Abstract
The rapid depletion of water quality in reservoirs is considered as a global ecological and health consequences. Hence, the regular monitoring of water quality status in such reservoirs is mandatory to ensure a safe water supply for the general public. The present study was conducted to evaluate the water quality, contamination status of cyanotoxins, species composition and abundance of phytoplankton in 43 selected major reservoirs in Sri Lanka. The water temperature, pH, Dissolved oxygen (DO), and Electrical Conductivity (EC) were measured at the site and the other water quality parameters, Total Nitrogen (TN), Total Phosphates (TP), Chemical Oxygen Demand (COD), Biological Oxygen Demand (BOD), Fluoride, Total Hardness (TH), Chlorophyll-a (Chl-a), Cyanotoxins; Microcystin-LR (MC-LR), Cylindrospermopsin (CYN), Saxitoxin (SAX), Nodularin (NOD), Anatoxin-a (ANA-a) concentrations and Faecal Coliform were measured following the standard methods. The water temperature, pH, DO, EC, TN, TP, COD, BOD, Fluoride, TH, and Chl-a were ranged from 20.5 to 29.7 ℃, 6.5 to 8.8, 2.1 to 7.9 mg/L, 25.6 to 1752 µS/cm, < 0.05 to 3.42 mg/L, < 0.05 to 20.55 mg/L, 10.2 to 220.6 mg/L, 0.6 to 10.6 mg/L, < 0.01 to 0.23 mg/L, 13.67 to 122 mg/L and 0.66 to 48.9 µg/L respectively. Further, each water body was contaminated with at least one algal toxin tested. Overall, MC-LR was the most dominant toxin variant (37%) in the collected water samples compared to CYN (10%), SAX (10%), NOD (10%), and ANA-a (10%). Moreover, 39 reservoirs tested out of 43 recorded fecal coliform greater than 200 (CFU)/100 mL. Among all the selected water bodies, Beira Lake in Colombo was the most polluted recreational water body. Furthermore, in Beira Lake, almost all measured water quality parameters exceeded the standard levels set by the Sri Lanka Standards Institute (SLSI) and the Environmental Protection Agency (EPA), USA for aquatic life. The measured water quality data indicated that most of the reservoirs are polluted, and the overall water quality has deteriorated. It suggests the negative impacts on the ecological balance of the ecosystem and these results indicate the necessity of proper treatment facilities for the reservoirs in Sri Lanka.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sri Lanka is an island in the Indian Ocean, southeast of the Bay of Bengal, with mean temperatures of 17 ℃ in the central highlands to 27 ℃ in the lowlands (Wijewardena 2021). Four monsoons dominate the rainfall distribution throughout the island: the southwest monsoon, the northeast monsoon, and inter-monsoons prevailing throughout the year. There are two primary climatic regions in Sri Lanka dry zone and the wet zone, based on the availability of rain (Ampitiyawatta et al. 2022). Additionally, many historically significant manmade reservoirs are primarily in the dry zone to collect rainwater for agricultural and recreational uses (Siriwardana et al. 2019). High populations in Sri Lanka rely on these water reservoirs to meet their water requirements (Mahagamage et al. 2014). Importantly, most of the urban and suburban human population in Sri Lanka directly consumes the reservoir water without any treatment.
The water requirement in Sri Lanka has gradually increased in recent decades due to the expansion of industrial and agricultural activities (Mahagamage and Manage 2019; Thadshayini et al. 2020). In addition, the direct discharge of untreated wastewater into natural water bodies, and excessive use of nitrogenous fertilizers for agricultural purposes, mainly contribute to the rapid water quality depletion in the reservoirs (Akhtar et al. 2021). The accumulation of excessive nitrate and phosphate in the reservoirs causes serious ecological concerns like eutrophication and increases toxin levels resulting in critical health problems (Bhateria and Jain 2016; Mahagamage et al. 2019). Furthermore, the accumulation of excess nitrate and nitrite ions in water can cause acute and chronic toxicity for both adults and children, leading to conditions such as methemoglobinemia and brain tumors (Karwowska and Kononiuk 2020). In addition, the increased nutrient concentrations in water can boost microalgae growth in tropical countries like Sri Lanka (Kulasoorita 2017; Manage 2019). As a result, cyanobacteria can pose an environmental and health risk to humans by forming dense agglomerations in freshwater habitats that produce and emit harmful cyanotoxins (Van Apeldoorn et al. 2007; Idroos and Manage 2014). Water quality depletion can be caused by metabolites released by cyanobacteria and microalgae degradation, which cause odor and taste problems in freshwater. Another critical aspect of cyanobacteria in drinking water quality is the formation of surface scums before the occurrence of blooms (Spoof et al. 2006). Anabaena circinalis, Aphanizomenonflos-aquae, Cylindrospermopsis raciborskii, and Microcystis aeruginosa, are among other cyanobacterial strains, that have been reported all over the world as cyanotoxins producers (Manage 2019). Wijewickrama and Manage (2019) documented that toxins can impact other flora and fauna.
The concentration of Chlorophyll-a present in a water body is used as a marker to measure the number of microalgae that can be present in that water body which can classify the trophic state of the water. Chlorophyll-a concentrations and algal density are higher in water, with high nutrients obtained from the dissolved chemicals. By promoting eutrophication, hypoxia, low DO in bottom waters, and anoxia through increased algal productivity, the increase of nutrients in the surface water bodies poses a hazard to the ecological balance in the water system. Increased nutrient levels commonly result in higher algal productivity, which increases Chlorophyll-a concentrations in water (Dahanayake et al. 2016).
Fecal contamination of drinking water is also a significant threat to public health, especially in developing countries. Contaminated drinking water is estimated to cause 485,000 diarrheal deaths yearly (WHO 2019). Further, microalgae can change fecal coliform concentrations in the aquatic system since microalgae are known to supply carbon, compete with fecal coliform for nutrients, modify the water chemistry, shelter fecal coliform from solar radiation, and release toxins and stimulants. Different types of microalgae can have different impacts on fecal coliform survival by altering fecal coliform populations. Microalgae can consequently affect any fecal coliform bacteria-based evaluation of the effectiveness of land-use practices, such as waste management, livestock feeding operations, stormwater control, recreational activities, and conservation practices on microbial water quality. In particular, practices causing or controlling eutrophication may influence microalgal populations and consequently affect the conditions fecal coliform has adapted for survival.
Hence, the mechanisms that underlie the source of water contamination variability are essential to study and monitor to enhance the safety of drinking water and preserve public health (Mahagamage et al. 2014). Further, making decisions about supplying safe water to the public is crucial. Hence, the current study focused on analyzing the water quality of a few critical reservoirs in Sri Lanka concerning nutrient levels, fecal coliform contaminations, algal composition, and various cyanotoxins.
Methodology
Study area and sampling
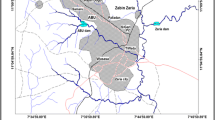
Water samples were collected from 43 major reservoirs (Fig. 1) in Anuradhapura, Polonnaruwa, Trincomalee, Ampara, Batticaloa, Kurunegala, Matale, Badulla, Hambanthota, Kandy, Nuwara Eliya, and Colombo districts in Sri Lanka during January to September 2021.
Water samples were collected from a 15 cm depth from the surface of the reservoir into pre-cleaned polypropylene bottles and sterilized amber colour glass bottles for chemical and microbial analysis, respectively. Samples were taken for two times from this period Then water samples were transported to the laboratory under cooling conditions (4–8 ℃) and stored in a cold room until analysis. The GPS coordinates were recorded via a hand-held Garmin eTrex 30 GPS receiver at the site. The User categories and climatic zones of the reservoirs sampled in the present study are mentioned in Table 1.
Physico-chemical analysis of water
Water temperature, DO, and water pH levels were measured using an HQD portable multiparameter (HACH–HQ 40D), and TDS, EC, and salinity were recorded using a portable conductivity meter (HACH–Sension EC5) at the site itself. COD was determined by using the closed reflux method where ammonia (as N-NH3), nitrates (as N-NO3), nitrite (as N-NO2), and TP concentrations were measured by using the Spectrophotometric (Spectro UV–VIS Double UVD 2960) methods. The total hardness was determined by using standard methods (APHA 1999). The SPADNS method was used to determine the fluoride in water using the colorimeter (HACH–DR 2700).
Total and fecal coliform analysis
The membrane filtration method was performed to determine the water's total coliform (TC), and E. coli counts. An aliquot of 0.1 dm3 from each water sample was filtered through a 0.45 µm filter paper (Whatman Cat No: 7001 0004, D-47 mm), and then the filter papers were kept on the Membrane Lactose Glucuronide Agar (MLGA) plates. Plates were incubated at 37 °C, and bacterial counts were taken after 18 ± 2 h (SLSI, 2013).
Determination of chlorophyll-a concentration
The Chlorophyll content was extracted from the concentrated algal sample into 10 mL of methanol (98%). The chlorophyll-a concentration was determined spectrophotometrically by measuring the absorbance (Optical Density-OD) at 665 nm and 750 nm wavelengths before and after acidification (1% HCl). The resulting absorbance measurements were then applied to a standard equation to detect Chlorophyll-a concentrations (Pathmalal et al. 2023; Gunawardana et al. 2022).
Determination of Cyanobacteria species composition and abundance
About 100 L of water was filtered through a plankton net (55 µm) to collect phytoplankton samples. Filtered samples were collected into plastic bottles (100 mL), fixed with a 1% final concentration of acidified Lugol's solution, transferred to a measuring cylinder at the laboratory, and allowed to settle for 24 h in the dark. The upper water column was gently drawn off using a Pasteur pipette, leaving a 5–10 mL sample in the cylinder, assuming all microalgae had settled to the bottom (Guiry and Guiry 2014; Sahu, 2013). Identification and enumeration were made using the Sedgewick-rafter counting chamber under the epifluorescence microscope. Cyanobacteria species were identified using a standard algae taxonomic guideline (Moestrup et al. 2009).
Determination of cyanotoxins by using ELISA
Commercially available test kits were used to quantify intracellular and extracellular MC-LR, CYN, NOD, SAX, and ANA-a (Beacon, USA). 25 mL from each water sample was frozen and thawed three times to lyse the cells and liberate the intracellular toxin. Each sample was analyzed in triplicate, and the procedure was followed exactly as directed by the manufacturer. In a plate reader, the absorbance of total cyanotoxin was measured at 450 nm (ELISA, USA) (Abeysiri and Manage 2022a, b).
Statistical analysis
The dataset was processed using the Minitab (version 15) statistical software. Principal Component Analysis (PCA) was carried out for 14 physico–chemical parameters and 43 surface water sampling locations. Pearson correlation was performed to compare the association between the water quality parameters in reservoirs (Mahagamage and Manage 2019).
Results
Physico-chemical analysis of water
The pH of all the reservoirs ranged between 6.5 ± 0.2 and 8.8 ± 0.1. The highest pH was recorded from the Beira Lake Colombo, and the lowest was recorded from the Dambulu oya, Kurunegala district. The lowest EC was 25.6 μScm–1 recorded from the Kandeela reservoir, and the highest EC was 1862.5 μScm–1 in the Nachchaduwa reservoir. The DO of the selected reservoirs ranged between 2.12 and 10.23 mg/L, and the highest and lowest values were recorded at Beira Lake and Gregory Lake, respectively. The highest total inorganic nitrogen, phosphate, COD, and BOD level was recorded at the Beira lake Colombo at 3.42 ± 0.08 mg/L, 20.55 ± 0.07 mg/L, 1220.6 ± 0.2 mg/L and 10.6 mg/L values, respectively. In this study, Chlorophyll-a concentrations in the studied water bodies ranged from 0.66 ± 0.0 to 48.9 ± 0.3 mg/L. The highest Chlorophyll-a was recorded from Beira Lake, and the lowest was on the Rantabe reservoir (Table 2).
Total and fecal coliform contamination of water
Fecal coliform (E.coli) is considered a critical water quality indicator that determines water quality for drinking purposes. According to the recorded CFU values (Table 2) the Rathkida, Ulhitiya, and Kandeela reservoirs were not contaminated with the fecal coliform. However, the fecal coliform count was recorded at more than 200 CFU in 100 mL of water in the reservoirs of Mahakanadara Wewa, Vahalkada Weva, Baire Lake, Polgolla Lake, Castelere Lake, Ridiyagama, Kandy Lake, Maussakele Lake, and Gregory Lake.
Cyanobacteria species composition and abundance
Five different cyanobacteria species were recorded; Microsystis sp., Oscillatoria sp., Cylindrspermopsis sp., and Anabaena sp., The Merismopedia sp. is the most abundant species in the reservoirs. During the study, the highest abundance of cyanobacteria cell densities was detected in Beira Lake, Colombo (Table 3).
Cyanotoxin contamination
At least one algal toxin (Microcystin-LR (MC-LR), Cylindrospermopsin (CYN), Saxitoxin, Nodularin, and Anatoxin-a) was detected in the 43 reservoirs. 37% of samples were positive for the MC-LR and the highest concentration of MC-LR was recorded in the Beira Lake (3.31 µg/L), where other reservoirs recorded at less than 1 µg/L. In the Anuradhapura district, MC-LR was detected in Basawakkulama and Padaviya reservoirs at 0.10 µg/L, 0.13 µg/L respectively, whereas in Polonnaruwa district; Ambagaswewa and Parkkrma Samudraya reservoirs were at 0.14 µg/L and 0.11 µg/L respectively. In the Badulla district, the Rathkinda reservoir was recorded at 0.10 µg/L MC-LR, and the Moragahakanda reservoir in the Matale district was 0.13 µg/L. Further, Maduru Oya, Jayanthi Wewa, Sagama tank, Senanayake Samudraya, Kandy Lake, Randenigala, Gregory, Kothmale, and Unnichchi reservoirs, MC-LR concentrations recorded at 0.12 µg/L, 0.10 µg/L, 0.10 µg/L, 0.11 µg/L, 0.11 µg/L, 0.10 µg/L 0.18 µg/L, 0.10 µg/L and 0.10 µg/L respectively during the study period. However, MC-LR was not recorded in reservoirs in Trincomalee, Kurunagala and Hambanthota districts. According to the established guidelines by the WHO and Sri Lanka Standard Institute (SLSI), MC-LR in drinking water should be below 1.0 µg/L. Except for the Beria Lake (3.31 µg/L) 15 irrigation tanks were contaminated with MC-LR and the concentrations remained below the WHO and SLSI concentrations given for drinking.
The concentrations of CYN in reservoirs ranged from 0.05 µg/L to 0.07 µg/L during the sampling. Compared to the other districts, high CYN concentrations were recorded in the Anuradhapura district. Further, the highest concentration of saxitoxin was recorded in the Padaviya tank (0.09 µg/L) in the Anuradhapura district following the Beira Lake (0.08 µg/L), Parakkrma Samudraya (0.08 µg/L) and Ulhitiya (0.08 µg/L) respectively. All the recorded saxitoxin concentrations were lower than the drinking guidelines given by the WHO (0.1 µg/L). Nodularin was only detected in four reservoirs, namely, Balalau (0.07 µg/L), Padaviya (0.05 µg/L), Ulhitiya (0.08 µg/L), Uninichchi (0.06 µg/L)and the Anatoxin-a was recorded in Nuwara Wewa (0.17 µg/L), Padaviya (0.19 µg/L), Parakkrama Samudraya (0.15 µg/L) and Unnichchi reservoirs (0.16 µg/L) respectively.
Statistical analysis
The first principal component of the PCA analysis is strongly correlated with average chlorophyll-a, MC-LR, COD, BOD, total phosphate, and total inorganic nitrogen. Furthermore, the second principal component is strongly correlated with water temperature, pH, total hardness, nodularin, and cylindrospermopsin parameters. However, dissolved oxygen negatively correlates with the first and second principal components. EC, anatoxin-a, saxitoxin, and fluoride parameter concentration are correlated with the first and second principal components (Fig. 2).
Padaviya wewa has a very high value for the second principal component and high values for the water temperature, pH, total hardness, nodularin and cylindrospermopsin. Similar results were shown in the Ambagaswewa. However, Castlereagh Lake, Maussakele and Gregory Lake negatively correlated with the water quality parameters. The Beira Lake was well correlated with the first component. It suggested that it has recorded very high values for the average chlorophyll-a, MC-LR, COD, BOD, total phosphate and total nitrogen. Tanks in the Anuradhapura district, except Padaviya Wewa, were clustered together, while lakes in the Nuwara Eliya district were clustered together. Furthermore, the lakes in the Nuwara Eliya district have recorded high values for dissolved oxygen. Kandeela, Ulhitiya, Rathkinda, Thissa wewa and Nuwara wewa, which are used for drinking, are well correlated with the parameters of dissolved oxygen while negatively correlated with the concentration of fluoride, electrical conductivity, the concentration of anatoxin and saxitoxin (Fig. 1).
Discussion
The water quality analysis with a combined sanitary inspection can be used to identify the most critical causes of and control measures for contamination of water bodies (Lu et al. 2015). This is important to support effective and national decision-making. Many national and international institutes have published the standard values of water quality, which is a generally applicable approach to ensuring the safety of drinking water in communities and recreational activities (Akhtar et al. 2021). In the present study, water quality, contamination status of cyanotoxins, species composition and abundance of phytoplankton in 43 selected major reservoirs in Sri Lanka to determine the deterioration of reservoir water quality. Reservoirs play a crucial role in supplying water for drinking, agriculture, fishing, industry, recreational activities, and the generation of hydroelectricity. Hence, it is important to determine the contamination levels in reservoir water which precedes taking necessary measures to continue the proper water quality status in reservoirs. It helps to maintain the good health and ecological balance. Furthermore, the degradation of reservoir water quality occurs gradually over an extended period. Once the degradation state reaches beyond the threshold level, returning it to its previous state in terms of ecological integrity and quality is impossible. Hence, the continuous monitoring of the water quality and the contamination status in reservoirs is important.
In the present study, 43 selected major reservoirs in Sri Lanka were investigated and the highest deterioration was determined in the Beira lake in Colombo district. According to Kasthuriarachchi et al. (2016), the MaduruOya reservoir has recorded a high accumulation of nutrients which leads to eutrophication and growth of toxin-producing algal species such as Microcystis and it affected the ecosystem health and human livelihood.
The pH is one of the fundamental water quality parameters. According to Sri Lankan drinking water quality standards, the pH of the drinking water should have remained within the range of 6.5–8.5 (EPA 2007). Notably, the pH level of selected water reservoirs has remained within Environmental Protection Agencies’ (EPA’s) maximum permissible levels for drinking and recreational activities. EC comprises different inorganic salts and some organic matter that are dissolved in water (Mahagamage et al. 2019). The reservoirs like Beira, Lunugamvehera, Sagama, and Mahakanadara wewa have recorded high EC values which were 1752 μScm–1, 1552 μScm–1, 1250 μScm–1 and 1666 μScm–1 respectively indicating the presence of the high amount of ions in the water.
Nitrate (N-NO3−) is another essential water quality parameter for aquatic life. Recently, the nitrate levels of the surface water reservoirs have increased due to improper anthropogenic activities (Mahagamage et al. 2019). The health impact of high nitrate and nitrite-contaminated water caused the formation of methemoglobinemia, and blue baby syndrome was recorded in different regions of the world (Singh et al. 2022). Therefore, the nitrate levels of the studied reservoirs have remained below the standard levels except for Beira Lake.
The total phosphorous (TP) concentrations in the surface water are relatively low due to the low solubility of the phosphorous (Varol et al. 2020). However, phosphate concentration can increase due to accumulating phosphate detergents in reservoirs (Sirisena and Suriyagoda 2018). Nitrates and phosphates are the significant nutrients contributing to reservoirs’ eutrophication phenomenon. In many cases, phosphorous is the limiting factor for the growth of algae and cyanobacteria (Zeng et al. 2016). To control eutrophication, the Environmental Protection Agency has established a recommended limit of 0.05 mg/L for total phosphates in streams that enter lakes (EPA 2007). According to the recorded values of total phosphate of the reservoirs, Polgolla, Lunugamvehera, Maussakele, Gregory, and Beira lakes have recorded the values 0.05 mg/L, 0.08 mg/L, 0.015 mg/L, 0.105 mg/L, 1.22 mg/L respectively. Water hardness is another vital water quality parameter generally expressed as the total concentration of calcium and magnesium ions in water. The Ca2+ and Mg2+ ions are the most important ions for hardness, and the unique reservoirs located in the northwestern province showed a greater hardness level (Mahagamage et al. 2019). In the present study, the highest total hardness values were recorded in Ambagaswewa (Value) and Dambulu oya (Value).
The coliform count is one of the critical microbiological parameters widely used to determine the contamination potential of drinking water with fecal matter (Mahagamage et al. 2020). E. coli is a coliform species always found in warm-blooded animals' guts (Edokpayi et al. 2018). Suppose the water is contaminated with fecal coliform. In that case, it is more likely to present some pathogenic bacterial species, such as Salmonella sp., Shigella sp., Campylopytor spp., etc., which can cause severe waterborne illnesses. According to the Sri Lankan drinking water standards, the maximum permissible level of fecal coliform in 100 mL of water is zero. The results indicated that 58% of studied reservoirs recorded a coliform count greater than 200 CFU/100 mL, indicating the water of those reservoirs was microbiologically unsatisfactory for direct consumption.
The mechanism of water contamination is critically important for the proper legislation and monitoring policies of the reservoirs in the country. Based on the overall water quality analysis it can clearly stated that the Baira Lake is the most polluted water body situated in Colombo (wet zone), Sri Lanka's capital. The water of Baira Lake has been contaminated with toilet effluents from the adjacent toilet pits of the city. Further, the poor sanitary facilities of the city, higher population density and the accumulation of the rainwater run off with may result from the high nitrate concentration, leading to a severe eutrophication problem (Madarasinghe et al. 2020). The Lunugamvehera and Mahakanadara reservoirs which situated in intermediate and dry zones respectively, were recorded high EC values which may be attributed to the geographical variation of the soil beds associated with the water body. Particularly, the abundance of the agricultural lands and frequent usage of nitrogen fertilizers around the Sagama and Lunugamvehera reservoirs may positively impact to concentrate the nitrate and phosphate ions in the reservoirs increasing the EC, TP and in the reservoirs. Additionally, Maussakele (Wet zone) and Gregory Lake (wet zone) have recorded high TP which may be due to the direct discharge of detergents which are used by the surrounding rural community that have associated with tea estates and the tourism-related anthropogenic activities respectively.
Regarding the surveillance for cyanotoxins, previous studies reveal that MC-LR is well distributed while the CYN was circumscribed to limited locations (Abeysiri and Manage 2019). However, there are no reports on the contamination status of reservoirs with anatoxin-a, saxitoxin, and nodularin in Sri Lanka. In the present study, MC-LR was detected as the dominant toxin (> 50%) in collected reservoir samples compared to the CYN (< 10%), SAX (< 10%), NOD (< 10%), and Ana-a (< 10%). MC-LR occurrence is well supported by the presence of Microcystis sp. in more than 70% of studied reservoirs in Sri Lanka (Abeysiri and Manage 2019). Freshwater ecosystems carry those toxins and have several impacts on drinking, irrigation, and recreation, and several socio-economic activities have been attributed, such as agriculture, sports, and even tourism. Moreover, dermal, inhalation, or ingestion of contaminated water, directly or indirectly, can occur if populations are misinformed. Very high concentrations were found in surface cyanobacterial scum in Germany (up to 120 ug/L) and Algeria (711.8 ug/L), but these represent a different type of sample and also a different type of analytical method (protein-phosphate assay inhibition) was employed than that used in the present study. Comparatively lower values were reported from Finland (0.21 µg/L), where water blooms are often dominated by different cyanobacterial species than Microcystis sp., which is common in Europe.
Although the most usual route of human ingestion of toxins is drinking contaminated water, the accumulation of toxins in vegetables may increase the number of intoxications and the long-term effects, including the risk of cancer (Gupta et al. 2019). Cattle and wildlife, including protected species, may also suffer from the presence of the toxin. Cases of mortality in wild birds due to drinking contaminated water have been recorded in Japan and Denmark (Rattner et al. 2022).
Cyanobacteria breakdown products are significant in providing nutrients for total coliforms existing in the biofilm. When these nutrients are present in small quantities, as is the case for much of the year, the chlorine residual can control any potential growth and cyanobacterial breakdown, providing the nutrition for total coliform regrowth (Kulasooriya 2017). The results of these studies indicate a strong link between the end of the cyanobacteria bloom and the cyanotoxin levels and coliform occurrences in the distribution system. There is likely an increase in available carbon from the cyanobacteria breakdown products, enabling coliforms to regrow (Kollu and Ormeci 2015). Ramanan et al. (2016) detail the role of cyanobacteria-derived toxins and other nutrients in the aquatic environment; cyanobacteria photosynthetic and decomposition processes release hazardous toxins, carbon, and other nutrients are used by bacteria in various mutualistic and competitive relationships.
Moreover, the loading plot of PCA indicated a clear correlation among chlorophyll-a, MC-LR, COD, BOD, total phosphate, and total inorganic nitrogen parameters in the reservoirs. Chlorophyll-a is regularly used as an estimate of algal biomass, and it is estimated that algal blooms may occur when the chlorophyll-a concentration increases by more than 40 μgL−1 (Balali et al. 2013). Nitrogen and phosphorous are vital macronutrient for algal growth and plays an important role in the stability of the ecosystem (Yaakob et al. 2021). Accordingly, Beira Lake has recorded very high total nitrogen and phosphate values, and Chlorophyll-a, MC-LR, COD, and BOD may increase due to rapid algae growth. Similar results were obtained in the Weerasinghe and Handapangoda (2019) study to analyze surface water quality in Beira Lake from 08 judgmental sampling sites for 06 months period from May to October 2017.
In the present study, the lakes in the Nuwara Eliya district were clustered together, and the dissolved oxygen values were high for the lakes. At the same time, low water temperatures were recorded in these lakes. The dissolved oxygen amount in a given volume of water depends on several reasons, including atmospheric pressure, water temperature, and the amount of other substances dissolved in the water. Accordingly, these lakes may have high dissolved oxygen amounts due to low water temperature. Weerasinghe and Handapangoda (2019) recorded similar results in the study, which analyzed water quality in Gregory Lake, Nuwara Eliya, concerning the dynamic input and output.
The presence of cyanotoxins in water sources poses serious health risks to both humans and animals if ingested or exposed to affected water. These toxins can cause various health issues, ranging from skin irritation to gastrointestinal problems and, in severe cases, potential liver damage or neurotoxic effects. Monitoring cyanotoxin contamination involves routine testing of water bodies, especially those used for drinking water, recreational activities, or agricultural purposes. Therefore, the status of cyanotoxin contamination represents nutrient pollution, poor land use, and inadequate water quality management in these reservoirs. Frequently, water quality monitoring and assessment for cyanotoxin presence is timely important.
The impairment of water quality in reservoirs depends on various factors, including seasonal variations, elevation, geographical distribution, and urban or rural metrics. When considering climate and elevation, only a few selected reservoirs were located in the Nuwara Eliya, Badulla, Kandy, and Matale districts, which have higher elevations in the country. Importantly, there was no noticeable difference in physicochemical parameters, cyanotoxins, and phytoplankton data between reservoirs at high and low elevations (Table 1).
However, urban and rural metrics affected the water quality of certain reservoirs. Particularly, Baire Lake and Gregory Lake, located in the densely populated Colombo and Nuwara Eliya cities, were severely contaminated with high nitrate and phosphate levels, resulting in elevated levels of fecal coliforms and phytoplankton in the water due to extensive population density and effluent discharge into the reservoirs (Table 1).
Nevertheless, some reservoirs in rural areas also recorded lower water quality, especially Basawakkulama Reservoir and Maussakele Reservoir. This may be attributed to poor agricultural practices linked to the excessive use of chemical fertilizers on agricultural lands.
Based on the results of the present study, it is encouraged to adopt sustainable agricultural practices to reduce the runoff of nutrients and pesticides into reservoirs, invest in wastewater treatment facilities to prevent the discharge of untreated water into reservoirs, establish comprehensive water quality monitoring programs, create buffer zones around reservoirs to reduce the impact of human activities and increase public awareness programs.
Conclusion
According to the overall result of the present study, most of the reservoirs are polluted and the water quality has deteriorated. Beira Lake in Colombo district which is situated surrounded by highly urbanized areas is a highly polluted reservoir and each water body was contaminated with at least one algal toxin tested. Furthermore, most of the reservoirs have recorded the presence of fecal coliform indicating high health risks in humans. The water quality measurements in most of the reservoirs indicated negative impacts on the ecological balance of the ecosystem and these results indicate the necessity of proper treatment facilities for the reservoirs in Sri Lanka.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.
References
Abeysiri HASN, Manage PM (2019) Water quality, cyanobacteria and cyanotoxin contamination in some water bodies in Anuradhapura, Polonnaruwa, Ampara and Badulla Districts, Sri Lanka. Proceedings of 6th International Symposium on Water Quality and Human Health: Challenges Ahead at University of Peradeniya, Sri Lanka, December 2019. p. 20.
Abeysiri HASN, Manage PM (2022a) Microcystin-LR contamination in Nile tilapia (Oreochromis niloticus) in some water bodies in Sri Lanka. Sri Lanka J Aquat Sci 27(2):91–105
Abeysiri HASN, Manage PM (2022b) Water quality, microcystin-LR, and Cylindrospermopsin contamination status of spring and dug well water in CKDu high, low, and non-prevalent areas of Sri Lanka. Sri Lanka J Aquat Sci 27(2):75–89
Akhtar N, Ishak MIS, Ahmad MI, Umar K, Md Yusuff MS, Anees MT, Qadir A, Ali Almanasir YK (2021) Modification of the water quality index (WQI) process for simple calculation using the multi-criteria decision-making (MCDM) method: a review. Water 13(7):905
Ampitiyawatta AD, Nilmalgoda EPRHHW, Wimalasiri EM (2022) A Gaussian model approach to determine the commencement, termination and length of the major growing season over the dry zone of Sri Lanka. Theoret Appl Climatol 148(1):739–750
Balali S, Hoseini A, Ghorbnia R, Kordi H, Khozani EA (2013) Relationships between nutrients and chlorophyll a concentration in the international Alma Gol Wetland. Iran Int J Aquat Biol 1(2):68–75
Bhateria R, Jain D (2016) Water quality assessment of lake water: a review. Sustain Water Resourc Manag 2(2):161–173
Dahanayaka DDGL, Perera B, Wijeyaratne MJS, Tonooka H (2016) Monitoring eutrophication trends in Bolgoda North Lake, Sri Lanka by satellite remote sensing. Turk J Fish Aquat Sci 16(3):563–570
Edokpayi JN, Odiyo JO, Popoola EO, Msagati TA (2018) Evaluation of microbiological and physicochemical parameters of alternative source of drinking water: a case study of nzhelele river. South Africa Open Microbiol J 12:18
Guiry MD, Guiry GM (2014) Algae Base. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. Accessed 10 Oct 2023
Gunawardana MHMASV, Wijesooriya MM, Randima GWAP, Atapaththu KSS, Sanjaya K, Masakorala K, Gamage SMKW (2022) Co-occurrence of microcystin and cylindrospermopsin in hypereutrophic Mahakanadarawa and Nachchaduwa reservoirs. J Sci Univ Kelaniya Sri Lanka 15(1):67–97
Gupta N, Yadav KK, Kumar V, Kumar S, Chadd RP, Kumar A (2019) Trace elements in soil-vegetables interface: translocation, bioaccumulation, toxicity and amelioration-a review. Sci Total Environ 651:2927–2942
Idroos SF, Manage PM (2014) Seasonal occurrence of Microcystin-LR with respect to physico-chemical aspects of Beira lake water. Int J Multidiscip Stud. 1(2):27
Karwowska M, Kononiuk A (2020) Nitrates/nitrites in food—Risk for nitrosative stress and benefits. Antioxidants 9(3):241
Kasthuriarachchi TDW, Wickramaarachchi WDN, Premaratne WAPJ (2016) Assessment of water quality status and pollution levels in maduru oya reservoir in Sri Lanka. In: International postgraduate research conference 2016, University of Kelaniya, p 161
Kollu K, Örmeci B (2015) Regrowth potential of bacteria after ultraviolet disinfection in the absence of light and dark repair. J Environ Eng 141(3):04014069
Kulasooriya SA (2017) Toxin producing freshwater cyanobacteria of Sri Lanka. Ceylon Journal of Science 46(1):3–16
Lu Y, Song S, Wang R, Liu Z, Meng J, Sweetman AJ, Jenkins A, Ferrier RC, Li H, Luo W, Wang T (2015) Impacts of soil and water pollution on food safety and health risks in China. Environ Int 77:5–15
Madarasinghe SK, Yapa KK, Satyanarayana B, Udayakantha PMP, Kodikara S, Jayatissa LP (2020) Inland irrigation project causes disappearance of coastal lagoon: the trajectory of Kalametiya Lagoon, Sri Lanka from 1956 to 2016. Coast Manag 48(3):188–209
Mahagamage MGYL, Manage PM (2019) Water quality and microbial contamination status of Madawachchiya, Padaviya and Kebathigollewa areas in Anuradhapura District, Water and Land Development. J Water Land Dev. 42(VII–IX):1–11
Mahagamage MGYL, Chinthaka SDM, Manage PM (2014) Multivariate analysis of physico-chemical and microbial parameters of surface water in Kelani river basin. Int J Multidiscip Stud (IJMS). 1(1):55–61
Mahagamage MGYL, Pathirage M, Manage PM (2020) Contamination Status of Salmonella spp., Shigella spp. and Campylobacter spp. in Surface and Groundwater of the Kelani River Basin Sri Lanka. Water. 12(8):2187
Manage PM (2019) Cyanotoxins: a hidden cause of chronic kidney disease of unknown etiology (CKDu) in Sri Lanka-a review. Sri Lanka J. Aquat. Sci. 24(1):1–10
Moestrup O, Lindberg K, Daugbjerg N (2009) Studies on woloszynskioid dinoflagellates IV: The genus Biecheleria gen. nov. Phycol Res 57(3):203–220
Pathmalal MM, RSKWD H, Dilena PK, Liyanage GY, Chalani HTR, Bandara KRV, Wijerathna PAKC, Abeysiri HASN (2023) Impact of the MV X-press pearl ship disaster on the coastal environment from Negambo to Benthota in Sri Lanka. Reg Stud Marine Sci 58:102788
Ramanan R, Kim BH, Cho DH, Oh HM, Kim HS (2016) Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnol Adv 34(1):14–29
Rattner BA, Wazniak CE, Lankton JS, McGowan PC, Drovetski SV, Egerton TA (2022) Review of harmful algal bloom effects on birds with implications for avian wildlife in the Chesapeake Bay region. Harmful Algae. 120:102319
Sahu AK, Siljudalen J, Trydal T, Rusten B (2013) Utilisation of wastewater nutrients for microalgae growth for anaerobic co-digestion. J Environ Manage 122:113–120
Shaw D (2007) EPA’s 2007 report on the environment: Science report (SAB review draft).
Singh S, Anil AG, Kumar V, Kapoor D, Subramanian S, Singh J, Ramamurthy PC (2022) Nitrates in the environment: a critical review of their distribution, sensing techniques, ecological effects and remediation. Chemosphere 287:131996
Sirisena D, Suriyagoda LD (2018) Toward sustainable phosphorus management in Sri Lankan rice and vegetable-based cropping systems: a review. Agric Nat Res 52(1):9–15
Siriwardana C, Cooray AT, Liyanage SS, Koliyabandara SMPA (2019) Seasonal and spatial variation of dissolved oxygen and nutrients in Padaviya reservoir, Sri Lanka. J Chem 2019:1–11
Spoof L, Berg KA, Rapala J, Lahti K, Lepistö L, Metcalf JS, Codd GA, Meriluoto J (2006) First observation of cylindrospermopsin in Anabaena lapponica isolated from the boreal environment (Finland). Environ Toxicol Int J 21(6):552–560
Thadshayini V, Nianthi KWG, Ginigaddara GAS (2020) Climate-smart and-resilient agricultural practices in eastern dry zone of Sri Lanka. Global climate change: resilient and smart agriculture. Springer, Singapore, pp 33–68
Van Apeldoorn ME, Van Egmond HP, Speijers GJ, Bakker GJ (2007) Toxins of cyanobacteria. Mol Nutr Food Res 51(1):7–60
Varol M (2020) Spatio-temporal changes in surface water quality and sediment phosphorus content of a large reservoir in Turkey. Environ Pollut 259:113860
Weerasinghe VPA, Handapangoda K (2019) Surface water quality analysis of an urban lake; East Beira, Colombo, Sri Lanka. Environ Nanotechnol, Monit Manag 12:100249
WHO (2019) Fact sheet on water. Retrieved from. https://www.who.int/en/news-room/fact-sheets/detail/drinking-water. (Accessed 10 April 2020)
Wijewardena KAILG (2021) Beautiful, natural places and tourist attractions in Sri Lanka. The Institute of biopaleogeography named under Charles R. Darwin, pp 1–44. ISBN 978-83-949342-2-4
Wijewickrama MM, Manage PM (2019) Accumulation of Microcystin-LR in grains of two rice varieties (Oryza sativa L.) and a leafy vegetable Ipomoea aquatica. Toxins 11(8):432
Yaakob MA, Mohamed RMSR, Al-Gheethi A, Aswathnarayana Gokare R, Ambati RR (2021) Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: an overview. Cells 10(2):393
Zeng Q, Qin L, Bao L, Li Y, Li X (2016) Critical nutrient thresholds needed to control eutrophication and synergistic interactions between phosphorus and different nitrogen sources. Environ Sci Pollut Res 23(20):21008–21019
Acknowledgements
The authors would like to acknowledge the University of Sri Jayewardenepura, Sri Lanka, and the Centre for Water Quality and Algae Research for providing the laboratory facilities for this study.
Author information
Authors and Affiliations
Contributions
P. A. K. C. Wijerathna- samples collection, microbiological analysis, water quality analysis manuscript writing and editing. K. T. Dilrukshi- samples collection, water quality analysis, manuscript writing and editing. G. Y. Liyanage cyanotoxin analysis, manuscript writing editing and proofreading. K. R. V. Bandara- sample collection, chlorophyll-a analysis, Cyanobacteria and algae enumeration, manuscript writing and editing. M. G. Y. L. Mahagamage- MS writing correction and proofreading. P. M. Manage conceptualization, sample collection, manuscript writing, editing and proofreading.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wijerathna, P.A.K.C., Dilrukshi, K.T., Liyanage, G.Y. et al. Status of cyanotoxin contamination and water quality in major irrigational and recreational reservoirs in Sri Lanka. Sustain. Water Resour. Manag. 10, 116 (2024). https://doi.org/10.1007/s40899-024-01091-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-024-01091-6