Abstract
Blooms of toxic cyanobacteria in Algerian reservoirs represent a potential health problem, mainly from drinking water that supplies the local population of Ain Zada (Bordj Bou Arreridj). The objective of this study is to monitor, detect, and identify the existence of cyanobacteria and microcystins during blooming times. Samples were taken in 2013 from eight stations. The results show that three potentially toxic cyanobacterial genera with the species Planktothrix agardhii were dominant. Cyanobacterial biomass, phycocyanin (PC) concentrations, and microcystin (MC) concentrations were high in the surface layer and at 14 m depth; these values were also high in the treated water. On 11 May 2013, MC concentrations were 6.3 μg/L in MC-LR equivalent in the drinking water. This study shows for the first time the presence of cyanotoxins in raw and treated waters, highlighting that regular monitoring of cyanobacteria and cyanotoxins must be undertaken to avoid potential health problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria have been around for three billion years and have an important role in the formation of the earth’s oxygen and nitrogen fixation (Mur et al. 1999). Cyanobacteria can develop high proliferation in freshwater ecosystems, thus disrupting the ecosystem functioning and the water usages (drinking water, fishing, and livestock watering). Their strong biomasses are associated with eutrophication due to the enrichment of water by nutrients mainly phosphorus from urban and domestic waste, agricultural practices, and erosion of fertile soils (Hamilton et al. 2016). Climate change also contributes in increasing their frequency and persistence (Paerl and Paul 2012; Carey et al. 2012). The scientific community has become more interested in the causes and consequences of cyanobacteria blooms around the 1980s (Merel et al. 2013).
Nowadays, these photosynthetic microorganisms are most often associated with toxic impacts on all biological organisms (Codd et al. 2005). Several toxins include neurotoxins, hepatotoxins, and dermatotoxins with inflammatory and cytotoxic effects on animals and humans (Pouria et al. 1998; Chorus and Bartram 1999; Lance et al. 2010a, b; Carmichael and Boyer 2016). Hepatotoxins in drinking water have been connected to several health incidents in Brazil, Australia, China, and Serbia. Results from China (Yu 1995) and Serbia (Svircev et al. 2013) indicate that cyanobacterial hepatotoxins can lead to higher incidence of primary liver cancer. In addition to the toxicity associated with cyanotoxins, cyanobacteria have negative consequences on the production of drinking water such as the obstruction of filtration systems, excess of organic matter, and production of other secondary metabolites (geosmin, 2-methylisoborneol, beta-cyclocitral) that give odorous unpleasant taste to water and fish (Falconer 1999; Merel et al. 2013).
In Algeria, the water shortages compared to the needs of the population is increasing and is likely to rise in the future due to the impact of climate change as reported by many authors (Heisler et al. 2008; Reichwaldt and Ghadouani 2012). Knowledge of the cyanobacteria dynamics is important in the Algerian water bodies used for drinking water especially because most of the proliferations are dominant throughout the year (Amrani et al. 2014).
Various potentially cyanotoxin-producing species Cylindrospermopsis raciborskii, Microcystis sp., Pseudanabaena sp., and Lyngbya sp. were described in earlier studies (Nasri et al. 2004, 2007, 2008). Microcystins were also detected in some water bodies (Amrani et al. 2014). Since then, the health hazards of cyanobacteria have become more significant and several studies have been conducted in various freshwater bodies (Ouartsi et al. 2011; Djabourabi et al. 2014; Boussadia et al. 2015; Saoudi et al. 2015; Bidi-Akli et al. 2017). To limit organic matter, most of the drinking water treatment plants in Algeria are equipped with activated charcoal and in particular that of Ain Zada. This method is also used to remove the cyanotoxins (in particular MC) from the water (Meriluoto et al. 2017).
The aim of this study is to characterize the Ain Zada water body at times of cyanobacterial blooms by measurements of physico-chemical parameters, identification and enumeration of cyanobacteria, and evaluation of the toxicity of the raw and treated drinking water by the determination of microcystins and cylindrospermopsins. We also propose a health risk management plan to control the development of cyanobacteria.
Materials and methods
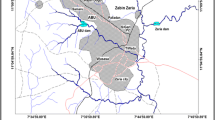
The dam of Ain Zada is located on the high plains of Setif at 815 m above sea level. The surface of the catchment area is approximately 2080 km2 (ANB BBA 2013). It is supplied by three main valleys: Bousselem (with industrial discharge), Malah, and Ain Taghrout (with urban water discharge). The dam water is used for drinking water supplies, for irrigation, and as an extensive aquaculture of the royal carp (Cyprinus carpio).
The climate of the region is semi-arid (harsh winters and dry hot summers), and average rainfall ranges between 300 and 600 mm, while air temperatures ranges from 38 °C (in July) and 0 °C (in December). The prevailing wind comes from the northwest with the exception of the summer when the sirocco is more recurrent. The drying wind increases evapotranspiration and thus enhances the effects of temperature. The hydraulic characteristics of the dam can be found in Table 1.
Sampling
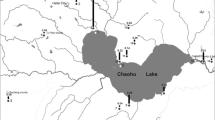
Monthly samples were taken from February to June 2013, from stations A1, A2, and A3 that corresponded to the valleys (Fig. 1) due to the easy access of those stations. Station A4 was sampled from the bank, and station A6 was near the dam wall. A7 was located at the entrance of the treatment plant 700 m from the reservoir, A8 near the treatment plant exit. Pumping raw water into the plant is carried out at 12 m above the reservoir sediment. Profiles and analyses were carried out at A5 station (Table 2) on 11 May 2013 during a large cyanobacterial bloom throughout the reservoir (Fig. 2) and associated with fish mortality.
Field measurements
Several physical and chemical parameters were recorded in the dam at each station. Temperature, dissolved oxygen, pH, and conductivity were measured “in situ” with a multi-parameter probe (WTW 340i Model). Turbidity was measured using the turbidimeter according to ISO 7027 standard and expressed in FNU (formazine nephelometric unit). Phycocyanin (PC) fluorescence, characteristic of a pigment specific of cyanobacteria, was measured with a TriOS microFlu-blue probe (DL = 0.02 μg/L PC) during vertical profiles from the surface down to 14 m depth and for the detection of cyanobacteria for samples on the bank (Brient et al. 2007; Bastien et al. 2011; Kong et al. 2014). Water samples were collected with a 1-m-long tube sampler for surface sampling and a Van Dorn bottle for vertical profiles. Water samples were kept on ice until analysis in the laboratory within 24 h.
Phytoplankton analyses
Samples collected for taxonomic analyses were conserved in Lugol’s iodine. Cyanobacteria identification was carried out on the basis of microscopic observation of the morphological characters according to the identification keys used by Bourrelly (1966), Komárek and Anagnostidis (1989–2005), Cronberg and Annadotter (2006), and Komárek (2013). Cyanobacteria counts were performed directly from the raw sample or by a concentration of at least 100 mL filtered through a polycarbonate membrane (CYCLPR PC 47 mm, porosity 5 μm). The counting was performed using a Nageotte cell with a minimum of 40 algal units as described in Brient et al. (2007).
Nutrient analyses
The raw water samples were filtered using a GF/C filter (1.2 μm) before being analyzed for dissolved nutrients. Nitrogenous nutrient elements (ammonium (ISO 7150/1984), nitrates (ISO 7890/1986), and nitrites (ISO 6777/1984)) and orthophosphates (ISO 6878/1986) were measured according to ISO/TC 147 (1994), by colorimetric method spectrophotometry HACH DR/4000 U (UV visible). Detection limits are 0.5 mg/L NO3-N, 0.004 mg/L NO2-N, 0.031 mg/L NH3-N, and 0.04 mg/L PO4 3−.
Microcystins and cylindrospermopsin analyses
The raw water samples were filtered using a GF/C filter, frozen, and sent for analysis to France (University of Rennes 1). The intracellular cyanotoxins were extracted using 75% MeOH for 1 h (Chorus and Bartram 1999; Meriluoto et al. 2017) for their analyses by HPLC for microcystins or ELISA for cylindrospermopsins. Analyses of microcystins were performed using a HPLC coupled with a diode array detection (DAD) method. A P4000 solvent delivery system from Thermo Scientific equipped with a UV6000LP detector from Thermo Finnigan was used for all analyses. Separation was carried out on a Kinetex C18 column from Phenomenex (4.6 mm i.d. × 100 mm long; 2.6 μm particle size) which was maintained at 35 °C. Samples (20 μL) were eluted with acetonitrile/ammonium acetate 0.01 M (24/76) over 30 min at a flow rate of 1 mL/min. Eluent was monitored from 200 to 300 nm, and microcystins were quantified by its characteristic UV spectrum at 238 nm. External standards (mix of MC LR, MC YR, and MC RR, 5 μg/L) are purchased from Abraxis. Instrument control, data acquisition, and processing were achieved using Chromeleon. The limit of quantification is 0.05 μg/L MC-LR with an injection of 20 μL. Microcystin analyses in the drinking water were performed using a ELISA kit Microcystin “ABRAXIS” PN 522015 product with a detection limit of 0.1 μg/L MC-LR.
Cylindrospermopsin analyses were performed using an ELISA kit Cylindrospermopsin “ABRAXIS” PN 522011 for raw and drinking water, with a detection limit more sensitive than HPLC (DL = 0.04 μg/L CYN). All toxin analyses were performed in the University of Rennes 1, laboratory.
Results
Abiotic factors
During 2013, the waters of Ain Zada dam supplied by Boussellem, Karoua, and Taghrout valleys (Table 3) were characterized by an alkaline pH of around 8, a conductivity of 833 to 2450 μS/cm, high ammonium concentrations (>5 mg/L NH4-N) and nitrate values of <7.75 mg/L NO3-N, and high phosphate values of 4.76 mg /L PO4. The water at the intake point used for potabilization has met the water standards in Algeria during this short period of 5 months of regular monitoring. It is noteworthy that in May 2013, nitrate values were the highest with 7.25 mg/L NO3-N at station 8 and with the presence of 0.12 mg/L PO4.
Cyanobacteria
During this period from February to June 2013, Oscillatoriales developed between late April and mid-May at stations 4 and 7 (Fig. 3) represented by Pseudanabaena sp. and Planktothrix agardhii, and Nostocales represented by Aphanizomenon sp., at all the sampling sites of the dam (Fig. 4). Pseudanabaena sp. and P. agardhii showed a persisted dominance.
On 11 May 2013, a more detailed sampling campaign on the dam revealed a dominant population of P. agardhii (Fig. 5). Phycocyanin profiles (Fig. 6) reveal the presence of cyanobacteria over the entire dam with a high biomass in the top 5 m of the water column. The biomass was so high that the signal from the PC probe was saturated in the top 5 m at a value of 200 μg/L PC. This PC concentration corresponded to an equivalent biomass of 600,000 cells/mL using the factory calibration. The three cyanobacterial species remained present with the dominance of P. agardhii. The vertical profiles of PC at A5 in the dam center are similar to A6.
Microcystins and cylindrospermopsins
Of the 21 samples (analyzed by HPLC) obtained from stations A4 and A6 and from the period February to June 2013, microcystins were present in all raw water samples except for those of 12 March 2013 with concentrations reaching 72.4 μg/L of the intracellular MC on 7 May 2013. The MC-LR equivalent is present mainly in these samples with a value of 69.25 μg/L on 7 May 2013 (Table 4). In the drinking water, microcystin is present in samples of 9 April 2013, with 0.97 μg/L of MC LR equivalent and of 7 May with 0.62 μg/L MC-LR equivalent.
During the massive cyanobacterial bloom on 11 May 2013, microcystins were present at different depths at stations A5, A6, and A7 with concentrations ranging between 12.1 and 19.6 μg/L of the intracellular microcystins. The 6.3 μg/L of extracellular MC-LR equivalent is found in drinking water at that date (Table 5).
ELISA tests detected no trace of cylindrospermopsin (intracellular or extracellular) in the 18 samples analyzed in raw and drinking water during the entire study period even during the bloom. This cyanotoxin has been analyzed because there are few species producers in the dam which include C. raciborskii (Hawkins et al. 1985, 1997), and Raphidiopsis curvata (Li et al. 2001) which were also found in other Algerian reservoirs and lakes.
Discussion
Cyanobacteria were dominating over other phytoplankton groups in Ain Zada dam during the sampling campaigns in accordance with the nutrient status and the climatic conditions of the region (Paerl and Huisman 2008; Carey et al. 2012). A diverse cyanobacterial community was identified in the water body and was composed of three genera, all of which are potentially toxic.
In Ain Zada, P. agardhii was the dominant species followed by species from the genus Aphanizomenon. This temporal succession suggests a response to change in mixing conditions and temperature as Oscillatoriales are known to dominate in cold mixed conditions (Reynolds 1984; Mantzouki et al. 2016). The Aphanizomenon sp. is stable in warm conditions of the water column together with the lack of rain (Huisman et al. 2004) and common during the summer in Mediterranean climate reservoirs (Fadel et al. 2015; Cirés and Ballot 2016).
In Ain Zada, the highest biomass of cyanobacteria (as indicated by the phycocyanin fluorescence) measured on 11 May 2013 was located above the water intake level but decreased only to a concentration of 50 μg/L at the intake depth in the center of the water body at station A5. This relatively high concentration of cyanobacteria throughout the water column resulted in the presence of microcystin in the raw water taken at the entrance of the treatment plant (12.1 μg/L MC LR equivalent) and its persistence in the treated water (6.3 μg/L MC-LR equivalent). This high microcystin concentration associated with high cyanobacterial biomass occurred during a drought period (lack of rain) with calm conditions, rising temperatures, and nutrient enrichment. Microcystin toxin production is positively correlated to cell growth rate (Briand et al. 2005, 2012). However, different genotypes with various toxin-producing abilities may coexist within the same lake (Sabart et al. 2015) and even during a bloom (Briand et al. 2008) reporting that there is not necessarily a relationship between cyanobacterial biomass and MC concentrations.
During the bloom on 11 May 2013, the concentrations of microcystin found in the dam ranged from 19.6 μg/L MC-LR equivalent in raw water (A5) to 6.3 μg/L in drinking water, showing that although the treated water decreased MC concentrations, it was not effective enough to decrease it to 1 μg/L MC-LR which is the threshold limit for drinking water determined by the WHO (1998). Hence, the MC concentration found in the treated water exceeded the WHO threshold also reported in Chorus and Bartram (1999) and in the Official Journal of the Algerian Republic (JORA 2014) as harmful when consumed by humans and as potentially causing cancer (Zhou et al. 2002; Zanchett and Oliveira-Filho 2013). Moreover, degradation products by oxidation may also contribute to some health problems. To avoid them, mainly when blooms persist, the most comprehensive physical removal is required before any chemical treatment (Zamyadi et al. 2012a, b; Roegner et al. 2014).
This study demonstrated the occurrence of cyanobacterial blooms in Ain Zada dam which is most likely associated with high nutrient inputs from the catchment area (domestic and industrial discharge and fertilizers related to agricultural activity) and favorable climatic conditions (low water renewal rate and high water temperatures). Increased nutrient inputs and increased temperature are believed to be the two most important factors driving recent changes in phytoplankton in lakes towards dominance of cyanobacteria (Carey et al. 2012) with possible selection of toxic producing species (Davis et al. 2009).
The water body of Ain Zada displayed different responses to abiotic factors. Ain Zada is considered an ancient dam, and is characterized by a large volume of 120 millions m3, as well as low rainfall, which results in a slow renewal time. These conditions favor the development of the cyanobacteria as the only phytoplankton group in this dam.
The recurrence of cyanobacterial blooms in these water bodies will negatively impact the environment. Protection of catchment area and providing information to the inhabitants should be considered to prevent human and animal health problems caused by these developments of cyanobacteria and their toxins.
Fish mortality, as was observed in the Ain Zada dam during the bloom on 11 May 2013, associated with cyanobacterial blooms has been reported in several countries, and cyanobacterial toxins have been found to bioaccumulate in the flesh of fish (Ibelings and Chorus 2007; Wu et al. 2011; Gutiérrez-Praena et al. 2013; Jia et al. 2014; Hardy et al. 2015).
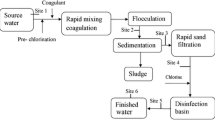
A health risk management plan in the form of a flow chart is proposed in Fig. 7, with the establishment of an identity card for the water body intended for drinking water production and its catchment (Fig. 8). Specification of an appropriate monitoring program for sampling and analyses on sites and in the laboratory together with effective treatment methods for toxin removal and drinking water production are presented.
Conclusion
The presence of cyanobacteria and cyanotoxins in raw and drinking waters of Ain Zada reservoir highlights the need for monitoring of cyanobacteria and cyanotoxins to avoid potential human health problems. Regular monitoring of cyanobacteria and cyanotoxins as proposed in the health risk management plan should be undertaken, due to its potential health risk for drinking water and for bioaccumulation of cyanobacterial toxins in the flesh of fish. The PC fluorescence probe can be used for monitoring cyanobacteria, as it is easily operated by people monitoring the lakes and enables a higher sampling frequency than traditional methods. The proposed monitoring will identify the cyanobacterial taxa, and therefore direct the analysis of other cyanotoxins besides microcystins. This monitoring will also help to raise the awareness of the Algerian water authorities to provide inhabitants with some actions to reduce nutrient loads discharged in the watershed in order to prevent the development of these toxic cyanobacterial blooms.
References
Amrani, A., Nasri, H., Azzouz, A., Kadi, Y., & Bouaïcha, N. (2014). Variation in cyanobacterial hepatotoxin (microcystin) content of water samples and two species of fishes collected from a shallow lake in Algeria. Archives of Environmental Contamination and Toxicology, 66(3), 379–389.
ANB BBA (Agence National des Barrages Borj Bou Arreridj) (2013). Données des caractéristiques techniques du Barrage Ain Zada.
Bastien, C., Cardin, R., Veilleux, E., Deblois, C., Warren, A., & Laurion, I. (2011). Performance evaluation of phycocyanin probes for the monitoring of cyanobacteria. Journal of Environmental Monitoring, 13(1), 110–118.
Bidi-Akli, S., Hacene, H., & Arab, A. (2017). Impact of abiotic factors on the spatio-temporal distribution of cyanobacteria in the Zeralda’s dam (Algeria). Revue d'Ecologie (la Terre et la Vie), 72(2), 159–167.
Bourrelly, P. (1966). Les Algues d’Eau Douce. I Les Algues Vertes. Paris: Boubée.
Boussadia, M. I., Sehli, N., Bousbia, A., Ouzrout, R., & Bensouilah, M. (2015). The effect of environmental factors on cyanobacteria abundance in Oubeira lake (Northeast Algeria). Research Journal of Fisheries and Hydrobiology, 10(14), 157–168.
Briand, J. F., Jacquet, S., Flinois, C., Avois-Jacquet, C., Maisonnette, C., Leberre, B., & Humbert, J. F. (2005). Variations in the microcystin production of Planktothrix rubescens (cyanobacteria) assessed from a four-year survey of Lac du Bourget (France) and from laboratory experiments. Microbial Ecology, 50(3), 418–428.
Briand, E., Gugger, M., Francois, J. C., Bernard, C., Humbert, J. F., et al. (2008). Temporal variations in the dynamics of potentially microcystin-producing strains in a bloom-forming Planktothrix agardhii (cyanobacteria) population. Applied and Environmental Microbiology, 74, 3839–3848.
Briand, E., Bormans, M., Quiblier, C., Salencon, M. J., & Humbert, J. F. (2012). Evidence of the cost of the production of microcystins by Microcystis aeruginosa under differing light and nitrate environmental conditions. PloS One, 7(1), e29981.
Brient, L., Lengronne, M., Bertrand, E., Rolland, D., Sipel, A., Steinmann, D., Baudin, I., Legeas, M., Le Rouzic, B., & Bormans, M. (2007). A phycocyanin probe as a tool for monitoring cyanobacteria in freshwater bodies. Journal of Environmental Monitoring, 10, 248–255.
Carey, C. C., Ibelings, B. W., Hoffmann, E. P., Hamilton, D. P., & Brookes, J. D. (2012). Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Research, 46, 1394–1407.
Carmichael, W. W., & Boyer, G. L. (2016). Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae, 54, 194–212.
Chorus, I., & Bartram, J. (1999). Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. Geneva: World Health Organization 416 p.
Cirés, S., & Ballot, A. (2016). A review of the phylogeny, ecology and toxin production of bloom-forming Aphanizomenon spp. and related species within the Nostocales (cyanobacteria). Harmful Algae, 54, 21–43.
Codd, G. A., Morrison, L. F., & Metcalf, J. S. (2005). Cyanobacterial toxins: risk management for health protection. Toxicology and Applied Pharmacology, 203, 264–272.
Cronberg, G., & Annadotter, H. (2006). Manual on aquatic cyanobacteria: a photo guide and a synopsis of their toxicology. Copenhagen: International Society for the Study of Harmful Algae and the United Nations Educational, Scientific, and Cultural Organization 106 p.
Davis, T. W., Berry, D. L., Boyer, G. L., & Gobler, C. J. (2009). The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae, 8, 715–725.
Djabourabi, A., Sehili, N., Boussadia, M., Samar, F., & Bensouilah, M. (2014). Fluctuations des Paramètres Physico Chimiques et des Communautés Phytoplanctoniques dans le lac Oubeira (Nord-Est Algérien). European Journal of Scientific Research, 118(2), 183–196.
Fadel, A., Atoui, A., Lemaire, B. J., Vinçon-Leite, B., & Slim, K. (2015). Environmental factors associated with phytoplankton succession in a Mediterranean reservoir with a highly fluctuating water level. Environmental Monitoring and Assessment, 187, 633.
Falconer, I. R. (1999). Safe levels and safe practices. Chapter 5, pp. 155–178. In I. Chorus & J. Bartram (Eds.), Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management. London: E&FN Spon 416 p.
Gutiérrez-Praena, D., Jos, Á., Pichardo, S., Moreno, I. M., & Cameán, A. M. (2013). Presence and bioaccumulation of microcystins and cylindrospermopsin in food and the effectiveness of some cooking techniques at decreasing their concentrations: a review. Food and Chemical Toxicology, 53, 139–152.
Hamilton, D. P., Salmaso, N., & Paerl, H. W. (2016). Mitigating harmful cyanobacterial blooms: strategies for control of nitrogen and phosphorus loads. Aquatic Ecology, 50, 351–366.
Hardy, F. J., Johnson, A., Hamel, K., & Preece, E. (2015). Cyanotoxin bioaccumulation in freshwater fish,Washington State, USA. Environmental Monitoring and Assessment, 187(11), 667.
Hawkins, P. R., Runnegar, M. T. C., Jackson, A. R. B., & Falconer, I. R. (1985). Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Applied and Environmental Microbiology, 50, 1292–1295.
Hawkins, P. R., Chandrasena, N. R., Jones, G. J., Humpage, A. R., & Falconer, I. R. (1997). Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon, 35, 341–346.
Heisler, J., Glibert, P. M., Burkholder, J. M., Anderson, D. M., Cochlan, W., Dennison, W. C., Dortch, Q., Gobler, C. J., Heil, C. A., Humphries, E., Lewitus, A., Magnien, R., Marshall, H. G., Sellner, K., Stockwell, D. A., Stoecker, D. K., & Suddleson, M. (2008). Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae, 8, 3–13.
Huisman, J., Sharples, J., Stroom, J. M., Visser, P. M., Kardinaal, W. E. A., Verspagen, J. M. H., & Sommeijer, B. (2004). Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology, 85(11), 2960–2970.
Ibelings, B. W., & Chorus, I. (2007). Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: a review. Environmental Pollution, 150(1), 177–192.
ISO/TC 147 (International Organization for Standardization) (1994). Environment: water quality. Chemical methods ISO Standards Compendium, Volume 2. 1st edition.
J.O.R.A (Official Journal of the Algerian Republic) (2014) n°13.
Jia, J., Luo, W., Lu, Y., & Giesy, J. P. (2014). Bioaccumulation of microcystins (MCs) in four fish species from Lake Taihu, China: assessment of risks to humans. Science of the Total Environment, 487, 224–432.
Komárek, J. (2013). Cyanoprokaryota: part 3: heterocytous genera. SüBwasserflora von Mittelleuropa freshwater flora of Central Europe. Germany: Springer Spektrum.
Komárek, J., & Anagnostidis, K. (1989). Modern approach to the classification system of cyanobacteria 4 Nostocales. Archiv fur Hydrobiologie Algological Studies, 56, 247–345.
Komárek, J., & Anagnostidis, K. (2005). Cyanoprokaryota: part 2: Oscillatoriales SüBwasserflora von Mittelleuropa freshwater flora of Central Europe. Germany: Spektrum Akademischer Verlag Heidelberg.
Kong, Y., Zhang, Y., & Lou, C. U. (2014). Using an online phycocyanin fluorescence probe for rapid monitoring of cyanobacteria in Macau freshwater reservoir. Hydrobiologia, 741, 33–49.
Lance, E., Josso, C., Dietrich, D., Ernst, B., Paty, C., Senger, F., Bormans, M., & Gerard, C. (2010a). Histopathology and microcystin distribution in Lymnaea stagnalis (Gastropoda) following toxic cyanobacterial or dissolved microcystin-LR exposure. Aquatic Toxicology, 98, 211–220.
Lance, E., Brient, L., Carpentier, A., Acou, A., Marion, L., Bormans, M., & Gérard, C. (2010b). Impact of toxic cyanobacteria on gastropods and microcystin accumulation in a eutrophic lake (Grand-Lieu, France) with special reference to Physa (= Physella) acuta. Science of the Total Environment, 408, 3560–3568.
Li, R. H., Carmichael, W. W., Brittain, S., Eaglesham, G. K., Shaw, G. R., Liu, Y. D., & Watanabe, M. M. (2001). First report of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from Raphidiopsis curvata (Cyanobacteria). Journal of Phycology, 37, 1121–1126.
Mantzouki, E., Visser, P. M., Bormans, M., & Ibelings, B. W. (2016). Understanding the key ecological traits of cyanobacteria as a basis for their management and control under expected environmental changes. Aquatic Ecology, 50, 333–350.
Merel, S., Walker, D., Chicana, R., Snyder, S., Baures, S., & Thomas, O. (2013). State of knowledge and concerns on cyanobacteria blooms and cyanotoxins. Environment International, 59, 303–327.
Meriluoto, J., Spoof, L., & Codd, G. A. (Eds.). (2017). Handbook of cyanobacterial monitoring and cyanotoxin analysis. Hoboken: Wiley & Sons, Ltd 548 p.
Mur, L. R., Skulberg, O. M., & Utkilen, H. (1999). Cyanobacteria in the environment. Chapter 2, pp.15–40. In I. Chorus & J. Bartram (Eds.), Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management. London: E&FN Spon 416 p.
Nasri, A. B., Bouaïcha, N., & Fastner, J. (2004). First report of a microcystin-containing bloom of the cyanobacteria Microcystis spp. I lake Oubeira, eastern Algeria. Archives of Environmental Contamination and Toxicology., 46(2), 197–202.
Nasri, H., Bouchaïcha, N., & Harche, M. K. (2007). A new morphospecies of Microcystis sp forming a bloom in the Cheffia dam (Algeria): seasonal variation of microcystin concentrations in raw water and their removal in a full scale treatment plant. Environmental Toxicology, 22(4), 347–356.
Nasri, H., El Herry, S., & Bouaicha, N. (2008). First reported case of turtle deaths during a toxic Microcystis spp. bloom in Lake Ubeira, Algeria. Ecotoxicology and Environmental Safety, 71(2), 535–544.
Ouartsi, A., Saoudi, A., & Chekireb, D. (2011). Etude des efflorescences toxiques à cyanobactéries dans le barrage de Mexa, Algérie. Revue de Microbiologie Industrielle Sanitaire et Environnementale, 5(1), 81–100.
Paerl, H. W., & Huisman, J. (2008). Climate. Blooms like it hot. Science, 320(5872), 57–58.
Paerl, H. W., & Paul, V. J. (2012). Climate change: links to global expansion of harmful cyanobacteria. Water Research, 46, 1349–1363.
Pouria, S., Andrade, A., Barbosa, J., Cavalcanti, R. L., Barreto, V. T., Ward, C. J., Preiser, W., Poon, G. K., Neild, G. H., & Codd, G. A. (1998). Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet, 352(9121), 21–26.
Reichwaldt, E. S., & Ghadouani, A. (2012). Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: between simplistic scenarios and complex dynamics. Water Research, 46(5), 1372–1393.
Reynolds, C. S. (1984). Phytoplankton periodicity: the interactions of form, function and environmental variability. Freshwater Biology, 14, 111–142.
Roegner, A. F., Brena, B., González-Sapienza, G., & Puschner, B. (2014). Microcystins in potable surface waters: toxic effects and removal strategies. Journal of Applied Toxicology, 34, 441–457.
Sabart, M., Misson, B., Jobard, M., Bronner, G., Donnadieu-Bernard, F., Duffaud, E., Salençon, M. J., Amblard, C., & Latour, D. (2015). Genetic diversity along the life cycle of the cyanobacterium Microcystis: highlight on the complexity of benthic and planktonic interactions. Environmental Microbiology, 17(3), 901–911.
Saoudi, A., Barour, C., Brient, L., Ouzrout, R., & Bensouilah, M. (2015). Environmental parameters and spatio-temporal dynamics of cyanobacteria in the reservoir of Mexa (Extreme North-East of Algeria). Advances in Environmental Biology, 9(11), 109–121.
Svircev, Z., Drobac, D., Tokodi, N., et al. (2013). Epidemiology of primary liver cancer in Serbia and possible connection with cyanobacterial blooms. Journal of Environmental Science and Health Part C, 31(3), 181–200.
WHO. (1998). Guidelines for drinking-water quality. Addendum to volume 2, health criteria and other supporting information (second ed.). Geneva: World Health Organization.
Wu, Q., Li, M., Gao, X., Giesy, J. P., Cui, Y., Yang, L., & Kong, Z. (2011). Genotoxicity of crude extracts of cyanobacteria from Taihu Lake on carp (Cyprinus carpio). Ecotoxicology, 20(5), 1010–1017.
Yu, S. Z. (1995). Primary prevention of hepatocellular carcinoma. Journal of Gastroenterology and Hepatology, 10(6), 674–682.
Zamyadi, A., Ho, L., Newcombe, G., Bustamante, H., & Prevost, M. (2012a). Fate of toxic cyanobacterial cells and desinfection by products formation after chlorination. Water Research, 46(5), 1524–1535.
Zamyadi, A., MacLeod, S. L., Fan, Y., McQuaid, N., Dorner, S., Sauve, S., & Prevost, M. (2012b). Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: a monitoring and treatment challenge. Water Research, 46(5), 1511–1523.
Zanchett, G., & Oliveira-Filho, E. C. (2013). Cyanobacteria and cyanotoxins: from impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins, 5(10), 1896–1917.
Zhou, L., Yu, H., & Chen, K. (2002). Relationship between microcystin in drinking water and colorectal cancer. Biomedical and Environmental Science, 15(2), 166–171.
Acknowledgments
We would like to thank Mrs. Chorin Marion for her help in the analyses of microcystins by HPLC and ELISA and cylindrospermopsin by ELISA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saoudi, A., Brient, L., Boucetta, S. et al. Management of toxic cyanobacteria for drinking water production of Ain Zada Dam. Environ Monit Assess 189, 361 (2017). https://doi.org/10.1007/s10661-017-6058-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6058-4