Abstract
The heat treatment behavior of semi-multi-alloyed white cast iron with relatively lower carbide-forming elements compared with regular multi-alloyed white cast iron was investigated. The semi-multi-alloyed white cast iron with carbon balance (Cbal) of − 0.68 to + 0.53 wt% was prepared by varying chromium (Cr) content from around 3–9 wt% under the fixed contents of 2 wt% molybdenum (Mo), 1 wt% tungsten (W) and 5 wt% vanadium (V). After annealing, the test specimens were hardened after austenitizing at 1050 °C and 1100 °C. The tempering was carried out between 400 and 600 °C. In as-hardened state, the macro-hardness and micro-hardness increased greatly as the Cbal rose from − 0.68 to − 0.01 wt% and then decreased gradually with an increase in the Cbal value. The volume fraction of retained austenite (Vγ) increased continuously as the Cbal increased, and more Vγ was obtained in the case of hardening from higher austenitizing temperature. In tempered state, the hardness curve showed an evident secondary hardening due to the precipitation of fine secondary carbides during holding and the transformation of retained austenite to martensite during post-cooling. The maximum tempered macro-hardness (HTmax) was obtained by tempering at 500–550 °C. The highest values, 862 HV30 and 795 HV0.1, were obtained in the specimen with − 0.01 wt%Cbal where the Vγ was about 2%. As the Cbal value increased, the maximum tempered micro-hardness (HTmax-M) increased remarkably toward − 0.01 wt%Cbal and then it decreased regardless of austenitizing temperature. At the same Cbal value, the HTmax-M of specimens hardened from 1100 °C were larger than those hardened from 1050 °C. It was found that the 10–50% Vγ in as-hardened state was necessary to obtain hardness over 800 HV30 and 750 HV0.1 by tempering. The degree of secondary hardening (ΔHs) increased with an increase in the Cbal and the Vγ in the as-hardened state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the steel-making, mining and cement industries, various kinds of machines are used for steel rolling, crushing and pulverizing of minerals and their products. Severe abrasive wear takes place frequently in the parts or components of such machines. The Ni-hard and high Cr cast irons have been availed as such materials because of both the higher hardness and wear resistance compared with steels.1,2,3,4 According to the demand for upgrading the productivity and quality of product, a new alloy with higher performance is required.5,6,7 A multi-alloyed white cast iron containing several kinds of strong carbide-forming elements such as chromium (Cr), molybdenum (Mo), vanadium (V) and tungsten (W) was developed for the purpose.5,6 This cast iron has been preferably applied to work rolls of hot strip mills in steel-making industry and some parts of pulverizing mills in cement industry. Typical microstructure consists of plural kinds of complex eutectic carbides with extremely high hardness such as MC, M2C, M6C types and the matrices with secondary carbides, martensite and some austenite.5,6,7,8,9 The roll made of multi-alloyed white cast iron is called “high-speed steel (HSS) roll” in the trade in spite of its cast iron composition.5 Compared with conventional rolls made by high Cr and Ni-hard cast irons, the roll made of multi-alloyed white cast iron shows better wear resistance and longer service life in spite of less volume fraction of eutectic carbides.6,7
The basic chemical composition of multi-alloyed white cast iron is 5 wt% of Cr, Mo, W, V each and 2 wt%C (hereafter wt% is expressed by %). The C content of the cast iron is higher than that of high-speed tool steel in order to obtain suitable amounts of hard eutectic carbides for superior wear resistance.5,9 The mechanical and wear properties of multi-alloyed white cast iron depend on the kind and amount of carbides as well as the matrix structure. Therefore, a heat treatment must be provided to improve the matrix structure for desirable properties. Generally, the heat treatment process for multi-alloyed white cast iron consists of annealing, hardening and tempering in the same manner as steels and other alloyed cast irons.10,11,12,13,14
The role of C is classified into two parts, formation of eutectic carbides during solidification and dissolution of the remainder into the matrix. The latter affects on the transformation of matrix. Therefore, a parameter of carbon balance (Cbal), which is defined as the amount of C dissolving in the matrix in equilibrium state, was introduced and expressed by Eqn. 1.5,10,11,12,13,15
where the %C is the C content of cast iron and the %Cstoich is the stoichiometric amount of C which combines with all carbide-forming elements to form their own carbides.
When M7C3 eutectic carbide does not crystallize during solidification, the Cstoich value can be theoretically calculated using Eqn. 2.
In the case that M7C3 eutectic carbides exist in the cast iron, the Cstoich must be calculated by Eqn. 3.
Here, the amount of C dissolved in the matrix more or less than that in equilibrium state can be estimated by the Cbal value. It was reported that the Cbal showed great effect on the phase transformation of multi-alloyed white cast iron.10,11,12,13
In most of the countries, centrifugal casting (CF) or spin casting (SP) process has been adopted to produce composite or bimetal rolls. When a roll of multi-alloyed white cast iron is manufactured by the CF process, the segregation of eutectic carbides occurs in the outer shell by the centrifugal force.16 To overcome this problem, the kind and amount of carbide-forming elements must be controlled. Normally, extremely heavy or light alloying elements should be reduced to decrease the difference in densities among the crystallized carbides. In the case of rolls made by CF casting method, alloy composition must be designed with lower contents of carbide-forming elements than those of regular or basic multi-alloyed white cast irons. For this purpose, the semi-multi-alloyed white cast iron had to be developed.
Up to the present, there has not been any research on heat treatment behavior of semi-multi-alloyed white cast iron. In this work, therefore, the semi-multi-alloyed white cast irons containing Cbal of − 0.68 to + 0.53% were prepared by controlling the Cr content from 3 to 9% under the fixed contents of 2%Mo, 1%W and 5%V and the behaviors of hardness and retained austenite during heat treatment were clarified.
Experimental Procedures
Preparation of Specimens
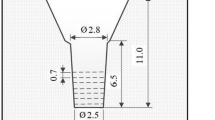
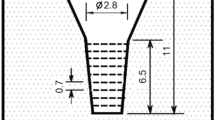
The charge materials of 30 kg consisted of steel scrap, pig iron, ferro-alloys and pure metals were melted in a high-frequency induction furnace. The melt was superheated to 1580 °C and held there for 10 min. After holding, the melt was poured at 1500–1520 °C into preheated CO2 bonded sand molds in a round bar shape. The mold is shown schematically in Figure 1. The substantial cavity size of the mold is 2.5 cm in diameter and 6.5 cm in length with the sufficient riser. After pouring, the surface of the riser was covered immediately by dry exothermic powder to prevent the top of melt from fast cooling. The chemical composition, Cbal value and the type of crystallized eutectic carbide in each specimen are summarized in Table 1. Equation 2 is applied to calculate the Cbal values for specimens No.1 and No.2, and Eqn. 3 for specimens No.3–No. 5.
Heat Treatment Procedures
First of all, the round bar ingots were annealed at 950 °C for 5 h in an electric furnace to relieve the stress and homogenize the micro-segregation of elements. The annealed ingot was sectioned by wire-cut machine to obtain test pieces with disk shape in 0.7 cm thickness. The test pieces used for heat treatment were coated with an anti-oxidation solution to prevent them from oxidizing and decarburizing. The hardening was carried out by fan air cooling (FAC) after austenitizing at 1050 and 1100 °C for 1 h in a vacuum furnace. The hardened test pieces were tempered between 400 and 600 °C at 50 °C intervals for 200 min in electric furnace and cooled in still air.
Investigation of Microstructure
Microstructure of test specimens was observed by optical microscopy (OM) and scanning electron microscopy (SEM). The test specimen was polished and etched by Groesbeck’s reagent to identify the carbide type. The Villella’s reagent was used to reveal the matrix microstructure. Particularly, the microphotographs of transformed matrix were taken with high magnification focusing on the matrix area.
Measurement of Hardness and Volume Fraction of Retained Austenite (Vγ)
The macro-hardness of specimens was measured using a Vickers hardness tester with a load of 30 kgf, and matrix hardness or micro-hardness was measured with a load of 0.1 kgf. The measurement was taken randomly at five points, and the average value was adopted. The X-ray diffraction method (XRD) which was developed for alloyed white cast iron was applied to measure the volume fraction of retained austenite (Vγ) quantitatively.10,11,12,13,14,17 A goniometer with special simultaneously rotating and swinging sample stage was used in order to cancel the preferred orientation of austenite. The Mo-Kα characteristic line with wave length of 0.007 nm filtered by Zr foil was utilized for measurement. The goniometer was scanned from 24° to 44° by 2θ. The four diffraction peaks which were independent from unnecessary phases were selected for Vγ calculation. The diffraction peaks of α(200), α(220), γ(220) and γ(311) were picked up, respectively. The integrated area of the inner side of each peak was measured using an image analyzer. The Vγ values were calculated from total area of peaks in three combinations of α200–γ311, α200–Σγ(220,311) and Σα(200,220)–γ311, and then the values were averaged.
Experimental Results and Discussions
As-cast Microstructure
The typical microstructures of as-cast specimens with different Cbal values are shown in Figure 2. All the specimens showed hypoeutectic composition which consisted of primary austenite dendrite and eutectic structures. The eutectics crystallize at the boundary region of primary austenite dendrites. The type and morphology of eutectic carbides in multi-alloyed white cast iron were clarified by Wu et al.8 and Hashimoto et al.9 According to their reports, the eutectic carbides of as-cast specimen with basic alloy composition of 2%C–5%Cr–5%Mo–5%W–5%V are mainly MC and M2C types with some amount of M7C3 type. In addition, each carbide shows different morphology. The MC carbide has nodular morphology, while the M2C carbide shows fine lamellar morphology. By contrast, the eutectic M7C3 carbide is rod-like or ledeburitic morphology solidifying in the same areas as M2C eutectic carbide. It is known that these carbides can be distinguished by Groesbeck’s reagent. The M2C and M7C3 carbides are colored, but MC carbide is not.18 Therefore, the eutectic carbides crystallized in specimens of No.1 with + 0.53%Cbal and No.2 with + 0.42%Cbal are mainly MC and M2C types. Besides, the carbides in specimens of No.3 with − 0.01% Cbal to No.5 with − 0.68% Cbal are mostly MC and M7C3 types coexisting small amount of M2C type. These results agree well with those of the references.8,9 As the Cbal value decreases, the amount of M7C3 carbide seems to increase but that of M2C carbide decreases. It can be understood that a decrease in the Cbal value or an increase in the Cr content of specimen promotes the crystallization of M7C3 carbide but inhibits that of M2C carbide.
As-hardened Microstructure
In general, the morphology of eutectic carbides in cast iron does not change during austenitizing except for the M2C carbide with low thermal stability.9 By contrast, the matrix structure varies widely depending on the heat treatment condition. To investigate the matrix structure in as-hardened state, the polished specimens were etched by Villella’s reagent and observed by SEM focusing on the matrix. As examples, the matrix structures in specimens hardened from 1100 °C austenitizing are shown in Figure 3. It was found that the matrix is composed of fine secondary carbides (Sc), martensite (M) and retained austenite (γ) in all of specimens except for specimen No.5 with − 0.68% Cbal of which matrix is mostly ferrite and coarse secondary carbides. This result suggests that the specimen with large negative Cbal value has poor hardenability due to insufficient C concentration in austenite. The martensite phases can be found throughout the matrix. It is considered that the austenite is destabilized by the precipitation of secondary carbides during austenitizing and transforms to martensite during quenching. The amount of retained austenite seems to decrease qualitatively as the Cbal value of specimen decreases. It is clear that the precipitation of secondary carbides occurs during austenitizing and they are evidently revealed. It seems that the secondary carbides lower in amount but enlarge in size as the Cbal value decreases. In addition, the types of secondary carbides could not be identified using SEM but only TEM analysis because the sizes are too fine to analyze alloy concentration quantitatively. However, it was reported that the precipitated secondary carbides in as-hardened state of multi-alloyed white cast iron with basic chemical composition are mostly MC and M6C with some M7C3 types.19
Behavior of Hardness and Volume Fraction of Retained Austenite (Vγ) in As-hardened State
As mentioned previously, the eutectic carbides generally change little during heat-treating. Therefore, the variation of hardness due to the heat treatment depends on the phase transformation of the matrix, that is, the hardness of matrix or micro-hardness. The effect of Cbal on hardness and Vγ in as-hardened state is displayed in Figure 4a and b, respectively. In Figure 4a, the macro-hardness and micro-hardness increased first remarkably to the highest value but beyond that point, the hardness decreased as the Cbal increased regardless of austenitizing temperature. The micro-hardness showed similar trend to the macro-hardness but overall lower. The hardness peaks are obtained near 0%Cbal, and the values are 868 HV30 and 816 HV0.1 in 1050 °C and 854 HV30 and 801 HV0.1 in 1100 °C austenitizing, respectively. The increase in hardness associated with Cbal values is due to a rise of secondary carbides together with more martensite in the matrix. Other reasons might be an increase in M2C eutectic and a decrease in M7C3 eutectic. It is considered that the decrease in hardness over the peak is because of more increase in retained austenite.
As shown in Figure 4b, the Vγ value in each austenitizing temperature increased progressively as the Cbal rose. At the same Cbal value, the Vγ is much greater in the case of higher austenitizing temperature. This proves that the C concentration in the matrix increases with an increase in the Cbal as well as that in the austenitizing temperature, and resultantly that the Ms temperature is lowered.
In order to consider the effect of Vγ on the hardness in as-hardened state, the relationship between hardness and Vγ for all of specimens was obtained and is shown in Figure 5. The macro-hardness and micro-hardness increased steeply to the highest point at around 12%Vγ. It is considered that the amounts of secondary carbides, martensite and retained austenite were well balanced at this Vγ value. Over 12%Vγ, by contrast, the hardness lowers because an excess of soft retained austenite and a decrease in martensite reduced the matrix hardness.
From above results, it is found that hardness and Vγ are quite low in the specimen with − 0.68% Cbal. This is because the C in this specimen was mostly consumed to form eutectic carbide and very much less C was dissolved in the matrix. As a result, the austenite could not transform to martensite but to pearlite or ferrite even by quenching. In specimens with Cbal of − 0.2 to 0.4%, on the other side, the sufficient amount of C dissolves in the matrix to form the secondary carbides and martensite. This causes good results of high hardness over 800 HV30 in the Vγ value of 6–34%.
Behavior of Hardness and Volume Fraction of Retained Austenite (Vγ) in Tempered State
During tempering, the stability of retained austenite is reduced by the precipitation of secondary carbides and transforms to martensite during post-cooling.10,11,12,13,14 Simultaneously, the martensite formed by quenching is tempered. Therefore, the transformation of matrix affects the tempered hardness significantly. In this work, the specimens hardened from 1050 and 1100 °C austenitizing were tempered at 400–600 °C. The effect of tempering temperature on the hardness and Vγ for all the specimens is illustrated in Figure 6a–e. The hardness and Vγ in as-hardened state are added into the diagrams for better understanding of the tempering behavior.
The hardness of each specimen dropped greatly when the specimen was tempered even at the lowest tempering temperature of 400 °C. After that, it began to rise to the maximum point and then lowered with an increase in the tempering temperature. It was found that the tempered hardness curve of each specimen showed an evident secondary hardening. The increase in hardness is due to the precipitation of secondary carbides from retained austenite during tempering and the transformation of martensite from the rest of austenite during post-cooling. The simultaneous decrease in the amount of Vγ could be one reason. Finally, it can be considered that the mutual relationship among the increase in tempered martensite, that of secondary carbides and the decrease in retained austenite determined the matrix hardness. It was reported that the secondary carbides in tempered state were MC, M6C and M7C3 types.19 In this experiment, the maximum tempered macro-hardness (HTmax) was obtained at 500 °C tempering for specimens hardened from 1050 and 550 °C tempering for those hardened from 1100 °C austenitizing. In the tempering at 500–550 °C, it is believed that a carbide reaction occurs in martensite to form nearly pure carbide of each alloying element.20 These carbides have very high hardness and strengthen the matrix.
When the behavior of Vγ in the tempering is considered, the Vγ decreased gradually at first and then decreased remarkably over 450 °C as the tempering temperature increased. This behavior could be due to the fact that the precipitation of secondary carbides from retained austenite was accelerated with an increase in the temperature. When the specimen is tempered at the temperature over the HTmax, the hardness continues to decrease due to the cohesion or coarsening of secondary carbides that is called over-tempering. The highest values of HTmax, 830 HV30 in hardening from 1050 °C and 862 HV30 from 1100 °C, are obtained in the specimen with Cbal of − 0.01%. The decomposition of austenite was almost completed near 550 °C at which the Vγ value is almost zero. In the case of hardening from 1100 °C, the tempering temperature to obtain the HTmax shifted to the high-temperature side. This is because higher tempering temperature is necessary for a larger amount of retained austenite in as-hardened state to decompose into each phase at the same tempering time.
Since the volume fraction of eutectic carbide in semi-multi-alloyed white cast iron is quite low compared with Ni-hard and high Cr cast irons,5,8 the constituent phases in matrix give significant effects on the tempered hardness. The relationship between macro-hardness, micro-hardness and Vγ of all the tempered specimens is shown in Figure 7a for 1050 °C and Figure 7b for 1100 °C hardening, respectively. The hardness behaves similarly in both cases. The hardness increased to the highest values at 2–6% Vγ and then decreased approximately in proportion to the Vγ value. The reason why the hardness lowers with a rise of the Vγ value must be due to an increase in soft retained austenite. Though the data are scattered within certain range, the macro-hardness and micro-hardness displayed similar behavior and the distribution of macro-hardness is higher than that of micro-hardness. Even if the austenitizing temperature is changed, the decreasing rate of hardness does not make much difference. Tempered hardness is a little higher in the case of austenitizing at 1100 °C compared with the case of 1050 °C austenitizing. This is because the Vγ in as-hardened state is increased since the solubility of C and other alloying elements in austenite is greater at higher austenitizing temperature and resultantly, more precipitation of secondary carbide and more formation of martensite from retained austenite occurred during tempering.
From Figure 7, it is found that the hardness scattered in wide range at the Vγ values less than 2%. In order to clarify this reason, the macro-hardness in the range of Vγ less than 2% are connected to the Cbal value of specimens and the relationship is shown in Figure 8. Macro-hardness increased greatly to the maximum value at near 0% Cbal and then decreased gradually as the Cbal value increased. In the region of high hardness, it is considered that the retained austenite should be destabilized mostly by the precipitation of secondary carbides and finally transformed to martensite. In the lower hardness region, conversely, the matrix became over-tempering where the coarsening of secondary carbides and transformation of austenite to ferrite or pearlite took place. The lowest hardness was obtained in the specimen with − 0.68%Cbal because the lack of C in austenite and could not be hardened.
It was mentioned that the macro-hardness in tempered state was directly related to the amount and kinds of constituent phases in the matrix or to the matrix hardness. The relationships among the maximum tempered micro-hardness (HTmax-M), Vγ of the specimens with HTmax-M and Cbal value are shown in Figure 9. In the specimens hardened from each austenitizing temperature, the HTmax-M showed similar trend corresponding with the Cbal value. The HTmax-M increased steeply to the highest value at about 0% Cbal and then decreased slowly to 0.53% Cbal. The remarkable increase in HTmax-M is due to the fact that an increase in C concentration in austenite caused by a rise of Cbal value improves not only the hardenability but also the precipitation of secondary carbides in the matrix. According to these reasons, more martensite and more special carbides with high hardness could be obtained in the matrix during tempering. A decrease in HTmax-M over the maximum point is due to the excess amount of austenite left in the matrix. At the same Cbal value, the HTmax-M is a little high in the specimens hardened from higher austenitizing temperature because a larger amount of retained austenite formed by hardening from high austenitizing temperature produces greater amount of secondary hardening.
As for the behavior of Vγ, the Vγ values did not change up to near 0%Cbal. Over the 0% Cbal value, however, the Vγ value increased steeply as the Cbal value rose. This suggests that even in the specimens with the HTmax-M, a certain amount of austenite is still left. The reason could be that the tempering time may not be sufficient to decompose the retained austenite completely. The Vγ values of the specimens hardened from 1100 °C are a little higher than those hardened from 1050 °C austenitizing. The Vγ values at HTmax-M were almost the same up to near 0%Cbal. Then, the difference of Vγ values between both hardening temperatures expanded as the Cbal value increased. The Vγ values at HTmax-M ranged in 11–14% at the largest Cbal value of + 0.53%.
It is considered from previous results that the Vγ in as-hardened state is closely related to the tempered hardness. The maximum tempered hardness (HTmax and HTmax-M) was connected to the Vγ values in as-hardened state. The relationship is shown in Figure 10. The variation of HTmax and HTmax-M associated with Vγ value in as-hardened state is quite similar irrespective of austenitizing temperature. Each maximum tempered hardness increased to the highest point at around 24%Vγ at which the hardness was 862 HV30 and 795 HV0.1, respectively. The reason is due to more reactions to increase the hardness. The increasing of Vγ in as-hardened state promoted an increase in the secondary carbides precipitation and the transformation of retained austenite to martensite during tempering. Therefore, it can be said that the more Vγ, the more precipitation of secondary carbides. Over the highest point, both maximum tempered hardness decreased a little as the Vγ value increased. The decrease in the hardness is due to an increase in the residual austenite after tempering reduced the matrix hardness greater than the secondary hardening. It is clear that the 10–50%Vγ in as-hardened state is necessary to obtain the tempered hardness over 800 HV30 and 750 HV0.1. From these results, it can be concluded that the Vγ value in as-hardened state which is controlled by Cbal value contributes greatly to the tempered hardness.
From the tempered hardness curves in Figure 6, the secondary hardening is recognized evidently. The secondary hardening shows more or less depending on the chemical composition and heat treatment condition. The secondary hardening occurred in alloyed white cast iron during tempering is generally explained by the following reactions;
- (1)
Precipitation of secondary carbides in the complex form of M type from some tempered martensite and in the stage of approaching the HTmax, the nearly pure carbides of carbide formers with much higher hardness (carbide reaction).
- (2)
Precipitation of carbides from austenite retained in matrix after hardening.
- (3)
Martensite formation from residual destabilized austenite during post-cooling.
Every reaction takes a certain part of increasing hardness in tempering and cooling after tempering. In the multi-alloyed white cast iron, however, the increase in the tempered hardness is considered to be covered by reactions (1) and (3).10,11,12,13 It is the fact that the carbide reaction occurs in martensite at 500–550 °C tempering.20 It raises the matrix hardness significantly in the same way as tool steels as reported by several works.10,11,12,13,14 There is a paper to investigate the identification of secondary carbides in matrix using XRD and TEM.19 The paper clarified that the MC, M6C and M7C3 carbides precipitated in heat-treated state. The carbides formed by carbide reaction during tempering can be predicted as following example for Mo;
The secondary carbides existing in matrix after tempering can be considered to be MC, M6C and some M7C3 coexisting with some of their pure carbides, and they should improve the matrix hardness significantly.
To clarify the effect of Cbal on the secondary hardening, the degree of secondary hardening (ΔHs), which was defined as the difference in the hardness between the HTmax and the hardness at which the secondary hardening began, was calculated from each hardness curve. The relationship between ∆Hs and Cbal of specimens is shown in Figure 11. The ∆Hs increased progressively as the Cbal value rose. The ∆Hs of specimen hardened from 1100 °C was overall higher than that hardened from 1050 °C. It can be said that an increase in the secondary hardening is accomplished by an increase in both the precipitation of secondary carbides and transformation of martensite from the residual austenite. Therefore, the relation of the ∆Hs versus Vγ in as-hardened state was obtained and it is shown in Figure 12. The ∆Hs increased in proportion to Vγ in as-hardened state. Here, it is concluded that relatively large amount of Vγ in as-hardened state is required to get a large degree of secondary hardening. In other words, the greater amount of retained austenite in the as-hardened state contributes more to the secondary hardening in the tempered state and results in the high hardness of the specimen. In addition, it can be also concluded that through the Vγ, the ∆Hs is indirectly related to the Cbal of specimens.
In view of all the previous results, it is clear that the highest hardness in the tempered state is obtained at around 0% Cbal where the concentration of C and alloying elements in austenite attained the most suitable value whether the coarsening of precipitated carbides occurs or not. Therefore, it can be reasoned that low Cbal value like near zero is adequate to maintain the Vγ value necessary to get the maximum hardness by tempering. As for work rolls in steel rolling mill, the hardness ranges from 720 to 880 HV depending on the stand used.6,7 Therefore, the semi-multi-alloyed white cast iron with − 0.20% to + 0.53% Cbal can be applied to the hot work rolls with necessary hardness by selecting the appropriate heat treatment.
Conclusions
In this research, semi-multi-alloyed white cast irons with − 0.68 to + 0.53%Cbal were prepared by varying Cr content from 3 to 9% under the fixed values of 2%Mo, 1%W and 5%V. After annealing, the test specimens were hardened by fan air from 1050 and 1100 °C austenitizing. The tempering was carried out at 50 °C interval between 400 °C and 600 °C. Then, the effect of carbon balance (Cbal) value on the variation of hardness, volume fraction of retained austenite (Vγ) and the degree of secondary hardening (ΔHs) was clarified. The obtained results are summarized as follows;
As-hardened State
-
1.
When the Cbal value increased from − 0.68 to − 0.01%, the hardness rose greatly. When the Cbal value got over − 0.01%, the hardness decreased gradually. The hardness in case of hardening from 1050 °C was greater than that of hardening from 1100 °C. The micro-hardness showed similar behavior to the macro-hardness.
-
2.
As the Cbal value rose, the Vγ increased progressively regardless of austenitizing temperature. At the same Cbal, the Vγ was greater in the case of higher austenitizing temperature.
-
3.
The macro-hardness and micro-hardness increased greatly to the maximum value at 12%Vγ and subsequently decreased gradually as the Vγ rose.
Tempered State
-
1.
The hardness curve showed an evident secondary hardening due to precipitation of fine secondary carbides during holding and the transformation of retained austenite to martensite during post-cooling.
-
2.
Regardless of Cbal value, the maximum tempered macro-hardness (HTmax) was obtained at 500 °C tempering in hardening from 1050 and 550 °C tempering in hardening from 1100 °C austenitizing. When Cbal of specimen near 0%, the highest values of HTmax, 830HV30 in 1050 °C and 862 HV30 in 1100 °C hardening, were obtained.
-
3.
In each specimen, the Vγ value tended to decrease as the tempering temperature increased. The Vγ value reduced greatly in tempering from 450 to 550 °C. The Vγ values at HTmax ranged from 2 to 14%.
-
4.
The tempered hardness increased to the maximum value at about 2%Vγ and over 2%Vγ; it decreased gradually as the Vγ increased. At the Vγ value less than 2%, the macro-hardness and micro-hardness scattered broadly.
-
5.
Up to the Cbal near 0%, the maximum tempered micro-hardness (HTmax-M) value increased remarkably and then decreased in a small rate as the Cbal rose. The relation of Cbal versus HTmax-M was similar in both cases of hardening from 1050 and 1100 °C.
-
6.
The 10–50%Vγ in as-hardened state was necessary to obtain the hardness over 800 HV30 and 750 HV0.1 by tempering.
-
7.
The degree of secondary hardening (ΔHs) was closely related through Vγ in as-hardened state to the Cbal value, and it increased with an increase in the Cbal value. The ΔHs was larger in the specimens hardened from higher austenitizing temperature.
-
8.
The Cbal value near 0% was adequate to maintain the Vγ value necessary to obtain the maximum hardness by tempering.
References
S. Inthidech, P. Sricharoenchai, Y. Matsubara, In. Metalcast. 6, 25–34 (2012)
I. Chakrabarty, Int. Metalcast. 5, 49–56 (2011)
D. Li, C. Sloss, Int. Metalcast. 9, 7–19 (2015)
A. Hadji, K. Bouhamla, H. Maouche, Int. Metalcast. 10, 43–55 (2016)
Y. Matsubara, N. Sasaguri, M. Hashimoto, Presented in Part at the 4th Asian Foundry Congress, Queensland (1996)
M. Hashimoto, S. Otomo, K. Yoshida, K. Kimura, R. Kurahashi, T. Kawakami, T. Kouga, ISIJ Int. 32, 1202–1210 (1992)
M. Hashimoto, T. Tanaka, T. Inoue, M. Yamashita, R. Kurahashi, R. Terakado, ISIJ Int. 42, 982–989 (2002)
H.Q. Wu, N. Sasaguri, Y. Matsubara, M. Hashimoto, AFS Trans. 104, 103–108 (1996)
M. Hashimoto, O. Kubo, Y. Matsubara, ISIJ Int. 44, 372–380 (2004)
W. Khanitnantharak, M. Hashimoto, K. Shimizu, K. Yamamoto, N. Sasaguri, Y. Matsubara, AFS Trans. 117, 435–444 (2009)
J. Opapaiboon, P. Sricharoenchai, S. Inthidech, Y. Matsubara, Mater. Trans. 56, 720–725 (2015)
T. Meebupha, S. Inthidech, P. Sricharoenchai, Y. Matsubara, Mater. Trans. 58, 655–662 (2016)
J. Opapaiboon, M.S.N. Ayudhaya, P. Sricharoenchai, S. Inthidech, Y. Matsubara, Mater. Trans. 60, 346–354 (2019)
S. Inthidech, P. Sricharoenchai, Y. Matsubara, Mater. Trans. 47, 72–81 (2006)
G. Steven, A.E. Nehrenberg, T.V. Philip, Trans. ASM 57, 925–948 (1964)
H. Fu, Q. Xiao, J. Xing, Mater. Sci. Eng. A 479, 253–260 (2008)
C. Kim, J. Heat Treat. 1, 43–51 (1979)
G. Laird, R. Gundlach, K. Rohrig, Abrasion-Resistant Cast Iron Handbook (Schaumburg, AFS, 2000), pp. 203–204
M. Hashimoto, O. Kubo, N. Sasaguri, Y. Matsubara, J. JFS 76, 205–211 (2004)
G. Roberts, G. Krauss, R. Kennedy, Tool Steel, 5th edn. (Materials Park, ASM International, 1998), pp. 97–103
Acknowledgements
Authors gratefully acknowledge to the Faculty of Engineering, Mahasarakham University and Mahasarakham University for research funding (Academic year 2017), for the financial support to this research. In addition, the authors appreciated Dr. Adrian Roderick Plant, native English speaker, for revision of English sentence.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Inthidech, S., Matsubara, Y. Effects of Carbon Balance and Heat Treatment on Hardness and Volume Fraction of Retained Austenite of Semi-multi-alloyed White Cast Iron. Inter Metalcast 14, 132–143 (2020). https://doi.org/10.1007/s40962-019-00343-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-019-00343-y