Abstract
Purpose
Breast cancer is the most common cancer in women. In recent research works, the role of cytokines in development of cancer has attracted attention. Therefore, this paper intends to comprehensively assess the association between breast cancer and polymorphism A/T 251 from the IL-8 gene using meta-analysis.
Methods
In this study, to find articles published electronically between 2004 and 2020, national and international databases of SID, MagIran, Embase, ScienceDirect, Scopus, PubMed, and Web of Science (WoS).
Results
The odds ratio of AA genotype in patients with breast cancer based was obtained as 1.42 (1.11–1.82: 95% confidence interval), which shows the increasing effect of AA genotype by 0.42, the odds ratio of the AT genotype in patients with breast cancer based on a meta-analysis of studies was obtained as 1.01 (0.84–1.22: 95% confidence interval), which demonstrates the increasing effect of the AT genotype by 0.01, and the odds ratio of the TT genotype in patients with breast cancer was similarly obtained and was 0.95 (1.08–0.24: 95% confidence limit), which shows the decreasing effect of TT genotype by 0.05.
Conclusion
According to this meta-analysis, allele A has a significant relationship with breast cancer, which can be used as a predictor of the course of the disease and the clinical outcome of breast cancer. Identifying polymorphism A/T 251 from the IL-8 gene, as a genetic marker and predictor, can also be effective in treating breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer in women is a commonly developed cancer and is a serious problem in today’s world. It is the second most common cancer in human (men and women) and the first most common cancer in women worldwide. Breast cancer accounts for 23% of all new cancers and 14% of all cancers. Risk factors for breast cancer include: family history of breast cancer, genetic status, personal history of breast cancer, formation of abnormal cells in the lobules or mammary glands in breast tissue, breast volume, androgen levels, menstrual cycles, pregnancy, breastfeeding baby, bone density, lifestyle factors such as postmenopausal hormone use, obesity and overweight, physical activity, diet, alcohol and tobacco use, birth control pills and other risk factors such as radiation, use of certain medications, infections, and environmental and occupational pollutions and genetic factors associated with cancer [1,2,3]. Breast cancer is a highly heterogeneous disease caused by the interaction of hereditary and environmental risk factors and leads to the progressive accumulation of genetic and epigenetic changes in breast cancer cells. Although epidemiological evidence emphasizes on the existence of specific risk factors (such as age, obesity, and alcohol consumption), having a family history of breast cancer is the greatest risk factor for development of this cancer [4].
Lately, the role of cytokines in cancer has been discussed in research works. Cytokines are glycoproteins that are secreted by the innate and acquired immune system and other cells and mediate many functions of these and other cells [5]. It has recently been reported that at the tumor site, the internal secretion of some cytokines decreases through the interaction with the tumor cells, leading to a weakening of the immune system, leading to tumor proliferation, metastasis, and tumor malignancy [5]. The importance of interleukin-8 receptor signaling pathways and this commocaine itself has been shown to boost the progression of malignant cancers [6, 7].
The IL-8 gene is located on chromosome 4q12-q13 [8], and the host's ability to produce IL-8 can be controlled by polymorphism A/T 251 in the promoter region of this chemokine gene [9]. Allele A has been shown to be associated with high levels of IL-8 production in this single-nucleotide polymorphism [9]. Further increase in IL-8 gene expression in tumor cells is of great importance in the survival of this type of tumor through the role of CXCR2, CXCR1 gene receptors (subfamily of chemokines 1, 2 genes) in cancer cells, endothelial cells and neutrophils, and tumors associated with macrophages [10]. The importance of IL-8 is related to the modulation between different cell types in tumors and microenvironments [6]. Tumor cells have been shown to maintain their mesenchymal state using continuous autocrine signaling rings [11]. IL-8 stimulates the tumor by activating various signaling pathways that ultimately affect the transcription factors associated with the tumor [12].
Studies on the effects of IL-8 and breast cancer have shown that there is a significant relationship between the metastatic potential of breast cancer cells and the expression of IL-8 gene expression. Based on these studies, it is shown that high-metastatic cell lines express more IL-8 genes than low-metastatic cells. This could be due to epigenetic changes such as an inappropriate methylation pattern in the IL-8 gene, which may be responsible for the difference in metastatic cells with other cells [13]. Recent research works have demonstrated that there is an association between polymorphism in IL-8 and CXCR2 genes with an increased risk of breast cancer in the Chinese female population [14,15,16,17]. According to clinical studies, serum levels of this interleukin in the serum of patients with breast cancer were higher than in healthy individuals, especially patients who were in the process of developing the disease. Single-nucleotide polymorphisms are important in cytokine genes and affect the expression of these genes and cell activity. The polymorphism in region 251 of the IL-8 gene promoter plays an important role in the production of IL-8, or its protein expression, both in the living organism and in the laboratory. Previous studies have shown that polymorphism A/T 251 of the IL-8 gene in various populations is associated with an increased risk of developing cancerous tumors [14, 15, 18,19,20,21].
With regard to the relationship between breast cancer and polymorphism A/T 251 of the IL-8 gene, several preliminary studies have been conducted and there are contradictions between the results of these studies. This meta-analysis attempts to clarify these contradictions and inconsistencies; therefore, the aim of this study is to determine the relationship between breast cancer and polymorphism A/T 251 of the IL-8 gene.
Methods
Method for Searching Articles
The study searched the SID and MagIran Persian databases, and the international databases of Embase, ScienceDirect, Scopus, PubMed, and Web of Science (WoS) to find resources between 2004 and 2020. The lists of references within all searched articles and reports were also manually evaluated to find other possible sources. The keywords used to search for resources were selected from the MeSH medical topics database. The keywords used were interleukin-8, IL-8, A/T 251, interleukin, breast cancer, and cancer.
Article Selection Criteria
Articles with the following characteristics were considered for selection of articles for our meta-analysis: 1) original research articles, 2) clinical trial studies, 3) availability of the full text of the article. and 4) studies that had assessed the association between breast cancer and polymorphism A/T 251 of the IL-8 gene.
Article Exclusion Criteria
The collected research works were examined in more details. Articles that were review papers, articles in which samples were not selected from breast cancer patients, and research works that only examined previous secondary data were excluded from the meta-analysis. Finally, 17 studies entered the quality evaluation stage.
Article Quality Evaluation
The quality of the articles was evaluated based on the criteria used within the CONSORT checklist (i.e., study plan, background and review of texts, place and time of study, consequence, entry criteria, sample size and statistical analysis). Articles that fulfilled 6 or 7 criteria were considered as high-quality articles, articles that achieved 3–5 criteria, and articles that only fulfilled 2 or less criteria were categorized as medium- and low-quality articles respectively [19,20,21,22]. In this study, 9 papers entered the systematic review and meta-analysis as high-quality and medium-quality studies, and 8 papers were classed as low-quality articles and they were removed.
Data Extraction
All final papers entered into the meta-analysis process were prepared by another checklist. This checklist included fields of: the title of the article, the name of the first author, the year of publication, the place of study, the sample size of the patient and control group, the frequency of the patient group genotype and the probability controls for case and patients groups, the frequency of A and T alleles and the mean age.
Statistical Analysis
Since the focus of this study was to assess the relationship between breast cancer and polymorphism A/T 251 of the IL-8 gene, frequency and percentage, as well as the standardized mean difference index in each study, were used to amalgamate the results of various studies. To investigate the homogeneity between the studies, the I2 index was used to measure the percentages of variation, and since heterogeneity was found among the studies, the random effects model was used to combine studies and perform the meta-analysis. When the I2 index was less than 25%, the heterogeneity was considered as low, between 75 and 25% as moderate heterogeneity and more than 75% as high heterogeneity. P levels less than 0.05 were considered significant. The funnel diagrams and the Egger’s test were also employed to investigate the publication bias. Data analysis was performed using the Comprehensive Meta-Analysis software.
Results
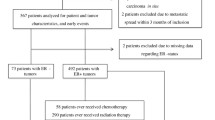
In this study, all research works that has investigated the relationship between breast cancer and polymorphism A/T 251 of the IL-8 gene were systematically reviewed without time constraints, and in accordance with the PRISMA guidelines. In the initial search, 383 articles were identified; after inputting all the articles in the classification database, all duplicate articles, articles with irrelevant contents, and abstracts of articles published in conferences were further removed. A total of 81 articles was fed into the second stage for further examination. At this stage, while reviewing the titles and abstracts, 64 unrelated articles were excluded from the study. In the next step, the full text of 17 articles was reviewed. At this stage, 8 low-quality articles were removed. Finally, 9 articles that were published between 2004 and April 2020 entered the final analysis stage (Fig. 1).
Among the initial studies included in the meta-analysis, the samples were 2429 and 2367 in the patients and control groups respectively. The characteristics of the studies that were systematically reviewed are provided in Table 1. Note that, all of the final studies were of the clinical trial type, and out of 9 final articles, one article was published in Persian and 8 articles were published in English (Table 1).
Investigating Heterogeneity and Publication Bias (AA Genotype)
The heterogeneity of the studies was investigated using the I2 test, and based on this test (I2 = 59.8%), the heterogeneity in the studies was evident; therefore, the random effects model was used to amalgamate the results of the studies together. Publication bias was measured using the Egger’s test (Fig. 2), and this was not statistically significant (p = 0.444).
The total number of samples entered in the study was 2429 in the group of patients and 2367 in the control group. The odds ratio of AA genotype in patients with breast cancer based on meta-analysis of studies was obtained as 1.42 (1.11–1.82: 95% confidence interval), which indicates the increasing effect of AA genotype by 0.42. This means that people with this genotype are 42% more likely than others to have breast cancer (Fig. 3). In Fig. 3, the odds ratios based on the random effects model are illustrated. Each black square represents the odds ratio and the length of the line on which the square is located demonstrated the 95% confidence interval in each study. The diamond symbol represents the odds ratio for all studies put together.
Investigating Heterogeneity and Publication Bias (AT Genotype)
The heterogeneity of the studies was investigated using the I2 test, and based on this test (I2 = 57.6%), the heterogeneity in the studies was evident; therefore, the random effects model was used to amalgamate the results of the studies together. Publication bias was measured using the Egger’s test (Fig. 4), and this was not statistically significant (p = 0.094).
The total number of samples entered in the study was 2429 in the group of patients and 2367 in the control group. The odds ratio of AT genotype in patients with breast cancer based on meta-analysis of studies was obtained as 1.01 (0.84–1.22: 95% confidence interval), which indicates the increasing effect of AT genotype by 0.01. This means that people with this genotype are 1% more likely than others to have breast cancer (Fig. 5). In Fig. 5, the odds ratios based on the random effects model are illustrated. Each black square represents the odds ratio, and the length of the line on which the square is located demonstrated the 95% confidence interval in each study. The diamond symbol represents the odds ratio for all studies put together.
Investigating Heterogeneity and Publication Bias (TT Genotype)
The heterogeneity of the studies was investigated using the I2 test, and based on this test (I2 = 90.8%), the heterogeneity in the studies was evident; therefore, the random effects model was used to amalgamate the results of the studies together. Publication bias was measured using the Egger’s test (Fig. 6), and this was not statistically significant (p = 0.705).
The total number of samples entered in the study was 2068 in the group of patients and 2006 in the control group. The odds ratio of TT genotype in patients with breast cancer based on meta-analysis of studies was obtained as 0.95 (1.08–2.04: 95% confidence interval), which indicates the decreasing effect of TT genotype by 0.05. This means that people with this genotype are 5% less likely than others to have breast cancer (Fig. 7). In Fig. 7, the odds ratios based on the random effects model are illustrated. Each black square represents the odds ratio, and the length of the line on which the square is located demonstrated the 95% confidence interval in each study. The diamond symbol represents the odds ratio for all studies put together.
Discussion
Cancer is one of the major health threats worldwide and is one of the leading causes of death and illness in children and adults. Cancer is a term that is referred to a variety of diseases (more than 200 diseases) [20]. Breast cancer is the most common type of cancer and is the foremost health threat for women today, accounting for about one-third of all cancers in the Western countries. The results of many existing research works suggest that single-nucleotide polymorphisms can increase the susceptibility to cancer by altering the DNA sequence. A number of epidemiological studies have found links between A/T 251 polymorphism in the IL-8 gene and the risk of breast cancer. This polymorphism, which is located in the 251 region of the IL-8 gene promoter, plays an important role in the production of IL-8, and it has been shown that this polymorphism in different populations is associated with tumor formation and metastasis in various cancers [20,21,22,23,24,25]. Therefore, this study was conducted to investigate the relationship between A/T 251 polymorphism of IL-8 gene and breast cancer using meta-analysis.
According to our systematic review and the meta-analysis, the odds ratio of AA genotype in patients with breast cancer based on meta-analysis of studies was obtained as 1.42, indicating an increasing effect of the AA genotype with the value of 0.42; the odds ratio of the AT genotype in patients with breast cancer based on a meta-analysis of studies was 1.01, indicating an increasing effect of the AT genotype by 0.01, and the odds ratio of TT genotype in patients with breast cancer based on meta-analysis of studies was obtained as 0.95, demonstrating the decreasing effect of TT genotype by 0.05. In other words, with the presence of heterozygous and homozygous genotypes in this polymorphism in the IL-8 gene, the factors for projecting the prognosis of the disease and the potential clinical outcome of breast cancer can be predicted. Given the prevalence of breast cancer in women and the threat to the patients, identifying IL-8 gene polymorphism as a genetic marker and in preventing the disease and diagnosing patients can be effective in restraining the progression of breast cancer.
Cytokines are small molecular weight regulatory proteins or lipoproteins that play an important role in regulating one’s immune function [24]. Such immunity effects can be achieved through stimulation, restricting activation, and proliferation or cell differentiation of the target cells. Different types of cytokines play different roles in the start or the spread of cancer and can, on one hand, pave the way for the occurrence and even proliferation and metastasis of cancer, yet on the other hand, prevent the development of cancer through their anti-inflammatory and anti-tumor effects.
Research works in recent years have shown the role of interleukins in breast cancer, where high levels of inflammatory cytokines are found in the serum and tumor tissue of breast cancer patients. The high levels of some of these cytokines in the serum of cancer patients are associated with the progression of the disease stage, the invasion of cancer cells and the development of metastasis [25,26,27,28]. Research has also shown that the progression and development of several types of breast cancer is associated with inflammation and the irregular and improper production of chemokines, especially IL-8, which appears to be a key step in metastasis of the cancer cells [28]. Previous research works has demonstrated that IL-8 controls the activating apoptotic pathways. Therefore, decreased expression of the IL-8 gene by tumor cells can stimulate chemokine receptors and thus impair the function of neutrophils and macrophages in suppressing inflammation [30]. It also alters IL-8 gene expression due to the presence of polymorphism in the gene's promoter area, which causes angiogenesis, resulting in metastasis and tumor malignancies, especially in breast cells, which can be directly linked to breast cancer [30]. IL-8 is one of the most important regulatory cytokines that has a central function in initiating and regulating cellular immune responses. IL-8 is expressed in macrophages and fibroblasts derived from intercellular cells and is known as a mediator derived from macrophages that play a role in angiogenesis. This cytokine is a chemotactic factor that can activate white blood cells [29, 30]. The role of this interleukin has also been demonstrated in a range of diseases such as psoriasis, rheumatoid arthritis, and ribosomal fibrosis. Numerous studies have demonstrated that IL-8 is an activating factor for angiogenesis, and can directly or indirectly cause tumor cell proliferation and the formation of new blood vessels through the superficial receptor of tumor cells in endothelial cells, and this can ultimately result in tumor’s growth and metastasis [30].
IL-8 is produced by breast cancer cells. It aids breast cancer cells in the Epithelial Mesenchymal Transition (EMT) phenotype, which stimulates, augments and enhances angiogenesis. IL-8 increases the proliferation and survival of endothelial cells and breast cancer, and enhances the migration of breast cancer cells, endothelial cells, and penetrating neutrophils at the tumor site. Thus, IL-8 expression is associated with angiogenesis, tumor formation, and breast cancer metastasis. In addition, IL-8 results in the resistance to chemotherapy in breast cancer cells (Fig. 8).
One of the limitations of this research work is that some samples were not based on random selection. Moreover, many articles were not selected due to the lack of uniform reporting in articles, the non-uniformity of the implementation methods, the unavailability of the full text of the articles, and articles presented in the conferences.
Conclusion
Considering the findings of this meta-analysis, allele A has a significant relationship with breast cancer, which can predict the progression course of the disease and the potential clinical outcome of breast cancer. Identifying polymorphism A/T 251 from the IL-8 gene, as a genetic marker and predictor, can also be effective in treating breast cancer.
Code Availability
Datasets are available through the corresponding author upon reasonable request.
References
Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, et al. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;138(1):291–301.
Sharma GN, Dave R, Sanadya J, Sharma P, et al. Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 2010;1(2):109–26.
Pero CM, Borresen-Dale AL. Systems biology and genomics of breast cancer. Cold Spring Harb Perspect Biol. 2011;3(2):a003293.
Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene. 2006;25(43):5898–905.
Vacchelli E, Aranda F, Bloy N, Buqu A, et al. Trial watch: immunostimulatory cytokines in cancer therapy. Oncoimmunology. 2014;3(6):290–4.
Singh JK, Simões BM, Howell SJ, Farnie G, et al. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15(4):210.
Chia CY, Kumari U, Casey P. Breast cancer cell invasion mediated by Gα12 signaling involves expression of interleukins-6 and−8, and matrix metalloproteinase-2. J Mol Signal. 2014;9(1):6.
He Y, Liangb X, Wua X, Menga C, Wua B, Fua D, et al. Association between interleukin 8–251 A/T and +781 C/T polymorphisms and osteoarthritis risk. Immunol Lett. 2014;162:207–11.
Ohyauchi M, Imatani A, Yonechi M, Asano N, Miura A, Iijima K, et al. The polymorphism interleukin 8 2251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005;54(3):330–5.
Snoussi K, Mahfoudh W, Bouaouina N, Fekih M, et al. Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness. Biomed Cancer. 2010;10:283.
Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–40.
Long X, Ye Y, Zhang L, Liu P, Yu W, Wei F, et al. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways. Int J Oncol. 2016;48(1):5–12.
Lin Y. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Publication of the international union Against Cancer (uicc). Int J Cancer. 2004;109:507–15.
Huang Q, Wang C, Qiu LJ, Shao F, et al. IL-8-251A>T polymorphism is associated with breast cancer risk: a metaanalysis. J Cancer Res Clin Oncol. 2011;137(3):1147–50.
Taheri M, Hashemi M, Eskandari-Nasab E, Fazaeli A, Arababi F, Bahrani-Zeidabadi M, et al. Association of-607 C/A polymorphism of IL-18 gene (rs1946518) with breast cancer risk in Zahedan. Southeast Iran Prague Med Rep. 2012;113(3):217–22.
Zhang J, Han X, Sun Sh. IL-8 -251A/T and +781C/T polymorphisms were associated with risk of breast cancer in a Chinese population. Int J Clin Express Pathol. 2017;10(7):7443–50.
He Y, Sun S, Liu Y, Tian K. Association of IL-8 genetic polymorphisms and breast cancer risk in a Chinese population. Biomed Res. 2017;28(18):7892–8.
Xiuyu C, Weihan H, BeiZh Ni D, et al. Genotyping of IL-8-251 T > A yields prognostic information in patients with gastric carcinoma. Biomarkers. 2013;18(7):559–64.
Chen Y, Yang Y, Liu S, Zhu S, et al. Association between interleukin 8–251 A/T and+ 781 C/T polymorphisms and osteosarcoma risk in Chinese population: a case–control study. Tumor Biol. 2016;37(5):6191–6.
Wang Z, Gao ZM, Huang HB, Sun LS, et al. Association of IL-8 gene promoter -251 A/T and IL-18 gene promoter-137 G/C polymorphisms with head and neck cancer risk: a comprehensive metaanalysis. Dovepress. 2018;10:2589–604.
Jessica C, Alwadris TT, Prasetyo SR, Puspitawati R, et al. Association of interleukin 8–251 A/T gene polymorphism with periodontitis in Indonesia. J Phys. 2018;1025:1–5.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18.
Siasi E, Gholami M, Ashrafi F. Investigation of the relationship between breast cancer and polymorphism A / T 251 of the IL-8 gene in the Iranian female population by tetra arms-PCR method. Cell Tissue Mag Cover. 2019;10(1):12–23 ([In Persian]).
Kamali-Sarvestani E, Aliparasti M, Atefi S. Association of interleukin-8 (IL-8 or CXCL8)-251T/A and CXCR2+ 1208C/T gene polymorphisms with breast cancer. NEOPLASMA-BRATISLAVA-. 2007;54(6):484.
Snoussi K, Mahfoudh W, Bouaouina N, Fekih M, Khairi H, Helal AN, et al. Combined effects of IL-8 and CXCR2gene polymorphisms on breast cancer susceptibility and aggressiveness. BMC Cancer. 2010;10(1):283.
Smith K, Bateman A, Fussell H, Howell W. Cytokine gene polymorphisms and breast cancer susceptibility and prognosis. Eur J Immunogenet. 2004;31(4):167–73.
Snoussi K, Mahfoudh W, Bouaouina N, et al. Genetic variation in IL-8 associated with increased risk and poor prognosis of breast carcinoma. Hum Immunol. 2006;67(1–2):13–21.
Vogel U, Christensen J, Nexo BA, et al. Peroxisome proliferator-activated [corrected] receptor-gamma2 [corrected] Pro12Ala, interaction with alcohol intake and NSAID use, in relation to risk of breast cancer in a prospective study of Danes. Carcinogenesis. 2007;28:427–34.
Jehn C, Flath B, Strux A, Krebs M, Possinger K, Pezzutto A, et al. Influence of age, performance status, cancer activity, and IL-6 on anxiety and depression in patients with metastatic breast cancer. Breast Cancer Res Treat. 2012;136(3):789–94.
Norii Daloii M, Tabarestani S. Molecular Genetics Diagnosis and treatment of breast cancer, review. J Sabzevar Univ Med Sci. 2010;17(2):74–87.
Acknowledgements
The authors thank the Student Research Committee of Kermanshah University of Medical Sciences.
Funding
This study is funded by the Deputy for Research and Technology, Kermanshah University of Medical Sciences (990398).
Author information
Authors and Affiliations
Contributions
NS and KM contributed to the design, MM statistical analysis, participated in most of the study steps. MM and MK prepared the manuscript. AHF and KM and FAN assisted in designing the study and helped in the interpretation of the study. All authors have read and approved the content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics Approval
Ethics approval was received from the Ethics committee of Deputy of Research and Technology, Kermanshah University of Medical Sciences (IR.KUMS.REC.1399.199).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salari, N., Kazeminia, M., Hosseinian-Far, A. et al. The Effect of Polymorphism A/T 251 of the IL-8 Gene on Breast Cancer: A Systematic Review and Meta-Analysis. Indian J Gynecol Oncolog 19, 70 (2021). https://doi.org/10.1007/s40944-021-00571-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-021-00571-3