Abstract
The trend of growth and aging of population worldwide will pose new challenges in health care, which will require faster solutions addressed to specific pacient needs. In this regard, additive manufacturing (AM) is a group of promising technologies capable of delivering custom biomedical parts of high complexity in reduced lead time. Although it has emerged commercially in the 1980s as a rapid prototyping and modeling technique, it is now applied to production of a wide range of shapes with various possible materials. In this work, the technological aspects of each type of AM process were reviewed according to their advantages, limitations and potential or current applications for the production of medical devices. Direct comparisons of resolution, price and printing speed made possible to identify the most important niche for each AM process in health care sciences. In one hand, the many variables involved make these processes difficult to model and control, but in the other hand, they allow fine tuning of the microstructure to produce purposeful anisotropy, porosity and varying chemical composition, which may be desired in many medical devices. In addition, since the AM technologies have different working principles and feedstock requirements, the historic concept and classification of biomaterials were also assessed in view of their application for tissue engineering, implantable devices and surgery equipment among other uses. The discussion of materials and manufacturing methods was based on several research works and commercial products, which show a extremely fast developing field with a broad range of current and future possibilities in terms of biomedical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Facing the speed with which the world’s population ages is a major challenge for many countries. Besides an increase in the world’s population of almost 8 billion today to over 10 billion expected by 2050, life expectancy has been consistently increasing, from 65 years in 1990 to 73 years in 2019 and possibly 77 years in 2050, as shown in Fig. 1. The elderly population (above 64 years old) is about 700 million at present, however, it could increase to more than 2 billion before the end of this century according to UN estimations [1].
Average life expectancy at birth by of world population since 1950 [1]
Such an evolution is being noticed as a result of recent advances in medicine. There is a growing concern about the diet and comfort produced by the typical “modern life”. Consequently, there is an increase in the older population, leading to the onset of diseases related to old age, obesity, and lack of physical activity [2]. With this average increase in population age, some factors are gaining importance in the human lifestyle, namely the musculoskeletal condition and osteoporosis, causing devastating effects. This further gives rise to the need of extensive research in this area to improve the quality of human life [3]. Table 1 shows the distribution of musculoskeletal problems as a function of age.
The latest projections revealed that one of the main factors causing problems in the musculoskeletal system was obesity. In 2005, 400 million obese inhabitants were considered globally, out of which 20 million were children under 5 years [4]. By 2018, one in eight adults in the world were found to be obese. Treatments for musculoskeletal system problems are extremely costly, representing an average 3% of gross domestic product (GDP) in more developed countries [1].
Another condition that is closely related to the modern lifestyle, aging, and numerous systemic complications is the diagnosed diseases, such as diabetes. About 415 million people were estimated to have this disease in 2017 with a projection of it reaching 642 million people by 2040. In addition, this infirmity represents the leading cause of hospitalization and increases the risk of amputation in diabetic patients by more than tenfold [5]. Fractures and diseases related to the musculoskeletal system are the main causes of death in the first 38 years of life. Further, they become responsible for a greater reduction in the productive years when compared to heart disease and cancer, altogether.
The most important factor that distinguishes a biomaterial from any other material is its ability to be in contact with human body tissues without causing any harm to the body. Most of the “materials for use in health” are classified as biomaterials and used for the manufacturing of: prostheses, lenses, grafts, stents, catheters, extracorporeal circulation tubes, tissue engineering frameworks, dental implants, orthopedic screws etc. In recent years, biomaterials have found their immense applications in the fields of joint and limb replacement, eye implants, artificial arteries, and skin surgery as well [6]. Therefore, the durability and comfort of human life can be considerably enhanced with the application of biomaterials.
Numerous challenges can be faced when performing the implantation of intracorporeal prosthesis. Some of the major issues inherent to implant placement are the difficulties in positioning and aligning the prosthesis and also the selection of model according to patient body and structure, as stated by Ranawat [7]. Moreover, improper implant design can lead to implant deterioration followed by its failure. This may further require a secondary surgery, as in the case of premature wear or stress induced bone remodeling, two of the most common failure causes of total arthroplasties [8]. Thus, there is a growing interest in developing personalized implants which can promise a comfortable life to the patient.
The need of fabrication of 3D anatomical models rises from the possibility of visualizing an anatomical replica of the patient, which further allows the evaluation and simulation of surgical techniques. Figure 2 presents a methodology for manufacturing prostheses in patients with some type of disease. This structure consists of: (1) acquiring two-dimensional medical images by CT (Computed Axial Tomography) or NMR (Nuclear Magnetic Resonance), (2) transforming medical images into three-dimensional virtual models; (3) modeling the virtual prosthesis and 3D CAD fixation systems, (4) fabricating the model (prosthesis and fixation systems) by AM technologies, and (5) fabricating the prostheses with biocompatible materials.

Adapted from Devgan and Sidhu [6]
Methodology for manufacturing anatomical models.
In certain cases, it may become essential for the medical team to plan the surgery with the use of a biomodel, since this allows the palpable verification of the area which has to be operated and replaced by the implant. The medical practitioners involved can also manipulate the physical object and perform surgery simulations, handling all surgical instruments and the implant itself. They can also make prosthesis connections with the region of interest to be replaced in the human anatomy (biomodel) [9]. Therefore, the development of highly biocompatible materials is appearing to be a greater need of the hour so as to improve reliability and reduce the risk of rejection by the human body.
Advanced manufacturing technologies are constantly being explored for the processing of biomaterials, mostly to reduce costs and facilitate customization as reviewed by Culmone et al. [10], and also to minimize inventory and maximize performance. These technologies are capable of producing single-component design and even structures with increasingly complex geometries [11]. Moreover, anatomical models such as the liver (as shown in Fig. 3), can be directly produced with the aid of various examination of the patient. By creating textures ranging from hard bones to soft tissues, healthcare professionals can plan, practice, and determine therapeutic approaches or surgical techniques, which are already in use for planning complex heart surgeries [12, 13].
Liver produced by additive manufacturing [17]
2 Additive manufacturing: evolution and concept
In the late 80s, some manufacturing technologies initially used for prototype manufacturing, were known as Rapid Prototyping (RP) [14]. Prototypes that took days, weeks or even months to develop, due to the high number of steps or phases, were performed within a few hours by RP. However, in the last 2 decades, advances in manufacturing have presented another possibility, known as three-dimensional printing or additive manufacturing (AM). It is noteworthy that despite being a technology on the rise in conjunction with Industry 4.0, additive manufacturing is not a new conecept. According to Zhou et al. [15], AM technologies have actually migrated from the Rapid Prototyping (RP) process to a direct digital manufacturing solution, applied to the production of final goods and not just prototypes. According to Miller [16], the industry began to use RP in prototype development, but the cost of equipment and materials as well as limited applications prevented the access for several companies in the 1980s. In the 1990s, the Wake Forest Institute for Regenerative Medicine in the United States used additive manufacturing to print structures in three dimensions for reproducing the human organs. In the 2000s, AM brought a revolution in the area of development of prostheses for human body. This technique allowed manufacturing industries to develop complex shapes and structures more efficiently that are often difficult to be developed with traditional injection molding or machining methods, as in the parts showed in Fig. 4. There is also less waste, resulting in shorter setup times and lower material costs [17]. These advantages could be so accentuated that even in traditional segments such as construction, there have been many researches exploring AM capabilities and possible applications, as reviewed by Paolini et al. [18].
In general, additive manufacturing technologies have as their basic operating principle of generation of three-dimensional (3D) objects through the process of adding material in a layer-by-layer fashion [19]. In the early stages of product development the mechanical characteristics, in many cases, may resemble with those of traditional processes. On the other hand, in some cases, AM technologies allow the construction of much freer forms than traditional processes with mechanical strength close to conventional ones.
According to Jamróz et al. [20], during this same period, studies focused on the identification of new materials including polymers, that could be used in additive manufacturing. According to Karunakaran et al. [21], one of the major concerns that emerged between the 1980s and 2000s, was the inflexibility of equipment and materials for AM. This concern led to the emergence of studies that provide hybrid technologies for materials. The major studies between the late 1980s and the 2010s dealt with the aspects of materials and machines of AM. However, no studies related to the production systems of AM were observed. After 2010, studies began to be conducted on the advantages of applying additive manufacturing over traditional manufacturing processes. An example of these studies is the work presented by Ford [22] that sought to expose how AM would impact the US companies.
Therefore, in short, the history of additive manufacturing can be divided into four phases. The first phase which is prototyping, has a limited use mainly by academic institutes for prototyping, but with high costs for both equipment and materials. The second phase deals with the applications of additive manufacturing. The research sought to show all the possible areas where AM could be employed. In verifying that the applications were pertinent, the third phase of research turned to materials and equipment. The demand for equipment expansion in AM always faces a challenge due to the high cost and variety of materials to be used. The most recent phase of the studies is concerned with the possible replacement of so-called “traditional” manufacturing processes by additive manufacturing.
Over the years, many authors [23,24,25,26] basically used the American Society for Testing and Materials (ASTM) concept of Additive Manufacturing [19] in the literatures. However, Ford [22] proposed a concept that complements ASTM, which actually did not present additive manufacturing as a manufacturing process contrary to traditional manufacturing. According to Ford [22], additive manufacturing is a set of emerging technologies that manufacture three-dimensional objects directly from digital models through a material addition process. Frazier [27] defined additive manufacturing as: “process of joining materials to make objects from information in the three-dimensional model, usually layer after layer, as opposed to subtractive manufacturing methodologies”. This definition is applicable to all classes of materials, including metals, ceramics, polymers, composites, and biological systems. Huang et al. [28] defined AM as a material adhesion process for making objects from 3D model data, usually layer by layer. It is also known as rapid manufacturing and unlike subtractive manufacturing processes (material removal), AM achieves the final shape by adding materials.
Thus, the rising demand for AM processes in health areas is increasingly imminent [29]. This technology is progressively gaining visibility not only in various manufacturing industries, but also in all the areas of market, society, and health. In biomedical applications, AM includes engineers to assist the medical and health professionals for treating the patients with damaged tissues or fractured bones, mostly in the cases of patient-specific implants. That is the case of printed organs such as liver, skull, urether, and ribs, among others, some of which have already been successfully implanted [30]. An example of this can be seen in medical applications, usually focused on implants and prosthetics. In dentistry, the advent of digital radiography has enabled orofacial scanning of fractured patients in three dimensions. AM has been proven to be efficient enough of producing implants and prostheses which are precisely adapted to patients. In addition to providing faster availability of prostheses and implants, AM enables dental surgeons to execute precise and safe surgeries with the aid of precisely customized parts [31]. However, this application is giving a way to a new branch in health sector by organ transplantation. At the Wake Forest Institute for Regenerative Medicine Institute, additive manufacturing equipment are being developed to replicate and generate human tissues and organs to be transplanted into patients (Fig. 4b) [32].
In this paper, the authors attempted to analyze the application of AM in the world, the desired variations as required by the manufacturing industries, and the effect of the same on the competitiveness among world’s manufacturing industries.
3 Technological aspects of AM
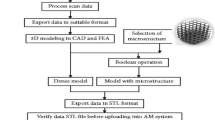
According to Huang et al. [28], additive manufacturing technology consists of three basic steps:
-
1.
A solid computerized 3D model developed and converted into a standard file with the traditional format and standard language (Fig. 5a).
-
2.
Exportation of this file to an AM equipment for its further manufacturing (Fig. 5b).
-
3.
Layer-to-layer construction in additive manufacturing equipment (Fig. 5c).
a Scan of the 3d model; b exploration of the file sent to equipment; c part manufactured by additive manufacturing [35]
According to Oliveira et al. [34], the classification of AM technologies is totally linked to three-dimensional printing equipment. Figure 6 presents the classification of AM technologies based on the state of the material to be used. Figure 7 shows schematic representations of the same processes, which are further discussed and compared.
Additive manufacturing technologies [34]
3.1 Material extrusion
The Fused Deposition Modeling (FDM) patent was granted on June 9, 1992, but the technique was previously described by Huang et al. [28]. This process is also called fused filament fabrication (FFF) and uses a thermoplastic and a heating chamber to liquify the polymer. Material deposition occurs through an extruder head that moves along the x and y axes by adding material filaments. After finishing a layer, the deposition platform moves down in z direction to build the next layer, and so on until the part is finished, as represented in Fig. 7a. The main variables are the temperature of the head and of the table, the scanning speed and path, and the wire speed.
Over the past few years, significant progress has been made in the finally produced parts. For example, nowadays many parts manufactured by FDM exhibit higher strength than the parts manufactured by classical processes of same material. This can be easily identified by comparing the yield stress values of Stratasys FDM thermoplastics material with those of molded materials. While the material values for FDM remain between 22 and 71 MPa, the equivalents for injection molds are between 20 and 60 MPa [42].
One of the main limitations is the resolution which depends on wire thickness, currently at the minimum of 0.127 mm [28]. In addition, the other limitation is the deposition rate which is very low compared to other AM technologies due to inertia of the printing table. Moreover, the process is limited to polymers, with thermoplastics among the most common.
Material extrusion is one of the oldest AM processes and its use for biomedical parts has been substantially researched. Its main application is the production of scaffolds with bioinert-resistant polymers such as PEEK [43], which can be combined with HA to induce cell attachment in bone repair. There are already several commercial applications of this technology for facial reconstruction [44] and joint pads in total arthroplasty, but full PEEK implants for more loaded joints such as knee and hip are not yet ready for commercial applications [45], although there have been many studies in this regard [46,47,48].
Biodegradble and porous scaffolds can also be extruded by employing PLA and PCL. Although their mechanical properties are usually lower, adjusting process parameters can help to minimize this drawback. de Ciurana et al. [49] showed that different FDM deposition paths can be chosen to tailor porosity and achieve a good combination between strenght and cell growth in the interconected pores [50]. This balance can be further improved using especial geometries [51] and topological optimization [52]. Waris et al. [41] produced porous biodegradable scaffolds to promote fibrous tissue growth in minipigs which could be useful for small human joints, such as thos in the fingers. Duan et al. [53] further filled the pores with stem cells and obtained faster osteochondral repair in rabbits. The convenient lower melting temperatures of the PCL wire also allow printing living cells with FDM technology as shown by Zheng et al. [54], who printed a goat meniscus within a hydrogel matrix rich in stem cells and connective tissue growth factors.
The major challenge in this regard is that there are still no degradable materials with the same resistance of high-performance polymers such as PEEK, however, composites containing ceramic nanoparticles and nanofibers could help to further improve resistance of resorbable polymers [55]. Moreover, the added material helps in the adhesion and proliferation of cells along the scaffolds, as in the case of bone cell growth induced by nanoHA crystals in PCL feedstock wire [56].
Other possible application is the construction of structure for in vitro studies of growth tissue. Rabionet et al. [57] studied the selection of printing parameters for FDM scaffolds used in cancer cell cultures. It was shown that three-dimensional culture supports are more appropriate to mimic physiological behavior [58]. The use of material extrusion was also investigated towards the manufacturing of biodegradable stents. Guerra et al. [59] combined FFF with a cylindrical spinning base to produce PCL stents and found that nozzle temperature, printing speed, and path were the most important factors for dimensional accuracy [60]. Moreover, it was discovered that an increase in these parameters could accelerate the degradation rate of the polymer in the body [61]. Furthermore, the drug release capability has stimulated commercialization of bioresorbable stents produced by FDM and other traditional technologies, such as laser cutting in human surgeries. However, while there is an advantage of these materials in avoiding in-stent restenosis compared to conventional nitinol stents [62], their development has been hampered by the lack of randomized clinical data guaranteeing its efficacy. The reason is that the parts could degrade within as little as 2 weeks. Currently, there is no unanimity among the medical professionals regarding the superiority of these biomaterials. However, their use is expected to increase once their mechanical and chemical properties are improved.
3.2 Stereolithography (SLA)
Stereolithography (SLA) is one of the most widespread additive manufacturing technologies, initially marketed by 3D Systems. Although the validation of its functional principle has been published by Kodama [63], SLA technology was initially pioneered by the founder of 3D Systems only in 1984 [64]. In addition, it is most widely used in relation to other additive manufacturing techniques. It is also known as photo-polymerization of liquid resin, which gets solidified as a result of electromagnetic irradiation.
The functional principle of this process is the localized curing of photosensitive resin by UV laser beam moving along the X and Y axes. It is possible to use other sources that promote polymerization, such as electron beam (EB), radiation, high-energy particle beam, X-ray, UV light beam, and conventional UV light [65]. The beam focuses on a resin-immersed container to construct the silhouette of the previous computationally calculated layer. Upon completion of each layer, a material support platform moves down along the Z axis to begin construction of the new layer, as represented in Fig. 7b. This process is repeated until the part is finished. Afterwards the platform is raised allowing the unpolymerized resin to drain [37]. The polymerization of the manufactured part is between 80 and 90% [65]. This further implies the completion of post-processing to finish the polymerization and increase mechanical strength. In this stage, the finished part is kept in an oven with a maintained UV light emission. Moreover, the removal of support material is also part of post-processing [66].
The materials typically used by this process are based on acrylates and epoxies, which provide suitable mechanical strength for manufacturing functional parts. Moreover, mechanical strength can be quantified and expressed as yield stress ranging from from 28 to 78 MPa [67]. With regard to the accuracy of this technology, layer resolution of up to 50 μm are currently found, while values about 25 μm are usual for sweeping accuracy. In this case, the construction speed of objects can reach up to 35 m/s. However, there are specific cases, such as microsterolithography, whose values of layer resolution (z) and scan accuracy (x–y) can reach upto 0.1 and 0.25 μm, respectively [68], although the printing speed becomes severely smaller.
The main advantage of the stereolithography process is the production time, which is shorter than FDM due to higher speed of laser scanning. Disadvantages include the size of the product which is limited to small dimensions (approximately the size of a 2-foot cube). Another disadvantage is the cost, as the photopolymer costs between $300 and $500, in addition to the value of the equipment itself. The materials used in SLA are even more limited compared to FDM, because not all thermoplastics can be easily processed from photocuring resin [28].
SLA is used in biomedical applications for production of scaffolds, with many works combining calcium phosphates dispersed in the resin to create porous ceramics for bone repair [69]. Zhou et al. [70] produced spine-shaped bodies from a mix of powdered β-TCP, resin, and dispersant. About 14% porosity and average grain size of 0.7 μm were obtained after sintering, reaching an adequate structure as bone scaffolds. Having allied to the better resolution of SLA, these characteristics are superior than other AM processes used for the same applications, such as SLM. However, the later may reach the same structure faster and without sintering, meaning a less expensive product. Pure polymeric scaffolds and models for surgery planning and didactic purposes are also a possible use of this technique, but FDM would be more advantageous due to versatility and price.
The most interesting feature of SLA is in the biofabrication of scaffolds containing living tissue and growth factors or drug delivery systems. Pereira et al. [71] explained how cells, proteins, and pharmaceutical components can be dispersed in hydrogels, which are crosslinked hydrophilic solid polymers that present physiochemical characteristics similar to soft tissues. Chartrain et al. [72] showed that microfeatures ranging from 5 to 250 µm can be produced by µSL, which would be ideal to produce vascular networks for tissue engineering. However, the current fast curing resins necessary to attain good resolutions do not exhibit good cell biocompatibility. Actually, most common feedstock material that possesses adequate mechanical resistance is not biocompatible according to Szymczyk-Ziółkowska et al. [73], and this limits the application to hard tissue. But the development of new resins and incorporation of microfibers might overcome this challenge. For instance, Kim et al. [74] designed a photocurable bioink mixed with silk fibers that have similar properties to cartilage and could be used to build complex organ structures, such as heart and vessels.
3.3 Powder bed fusion
This technology consists of the construction of 3D objects by melting or sintering material powder. The construction of the layers occurs by fusion of the metallic, ceramic or polymeric particulate through a high power energy source that scans the powder bed along the x–y axes. After completion of each layer, the construction platform moves along the z-axis and material is spread into the bed to form a new layer [39], as shown in Fig. 7d.
The two types of power sources are laser and electron beam, which have different process requirements and yield different part charactheristics for similar parameters. The first yields the commercial names Direct Metal Laser Melting (DMLM), Selective Laser Melting (SLM), and Selective Laser Sintering (SLS), whereas the second is known as Electron Beam Melting (EBM). In both cases, the powder bed may be pre-heated by scanning it between layers to minimize thermal distortion and facilitate fusion with the previous layer [28].
Laser-based equipment can achieve better resolution since the spot can be focused to a smaller diameter. This allows the production of very thin walls with tight dimensional tolerances. EBM on the other hand depends on electrons accelerated by voltage ranging from 30 to 60 kV [75]. It usually presents higher power output and power conversion efficiency compared to laser, producing a larger melt pool that allows it to produce high volume parts faster than the former process [76]. This process demands an expensive high vaccumm chamber to avoid dispersion of the electrons [39], however, it allows processing of highly reactive materials as in the case of pre-alloyed powder metals [77]. The scanning speed of about 1000 m/s achieved by electronically controlled solenoids is much larger than the 10 m/s of laser, which is mechanically controlled by moving mirrors. The later occurs under inert gas atmosphere to avoid oxidation of the melt pool and powder, which also helps to cool down the chamber and part [29]. This represents a better control of the melt pool for laser which helps to reduce surface roughness [78].
Both processes depend on many variables such as powder bed compactation, power and size of the beam [79], scanning path and speed [80], powder size distribution and chemical composition, and cooling rate of the melting pool. Due to the large number of parameters, there is still a lot of variation in the final characteristics of the parts. Achievable surface finish is not as good as stereolithography technology [64] and porosity level (according to process parameters and particulate material properties) can vary between 50 and 90% of the volume of the final object, with better results for EBM. On this account, the mechanical properties are noticeably varied, with yield stress ranging between 5.5 and 90 MPa [81]. This can be corrected by the use of hot isostatic pressing (HIP), but this process is also very expensive.
The advantages of these processes are: best resolution and tolerances for metal products among the AM processes; wide range of materials that can be processed, which include mainly metals and ceramics, enabling even the mixing of different powders. Nevertheless, the use of EBM requires power bed conductivity [82], of course. It is important to highlight that the most equipment manufacturers today also supply the recommended feedstock, and the lack of an established market hinders the autonomy of the buyer.
Some of the major drawbacks of EBM are the price of equipment and low productivity [83]. In addition, an important drawback of powder-based techonologies are the environmental and health risks [84]. The high surface to volume ratio makes it extremely flammable, requiring especial procedures for storing and transporting the material [85]. Besides, the fine metal particles are prone to get airborne and exposed operators could develop inflammations in the respiratory tract [86]. The high cost of the powders is also another concern because they are manufactured by energy intensive methods, such as gas or plasma atomization [87].
SLM and EBM have been widely employed to manufacture metal and metal-ceramic implants for hard tissue replacement, for which Dallago et al. [88] showed that adjusting process parameters to control dimensional errors is one of the main goals in this process. Many commercial implants are already manufactured by SLM, SLS, and EBM, such as acetabular components for hip arthroplasty [89], dental abutments [90], and knee implants [91], in which porosity proved to be beneficial for osseointegration. Furthermore, Zheng et al. [54] studied the influence of final porosity in the tendon growth along Ti–6Al–4V scaffolds during in vivo tests in rabbits and found that there is an ideal pore size which can be obtained according to laser scan speeds and powder size. These particularities show that there is still a need to improve process predictability to guarantee quality in customized products, because each one might require different printing parameters. It is expected that the improvement in FEM simulations might overcome this difficulty in the forthcoming years [92].
Another area of interest is the production of orthesis in patients with neurological diseases that affect muscle contraction of hand or fingers [93], in which the printed device acts as an exoskeletal apparatus. PBF processes have an advantage at producing metal parts tailored to patient morphology in this case, but cheaper conventional processes still have the advantage for the less complex components. SLM of engineering and biodegradable polymers has also been extensively studied, especially for bone-implant applications, whereas it is possible to combine HA powder to produce composite implants more favorable to osseointegration [94]. However, the lower mechanical properties of polymers compared to trustworthy alloys such as Ti–6Al–4V hinders their application in PBF techniques, especially when there are less expensive AM processes to work with polymers.
3.4 Directed energy deposition (DED)
DED processes work by directly depositing material in the form of powder or wire heated up to the melting temperature. The heat sources include laser and plasma arc in the same way used during welding operations. However, even the thinner wire feedstocks produce much larger melt pools than in the case of EBM and SLM.
The variation with better resolution and speed is Laser Engineered Net Shaping (LENS) with powder [28], in which the substrate is moved under the laser beam depositing a thin cross-section of material to create the desired geometry. Consecutive layers are deposited sequentially to build a 3D part, as shown in Fig. 7e. As an advantage, it can be used to repair old parts besides new ones, and good mechanical properties are easily achievable [28]. However, LENS technology requires some post-finishing processes to ensure better quality such as milling, turning, and polishing, for example. In addition, its geometric limitation is noteworthy for complex surfaces.
The main applications of this AM process in medical field is to produce titanium implants for orthopedic surgeries, being an alternative to PBF processes. Attar et al. [95] compared the mechanical properties of parts produced by LENS and SLM, and found the later yields better tensile resistance. This is due to the finer microstructure of titanium as result of the faster cooling rates in SLM, which was also better for corrosion and wear resistance. Nevertheless, these characteristics have still been found superior in comparison to titanium products manufactured by conventional methods such as casting or hot pressing [96]. A major concern is the fatigue strength as pointed by Harun et al. [97], due to the formation of microcracks between pores. DED processes are behind PBF for manufacturing biocomponents due to versatility and better tolerances and roughness achieved by SLM or EBM for instance. Therefore, the only advantage of LENS would be its easier ability to change between powder feedstock to produce composites with bioceramics. This has been done by Bandyopadhyay [98], and although the authors were more interested in the formation of a low friction tribofilm, this could prove most useful for incorporating HA progressively in the surface of the titanium implant within a single process, dispensing the traditional post-processing by plasma jetting.
3.5 3D binder jet printing (3DP)
The binder jet 3D printing technology is licensed by the Massachusetts Institute of Technology under the commercial name Prometal, and is based on the deposition of a binder onto a powder layer, generating a cluster. In this process, as shown in Fig. 7f, a dust-containing reservoir lifts a platform while a roller distributes over the workpiece construction platform. For layer generation, an inkjet head moves along x–y for printing the sticky material onto the dust layer. This process is called 3DP because of its similarity to the inkjet printing process that is used for two-dimensional paper printing. According to Huang et al. [28], the material is first stabilized by spraying with water droplets to avoid excessive disturbance when struck by the binder. In addition, an infiltration process may be performed during deposition, since the binder might not be enough to guarantee enough green resistance for handling. After the sequential application of layers, unbound dust is removed and the so-called ‘green’ part is subjected to debinding and sintering. At first, the temperature is kept at lower values, enough only to vaporize the binder, whereas later, a high temperature is used to promote diffusion and further strengthen the bonding of the material. In some cases, hot isostatic pressing is performed at post-processing to reduce porosity, which may further lead to yield stresses higher than 400 MPa.
This process can be applied to the production of metal, ceramics, and ceramic composites. As an advantage, it enables fast and low-cost material manufacturing. Regarding the deposition thickness, this technology provides layer thickness between 0.089 and 0.2 mm, while the resolution is between 600 × 540 DPI. In addition, it can be said that this overall accuracy is approximately 0.125 mm [28]. Just like in EBM and SLM, this process can be used with different powders within the same part to produce complex alloys, composites, and varying properties throughout the volume [64].
This AM technology is one of the fastest technologies in terms of printing time. However, there are some limitations, such as high porosity after sinterization, size limitation and too many post-processing stages. Although the consolidated inkjet technology lowers the cost of the printer, most sintering ovens and HIP equipment are quite expensive [28]. Just like in SLS and EBM, the powders require careful handling, and the many variables related to the binder and sintering further increase the complexity when adjusting material and process parameters [40].
The application of 3DP by binder jetting has been mostly applied to the production of metal and ceramic parts or scaffolds for bone and joint surgeries [99]. Most common materials for these purposes are Ti–6Al–4V alloy and calcium phosphates, but there has also been research with biodegradable iron alloys too [100]. Seidenstuecker et al. [101] produced composite scaffolds from bioglass and tricalcium phosphate which favored cell growth, but with poor mechanical resistance. Sun et al. [102] observed that the use of fine powders provide better resistance after sintering, but create more difficulty during printing because of low flowability in the powder bed. Very low resistance was obtained by de Melo et al. [103] in the production of TCP and silica scaffolds, but their results are still close to the lower strength of spongeous bone and the high porosity of 70% could be of benefit for replacing this tissue. On the other hand, Shao et al. [104] employed wollastonite containing magnesium and obtained flexural strength of 31 MPa, which is fairly close to that of human cortical bone, but at the cost of lower porosity. There is great variation in final mechanical properties since even the type of binder may interfere [105, 106].
Drug delivery systems are another major field for application of 3DP. Wu et al. incorporated antibiotics in polymeric bioresorbable bone implants and treated infected rabbits with success [107], but it is unlikely similar implants for larger animals would present adequate resistance. The drug release principle can be applied for subcutaneous implants and resorbable sutures aswell [108], which could increase in the next years to attend patient-specific needs. Pills and tablets can also be tailored to combine several pharmaceutical ingredients that are released in the body at different rates or in different organs of the digestive system [109], but most techniques are not yet fit for mass production at reasonable price.
The last application is the construction of 3D models for surgery planning and medical teaching purposes [110]. Kondo et al. [111] used this AM technology to produce transparent brain models showing colorful regions simulating tumors, taking advantage of the diverse color printing capability. Tai et al. also produced bone models with different volume composition to simulate resistance to drilling during surgery [112]. Although the production of composite models supersedes the other AM processes, the main goal for 3DP currently is to incorporate growth factors, stem cells or drug release capabilities within mechanical resistant parts [73], which has not been achieved yet [113].
3.6 Inkjet printing (IJP)
This technology consists of the use of inkjet printing to deposit thin layers of photopolymer (up to 16 microns) that are cured by heat or ultraviolet rays after each layer has finished printing [28]. The inkjet head moves on the “x” and “y” and after completion of each layer, the construction platform moves in z direction [114]. The inkjet technology was extensively developed for conventional 2D printing in paper using piezoelectric valves to deposit droplets of liquid material, and this same principle is employed in this AM technique, as displayed in Fig. 7c [38].
Materials typically used are based on acrylates, epoxes, thermoplastics and wax, which differ from each other in terms of the solidification process of these materials. This allows these technologies to be divided into two groups: photopolymerizable IJP and thermopolymerizable IJP. For example, the EDEN equipment, developed by Objet, is an IJP type of light-curing material and its inkjet head deposits material through 1536 individual nozzles arranged in line simultaneously in 15 μm layers. In this process the degree of polymerization of the final part is usually higher than SLA, and so the need for post-processing is not identified [115].
The main advantages of this process is its ability of producing parts with high degree of precision and surface finish at high resolution. The technology is well consolidated which lowers the price of equipment and feedstock. In addition, one great capability is to vary the ink source within the layers to produce colored parts, polymer composites, and seed living cells in polymeric scaffolds [116]. The disadvantages are the poor strength, durability of materials, and mechanical properties, besides the same limitations of SLA in terms of photocurable polymers. Products built on this technology are considered more fragile when compared to stereolithography and FDM.
The biomedical applications of IJP are very similar to some of binder jetting. The production of models for didactic purposes and surgery planning is a common trend [117], and there are studies for commercial application in fast production of dental trays [118] due to better geometric tolerances than SLA. There is usually a misconcept regarding IJP as an AM process for production of enhanced pharmaceutical pills and trablets [73]. The lack of UV light and photocurable resins in most reported cases [119] characterize the process as the binder jetting technique instead. Actually, the use of UV light can degrade the active principles in the pills or in other possible applications like bioprinting, and therefore, this process is fairly limited in medical field. However, it may prove more relevant in biosciences in the future for building lattice structures with low form errors [120] that could serve as ultra light structure for tissue engineering. As an example, Egan et al. [121] produced polymer beam-based lattices for bone fusion with spinal cage, presenting up to 213 MPa of elasticity modulus and 50% porosity.
3.7 Laminated object manufacturing (LOM)
This technology combines additive and subtractive techniques for building the layers made by adhesive laminate materials cut by laser [28], as displayed in Fig. 7g. Laser beam velocity and focus are adjusted so that the depth of cut corresponds exactly to the thickness of the layer, thus, avoiding damage to the underlying material.
A variety of materials may be used, including paper, metals, plastics, fabrics, synthetic materials and composites. LOM technology is cheap and can be automated to require little attention from an operator, making it easy to produce large parts. However, it does have some accuracy issues resulting in dimensional stability problems. It may generate some internal cavities that affect product quality. Besides that, postproduction time is required to eliminate waste and, in some cases, secondary processes are required to generate parts more accurately [122]. Disadvantages are related to material waste (resulting from the combination of additive and subtractive techniques), and the difficulty of producing complex internal cavities [28]. In addition, in the case of metals, the bonding has much lower resistance than the alloys itself, making it less attractive in comparions to the other AM processes.
Regarding biomedical applications, LOM is mostly restricted to manufacturing models for teaching and surgery planning [156], but mostly other methods are more suitable. One possible application that could develop in the future is the manufacturing of microporous scaffolds from ceramic materials. Zhang et al. [123] were able to produce alumina scaffolds with regular pores of 80 μm and 50% overall porosity. Although these could be useful as membranes, their stacking in LOM would have the same resistance issues typical from this AM process.
3.8 Summary and comparison of AM processes
Each additive manufacturing process provides unique and interesting features. Thus, through the comparative analysis among these technologies, it can become possible to identify the prime advantages and disadvantages of each of these processes. Table 2 summarizes the advantages and disadvantages of AM techniques presented in this text, and Table 3 presents a survey of the leading technologies marketed in Europe, showing cost and resolution ranges.
In addition, it can be observed that despite the high resolution, these technologies are subjected to process variations, resulting in dimensional distortions in the final product. This analysis can be clearly seen in Fig. 8, where the warping of a part manufactured by FDM at the end of the manufacturing process is presented [127]. In the study performed by Domingos et al. [56], it was possible to identify dimensional differences provided by the 4 most widespread AM processes today. The summary of the main results found was statistically discussed. In Table 4, this analysis is presented, where stereolithography is indicated as the most accurate process among the studied.
Bending of part manufactured by FDM process [127]
4 Biomaterials: evolution and application in manufacturing of biomedical devices
Biomaterials comprise a representative fraction of the products used in healthcare. Biomedical devices (such as biosensors, blood circulation tubes, hemodialysis systems etc.), implantable materials (such as sutures, plaques, bone substitutes, screens or meshes, heart valves, lenses, and teeth), drug delivery devices (in the form of films, subdermal implants, and particles), and artificial organs (such as heart, kidney, liver, pancreas, lungs, and skin) are some of the examples of healthcare products made up of various biomaterials. According to Paital and Dahotre [128], biomaterials are metallic, ceramic, polymeric or composite materials designed to function adequately in a bioenvironment. They are also used to replace or repair damaged structures, diseased or damaged tissues, and “diseased” organs.
The field of biomaterials gained due recognition after the first meeting on this subject at Clemson University, South Carolina in 1969 [128]. In 1974, at the request of the World Health Organization (WHO), the term “biomaterial” was defined as “a systemically and pharmacologically inert substance designed for implantation or incorporation into living tissue”, while it was later defined as “a nonviable material used in a medical device intended to interact with biological systems” in the 1986 Conference of the European Society for Biomaterials, held in England. Due to the wide range of products that emerged or are under research, the definition had to evolve. According to Bose et al. [11], “biomaterial” can be used to design the functional restoration of different tissues to improve human health and quality of life, whether natural or synthetic.
The use of these materials is not recent, and their application in correcting the most diverse types of problems related to human health goes back to antiquity [129]. For example, there are records of the use of flax and gold sutures in ancient Egypt (2000 BC), cat intestines in Europe during the middle ages, artificial teeth made by Mayan shells (600 BC), iron by the French (200 BC), gold and wood by the Romans, Chinese, and Aztecs. Bone substitutes made of wood were also found in ancient Egypt and Europe in the middle ages, and efficient osseointegration was observed. There are also records of biomaterials in the early twentieth century as an application of wound healing.
The characterization of a biomaterial does not enable its use as a biocomponent, but can and should be used as a pre-selection of conditions to be tested in the following steps. Materials “approved” at this stage will have to undergo laboratory tests in cell culture (in vitro tests) and then in vivo (animal) tests, and finally clinical tests. However, in this sequence, the tests become increasingly expensive and complex. Therefore, the same should be restricted to the fewest possible conditions. Zhou et al. [15] defined some criteria that a material must meet to be identified as a biomaterial:
-
The material must be biocompatible, i.e., its presence should not cause short or long-term damage to the implant site or the immune system.
-
Tissues should not cause degradation of implanted material, such as corrosion to metals unless tolerable.
-
Material must be biofunctional, i.e., it must have the proper characteristics to fulfill the desired function (static or dynamic) for the desired period of time.
-
The material must be sterilizable.
Usages and sale of biocomponents in each country is supervised by the pertinent regulatory agency(s). In the United States, the product must be approved by FDA before market introduction, who has already discussed and published that AM products must undertake the same regulatory pathways of the ones produced by other methods. As discussed by Di Prima et al. [130], the FDA certification is granted to products with specified intended uses, manufactured by determined methods and equipment, and not a material or technology with unspecified application. Nevertheless, the certification process can be simplified if it is proved the AM product is capable of fulfilling the functions of an already marketed similar item, which means a new model or adaptation of AM biocomponents might be more easily approved if the same material and technology have already been approved for a similar item.
According to Paital and Dahotre [128], surgical procedures with the application of biomaterials were not very successful, since they caused infections after surgery. The discovery of an aseptic surgical technique, developed by Joseph Lister in the 1960s, caused the turning point in the application of biomaterials. The first successful cases of orthopedic implant surgical applications were related to skeletal system corrections. They consisted of the application of bone fracture fixation plates, but at the given mechanical design deficiencies, these plates broke very easily due to their very thin thickness and flat geometry (they had straight corners, which are susceptible to stress concentration). Similarly, the use of materials such as vanadium steel (selected because of improved mechanical properties) had opposite consequences to the desired results. It got quickly eroded, subsequently, causing adverse effects on rehabilitation processes [131].
However, in the 1930s, with the introduction of stainless steels and cobalt–chromium (CoCr) alloys, there was an increase in successful cases of fracture fixation, which led to the first interventions to replace joints. Table 5 describes some of the most important developments at implant level chronologically.
In the beginning, bioinert materials (focus on the material itself) were sought. Over the time, the goal became the bioactivity of biomaterials. Recently, the goal has become the regeneration of a functional tissue indeed, with the focus on the biological aspect [129]. In other words, initially, the objective was to obtain biocompatible materials that could replace a damaged tissue and provide mechanical support, with minimal biological response of the patient. Over the years, attempts were made to increase the life of the implants by their interaction with the host tissue interface. Later, the focus became the development of biodegradable materials capable of being incorporated or absorbed (after dissolution). In recent years, biomimetic concept came into notice, looking for actively participating materials in the recovery process. It acts on the tissue specifically with stimulation at the cellular level.
Figure 9 shows the evolution in the development and use of biomaterials. It is possible to observe that the materials used for clinical purposes are mostly from biocompatible, bioactive, and biodegradable categories. However, the most researched are those which fall in the category of bioactive, biodegradable, and biomimetic materials.
Evolution of functionality and regenerative capacity of biomaterials throughout its development [111]
Biomaterials are used in the manufacturing of medical devices and can directly interact with the biological systems [131]. Table 6 presents the main application areas of biomaterials. The performance of biomaterials in relation to the human body can be classified depending on their perspective. In other words, it can be considered from the point of view of a particular area where there is a problem that needs to be solved. There can be several steps involved from identifying the need of a biomaterial to the use and final analysis of the product (Fig. 10). In general, the need may be the treatment of a disease, the replacement of an organ or the purely cosmetic use, etc.
Following is the design and synthesis of the materials for various tests (composition, structure, mechanical properties, toxicology, bioreaction of the material, and biostability). Based on the choice of the most appropriate ones, manufacturing followed by sterilization and packaging of the biomaterial (which is then directed to more detailed toxicology, in vitro and in vivo biointeraction testing) will be performed. Next focus is on the regulatory aspects related to pre-market approval, initial clinical studies, clinical screening and long-term follow-up. Development continues even after approval and clinical use of the biomaterial, with the analysis and registration of explants extracted from patients to understand possible failures for their correction.
The inclusion of biomaterials has a major impact on human life. These materials, for example, can be used as an excellent way to restore defects using facial prostheses when reconstruction by surgery is not optimal. Figures 11 and 12 present applications of biomaterials (produced by additive manufacturing) in various parts of the human body.
Dental device manufactured by additive manufacturing [17]
Titanium custom prosthesis design [132]
Figure 13 shows the application of a titanium alloy prosthesis to the maxillofacial reconstruction tailored to the patient. This jaw implant was developed to accommodate a bone graft inside, creating anchors on the surface, in which the graft evolves and expands into the existing healthy bone.
a Filling the interior of the titanium alloy implant with bone graft; b coverage with cortical bone; c implant placement [133]
Figure 14 shows the application of a metal prosthesis applied to the healthy part of the femoral. It fits into the acetabular pelvic cavity and is coated with ultra-high-molecular weight polyethylene (UHMWPE) to resist friction wear [134]. This type of prosthesis has a normal average life cycle of 10–15 years. Some more typical applications using the same materials described in the previous examples are the knee, elbow, ankle, and wrist prosthesis, as shown in Fig. 15.
Components in different models of hip joint implant [135]
Implant examples for total arthroplasty of knee, elbow, ankle, shoulder, and hip [136]
Other applications of biocompatible alloys are the immobilization of bone fractures through plates. They are usually marketed in standard models. The metal plates, screws, and threads are used during the healing process to join and consolidate the fractured bone segments (Fig. 16). Depending on the extent of the injury, these devices may or may not be removed after the bone has fully recovered, so there may be some interaction between the soft tissue and the screws, which is not harmful.
a Metal plates, threading, and screws used for bone fixation; b radiograph and outcome of tibial implantation of a fully recovered patient [137]
Still other implants serve to connect the spinal segments when the vertebral bones fracture due to osteoporosis or back injury. This procedure involves the implementation of a metal cage, which will accommodate the particles of the patient's own bone to allow the formation of new bone that will adjust with the adjacent vertebrae in future (Fig. 17).
Metal implants of metallic segments of the spine [134]
Orthodontics is another large area of large biomaterials acting as an alternative to traditional dentures. Dental implants are implanted directly into the bone and can replace each missing tooth. The implant is subsequently covered at the top by a ceramic crown (Fig. 18). The great advantage of these implants over dentures is that they transmit stress to the jaw, stimulate it, and thus, result into bone resorption and growth over time.
Dental implants [134]
Another application of biomaterials is their use as bone plates and meshes for cranial reconstruction (Fig. 19), both made to order (cranioplasty, maxillofacial, etc.). They are fixed to the skull with the help of titanium screws. Bone plates are ductile, come in a variety of shapes, and have sizes designed for the particular attachments.
a Titanium meshes for cranial reconstruction; b mesh implant and titanium mini-plate for craniofacial reconstruction [132]
Different biodegradable materials have been used lately for the manufacturing of these screws to eliminate a series of complications associated with the use of some metallic screws, such as the need to remove it after it is done with its purpose. This removal requires a second surgical intervention. The cost of removal procedures for such metal devices is enormous compared to treatment using biodegradable materials. On the other hand, the psychological advantages for the patient, in being able to treat his fracture with a single surgical intervention, are profoundly striking.
Biodegradability is also useful for stent making. These devices are used to treat cardiac patients with narrowing of the heart arteries. They are placed in arteries of the heart or in peripheral vessels in other parts of the body, which are partially obstructed by plaques of fat or calcium (Fig. 20). Over the time, this stent is absorbed by the cells to become part of the artery—an essential process for its success as removal would cause further damage to the artery. They can be made of metal alloys or polymers, depending on the characteristics required by each type of surgery and specifications from medical doctors [138]. Some require more flexibility to ease implantation, while others need more chemical stability, which is why the development of new and custom products depends on the joint work between physicians and engineers.
Heart stenting to improve blood flow around organs [139]
Laser cutting is a consolidated technique for stent manufacturing [140], but AM through selective laser melting (SLM) technique is a promising operation [141]. On the other hand, researchers at the Eindhoven University of Technology in the Netherlands published a proof of concept article on biodegradable, 3D printed, and self-expanding stents. Stents are designed to be minimally invasive, supporting narrow or weak heart arteries, especially in cases involving children. Eindhoven stents are made up of an absorbable polymer rather than a metal alloy (CoCr, NiTi, among others) to promote body comfort and acceptance. Using the design parameters of a typical nitinol stent, the researchers created a computerized design of a plastic polymer stent. Through simulated crush tests, the design of the Thermoplastic Co-Polyester (TPC) FDM printed stent is modified in degrees until it meets or exceeds the required response obtained on nitinol.
5 The trade-in biomaterials
Trade-related to the area of biomaterials is significant from the point of view of the number of units sold annually and the observed financial movement. This can be conveniently segmented based on two different criteria [142]:
-
1.
Types of compounds from which biomaterials are constituted, such as metals, ceramics, polymers, and materials of natural origin.
-
2.
Form of application of biomaterial, such as orthopedic, cardiovascular, dental, ophthalmic, plastic surgery, engineering, tissue, injury treatment, neurological, and central nervous system disorders. It also includes devices with other applications such as gastrointestinal and urinary, or as drug delivery systems, and for bariatric surgery.
According to Tofail et al. [142], trade-in orthopedic implants reached $ 57.9 billion in 2018. Although there is a conflicting record of prediction regarding this segment, orthopedic biomaterials are undoubtedly highly economic. Another noteworthy branch is biomaterials for cardiovascular applications, with an estimated market share of 34.5%. Although metallic biomaterials currently dominate the world market by about 50%. The sharp growth of the polymeric biomaterials market is expected in the near future due to more appropriate characteristics such as flexibility, elasticity, biological inertia, longevity, and biocompatibility. According to Ghasemi-Mobarakeh et al. [143], the term biocompatibility is defined as: “The ability of a material to perform its desired functions in relation to an organism to interact with living systems without having any risk of injury, toxicity or rejection by the immune system.” This means that the implant should not release substances in the body that could cause systemic damage to the patient.
In 2000, the world market for biomaterials was estimated at 16.9 billion euros, with a growth rate of 12% per year, which means that it exceeded 110 billion reais in 2010. In this sense, a considerable progress is expected in the field of biomedical engineering. This basically aims at applications in the field of regenerative medicine, which will certainly require significant improvements in the design and execution of the supports used for the growth of cells of normal tissues or even cells. Scaffolds for instance have functions that go far beyond providing a biocompatible matrix with porosity, roughness, three-dimensional structure, degradability, mechanical and mass-transport properties, including potential growth stimulation, cell migration, interaction, and differentiation. It may further dispense growth factors and other appropriate biochemical signals to cells, which can be incorporated into or adsorbed on them, to provide a microenvironment that refers to the extracellular matrix. Only in the stem cell-based therapies segment it is estimated that the global market will reach US $ 330 million by 2020 Xie et al. [132].
6 Classification of biomaterials
It is not possible to generalize what the required characteristics of biomaterials should be, as they depend fundamentally on their applications. Some properties are often evaluated so that the device design can be carried out effectively and economically. In this sense, biological properties are highlighted, such as biocompatibility, often associated with hemocompatibility, cytotoxicity, allergenicity, adhesion stimulation, and cell proliferation. According to Devgan and Sidhu [6], such properties have a vital requirement associated with the ability to attach tissues within the human body without causing unwanted discomfort. The posterior osseointegration process supports the regeneration of new bone by encapsulating new tissues that spread around the reconstructed bone and the implant surface.
Physical properties (such as surface morphology, surface energy, anatomical fit, roughness, porosity, color, transparency, and permeability), mechanical properties (such as tensile strength, elongation, and flexibility), and chemical properties (such as density, stability, sterilization resistance, and shape) also play their considerable role. This comprehensive set of parameters in represented in Fig. 21.
Demonstration of other factors that are considered for biomaterial selection [6].
In general, biomaterials encompass a broad class of natural or synthetic substances with mechanical, physical, and chemical properties suitable for the recovery of original functions of tissues, organs or systems. Synthetic materials are divided into metals and their alloys, polymers, composites, and ceramic materials. Tables 7 and 8 show, respectively, the types of materials, their classification, main applications, advantages and disadvantages of artificial and natural biomaterials.
6.1 Metallic biomaterials
Metallic biomaterials, compared to other ceramic and polymeric biomaterials, have the ability to withstand higher stresses, even of a dynamic nature. This is why various alloys are used as structural materials for skeletal reconstructions subjected to high load application. Major applications of metal biomaterials include fracture fixation wires, screws and plates, dental implants and joint replacement prostheses [144]. Moreover, metals can be used in the manufacturing of artificial heart valves and expandable stents, which require, in addition appropriate mechanical strength, durability, and visualization on X-ray images [145]. Good electrical conductivity, another common attribute of these materials, has facilitated neuromuscular stimulation devices, such as cardiac pacemakers [146].
The great versatility of metals for biomedical use is also due to the possibility of their surface polishing and abrasion. Sterilization is responsible for the extensive application of metals in surgical instrumentation, such as scissors, needles, forceps, and retractors. In addition, the following properties are highly desired in metallic biomedical devices:
(a) Corrosion resistance
Corrosion occurs when chemicals contained in human body fluids react with the metal implant, forming oxides or other compounds (resulting from a chemical reaction). This may weaken the implant and the particles produced may lodge in the tissues around the metallic implant, and in some cases may increase the concentration of metals in the blood [6]. Thus, selecting a material that has high corrosion resistance is crucial for the success of the implanted component.
(b) Biocompatibility
Biocompatibility is the surface exhibiting the formation of oxide films. The oxides also act as a passivation layer to protect the surface against corrosion. The examples are chromium oxide in stainless steels and CoCr (cobalt–chromium) alloys, or titanium dioxide in titanium alloys. The alumina-identical oxide films exhibit inert behavior in relation to the prosthesis processing environment up to its packaging or even during the surgical intervention [147].
(c) Biofunctionality
Biofunctionality is the relationship between the mechanical and physical properties of the material compatible with its specific function in the human body. Some mechanical properties such as modulus of elasticity, fatigue strength, fracture strength, tensile strength, and elongation, should be considered for the selection of biocompatible alloys.
To be safely applied to the human body, metals or alloys must meet other requirements: not producing inflammatory, toxic or allergic reactions, being chemically stable and preventing degradation in the biological environment. Table 9 presents some possible causes of degenerative and inflammatory problems due to the high rate of implant degradation.
In the case of bone implants, in addition to modulus of elasticity similar to that of human bone and fatigue strength, high adhesion strength between the osteoblasts and the implant should also be required. In general, the modulus of elasticity of bone is much smaller compared to metallic materials commonly used as biomaterials. Devgan and Sidhu [6] stated that depending on the type of human bone or joints, bone modulus values range from 4 to 30 GPa, which is considerably lower than the implanted material modulus (Table 10).
Research indicates that insufficient load transfer from the implant to adjacent areas may result in bone resorption and eventual implant loosening. Due to this fact, there is great interest in the production of biomaterials with low modulus of elasticity (closer to that of bone), which can stimulate a better stress distribution. This can be done by altering geometric and manufacturing parameters of sponge or lattice cell structures until the proper mechanical response is obtained.
In a recent work, Li et al. [149] studied that how varying geometry of cell units and their interconnection would change stiffness, energy absorption, maximum compressive strength, and modulus strength of cellular panels produced by SLM. Using AlSi10Mg alloy, the later property ranged from 99 to 305 MPa. Besides varying geometry of cells between different parts, Plocher and Panesar [150] also evaluated how lattices produced by FDM using carbon fiber-reinforced Nylon would behave with graded density within a single part. Apart from the effect of fine-tuning of implant mechanical properties, the porosity could enable cell growth intertwined in these structures. Moreover, active chemical compounds to further induce cell attachment and replication could be incorporated in such parts, either after additive manufacturing or mixed in the powder and wire stock used in SLM and FDM processes, respectively.
Once implanted, biomaterials remain in contact with body fluid, which consists of an aqueous solution containing dissolved oxygen, proteins, and various ions such as chloride and hydroxides [151]. In the case of dental implants or orthodontic materials, metal alloys are also susceptible to temperature and pH variations, presence of microbial biofilm, and the physical and chemical properties of ingested foods. These media can be aggressive to metals, causing their corrosion. In addition to these factors, most implants work under the action of mechanical loads that generate friction, slip and, consequently, the possible release of metallic particles [152].
Noble metals, such as gold and silver, are not susceptible to corrosive processes. However, other attributes such as high density, insufficient strength, and high cost make their orthopedic applications unfeasible [131].
In general, the resistance to this process comes from a thin oxide film formed spontaneously by exposure of the metal surface to air. This film, in the form of a passivation layer, prevents ion exchange, and thus, protects the surface. Some factors, however, may compromise the corrosion resistance, such as lack of homogeneity in the microstructure related to variation in composition, surface deformation, presence of impurities, precipitation, segregation, and inclusions. Thus, during the manufacturing process, to improve corrosion resistance by strengthening the protective film, implants may undergo further treatment and deposition of oxides on their surfaces.
According to Brockett et al. [153], a disadvantage of metals relates to the possible noise resulting from friction in implants composed of two metals in contact. The incidence of this problem in patients with hip implants, for example, can be as high as 10% and usually begins within 6 months–2 years after surgery. In addition, the high metal density may lead to high mass implants which are uncomfortable for the patient. Specific characteristics of some categories of metals most often used as biomaterials components are displayed in Table 11.
-
(i)
Co–Cr alloys
In the 1930s, Co–Cr–Mo (Vitallium) alloys first began to be used as casting alloys for dental applications. Later, in the 1940s, they were adapted for orthopedic applications. Nowadays, these materials have been mainly used in the manufacture of knee, shoulder, and hip orthopedic prostheses, as well as fracture fixation devices, maxillofacial, and dental implants [128]. These alloys are non-magnetic and highly resistant to wear, heat and corrosion. The wear resistance of Co–Cr alloys exceeds that of stainless steels and titanium alloys. The disadvantages of these alloys are related to their low plasticity and machinability [145].
According to ASTM, Co–Cr alloys used for implants can be divided into two distinct groups as shown in Table 11: cast and worked. Out of these alloys, Co28Cr6Mo and Co35Ni20Cr10Mo are the most used for the manufacturing of joint implants.
The ASTM F75 alloy is one of the most commonly used in implant casting. Its main feature is the high resistance to corrosion in harsh environments, particularly in contact with the fluids in the human body. The ASTM F75 alloy used in implant manufacturing requires a precision casting process. Unlike the melting temperature of Co–Cr binary alloys (with melting temperature ranges from 1450 ℃ at 1500 ℃), the carbon content (0.5%) of ASTM F75 alloy allows its melting temperature to be lowered to 1350 ºC, facilitating the melting and casting process. Higher quality castings can be obtained through the process of melting and vacuum casting to prevent oxidation [154]. Table 12 shows the chemical composition of the alloys mentioned in Table 11.
(ii) Stainless steels
Stainless steels are iron-based alloys, which contain carbon ranging from 0.03 to 1%, and at least 10.5% chromium. The most commonly used stainless steel in implants is rated 316L, which belongs to the group of austenitic stainless steels. The presence of chromium is important because it causes a corrosion resistant oxide layer [147]. The presence of molybdenum improves corrosion resistance at grain boundaries [155]. Nickel is the main element that stabilizes the austenitic form of iron and improves corrosion resistance. However, the presence of nickel alloys in implant manufacturing has been challenged due to the possible nickel toxicity with the human body [156] causing problems (in some situations) such as allergies, cancer, and genotoxic or mutagens [157]. Still, nickel and its alloys may be used in additive manufacturing of surgical equipment such as the instruments displayed in Fig. 22, which are used to manipulate knee ligaments during anterior cruciate ligament (ACL) repair surgery. In this case, the contact with the tissue is made only during surgery so there is no chance of it being released in the body.
Hence, investigations were performed to eliminate the use of nickel in the chemical composition of stainless steel alloys, such as the case of Carpenter Technology Corporation’s BioDur 108 alloy. This alloy does not contain nickel in its composition, but in comparison to alloy 316L, it has a high level of nitrogen to maintain the austenitic structure, which allows an improvement in its mechanical properties, namely the tensile strength, fatigue strength, and corrosion resistance [155].
These surgery guides for ACL repair were printed with Inconel 718 by DMLM [17]
(iii) Titanium and its alloys
Titanium is a low-density element (4.5 g/cm3), with approximately 60% of iron density. With the addition of alloying elements, its properties can be further improved. The increasing use of this material is mainly due to its low modulus of elasticity among metals (100 GPa), and superior biocompatibility and corrosion resistance compared to stainless steel and Co–Cr alloys. It was these attractive features that began their introduction, initially of pure titanium, later the α + β alloys (Ti–Al6–V4) and, recently, the β alloys.
Another additional advantage of this material is the greater tendency of osseointegration, an important feature for long-lasting implants. The reduced or nonexistent reaction of titanium with the tissues surrounding the implant is due to the passivation on the metal surface formed by titanium dioxide (TiO2) film, usually of nanometer thickness [145].
In recent years, a great effort has been made to formulate new titanium β alloys with biocompatible alloying elements such as niobium, tantalum, zirconium, and molybdenum for implant applications. The competitive advantage of β alloys over α + β alloys lies in their high strength and low modulus of elasticity. Another advantage in terms of precision casting of β alloys is that it is possible to obtain mechanical characteristics identical to the forged β alloys [158]. Other new β alloy implants include Ti15Mo5Zr3Al, Ti29Nb13Ta46Zr, and Ti29Nb13Ta4Mo. Their properties as well as microstructure are similar to those previously described [158].
Among the different alloys involving titanium, the Ni–Ti equiatomic alloy, known as Nitinol, has high prominence due to its remarkable shape memory properties, superelasticity, and fatigue and torsion resistance. Shape memory property refers to the ability of the material to return to its original shape after deformation by increasing temperature. Nitinol’s elastic behavior allows it to be deformed up to 20 times by re-engaging the original dimensions after stress release, which is ideal for stent implantation.
6.2 Ceramic biomaterials
Man discovered ceramics thousands of years ago when he found that the clay placed in fire turns into a rigid and hard mass. Ceramics can be defined as composed of metallic and non-metallic elements, for example Al2O3, MgO, and TiO2, which are ordinary ceramic materials.
Bioceramics have been used since 1969 to solve dental defects and orthopedics. These materials exhibit excellent properties such as oxidation and corrosion resistance, high elastic modulus, and excellent biological compatibility [132]. Applications cover the most diverse areas, such as diagnostic instruments (thermometers, endoscopy fibers), orthopedic prostheses, devices for dental and maxillofacial reconstruction, heart valves, artificial tracheas, and bone fillers. The wide field of application is largely due to the crystallographic properties and superior chemical compatibility of ceramics with the physiological environment and rigid tissues, such as bones and teeth [159].
From a chemical point of view, ceramics are inorganic compounds, usually formed of metallic and non-metallic elements, and joined by ionic and/or covalent bonds. In these bonds, the electrons are not free as in metals, but located between the ions/atoms. With this, ceramics tend to behave as materials of low electrical and thermal conductivity [160]. In general, ceramics are less dense than mostly metals and their alloys. These materials have good dimensional stability, are resistant to wear and compression and stable in corrosive environments. In addition, they are very sensitive to the presence of cracks and other defects, which can act as fracture initiation points and contribute to early material rupture during use. Due to these factors, ceramics are poorly suited for applications in regions subjected to high stress and requiring lift.
According to Santin and Phillips [161], the term bioinert is not appropriate, since all material induces some kind of host tissue response (even if minimal). There is no material implanted in living tissue that is completely inert, and for this reason, the term bioinert should be avoided. However, the term is still commonly used and is defined as a material that displays minimal interfacial response that does not result in binding or rejection of host tissue and forming a nonadherent fibrous capsule around the material. Examples of bioinert ceramics include alumina (Al2O3), zirconia (ZrO2), and titanium dioxide (TiO2).
Alumina (Al2O3) has a compact hexagonal crystalline (HC) structure, with characteristics of high hardness, compressive and abrasion resistance, and can be polished to a high surface finish. The strong ionic bonds and high oxygen ratio make it a chemically inert material with great stability in physiological and corrosive media [157]. High purity (α-Al2O3) polycrystalline alumina ceramics (> 99.5%) are the most commonly used implants. The toughness and tensile strength, and fatigue resistance of this type of material is associated with grain size and purity. Small amounts of MgO (< 0.5%) are often added to inhibit grain growth during sintering to improve mechanical properties. Alumina with an average grain size of less than 4 μm and purity of over 99.7% has good flexural and compressive strength. Grains larger than 17 μm can decrease alumina mechanical strength by up to 20% [159].
The main application of alumina is related to the production of acetabulum and femoral heads for hip arthroplasty, both elements that constitute hip prosthesis. When these two pieces are polished together and used as a pair, the joint friction coefficient decreases over time and the value tends to approach that of the normal joint. Thus, the wear of alumina-alumina surfaces is approximately 10 times less than that of metal-polyethylene surfaces, for example. Other clinical applications of alumina include knee prostheses and elements for maxillofacial reconstruction, bone screws, middle ear ossicle substitutes, corneal prostheses, segmental bone replacements, and dental implants. Monocrystalline alumina (sapphire) has mechanical strength about three times higher than polycrystalline alumina, good esthetics, and the possibility of obtaining devices with different sizes and shapes. Such material has wide application in the making of dental prostheses and crowns. However, its use decreased due to the low impact resistance [162].
Zirconia (ZrO2) belongs to the group of inert ceramics and presents a polymorphic structure with three distinct crystal forms: monoclinic, cubic, and tetragonal [163]. Table 13 presents the formation of zirconia polymorphic structures at different temperatures.
During cooling from the processing temperature, the tetragonal phase becomes the monoclinic phase accompanied by a volume expansion (3–4%) that causes internal tensions in the microstructure of the material, generating cracks which further make it extremely fragile. Thus, the mechanical and refractory properties of pure zirconia are impaired, limiting its applications [163]. To increase mechanical strength and toughness, the tetragonal and cubic crystalline phases can be stabilized at low temperatures by the use of additives such as magnesium, cerium, yttrium, and calcium oxides. Depending on the concentration of additives, this may result in polycrystalline tetragonal zirconia (TZP), fully stabilized zirconia (FSZ, usually in the cubic phase) and partially stabilized zirconia (PSZ), in which fine metastable tetragonal particles are dispersed in a matrix. Among the different modified forms, the yttria-stabilized polycrystalline tetragonal zirconia ceramics (Y-TZP) stands out for its very fine grains and low porosity. These factors make it possible to obtain a material with high flexural strength, toughness, and erosive wear resistance, which can be successfully used in applications subject to mechanical stress.
Compared to alumina, this ceramic has higher strength, lower hardness, and lower elastic modulus. The main applications of zirconia are as an alternative material to alumina in the manufacturing of femoral heads in hip prostheses, knee and shoulder prostheses, and dental materials. Calcium phosphate ceramics have high potential for applications as biomaterial due to their chemical and structural similarity to biological apatite, which is present in large proportions in the mineral phase of bones and teeth. These materials have excellent biocompatibility and bioactive behavior, enabling high levels of osseointegration and osteoconduction. Calcium phosphates have been widely studied and employed in skeletal system-wide applications such as craniomaxillofacial reconstruction and treatment of bone defects. The main limitations of the use of calcium phosphates come from the fact that they are very brittle and have low resistance to fatigue. As a result, dense or porous coatings of these ceramics are often applied to metal bearing implants so as to allow biological fixation or osseointegration.
Hydroxyapatite (HP) is one of the major mineral components of bones, enamel, dentin and is also present in urinary and dental calculi. As a biomaterial, it has the advantages of rapid bone adaptation, non-formation of fibrous tissue, short healing time and close implant/tissue adhesion. Another class that has aroused interest in the biomedical field is calcium phosphate (CFC) cements. These materials are biodegradable and multicomponent, consisting of an inorganic solid phase and a liquid phase which, when mixed, form a paste that spontaneously stiffens at room or body temperature as a result of precipitation of one or more calcium phosphates [164].
6.3 Polymeric biomaterials
These materials are widely used in the medical field. Their main advantages compared to ceramic and metallic materials, include the ease of producing various shapes (particles, films, wires, among others), secondary processing, reasonable cost, and availability with desired mechanical and physical properties for specific applications [165]. Figure 23a presents 3d-printed tracheal splints for babies suffering from breathing problems.
a 3D-printed tracheal splints for babies suffering from a congenial breathing condition were made out of polycaprolactone [17] and b artificial heart manufactured by additive manufacturing
Several criteria must be considered when selecting a polymer material, as each polymer may have particular properties that will direct it to a specific application. In this sense, the shape of the chains, arrangement of monomeric units, the presence or absence of particular atoms or functional groups, the structural rigidity, the polarity of the chain and the molar mass of the polymer, result in subclasses of compounds [165]. This way, the combination of different polymers might help to overcome deleterious characteristics of each separate material as demonstrated by Guerra et al. [166] in the combination of PLA and PCL to produce stents with intermediate elasticity module. Shape memory elasticity is also achievable for polymers through 4D printing, a technique in which material composition is carefully modified during deposition to achieve shape changing structures activated by heat, pH, light or electricity [80].
The polymers can be obtained from polymerization reactions or by living organisms. They are classified as synthetic and natural, and can be made into nanostrucutres such as nanoparticles, nanocapsules, and nanofibers that provide targeted delivery of drugs in the body [167]. The most used are synthetic ones because of their greater stability during use. Moreover they have well-defined reproducible properties and low cost. In particular, vinyl polychloride comprises about 40% of all polymeric materials applied for medical devices and is the preferred material for medical tubing and flexible containers due to its inertia, high transparency, sterility, and resistance [168]. Examples include catheters, oxygen masks, bags for blood, urine and medication, infusion sets, cannulae, and gloves.
On the other hand, polyhema is a transparent, biocompatible hydrogel with good mechanical properties and adequate stability, water absorption, and oxygen permeability. This further makes it particularly suitable for the production of contact lenses or drug delivery systems [169]. Since hydrogels are mostly employed in AM extrusion process, fibers can also be incorporated to improve mechanical support. Allograft or stem cells can be diluted in bioinks for manufacturing of complete tissues or integrated in scaffolds of more rigid polymers, with resolution up to a single cell via jet printing [170].
Figure 23b shows an artificial heart developed by the Federal Institute of Technology of Zurich, Switzerland. This organ was made up of silicon, which is a softer material. The shape resembles with the original organ and is composed of two compartments that play the role of ventricles. The pulse is made by a third chamber, which pumps compressed air, and weighs around 390 g (an average human heart weighs 310 g).
6.4 Composite biomaterials
Composites are a class of materials consisting of a continuous phase (matrix) and a dispersed phase (reinforcing component or modifier) separated by interfaces. The characteristics of which may incorporate combined properties of the individual constituents. The reinforcing or modifying material may be used in the form of fibers or particles and is added for various purposes, such as for improving mechanical properties, biocompatibility, bioactivity, degradation rate, even controlling drug release or growth factors incorporated into devices [171]. The main factors affecting the properties of composites are the characteristics of the constituent materials, their percentage and distribution, orientation of the fibers or particles in the matrix, and the interfacial interactions. Electrospinning is the preferred choice to produce nanofibers from bioinert polymers such as HDPE, PVC, and PA-12 or biodegradable such as PLA and PCL [82].
In polymeric bone-implant biomaterials, the addition of bioglass particles, hydroxyapatite or even eggshell powder [172] is intended to increase the biocompatibility and modulus of elasticity of the matrix. The mechanical properties of the composite become closer to those of bone, thus, contributing to the reduction of the stress-shielding phenomenon [173]. Combining bioactive glasses with polymeric materials, such as polyvinyl alcohol (PVA), PMMA, chitosan or even collagen, can minimize problems such as poor mechanical performance and limited machinability of these matrices. To increase the fracture resistance of calcium phosphate, bioglass and glass–ceramic matrices, fibers, and metallic particles of titanium or stainless steel can be incorporated as reinforcement material.
In orthopedic and dental metal implants, calcium phosphates, mainly hydroxyapatite, have been used as coatings to obtain a conductive microenvironment for bone formation and growth on the implant surface, and also to promote its stabilization. Most of the applications of metallic composites are based on mechanical properties. Silver, for example, has been evidenced by its potential antimicrobial effect as it can be effective against a wide range of bacteria, fungi, protozoa, and viruses. This metal is mainly used in the form of micro and nanoparticles incorporated into polymeric materials, aiming at the prevention of infections in skin lesions. In devices such as catheters, silver can prevent bacterial colonization during use [174].
7 General comments and conclusions
Due to numerous differences among people physiologies, any healing strategy that involves the use of a prosthesis, implants or even surgical material must consider the patient particular limitations for the design of said apparatus, such as size and shape. This means the capacity of producing custom-made medical devices is an important trait in this segment when analyzing the fabrication processes utilized. For this reason, the use of AM technologies has been widely studied for use in biomedical applications, since it can produce complex parts from digital models in a readily available manner.
These technologies apply different deposition strategies to produce a part layer by layer, and each one has its limitations regarding what type of material can be used, such as metals, ceramics, polymers, or a mix of these to form a composite. Different technologies were classified according to their working principle and reviewed according to their advantages and limitations in biomedical applications, considering current commercial applications and most researched topics.
FDM shows promising capabilities for printing organic tissue and scaffolds for cell growth, but parameters such as temperature and material delivery, must be better studied. It is a slow process with relatively poor resolution, but cheaper and more accessible compared to the other technologies. For these reasons, it has been mainly used for prototyping plastic components instead of producing commercial parts. MJ is the most obvious competitor regarding the same applications, with the advantage of better resolution and higher cost of equipment and feedstock as limitation. Vat photo-polymerization method also concur in the same niche, but is more adequate for printing scaffolds due to its higher resolution and speed compared to the other two. Its application in polymeric implants or prosthetic depends on the material availability as a photocurable resin, while generation of organic live tissue is more complicated due to cell sensitivity to UV light and requires more consistent investigation.
DED processes traditionally work with metals and have limited capabilities in terms of part complexity, resolution and geometric tolerance, usually requiring post-process machining. Because of this reason, even though they yield high deposition rate, they are not adequate for the majority of implants and prosthesis yet. The most suitable would be the BJ and PBF processes, which can produce parts with optimal resolution and good productivity. While PBF produces ready to use products, BJ pieces need to undergo heat treatment processes. Nevertheless, the second has much higher printing speed making it more appealing for large orders. Yet, they both have high acquisition and operating costs. PBF requires controlled atmosphere through vacuum chambers and inert gases, while the power sources, either laser or electron beam, are both costly equipment. On the other side, BJ requires controlled temperature ovens and parts often need to undergo hot isostatic pressure treatment for reducing porosity. The powders are expensive, come from limited suppliers, and are extremely delicate to handle and store due to health risks and fire hazard. Although, they are the most propitious AM methods for producing most of the joint replacement, dental, maxillofacial and cranial metallic implants, the business model comprising logistics and overall equipment effectiveness must be well developed so as to be economically sustainable.
The choice of material for the biomedical device is also an important design consideration and is tied with the manufacturing process characteristics. Biocompatibility demands that product must not cause any injury, toxicity or inflammatory reaction in the patient’s body and must fulfill the original functionalities of the replaced or modified tissue. At first, it was thought that biomaterials should be inert and resist any chemical degradation, but nowadays the trend is to develop components that stimulate tissue response and cell growth, while some are even designed to be biodegradable or bioresorbable. Various types of materials implemented in the medical industry have been discussed according to their advantages and limitations, which determine their usefulness for each type of device.
Metallic implants usually feature CoCr, stainless steel, or titanium alloys. They exhibit good corrosion resistance and fracture toughness, besides high mechanical and wear resistance. They undergo passivation due to the formation of oxide layers which make the first two types bioinert. However, for the third there are of evidences of induced cell activity by the titanium oxide phases. Since they withstand high temperatures without degradation they can be easily sterilized, which is an important trait. Apart from the traditional manufacturing processes such as casting, forging, and machining, these materials can be worked in BJ and PBF additive manufacturing processes, which further make them ideal for producing dental, bone, and joint implants or fixation plates, besides heart valves and electrodes for neural stimulation, among other products. A major drawback of metals is their complicated imaging, since in X-ray their translucence hinders observation of the tissue interface, while in MRI and CT scan they distort the image generated from their surrounding area. In addition, an important concern regarding metallic and ceramic implants is that due to their much higher elastic modulus in comparison to the bone, they might provoke a stress-shielding effect, which has been presented to cause bone resorption and aseptic loosening. Nevertheless, it might be overcome through the utilization of 3D sponges, which is an interesting research topic for present and future works.
Another option is the utilization of polymeric implants since their elastic modulus is closer to the bone while they can still reach good mechanical properties such as yield and fatigue strength, and fracture toughness. The most prominent is from the poly-aryl-ether-ketone family, which are ultra-high-performance polymers that have been gaining substantial attention for the use in implants due to chemical stability and high-temperature performance. They also render low friction and wear in joint applications and although they are more difficult to be processed via AM, they are more economical if they are to be produced by conventional injection molding. Other interesting polymers for less stress loaded applications are from the polylatic acid, polyacetone and polyamide fanilies, which could be applied for stents, drug-releasing devices, and organ repair or substitution, whereas the first two naturally degrade into nontoxic substances and can be absorbed by the body. Polysiloxanes and acrylate-based compounds are the artificial polymers that could be used for building lenses for corneal transplants, scaffolds, or sol–gel medium for cell growth, whereas collagen, chitin, and cellulose are naturally occurring polymers that can be used for the same goal. Not without reason, polymers are the most researched materials in the latest publications of AM biocomponents [10].
Most ceramic applications are limited by their low fracture toughness and tensile yield strength. Due to high compressive, wear and corrosion resistance, they are mostly applied to some parts of joint replacement and dental implants, with the advantage of being lighter than their metallic counterpart. Phosphate calcium and titanium oxide-based compounds are specifically relevant as coating materials to induce osteogenic growth and attachment in bone and joint arthroplasties. These have been and are still being extensively studied in conjunction with metal or polymer matrices, making up an important parcel of composite biomedical materials.
The most adequate set of material and the corresponding AM process depend on what kind of problem must be solved. On the other hand, each human body has its response depending on some other factors such as previous and actual diseases, like diabetes among others. Since a general guide is extremely difficult to find and recommend due to wide variables involved in this complex system like human body, the present review is capable of providing the academia and health and engineering professionals a set of relevant information related to the applications of AM and biomaterials with strong potential.
8 Future trends
Biomaterials and processes for 3D bioprinting have progressed exponentially in the last 20 years but there are still many developments to be made. The consolidation of new technologies takes a long time between laboratory and clinical tests, registry, approval and market introduction, which means the majority of applications is not ready for commercialization yet. Even after deployment, some issues can only be detected after several years, as was the case of the first generation of orthopedic implants and stents. Therefore, it is possible that many researches conducted from the last 10 years up to today will still take at least a few years before market insertion.
In this regard, the next section discusses some trending topics in the field of additive manufacturing of biomaterials and living tissues. Most are still in exploratory phase and require many experiments before producing viable parts ready for use in the human body. Nonetheless, the possibilities are limitless and after development this topics could push the boundaries of medical sciences to solve problems such as replacement of living organs by totally artificial ones, promotion of accelerated tissue regeneration withing the body, reduction or neutralization of side effects from chemotherapy and strong medicaments, or correct any other health issues.
8.1 Tissue engineering and bioprinting
According to Vanaei et al. [175], 3D bioprinting is the production of three-dimensional structures using a bioink, which is the mixture of hydrogels, polymers, living cells, drugs or other bioactive molecules. The main natural materials used include collagen, gelatin, chitosan, alginate and extra cellular matrix (ECM) [176], while most common synthetic polymers are PLA, PCL, PMMA, and PEEK [175]. In addition, bioactive polysaccharides can be cheaply produced by bacteria to form biocompatible and resorbable bioinks with great printing properties [177]. Although they are not the same as bioinks, spheroids are another interesting option for bioprinting. They are made of a densely groupments of cells which are usually injected in a hydrogel matrix where they can differentiate or interconnect to self-assemble in complex tissue structures [178]. They may be stimulated by bioactive compounds in the hydrogel and the process of spheroid assembly can be easily automated. Although production of large and complex organs would be possible, it is still difficult to control the assembly of multiple cell populations [179].
This process has fantastic possibilities regarding tissue engineering and it is one of the most important fields nowadays in medical sciences. Many types of tissue can be viabilized, allowing regeneration of bone, cartilage, muscle, skin and even nerves [180]. Although there is no immediate possibility yet of producing artificial organs for transplantation with the same functionality of natural ones, in vitro models may already fast-forward critical research that cannot be readily carried out in human tissue, such as new drugs for liver treatment [181]. Nevertheless, recent advances in the printing of vascularized tissue may one day enable transplantation of artificial organs, since the adequate delivery of oxygen is the major challenge for this purpose nowadays. Zhang et al. [182] states that this will require process capability of printing micrometer to millimeter scale integrated capillaries, not yet available. Other path is the use of bioactive bioinks to facilitate angiogenesis, which demands comprehensive knowledge of embryonic development, mechanobiology, cell–cell and cell–material interactions.
According to França et al. [180], the most researched AM processes for bioprinting are extrusion, inkjet and stereolitography. In the first class the bionk can be mechanically pumped, but this creates shear strain that can be deleterious to living cells. Therefore, only larger nozzles are currently used, which limit the resolution of the structures [183]. Thermal expansion is another possibility, but the temperature damage to cells is a severe limitant for the speed of the process. Inkjet has been growing substantially, due to the possibility of incorporating several types of cells and chemical compounds at the same times, but the resolution is still a problem because of the size of nozzles, in the same way of FDM. In addition, it might be necessary to use photocurable resins and UV light in some structures to improve mechanical resistance, which hinders cell viability. SLA has this same limitation, in spite of its improved resolution.
8.2 4D printing
According to Pei et al. [139], 4D printing is the use of an additive manufacturing process to achieve a gradational mixing of materials to fabricate freeform geometries with variable properties within one component. This way, complex implantable materials could be developed by varying chemical composition or type of material in bulk and surface during printing. This has intrinsic difficulties, such as adjusting scanning path and extrusion speed according to the characteristics of feedstock at each moment [187]. However, many different shape-shifting behaviors could be obtained such as self-folding, self-assembling, and self-dis-assembling structures [188]. This principle is already applied for manufacturing of coronary, tracheal and ureter stents, as mush as foam and coils for aneurism occlusion [189] based on bioresorbable SMPs. Further researches are being conducted to achieve better control over their behavior. Another very interesting field is controlled drug delivery, which can be activated by changes in temperature and pH, ultrasonic or infrared waves [190]. There are many future expectations about this technique towards improving chemotherapy treatments by activating the drugs specific in the tumor region, reducing many harmful side effects.
Other useful feature of 4D printing is expected to increase is the development of complex scaffolds for joint replacement. Biodegradable polymers containing bioglass [193] present a strong osseointegration response although their resistance is still very low. This could be further improved by combining degradable and engineering polymers with bioceramics and nanofibers [195] or using metallic special alloys [194]. The use of graphene and CFRP is widely researched and commercial products might be presented as a next generation of more resistant biodegradable implantable composites in the next years [200].
8.3 Nanostructured biomaterials
Nanotechnology is already contributing to development of advanced biosensors, bioimaging, gene delivery, drug delivery, smart nanorobots etc. Similarly, nanocomposites or nanoenhanced 3D bioprinted biomedical products can add value tremendously by influencing the physical, chemical, electrical, optical and eventual biological poperties of the implantable products. For instance, nanotubes can be used to house drugs or proteins that enhance tissue integration or prevent infections and immune system reactions [184]. Xiao et al. [185] recently produced nanocomposite scaffolds of natural aluminosilicate nanotubes, PLLA and Ag, which achieved a sustained-release of Ag + over 28 days for antibacterial property without compromising the cytocompatibility, biomineralization ability or mechanical properties. These nanotubes can also be produced from mesoporous bioactive glass [185] or carbon nanotubes (CNTs). The later are known for effective adhesion, growth, and differentiation of bone, muscle, and cardiac cells, but can cause in vivo toxicity due to loose CNTs. This can be overcome be merging them in a larger biocompatible substrate [186], which may favor their wider application in the following years. The nanostructured fillers may be dissolved in the feedstock material of SLA and FDM processes, or be produced by adjusting parameters in PBF, MJ, DED and inkjet processes [187]. Coatings may also be formed in post-processing stages through electrochemical treatments.
Graphene is another very promising nanomaterial, either for biosensors or tissue development. The later is more commonly researched in AM applied to scaffolds for bone, soft tissues and complex organs. Nie et al. [188] mixed reduced graphene oxide (rGO) with nanoHA to enhance osteogenic activity of rat BMSCs, while Magaz et al. [189] found that electroactive composite fibrous scaffolds mande of rGO exhibit potential to enhance the neuronal cell response and could be versatile supportive substrates for neural tissue engineering applications. The antimicrobial activity may be also obtained after the incorporation of Ag or TiO2 within the graphene structures [190]. Still, the most significant use for biomedical application is the regeneration of soft tissue and capillaries from existing blood vessel in an organized and controllable manner [191]. Graphene platelets and foams can be tuned to promot differentiation of stem cells into specific tissue, which could allow the creation of organized blood vessel networks for organ irrigation, the greatest challenge for organ engineering nowadays.
8.4 Metamaterials
Metamaterial is a designation for engineered artificial materials whose properties are not found in naturally occurring ones. They are usually produced in the form of lattice structure containing several repeating unities of micrometric scale, smaller than the wavelengths of the phenomenon they interact with. There are three main categories which comprise structures with multifunctional mechanical characteristics, intelligent shape-shifting features or multiwave field response [192].
The first includes materials with negative Poisson’s ratios, pentamode metamaterials with adjustable tensegrity and biomimetic structures [193], which can be easily produced by the current AM technologies. The possibility of tailoring elasticity modulus enables their use as complex scaffolds for artificial organs and bone regeneration. The second group is similar to SMPs in biomedical applications, such as stents and drug-releasing components [194] but their intelligent structures enable other possibilities such as obtaining negative hydration expansion for use in biosensors [195] or capturing specific ions [196] to remove heavy elements from the body. Currently, this kind of behavior requires complex chemical structures that are not easily obtainable through AM processes alone, but it is expected that future technological development may easy their bioprinting.
The third category is the most common research topic among metamaterials due to its capability of interacting with electromagnetic field to send and receive signals with frequencies at the order of THz. Han et al. [197] developed antenna like structures that could be used in imaging for medical sciences or sensors. [198] produced biosensors based on gold nanoparticles capable of changing their vibrational response in contact with specific microRNA, possibly useful for diagnosis of cancer or other diseases. In addition, Palai et al. [199] proposed advanced metamaterials for sensing urea, sodium chloride, and glucose present in the blood. This has special interest for the treatment of diabetes and high blood pressure, two of the most prevalent diseases nowadays. However, the micrometric or even nanometric structures made of polymers and metals are not easily produced through most AM processes. Only SLA can produce features at this scale, but new photocurable resins would have to be designed.
8.5 Autogenous tissue engineering
The use of autogenous bone extracted from the patient iliac crest, calvarium, mandibular, tibia or other bones is the gold standard in bone repair surgery nowadays. However, there might appear complications for the patient aside from the obvious inconvenience of bone extraction. Therefore, recent studies aimed at reducing the amount of autogenous bone graft or completely substituting it by enhanced scaffolds in bone repair surgery [200]. Cui et al. [201] employed a mix of bone powder, silicon-substituted calcium phosphate (Si–CaP) and bone marrow stem cells to produce a adequate bone graft with reduced amount of autogenous bone. Moreover, Pelttari et al. [202] engineered patient nasal cartilage in vitro for developing autologous cartilage repair in osteoarthritis joint surgery.
These examples of tissue engineering with bone from the patient could be combined with additive manufacturing to develop more complex bone structures before implantation. These would be useful for patients treating cancerous diseases affecting their skeleton. Indeed, the extraction, storing and later reinjection of BMSC is a consolidated procedure in health care nowadays, especially when patients are subjected to chemotherapy treatments. The combination of this type of treatment with AM processes has not yet been explored, but could be an interesting health resort in the future.
Abbreviations
- µSL:
-
Micro-stereolithography
- 3D:
-
3-Dimensional
- 3DP:
-
3-Dimensional printing
- 4D:
-
4-Dimensional
- ACL:
-
Anterior cruciate ligament
- AM:
-
Additive manufacturing
- ASTM:
-
American Society for Testing and Materials
- BMSC:
-
Bone mesenchymal stem cells
- BJ:
-
Binder jetting
- CAD:
-
Computer-assisted design
- CFC:
-
Calcium phosphate cements
- CFRP:
-
Carbon fiber-reinforced composite
- CT:
-
Computed tomography
- DED:
-
Direct energy deposition
- DMLM:
-
Direct metal laser melting
- DPI:
-
Dots per inch
- EBM:
-
Electron beam manufacturing
- ECM:
-
Extra celular matrix
- FDA:
-
Food and drug administration
- FDM:
-
Fused deposition modeling
- FEM:
-
Finite-element model
- FFF:
-
Fused filament fabrication
- FSZ:
-
Fully stabilized zirconia
- GDP:
-
Gross domestic product
- HA:
-
Hydroxyapatite
- HC:
-
Hexagonal crystalline
- HDPE:
-
High-density polyethylene
- HIP:
-
Hot isostatic pressing
- IJP:
-
Inkjet printing
- LENS:
-
Laser engineering net shaping
- LOM:
-
Laminated object manufacturing
- MJ:
-
Material jetting
- MRI:
-
Magnetic resonance imaging
- NMR:
-
Nuclear magnetic ressonance
- PA-12:
-
Polyamide 12
- PBF:
-
Powder bed fusion
- PCL:
-
Polycaprolactone
- PEEK:
-
Polyether-ether-ketone
- PLA:
-
Polylactic acid
- PMMA:
-
Polymethyl-methacrylate
- PVA:
-
Polyvinyl acetate
- PVC:
-
Polyvinyl chloride
- rGO:
-
Reduced graphene oxides
- RP:
-
Rapid prototyping
- SLA:
-
Stereolithography
- SLM:
-
Selective laser melting
- SLS:
-
Selective laser sintering
- SMP:
-
Shape memory polymer
- TCP:
-
Tricalcium phosphate
- TPC:
-
Thermoplastic co-polyester
- TZP:
-
Polycrystalline tetragonal zirconia
- UHMWPE:
-
Ultrahigh molecular weight polyethylene
- UV:
-
Ultra violet
- WHO:
-
World Health Organization
- Y-TZP:
-
Yttria-stabilized polycrystalline tetragonal zirconia
References
Nations U (2019) World Population Prospects 2019, Volume II: Demographic Profiles Department of Economic and Social Affairs, Population Division.
Mehrabani D, Seghatchian J, Acker JP (2019) Platelet rich plasma in treatment of musculoskeletal pathologies. Transfus Apheresis Sci 58(6):102675. https://doi.org/10.1016/j.transci.2019.102675
Yelin E, Weinstein S, King T (2014) The burden of musculoskeletal diseases in the United States, 3rd edn. United States Bone and Joint Initiative, Rosemont
Woolf AD, Brooks P, Akesson K, Mody GM (2008) Prevention of musculoskeletal conditions in the developing world. Best Pract Res Clin Rheumatol 22:759–772. https://doi.org/10.1016/j.berh.2008.07.003
Sundman EA, Cole BJ, Fortier LA (2011) Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med 39:2135–2140. https://doi.org/10.1177/0363546511417792
Devgan S, Sidhu SS (2019) Evolution of surface modification trends in bone related biomaterials: a review. Mater Chem Phys 233:68–78
Ranawat CS (1985) Total-condylar knee arthroplasty: technique, results, and complications, 1st edn. Springer-Verlag, New York. https://doi.org/10.1007/978-1-4612-5050-0
Hirakawa K, Jacobs JJ, Urban R, Saito T (2004) Mechanisms of failure of total hip replacements: lessons learned from retrieval studies. Clin Orthop Relat Res 420:10–17
Kim A, Kim A, Kim Y, Kim YE, Kim H-J, Jeon B (2019) Musculoskeletal problems in PD patients have no association with socioeconomic status. J Clin Neurosci 70:229–233
Culmone C, Smit G, Breedveld P (2019) Additive manufacturing of medical instruments: a state-of-the-art review. Addit Manuf 27:461–473. https://doi.org/10.1016/j.addma.2019.03.015
Bose S, Ke D, Sahasrabudhe H, Bandyopadhyay A (2018) Additive manufacturing of biomaterials. Prog Mater Sci 93:45–111
Guo H-C, Wang Y, Dai J, Ren C-W, Li J-H, Lai Y-Q (2018) Application of 3D printing in the surgical planning of hypertrophic obstructive cardiomyopathy and physician-patient communication: a preliminary study. J Thorac Dis 10:867
Batteux C, Haidar M, Bonnet D (2019) 3D-printed models for surgical planning in complex congenital heart diseases: a systematic review. Front Pediatr 7:23
Mahamood R, Akinlabi ET, Shukla M, Pityana S (2014) Revolutionary additive manufacturing: an overview. Lasers Eng 27(3):161–178
Zhou W, Li D, Chen Z, Chen S (2010) Direct fabrication of an integral ceramic mould by stereolithography. Proc Inst Mech Eng Part B J Eng Manuf 224:237–243
Miller R (2014) Additive manufacturing (3D printing): Past, present and future. Ind Heat 82:39–43
Weber A (2019) Additive manufacturing for medical device production assembly. Assembly 61(5):58–63. Available via http://digital.bnpmedia.com/publication/?i=492995&ver=html5&p=1&oly_enc_id=0828C6687690J1E. Accessed 29 April 2021
Paolini A, Kollmannsberger S, Rank E (2019) Additive manufacturing in construction: a review on processes, applications, and digital planning methods. Addit Manuf 30:100894
Standard A (2012) ISO/ASTM 52900: 2015 Additive manufacturing-General principles-terminology. ASTM F2792–10e1
Li D, Soar R (2007) Temperature measurements of monolithic 3003 samples during ultrasonic consolidation process. Rapid Manufacturing Research Group, Loughborough University, Loughborough, pp 1–11
Karunakaran KP, Bernard A, Simhambhatla S, Dembinski L, Taillandier G (2012) Rapid manufacturing of metallic objects. Rapid Prototyp J 18:264–280. https://doi.org/10.1108/13552541211231644
Ford S (2014) Additive manufacturing technology: potential implications for U.S. manufacturing competitiveness. J Int Commerce Econ 2014(9):1–35
Goodrich M (2020) 3D Revolution. Michigan Technological University. Available via https://www.mtu.edu/magazine/research/2014/stories/3d-revolution/. Accessed 29 April 2021
Minetola P, Iuliano L, Bassoli E, Gatto A (2015) Impact of additive manufacturing on engineering education—evidence from Italy. Rapid Prototyp J 21:535–555. https://doi.org/10.1108/RPJ-09-2014-0123
Mishra S (2013) Helping additive manufacturing ‘learn’. Met Powder Rep 68(4):38–39. https://doi.org/10.1016/S0026-0657(13)70129-2
Prince J (2014) 3D Printing: an industrial revolution. J Electron Resour Med Libr 11:39–45. https://doi.org/10.1080/15424065.2014.877247
Frazier WE (2014) Metal Additive Manufacturing: A Review. J Mater Eng Perform 23:1917–1928. https://doi.org/10.1007/s11665-014-0958-z
Huang SH, Liu P, Mokasdar A, Hou L (2013) Additive manufacturing and its societal impact: a literature review. Int J Adv Manuf Technol 67:1191–1203. https://doi.org/10.1007/s00170-012-4558-5
Mortara L, Hughes J, Ramsundar P, Livesey F, Probert D (2009) Proposed classification scheme for direct writing technologies. Rapid Prototyp J 15:299–309. https://doi.org/10.1108/13552540910979811
Munoz-Abraham AS, Rodriguez-Davalos MI, Bertacco A, Wengerter B, Geibel JP, Mulligan DC (2016) 3D printing of organs for transplantation: where are we and where are we heading? Curr Transplant Rep 3:93–99. https://doi.org/10.1007/s40472-016-0089-6
Zimmerman BA, Allen EE (2013) Analysis of the potential impact of additive manufacturing on Army logistics. Thesis, Naval Postgraduate School, Monterey, CA, United States. Available via https://calhoun.nps.edu/handle/10945/38870. Accessed 29 April 2021
Srinivasan V, Bassan J (2012) 3D Printing and the Future of Manufacturing. Computer Sciences Corporation, Leading Edge Forum. Available via https://leadingedgeforum.com/media/1917/3d-printing-and-the-future-of-manufacturing.pdf. Accessed 29 April 2021
Morgan JA, Prentiss JM (2014) An analysis of item identification for additive manufacturing (3-D printing) within the Naval supply chain. Dissertation, Naval Postgraduate School, Monterey, CA, United States. Available via https://calhoun.nps.edu/handle/10945/44623. Accessed 29 April 2021
Oliveira JP, Santos TG, Miranda RM (2020) Revisiting fundamental welding concepts to improve additive manufacturing: From theory to practice. Prog Mater Sci 107:100590. https://doi.org/10.1016/j.pmatsci.2019.100590
Sager A (2019) 3D scanning with artec spider for healthcare. Accucode3D. Available via https://accucode3d.com/3d-scanning-with-artec-spider-for-healthcare/. Accessed 29 April 2021
Ransikarbum K, Pitakaso R, Kim N (2020) A Decision-Support Model for Additive Manufacturing Scheduling Using an Integrative Analytic Hierarchy Process and Multi-Objective Optimization. Applied Sciences, 10(15):5159:1–16. https://doi.org/10.3390/app10155159
Coon C, Pretzel B, Lomax T, Strlič M (2016) Preserving rapid prototypes: a review. Heritage Sci 4(1):1–16. https://doi.org/10.1186/s40494-016-0097-y
Chen Z, Li Z, Li J, Liu C, Lao C, Fu Y, Liu C, Li Y, Wang P, He Y (2019) 3D printing of ceramics: a review. J Eur Ceram Soc 39:661–687. https://doi.org/10.1016/j.jeurceramsoc.2018.11.013
Sohrabpoor H, Negi S, Shaiesteh H, Ahad I, Brabazon D (2018) Optimizing selective laser sintering process by grey relational analysis and soft computing techniques. Optik 174:185–194
Ziaee M, Crane NB (2019) Binder jetting: a review of process, materials, and methods. Addit Manuf 28:781–801
Waris E, Ashammakhi N, Lehtimäki M, Tulamo R-M, Kellomäki M, Törmälä P, Konttinen YT (2008) The use of biodegradable scaffold as an alternative to silicone implant arthroplasty for small joint reconstruction: an experimental study in minipigs. Biomaterials 29:683–691
Cunico MWM (2019) 3D printers and additive manufacturing: the rise of the industry 4.0, 1st edn. Concep3D Pesquisas Científicas, Curitiba
Verma S, Sharma N, Kango S, Sharma S (2021) Developments of PEEK (Polyetheretherketone) as a biomedical material: a focused review. Eur Polymer J 147:110295. https://doi.org/10.1016/j.eurpolymj.2021.110295
Arif M, Kumar S, Varadarajan K, Cantwell W (2018) Performance of biocompatible PEEK processed by fused deposition additive manufacturing. Mater Des 146:249–259
Singh S, Prakash C, Ramakrishna S (2019) 3D printing of polyether-ether-ketone for biomedical applications. Eur Polymer J 114:234–248
Polacek M, Nyegaard CP, Høien F (2020) Day-case opening wedge high tibial osteotomy with intraosseous PEEK implant. Arthrosc Sports Med Rehabil 2:e145–e151
Kolb W, Guhlmann H, Windisch C, Marx F, Koller H, Kolb K (2010) Fixation of periprosthetic femur fractures above total knee arthroplasty with the less invasive stabilization system: a midterm follow-up study. J Trauma Acute Care Surg 69:670–676
Cowie RM, Briscoe A, Fisher J, Jennings LM (2016) PEEK-OPTIMATM as an alternative to cobalt chrome in the femoral component of total knee replacement: a preliminary study. Proc Inst Mech Eng [H] 230:1008–1015
de Ciurana J, Serenóa L, Vallès È (2013) Selecting process parameters in RepRap additive manufacturing system for PLA scaffolds manufacture. Proc Cirp 5:152–157
Rabionet M, Guerra AJ, Puig T, Ciurana J (2018) 3D-printed tubular scaffolds for vascular tissue engineering. Proc Cirp 68:352–357
Almeida HA, Bartolo PJ (2014) Design of tissue engineering scaffolds based on hyperbolic surfaces: Structural numerical evaluation. Med Eng Phys 36:1033–1040
Almeida HA, Bártolo PJ (2013) Topological optimisation of scaffolds for tissue engineering. Proc Eng 59:298–306
Duan P, Pan Z, Cao L, Gao J, Yao H, Liu X, Guo R, Liang X, Dong J, Ding J (2019) Restoration of osteochondral defects by implanting bilayered poly (lactide-co-glycolide) porous scaffolds in rabbit joints for 12 and 24 weeks. J Orthop Transl 19:68–80
Zheng Y, Han Q, Li D, Sheng F, Song Z, Wang J (2021) Promotion of tendon growth into implant through pore-size design of a Ti-6Al-4V porous scaffold prepared by 3D printing. Mater Des 197:109219
Dziadek M, Stodolak-Zych E, Cholewa-Kowalska K (2017) Biodegradable ceramic-polymer composites for biomedical applications: a review. Mater Sci Eng C 71:1175–1191
Domingos M, Gloria A, Coelho J, Bartolo P, Ciurana J (2017) Three-dimensional printed bone scaffolds: the role of nano/micro-hydroxyapatite particles on the adhesion and differentiation of human mesenchymal stem cells. Proc Inst Mech Eng [H] 231:555–564
Rabionet M, Polonio E, Guerra AJ, Martin J, Puig T, Ciurana J (2018) Design of a scaffold parameter selection system with additive manufacturing for a biomedical cell culture. Materials 11:1427
Rabionet M, Puig T, Ciurana J (2020) Manufacture of PCL scaffolds through electrospinning technology to accommodate Triple Negative Breast Cancer cells culture. Proc CIRP 89:98–103
Guerra A, Roca A, de Ciurana J (2017) A novel 3D additive manufacturing machine to biodegradable stents. Proc Manuf 13:718–723
Guerra AJ, Ciurana J (2018) 3D-printed bioabsordable polycaprolactone stent: the effect of process parameters on its physical features. Mater Des 137:430–437
Guerra AJ, Ciurana JD (2019) Three-dimensional tubular printing of bioabsorbable stents: the effects process parameters have on in vitro degradation. 3 D Print Addit Manuf 6:50–56
McMahon S, Bertollo N, O’Cearbhaill ED, Salber J, Pierucci L, Duffy P, Dürig T, Bi V, Wang W (2018) Bio-resorbable polymer stents: a review of material progress and prospects. Prog Polym Sci 83:79–96
Kodama H (1981) Automatic method for fabricating a three-dimensional plastic model with photo-hardening polymer. Rev Sci Instrum 52:1770–1773
Gibson I, Rosen DW, Stucker B (2009) Additive manufacturing technologies: rapid prototyping to direct digital manufacturing, 1st edn. Springer, Boston. https://doi.org/10.1007/978-1-4419-1120-9
Wu H, Cheng Y, Liu W, He R, Zhou M, Wu S, Song X, Chen Y (2016) Effect of the particle size and the debinding process on the density of alumina ceramics fabricated by 3D printing based on stereolithography. Ceram Int 42:17290–17294
Liou FW (2007) Rapid prototyping and engineering applications: a toolbox for prototype development, 1st edn. CRC Press, Boca Raton. https://doi.org/10.1201/9781420014105
Cooper K (2001) Rapid prototyping technology: selection and application, 1st edn. CRC press, New York. https://doi.org/10.1201/9780203910795
3DSYSTEMS (2011) 3DSYSTEMS.
Varma MV, Kandasubramanian B, Ibrahim SM (2020) 3D printed scaffolds for biomedical applications. Mater Chem Phys 255:123642
Zhou T, Zhang L, Yao Q, Ma Y, Hou C, Sun B, Shao C, Gao P, Chen H (2020) SLA 3D printing of high quality spine shaped β-TCP bioceramics for the hard tissue repair applications. Ceram Int 46:7609–7614
Pereira RF, Almeida HA, Bártolo PJ (2013) Biofabrication of hydrogel constructs. In: Coelho J. (ed) Drug delivery systems: advanced technologies potentially applicable in personalised treatment, 1st edn. Springer, Dordrecht, p 225–254. https://doi.org/10.1007/978-94-007-6010-3_8
Chartrain NA, Williams CB, Whittington AR (2018) A review on fabricating tissue scaffolds using vat photopolymerization. Acta Biomater 74:90–111
Szymczyk-Ziółkowska P, Łabowska MB, Detyna J, Michalak I, Gruber P (2020) A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern Biomed Eng 40:624–638
Kim SH, Yeon YK, Lee JM, Chao JR, Lee YJ, Seo YB, Sultan MT, Lee OJ, Lee JS, Yoon S-i (2018) Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat Commun 9:1–14
Wong KV, Hernandez A (2012) A review of additive manufacturing. In: ISRN Mechanical Engineering 2012
Negi S, Nambolan AA, Kapil S, Joshi PS, Manivannan R, Karunakaran K, Bhargava P (2019) Review on electron beam based additive manufacturing. Rapid Prototyp J 26(3):485–498. https://doi.org/10.1108/RPJ-07-2019-0182
Aboulkhair NT, Simonelli M, Parry L, Ashcroft I, Tuck C, Hague R (2019) 3D printing of aluminium alloys: additive manufacturing of aluminium alloys using selective laser melting. Prog Mater Sci 106:100578
Pupo Y, Monroy KP, Ciurana J (2015) Influence of process parameters on surface quality of CoCrMo produced by selective laser melting. Int J Adv Manuf Technol 80:985–995
Ciurana J, Hernandez L, Delgado J (2013) Energy density analysis on single tracks formed by selective laser melting with CoCrMo powder material. Int J Adv Manuf Technol 68:1103–1110
Delgado J, Ciurana J, Rodríguez CA (2012) Influence of process parameters on part quality and mechanical properties for DMLS and SLM with iron-based materials. Int J Adv Manuf Technol 60:601–610
Cunico MWM (2015) Impressoras 3D: O novo meio Produtivo, 1st edn. Concep3D Pesquisas Científicas, Curitiba
Bartolo P, Kruth J-P, Silva J, Levy G, Malshe A, Rajurkar K, Mitsuishi M, Ciurana J, Leu M (2012) Biomedical production of implants by additive electro-chemical and physical processes. CIRP Ann 61:635–655
Busachi A, Erkoyuncu J, Colegrove P, Martina F, Watts C, Drake R (2017) A review of additive manufacturing technology and cost estimation techniques for the defence sector. CIRP J Manuf Sci Technol 19:117–128. https://doi.org/10.1016/j.cirpj.2017.07.001
Hryha E, Shvab R, Bram M, Bitzer M, Nyborg L (2016) Surface chemical state of Ti powders and its alloys: effect of storage conditions and alloy composition. Appl Surf Sci 388:294–303
Horstkotte R, Heinrich F, Prümmer M, Arntz K, Bergs T (2021) Generation and evaluation of automation concepts of additive process chains with Laser Powder Bed Fusion (L-PBF). Procedia CIRP 96:97–102
Chen R, Yin H, Cole IS, Shen S, Zhou X, Wang Y, Tang S (2020) Exposure, assessment and health hazards of particulate matter in metal additive manufacturing: a review. Chemosphere, 259:127452. https://doi.org/10.1016/j.chemosphere.2020.127452
Powell D, Rennie A, Geekie L, Burns N (2020) Understanding powder degradation in metal additive manufacturing to allow the upcycling of recycled powders. J Clean Prod, 268:122077. https://doi.org/10.1016/j.jclepro.2020.122077
Dallago M, Zanini F, Carmignato S, Pasini D, Benedetti M (2018) Effect of the geometrical defectiveness on the mechanical properties of SLM biomedical Ti6Al4V lattices. Proc Struct Integr 13:161–167
Migaud H, Common H, Girard J, Huten D, Putman S (2019) Acetabular reconstruction using porous metallic material in complex revision total hip arthroplasty: a systematic review. Orthop Traumatol Surg Res 105:S53–S61. https://doi.org/10.1016/j.otsr.2018.04.030
Revilla-León M, Sadeghpour M, Özcan M (2020) A review of the applications of additive manufacturing technologies used to fabricate metals in implant dentistry. J Prosthodontics 29:579–593. https://doi.org/10.1111/jopr.13212
Johnson AJ, Harwin SF, Krackow KA, Mont MA (2011) Alignment in total knee arthroplasty: where have we come from and where are we going? Surg Technol Int 21:183–188
Song X, Feih S, Zhai W, Sun C-N, Li F, Maiti R, Wei J, Yang Y, Oancea V, Brandt LR (2020) Advances in additive manufacturing process simulation: residual stresses and distortion predictions in complex metallic components. Mater Des 193:108779
Almeida HA, Costa AF, Ramos C, Torres C, Minondo M, Bártolo PJ, Nunes A, Kemmoku D, da Silva JVL (2019) Additive manufacturing systems for medical applications: case studies. In: Pei E, Monzón M, Bernard A (eds) Additive manufacturing—developments in training and education, 1st edn. Springer, Cham. p 187–209. https://doi.org/10.1007/978-3-319-76084-1_13https://doi.org/10.1007/978-3-319-76084-1_13
Puppi D, Chiellini F (2020) Biodegradable polymers for biomedical additive manufacturing. Appl Mater Today 20:100700. https://doi.org/10.1016/j.apmt.2020.100700
Attar H, Bermingham M, Ehtemam-Haghighi S, Dehghan-Manshadi A, Kent D, Dargusch M (2019) Evaluation of the mechanical and wear properties of titanium produced by three different additive manufacturing methods for biomedical application. Mater Sci Eng, A 760:339–345
Buciumeanu M, Bagheri A, Shamsaei N, Thompson S, Silva F, Henriques B (2018) Tribocorrosion behavior of additive manufactured Ti-6Al-4V biomedical alloy. Tribol Int 119:381–388
Harun W, Manam N, Kamariah M, Sharif S, Zulkifly A, Ahmad I, Miura H (2018) A review of powdered additive manufacturing techniques for Ti-6Al-4V biomedical applications. Powder Technol 331:74–97
Bandyopadhyay A, Dittrick S, Gualtieri T, Wu J, Bose S (2016) Calcium phosphate–titanium composites for articulating surfaces of load-bearing implants. J Mech Behav Biomed Mater 57:280–288
Mostafaei A, Elliott AM, Barnes JE, Li F, Tan W, Cramer CL, Nandwana P, Chmielus M (2020) Binder jet 3D printing—process parameters, materials, properties, modeling, and challenges. Prog Mater Sci. https://doi.org/10.1016/j.pmatsci.2020.100707
Hong D, Chou D-T, Velikokhatnyi OI, Roy A, Lee B, Swink I, Issaev I, Kuhn HA, Kumta PN (2016) Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater 45:375–386
Seidenstuecker M, Kerr L, Bernstein A, Mayr HO, Suedkamp NP, Gadow R, Krieg P, Hernandez Latorre S, Thomann R, Syrowatka F, Esslinger S (2017) 3D Powder printed bioglass and β-tricalcium phosphate bone scaffolds. Materials (Basel, Switzerland). https://doi.org/10.3390/ma11010013
Sun C, Tian X, Wang L, Liu Y, Wirth CM, Günster J, Li D, Jin Z (2017) Effect of particle size gradation on the performance of glass-ceramic 3D printing process. Ceram Int 43:578–584
De Melo BR, Salmoria G, Wirth C, Hotza D, Stares S, Günster J (2018) Manufacturing of SiO 2-coated β-TCP structures by 3D printing using a preceramic polymer as printing binder and silica source. J Ceram Sci Technol 9:37–42
Shao H, Sun M, Zhang F, Liu A, He Y, Fu J, Yang X, Wang H, Gou Z (2018) Custom repair of mandibular bone defects with 3D printed bioceramic scaffolds. J Dent Res 97:68–76
Wei Q, Wang Y, Li X, Yang M, Chai W, Wang K (2016) Study the bonding mechanism of binders on hydroxyapatite surface and mechanical properties for 3DP fabrication bone scaffolds. J Mech Behav Biomed Mater 57:190–200
Wei Q, Wang Y, Chai W, Zhang Y, Chen X (2017) Molecular dynamics simulation and experimental study of the bonding properties of polymer binders in 3D powder printed hydroxyapatite bioceramic bone scaffolds. Ceram Int 43:13702–13709
Wu W, Ye C, Zheng Q, Wu G, Cheng Z (2016) A therapeutic delivery system for chronic osteomyelitis via a multi-drug implant based on three-dimensional printing technology. J Biomater Appl 31:250–260. https://doi.org/10.1177/0885328216640660
Chen G, Xu Y, Chi Lip Kwok P, Kang L (2020) Pharmaceutical applications of 3D printing. Addit Manuf 34:101209. https://doi.org/10.1016/j.addma.2020.101209
Jose P, Christoper P (2018) 3D printing of pharmaceuticals—a potential technology in developing personalized medicine. Asian J Pharm Res Dev 6:46–54. https://doi.org/10.22270/ajprd.v6i3.375
Salmi M (2016) Possibilities of preoperative medical models made by 3D printing or additive manufacturing. J Med Eng 2016:6191526–6191526. https://doi.org/10.1155/2016/6191526
Kondo K, Harada N, Masuda H, Sugo N, Terazono S, Okonogi S, Sakaeyama Y, Fuchinoue Y, Ando S, Fukushima D, Nomoto J, Nemoto M (2016) A neurosurgical simulation of skull base tumors using a 3D printed rapid prototyping model containing mesh structures. Acta Neurochir 158:1213–1219. https://doi.org/10.1007/s00701-016-2781-9
Tai BL, Kao Y-T, Payne N, Zheng Y, Chen L, Shih AJ (2018) 3D Printed composite for simulating thermal and mechanical responses of the cortical bone in orthopaedic surgery. Med Eng Phys 61:61–68. https://doi.org/10.1016/j.medengphy.2018.08.004
Trombetta R, Inzana JA, Schwarz EM, Kates SL, Awad HA (2017) 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Ann Biomed Eng 45:23–44. https://doi.org/10.1007/s10439-016-1678-3
MENDRICKY R, (2016) Accuracy analysis of additive technique for parts manufacturing. MM Sci J 2016:1502–1508
Stratasys (2011) Objet eden260VS catalogue. Available via https://pdf.directindustry.com/pdf/stratasys/objet-eden260vs/25745-793765.html. Accessed 29 April 2021
Santos ARC, Almeida HA, Bártolo PJ (2013) Additive manufacturing techniques for scaffold-based cartilage tissue engineering. Virt Phys Prototyp 8:175–186. https://doi.org/10.1080/17452759.2013.838825
Tappa K, Jammalamadaka U (2018) Novel biomaterials used in medical 3D printing techniques. J Funct Biomater. https://doi.org/10.3390/jfb9010017
Camardella LT, de Vasconcellos VO, Breuning H (2017) Accuracy of printed dental models made with 2 prototype technologies and different designs of model bases. Am J Orthod Dentofac Orthop 151:1178–1187
Kiefer O, Breitkreutz J (2020) Comparative investigations on key factors and print head designs for pharmaceutical inkjet printing. Int J Pharm 586:119561
Liu W, Song H, Huang C (2020) Maximizing mechanical properties and minimizing support material of PolyJet fabricated 3D lattice structures. Addit Manuf 35:101257. https://doi.org/10.1016/j.addma.2020.101257
Egan PF, Bauer I, Shea K, Ferguson SJ (2019) Mechanics of three-dimensional printed lattices for biomedical devices. J Mech Des 141(3):031703. https://doi.org/10.1115/1.4042213
Bourell D, Kruth J-P, Leu M, Levy G, Rosen D, Beese A, Clare A (2017) Materials for additive manufacturing. CIRP Ann Manuf Technol. https://doi.org/10.1016/j.cirp.2017.05.009
Zhang G, Guo J, Chen H, Cao Y (2020) Organic mesh template-based laminated object manufacturing to fabricate ceramics with regular micron scaled pore structures. J Eur Ceram Soc 41(4):2790–2795. https://doi.org/10.1016/j.jeurceramsoc.2020.11.012
Ahangar P, Cooke ME, Weber MH, Rosenzweig DH (2019) Current biomedical applications of 3D printing and additive manufacturing. Appl Sci 9:1713
Pham DT, Gault RS (1998) A comparison of rapid prototyping technologies. Int J Mach Tools Manuf 38:1257–1287
Krunić S, Perinić M, Maričić S (2010) Rapid Prototyping application. Eng Rev 30(2):91–100. https://hrcak.srce.hr/63642
Mahesh M, Wong Y, Fuh J, Loh H (2004) Benchmarking for comparative evaluation of RP systems and processes. Rapid Prototyp J 10:123–135
Paital SR, Dahotre NB (2009) Calcium phosphate coatings for bio-implant applications: materials, performance factors, and methodologies. Mater Sci Eng R Rep 66:1–70
Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2004) Biomaterials science: an introduction to materials in medicine, 2nd edn. Academic Press, Oxford. https://doi.org/10.1016/C2009-0-02433-7
Di Prima M, Coburn J, Hwang D, Kelly J, Khairuzzaman A, Ricles L (2016) Additively manufactured medical products–the FDA perspective. 3D Print Med 2:1–6
Park JB, Bronzino JD (2002) Biomaterials: principles and applications, 1st edn. CRC Press, Boca Raton. https://doi.org/10.1201/9781420040036
Xie H, Zhang L, Xu E, Yuan H, Zhao F, Gao J (2019) SiAlON–Al2O3 ceramics as potential biomaterials. Ceram Int 45(14):16809–16813. https://doi.org/10.1016/j.ceramint.2019.05.221
Singare S, Dichen L, Bingheng L, Yanpu L, Zhenyu G, Yaxiong L (2004) Design and fabrication of custom mandible titanium tray based on rapid prototyping. Med Eng Phys 26:671–676
Enderle J, Bronzino J (2012) Introduction to biomedical engineering, 3rd edn. Academic Press, Oxford. https://doi.org/10.1016/C2009-0-19716-7
Murr LE, Gaytan SM, Martinez E, Medina F, Wicker RB (2012) Next generation orthopaedic implants by additive manufacturing using electron beam melting. Int J Biomater 2012:245727:1–14. https://doi.org/10.1155/2012/245727
Webb M (2018) Market intel: innovation drives growth, draws younger patients in joint replacement implants market. Medtech insight, informa pharma intelligence. Available via https://medtech.pharmaintelligence.informa.com/MT123216/Market-Intel-Innovation-Drives-Growth-Draws-Younger-Patients-In-Joint-Replacement-Implants-Market. Accessed 29 April 2021
Ahn D-G, Lee J-Y, Yang D-Y (2006) Rapid prototyping and reverse engineering application for orthopedic surgery planning. J Mech Sci Technol 20:19
Ciurana J (2014) Designing, prototyping and manufacturing medical devices: an overview. Int J Comput Integr Manuf 27:901–918
Bukač M, Čanić S, Tambača J, Wang Y (2019) Fluid–structure interaction between pulsatile blood flow and a curved stented coronary artery on a beating heart: A four stent computational study. Comput Methods Appl Mech Eng 350:679–700
Guerra AJ, Farjas J, Ciurana J (2017) Fibre laser cutting of polycaprolactone sheet for stents manufacturing: a feasibility study. Opt Laser Technol 95:113–123
Demir AG, Previtali B (2017) Additive manufacturing of cardiovascular CoCr stents by selective laser melting. Mater Des 119:338–350. https://doi.org/10.1016/j.matdes.2017.01.091
Tofail SA, Koumoulos EP, Bandyopadhyay A, Bose S, O’Donoghue L, Charitidis C (2018) Additive manufacturing: scientific and technological challenges, market uptake and opportunities. Mater Today 21:22–37
Ghasemi-Mobarakeh L, Kolahreez D, Ramakrishna S, Williams D (2019) Key terminology in biomaterials and biocompatibility. Curr Opin Biomed Eng 10:45–50. https://doi.org/10.1016/j.cobme.2019.02.004
Poinern GEJ, Brundavanam S, Fawcett D (2012) Biomedical magnesium alloys: a review of material properties, surface modifications and potential as a biodegradable orthopaedic implant. Am J Biomed Eng 2:218–240
Hanawa T (2009) Materials for metallic stents. J Artif Organs 12:73–79
Davis JR (2003) Handbook of materials for medical devices, 1st edn. ASM International, Materials Park
Borah J, Webster J (2006) Measurement techniques for eye movement. Encycl Med Dev Instrum 3:263–286
Kadkhodapour J, Montazerian H, Darabi AC, Anaraki A, Ahmadi S, Zadpoor A, Schmauder S (2015) Failure mechanisms of additively manufactured porous biomaterials: effects of porosity and type of unit cell. J Mech Behav Biomed Mater 50:180–191
Li C, Lei H, Zhang Z, Zhang X, Zhou H, Wang P, Fang D (2020) Architecture design of periodic truss-lattice cellsfor additive manufacturing. Addit Manuf 24:101172. https://doi.org/10.1016/j.addma.2020.101172
Plocher J, Panesar A (2019) Review on design and structural optimisation in additive manufacturing: Towards next-generation lightweight structures. Mater Des 183:108164. https://doi.org/10.1016/j.matdes.2019.108164
Okazaki Y, Gotoh E (2005) Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 26:11–21
Manivasagam G, Dhinasekaran D, Rajamanickam A (2010) Biomedical implants: corrosion and its prevention—a review. Recent pat corros sci 2(1):40–54. https://doi.org/10.2174/1877610801002010040
Brockett CL, Harper P, Williams S, Isaac GH, Dwyer-Joyce RS, Jin Z, Fisher J (2008) The influence of clearance on friction, lubrication and squeaking in large diameter metal-on-metal hip replacements. J Mater Sci Mater Med 19:1575–1579
Murphy W, Black J, Hastings G (2016) Handbook of biomaterial properties, 2nd edn. Springer-Verlag, New York. https://doi.org/10.1007/978-1-4939-3305-1
Yaszemski MJ, Trantolo DJ, Lewandrowski KU, Hasirci V, Altobelli DE, Wise DL (2003) Biomaterials in orthopedics, 2nd edn. CRC Press, Boca Raton. https://doi.org/10.1201/b14227
Hin TS (2004) Introduction to biomatrerials engineering and processing-an overview. Eng Mater Biomed Appl, pp 1–16
Rasouli R, Barhoum A, Uludag H (2018) A review of nanostructured surfaces and materials for dental implants: surface coating, patterning and functionalization for improved performance. Biomater Sci 6:1312–1338
Lütjering G, Williams JC (2003) Titanium, 2, end. Springer, Clumbus, pp 17–156
Hench LL, Best SM (2013) Ceramics, glasses, and glass-ceramics: basic principles. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (eds) Biomaterials science: an introduction to materials in medicine, 3rd edn. Elsevier, p 128–151. https://doi.org/10.1016/B978-0-08-087780-8.00016-4
Dee KC, Puleo DA, Bizios R (2003) An introduction to tissue-biomaterial interactions, 1st edn. Wiley, Hoboken. https://doi.org/10.1002/0471270598
Santin M, Phillips GJ (2012) History of biomimetic, bioactive and bioresponsive biomaterials. In: Santin M, Phillips GJ (eds) Biomimetic, bioresponsive, and bioactive materials: an introduction to integrating materials with tissues, 1st edn. Wiley, Hoboken, p 1–34. https://doi.org/10.1002/9781118129906
Haubenreich JE, Robinson FG, West KP, Frazer RQ (2005) Did we push dental ceramics too far? A brief history of ceramic dental implants. J Long-term Effects Med Implants 15
Afzal A (2014) Implantable zirconia bioceramics for bone repair and replacement: a chronological review. Mater Express 4:1–12
Bohner M (2000) Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury 31:D37–D47
Heath DE, Cooper SL (2013) Polymers: basic principles. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (eds) Biomaterials Science: an introduction to materials in medicine, 3rd edn. Elsevier, p 64–79. https://doi.org/10.1016/B978-0-08-087780-8.00008-5
Guerra AJ, Cano P, Rabionet M, Puig T, Ciurana J (2018) 3D-printed PCL/PLA composite stents: towards a new solution to cardiovascular problems. Materials 11:1679
Ciurana J, Rodríguez CA (2017) Trends in nanomaterials and processing for drug delivery of polyphenols in the treatment of cancer and other therapies. Curr Drug Targets 18:135–146. https://doi.org/10.2174/1389450116666151102094738
Chiellini F, Ferri M, Morelli A, Dipaola L, Latini G (2013) Perspectives on alternatives to phthalate plasticized poly (vinyl chloride) in medical devices applications. Prog Polym Sci 38:1067–1088
Nogueira N, Conde O, Minones M, Trillo J, Minones J Jr (2012) Characterization of poly (2-hydroxyethyl methacrylate)(PHEMA) contact lens using the Langmuir monolayer technique. J Colloid Interface Sci 385:202–210
Francis MP, Kemper N, Maghdouri-White Y, Thayer N (2018) Additive manufacturing for biofabricated medical device applications. In: Zhang J, Jung YG (eds) Additive manufacturing, 1st edn. Elsevier, p 311–344. https://doi.org/10.1016/B978-0-12-812155-9.00009-8
Habraken W, Wolke J, Jansen J (2007) Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev 59:234–248
Biscaia SI, Viana TF, Almeida HA, Bártolo PJ (2015) Production and characterisation of PCL/ES scaffolds for bone tissue engineering. Mater Today Proc 2:208–216. https://doi.org/10.1016/j.matpr.2015.04.024
Katti KS (2004) Biomaterials in total joint replacement. Colloids Surf B 39:133–142
Niu G, Criswell T, Sapoznik E, Lee S, Soker S (2013) The influence of cross-linking methods on the mechanical and biocompatible properties of vascular scaffold. J Sci Appl Biomed 1:1–7
Vanaei S, Parizi M, Salemizadehparizi F, Vanaei H (2021) An overview on materials and techniques in 3D bioprinting toward biomedical application. Eng Regener 2:1–18
Mao H, Yang L, Zhu H, Wu L, Ji P, Yang J, Gu Z (2020) Recent advances and challenges in materials for 3D bioprinting. Progress Nat Sci Mater Int 30(5):618–634. https://doi.org/10.1016/j.pnsc.2020.09.015
McCarthy RR, Ullah MW, Booth P, Pei E, Yang G (2019) The use of bacterial polysaccharides in bioprinting. Biotechnol Adv 37:107448
Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR (2009) Organ printing: Tissue spheroids as building blocks. Biomaterials 30:2164–2174. https://doi.org/10.1016/j.biomaterials.2008.12.084
Kim EM, Lee YB, Kim S-j, Park J, Lee J, Kim SW, Park H, Shin H (2019) Fabrication of core-shell spheroids as building blocks for engineering 3D complex vascularized tissue. Acta Biomater 100:158–172. https://doi.org/10.1016/j.actbio.2019.09.028
França FS, Garrido dos Santos M, Prestes JP, Alcântara B, Borges MF, Pranke P (2021) Bioprinting: a promising approach for tissue regeneration. Bioprinting. https://doi.org/10.1016/j.bprint.2021.e00130
Wang J-Z, Xiong N-Y, Zhao L-Z, Hu J-T, Kong D-C, Yuan J-Y (2018) Review fantastic medical implications of 3D-printing in liver surgeries, liver regeneration, liver transplantation and drug hepatotoxicity testing: a review. Int J Surg 56:1–6
Zhang Y, Kumar P, Lv S, Xiong D, Zhao H, Cai Z, Zhao X (2021) Recent advances in 3D bioprinting of vascularized tissues. Mater Des 199:109398. https://doi.org/10.1016/j.matdes.2020.109398
Naghieh S, Chen D (2021) Printability—a key issue in extrusion-based bioprinting. J Pharm Anal. https://doi.org/10.1016/j.jpha.2021.02.001
Zadpoor A (2019) Meta-biomaterials. Biomater Sci. https://doi.org/10.1039/C9BM01247H
Xiao J, Wan Y, Yang Z, Huang Y, Yao F, Luo H (2019) Bioactive glass nanotube scaffold with well-ordered mesoporous structure for improved bioactivity and controlled drug delivery. J Mater Sci Technol 35:1959–1965. https://doi.org/10.1016/j.jmst.2019.04.027
Parikh SD, Dave S, Huang L, Wang W, Mukhopadhyay SM, Mayes DA (2020) Multi-walled carbon nanotube carpets as scaffolds for U87MG glioblastoma multiforme cell growth. Mater Sci Eng C 108:110345. https://doi.org/10.1016/j.msec.2019.110345
Velu R, Calais T, Jayakumar A, Raspall F (2020) A comprehensive review on bio-nanomaterials for medical implants and feasibility studies on fabrication of such implants by additive manufacturing technique. Materials 13:92
Nie W, Peng C, Zhou X, Chen L, Wang W, Zhang Y, Ma PX, He C (2017) Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 116:325–337. https://doi.org/10.1016/j.carbon.2017.02.013
Magaz A, Li X, Gough JE, Blaker JJ (2021) Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater Sci Eng C 119:111632. https://doi.org/10.1016/j.msec.2020.111632
Zhang C, Wang X, Liu A, Pan C, Ding H, Ye W (2021) Reduced graphene oxide/titanium dioxide hybrid nanofiller-reinforced electrospun silk fibroin scaffolds for tissue engineering. Mater Lett. https://doi.org/10.1016/j.matlet.2021.129563
Tiwari S, Patil R, Dubey SK, Bahadur P (2020) Graphene nanosheets as reinforcement and cell-instructive material in soft tissue scaffolds. Adv Coll Interface Sci 281:102167. https://doi.org/10.1016/j.cis.2020.102167
Yuan X, Chen M, Yao Y, Guo X, Huang Y, Peng Z, Xu B, Lv B, Tao R, Duan S, Liao H, Yao K, Li Y, Lei H, Chen X, Hong G, Fang D (2021) Recent progress in the design and fabrication of multifunctional structures based on metamaterials. Curr Opin Solid State Mater Sci 25:100883. https://doi.org/10.1016/j.cossms.2020.100883
Mohammadi K, Movahhedy MR, Shishkovsky I, Hedayati R (2020) Hybrid anisotropic pentamode mechanical metamaterial produced by additive manufacturing technique. Appl Phys Lett 117:061901
Babaee S, Pajovic S, Kirtane AR, Shi J, Caffarel-Salvador E, Hess K, Collins JE, Tamang S, Wahane AV, Hayward AM (2019) Temperature-responsive biometamaterials for gastrointestinal applications. Sci Transl Med 11(488):1–13. https://doi.org/10.1126/scitranslmed.aau8581
Wei Y-L, Yang Q-S, Ma L-H, Tao R, Shang J-J (2020) Design and analysis of 2D/3D negative hydration expansion Metamaterial driven by hydrogel. Mater Des 196:109084
Lee H-K, Choi J-W, Choi S-J (2020) Magnetic ion-imprinted polymer based on mesoporous silica for selective removal of Co(II) from radioactive wastewater. Sep Sci Technol. https://doi.org/10.1080/01496395.2020.1797798
Han X, Zhang Z, Qu X (2021) A novel miniaturized tri-band metamaterial THz absorber with angular and polarization stability. Optik 228:166086. https://doi.org/10.1016/j.ijleo.2020.166086
Yang K, Li J, de la Chapelle ML, Huang G, Wang Y, Zhang J, Xu D, Yao J, Yang X, Fu W (2021) A terahertz metamaterial biosensor for sensitive detection of microRNAs based on gold-nanoparticles and strand displacement amplification. Biosens Bioelectron 175:112874
Palai G, Kisan S, Das A (2018) A proposal for bio-medical device to measure GUS in human blood using metamaterial. Optik 164:138–142
Kamal M, Ziyab A, Bartella A, Mitchell D, Al-Asfour A, Hölzle F, Kessler P, Lethaus B (2018) Volumetric comparison of autogenous bone and tissue-engineered bone replacement materials in alveolar cleft repair: a systematic review and meta-analysis. Br J Oral Maxillofac Surg 56:453–462
Cui L, Xiang S, Chen D, Fu R, Zhang X, Chen J, Wang X (2021) A novel tissue-engineered bone graft composed of silicon-substituted calcium phosphate, autogenous fine particulate bone powder and BMSCs promotes posterolateral spinal fusion in rabbits. J Orthop Transl 26:151–161
Pelttari K, Rua LA, Mumme M, Manferdini C, Darwiche S, Khalil A, Buchner D, Lisignoli G, Occhetta P, von Rechenberg B (2020) Engineered nasal cartilage for the repair of osteoarthritic knee cartilage defects. Cytotherapy 22:S14
Author information
Authors and Affiliations
Corresponding author
Additional information
In memoriam. This paper is dedicated to Dr. Wisley Falco Sales, who lost his life to COVID19.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, L.R.R., Sales, W.F., Campos, F.d.R. et al. A comprehensive review on additive manufacturing of medical devices. Prog Addit Manuf 6, 517–553 (2021). https://doi.org/10.1007/s40964-021-00188-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40964-021-00188-0