposition modeling of thermoplastic polymer composites. Compos B Eng. https://doi.org/10.1016/j.compositesb.2020.108336
Dudek P (2013) FDM 3D printing technology in manufacturing composite elements. Arch Metall Mater 58:1415–1418
M Ahmadi, D Rahmatabadi, A Karimi, MHA Koohpayeh, R Hashemi (2023) The Role of Additive Manufacturing in the Age of Sustainable Manufacturing 4.0 BT - Sustainable Manufacturing in Industry 4.0: Pathways and Practices, in: H. Gholami, G. Abdul-Nour, S. Sharif, D. Streimikiene (Eds.), Springer Nature Singapore, Singapore, pp. 57–78. https://doi.org/10.1007/978-981-19-7218-8_4.
N Karimi, SAA Bozorgnia Tabary, H Fayazfar (2024) In‐depth investigation and industry plan for enhancing surface finishing of 3D printed polymer composite components: A critical review, J Appl Polym Sci e55494
A Sharma, PS Bharti, A Kaushik, U Punia, RK Garg, M Yadav, D Chhabra, A Bansal (2023) 3D Printable titanium alloys and their properties in biomedical applications: state of the art, Mechanical Properties and Characterization of Additively Manufactured Materials 107–120
Zander NE, Gillan M, Burckhard Z, Gardea F (2019) Recycled polypropylene blends as novel 3D printing materials. Addit Manuf 25:122–130
Oussai A, Bártfai Z, Kátai L (2021) Development of 3D printing raw materials from plastic waste, a case study on recycled polyethylene terephthalate. Appl Sci 11:7338
Vaucher J, Demongeot A, Michaud V, Leterrier Y (2022) Recycling of bottle grade PET: influence of HDPE contamination on the microstructure and mechanical performance of 3D printed parts. Polymers (Basel) 14:5507
Singh R, Singh BP, Singh AP, Kumar V, Kumar R, Bodaghi M, Serjouei A, Wei Y (2022) On 3D printing of low-cost sensors using recycled PET. Sādhanā 47:260
C Suescun Gonzalez, FA Cruz Sanchez, H Boudaoud, C Nouvel, JM Pearce (2024) Multi‐material distributed recycling via material extrusion: recycled high density polyethylene and poly (ethylene terephthalate) mixture, Polym Eng Sci
Exconde MKJE, Co JAA, Manapat JZ, Magdaluyo ER Jr (2019) Materials selection of 3D printing filament and utilization of recycled polyethylene terephthalate (PET) in a redesigned breadboard. Procedia CIRP 84:28–32
Sadeghi B, Marfavi Y, AliAkbari R, Kowsari E, Borbor Ajdari F, Ramakrishna S (2021) Recent studies on recycled PET fibers: Production and applications: a review. Mater Circ Econ 3:1–18
Zander NE, Gillan M, Lambeth RH (2018) Recycled polyethylene terephthalate as a new FFF feedstock material. Addit Manuf 21:174–182. https://doi.org/10.1016/j.addma.2018.03.007
Van De Voorde B, Katalagarianakis A, Huysman S, Toncheva A, Raquez J, Duretek I, Holzer C, Cardon L, Bernaerts KV, Van Hemelrijck D, Pyl L, Van Vlierberghe S (2022) Effect of extrusion and fused filament fabrication processing parameters of recycled poly ( ethylene terephthalate ) on the crystallinity and mechanical properties. Addit Manuf. https://doi.org/10.1016/j.addma.2021.102518
B Demirel, A Yaraş, H Elcicek (2011) Crystallization behavior of PET materials
Badía JD, Vilaplana F, Karlsson S, Ribes-Greus A (2009) Thermal analysis as a quality tool for assessing the influence of thermo-mechanical degradation on recycled poly (ethylene terephthalate). Polym Test 28:169–175
Achilias DS (2022) Thermal analysis in polymer recycling. Therm Anal Poly Mater Methods Develop 2:485–508
Ioakeimidis C, Fotopoulou KN, Karapanagioti HK, Geraga M, Zeri C, Papathanassiou E, Galgani F, Papatheodorou G (2016) The degradation potential of PET bottles in the marine environment: An ATR-FTIR based approach. Sci Rep 6:23501
Ghosh A (2021) Performance modifying techniques for recycled thermoplastics. Resour Conserv Recycl 175:105887
Bartolome L, Imran M, Cho BG, Al-Masry WA, Kim DH (2012) Recent developments in the chemical recycling of PET. Mater Recyc Trends Persp 406:576–596
All About ABS 3D Printing Filament: Materials, Properties, Definition | Xometry, (n.d.). https://www.xometry.com/resources/3d-printing/abs-3d-printing-filament/ (accessed May 6, 2024)
Acknowledgements
The financial support of NSERC and Ontario Tech Start-up grants is greatly appreciated. Special thanks to Dr. Michael Thompson for allowing us to utilize his laboratory at McMaster University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tabary, S.A.A.B., Fayazfar, H.R. Circular economy solutions for plastic waste: improving rheological properties of recycled polyethylene terephthalate (r-PET) for direct 3D printing. Prog Addit Manuf (2024). https://doi.org/10.1007/s40964-024-00784-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40964-024-00784-w