Abstract
Many places on Earth have been experiencing the impact of climate change. Due to a rise in the frequency of hydrological and climatic events, the worsening trend of potable water quality due to floods has increased, which has possible health repercussions. Hence, the present study was planned to examine the water quality following a flood event. This study determined water quality by estimating physicochemical parameters and quality indices. Moreover, health risks associated with water intake through ingestion and dermal contact were assessed. Consequently, all of the samples were found to be polluted to varying degrees, and the majority of them were found unsuitable for human consumption as the high values of total dissolved solids (TDS) 1120 mg/L, total hardness (TH) 575 mg/L, total alkalinity (TA) 3516.75 mg/L was observed. Further, 60% of the samples exceeded the recommended values of Mg+ and Cl− showing that the quality of floodwater, handpump and well water was found to be extensively poor. Furthermore, the Water Quality Index (WQI) revealed that 20% of samples had poor water quality with the highest value of 161. The elemental analysis revealed that arsenic (As) had a high value (0.0516 mg/L). Additionally, it was discovered that the oral (66.03) and dermal (201.43) pathways had high hazard quotients (HQs), indicating health risks to both adult and children. The Pearson correlation and principal component analysis revealed a positive association between physicochemical and heavy metals, indicating a similar origin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is one of the principal substances for humans, animals, plants, and other living things to survive. On this planet, no life is possible without water (Srivastava et al. 2011). It is a vital natural resource that is substantial and covers around two-thirds of the earth's surface. However, freshwater accounts for only 1% of the total water resources. (i.e., lakes, surface water-rivers, streams, and groundwater) for drinking and other purposes (Sangu et al. 1987). According to a study conducted by the World Health Organisation (WHO), ensuring access to potable water is the highest priority among all water requirements for human consumption (Reed et al. 2013; Udhayakumara et al. 2016).
With an increase in anthropogenic activities and rising events of climate change, water quality is continuously decaying (Jennerjahn et al. 2004). The climate influences the hydrological regimes in ecosystems, predominately riverine ecosystems (Krasner et al. 2008). Further, climatic variations also influence weather patterns like rainfall patterns and rainy days contributing to widespread flooding as a major natural disaster globally (Bates et al. 2008; Alfieri et al. 2017; Slater and Villarini 2016). Heavy rains cause floods along the course that pickup soil, waste materials and several pollutants that ultimately get deposited into the freshwater bodies and deteriorate water quality (Krasner et al. 2008; Fida et al. 2021).
Currently, all the populated areas worldwide are at risk of being flooded. In India, floods account for more than half of all natural disasters since the last decade, and it’s damage has been extremely severe, indicating that floods' intensity and frequency are increasing (Patankar 2019). Flood hazards have a wide range of vulnerabilities that are far more significant than most other hazards. Floods directly or indirectly affect human communities through water contact or damage to natural and built environments. Even if they are modest and confined, floods can significantly influence on individual’s physical and mental well-being. (Tapsell and Tunstall 2008; Ferrari et al. 2020; Masson et al. 2019).
India has seen an increase in the number of flood-related fatalities as well as financial damages (Mishra and Mishra 2010; Nanditha and Mishra 2021). According to data from the Central Water Commission (CWC) of India, from 1952 to 2018, floods claimed over 10,000 lives and caused 4.69 trillion INR in economic losses (Mahapatra 2020). Furthermore, it is predicted that floods may have a detrimental effect on the quality of water (Ching et al. 2015; Mohan et al. 2019; Saha et al. 2020). Many Indian cities such as Mumbai, Hyderabad, Chennai, Ahmedabad, Delhi, Surat, Patna, Kolkata, Srinagar, Jaipur and Kerala experienced flooding during the recent decades (2010–2020) (Nanditha and Mishra 2021). Despite the implementation of structural measures such as building levees, flood control reservoirs and river training works to manage flooding, there have been instances where these systems proved ineffective resulting in severe and catastrophic damages from floods (Kundzewicz et al. 2014; Rammelt et al. 2018). Due to the increase in flood frequency and intensity; the individual’s livelihood are affected in several ways. The primary effects of rising floods in India could seriously impact human life by disturbing access to and availability of clean water, resulting in severe health hazards (Sam et al. 2017).
Floods modify water quality by the direct effect of dilution or concentration of dissolved substances including ions and metals. There are several studies from Patna city examining the water quality and metal concentration and associated health risks (Chakraborti et al. 2003; Saha 2009; Chakraborti et al. 2016; Nath et al. 2013; Kumar et al. 2019; Dubey et al. 2018; Abhinav et al. 2019). However, in the context of Patna floodwater analysis, a comprehensive health risk assessment studies have not yet been carried out. In light of this knowledge gap and the public health concern, this study aims is to evaluate the water quality of post-flood in Patna. The main objective is to assess floodwater acceptability for human consumption and investigate any potential health risks by using the Water Quality Index (WQI) and health risk assessment (HRA) approach, especially in areas affected by flooding (2019) in Patna, Bihar, India. The water quality characteristics in a post-flood environment are fundamental in understanding the effects of flood and associated health risks. This study will be helpful for the development of more effective strategies to improve the water quality in flood-affected areas.
Materials and methods
Site specification
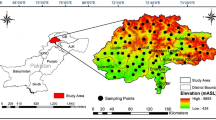
Patna is located in the alluvial plains of South Bihar. The district is bordered on the north by the river Ganga, on the south by the districts of Jahanabad and Nalanda, on the east by Lakhisarai, and on the west by Bhojpur. The district is located between 25° 13′ and 25° 45′ north latitude and 84° 43′ and 86° 44′ east longitude and inhabits 3202 km2 geographical areas with a population of 5,772,804 as per Patna population census (Census of India 2011). It is divided into 23 major blocks in the district. The Ganga is located in the north, the Sone in the west, and the Punpun and Phalgu, as well as their tributaries, are located in the centre. The district is majorly drained by the river Ganga. In the district, net irrigation covers 1,09,000 ha (Government of Bihar), with irrigation through groundwater accounting for about 80% of the total area. The weather is moderately extreme, being rather hot during summer and cold during winter. January is generally the coldest in this district; in March, the temperature begins to rise and reaches its peak in May. The rainy season starts in mid-June and lasts until mid-September. The monsoon months of July and August see the most rain. Winter showers occur of January and February. The average annual rainfall in the district is 1076 mm.
Hydrology and geology of the study area
The district is located over the Ganga basin and has a monotonously flat topography with height changes. The land surface elevation varies from 68 m in the south to 48 m in the north and from 67 m in the west to 45 m in the east in the western half of the district. In addition to providing a natural barrier, there is a strong natural embankment formation along the Ganga’s southern bank, allowing many of the streams to flow parallel to the river’s path until they meet the Ganga further east of the district line.
This district’s soil tends to be sandy loam and clay loam, usually in areas with low to medium nutritional status. It has a pH range of 6.3–8.2 and is usually alkaline. Traditionally, soils have been classed according to their deposition mechanism. Soils are classified into three categories: younger alluvium, older alluvium and tal. Most of the district’s soil is made up of younger alluvial ‘deposit’s formations due to physical weathering, resulting in soil being coarser in texture.
The area is covered with a quaternary alluvial formation comprising various grades of sand, silt, gravel and clay with the rare sandstone. The entire district comes into the good to excellent category regarding groundwater potential. The primary water supply for agriculture and drinking in this area is groundwater. The state has roughly 1700 deep tube wells and 0.9 million small tube wells (Sukumaran et al. 2015). In some areas, the combination of kankar (CaCO3 nodules) and fine sand make the top clay zone semi-permeable, allowing groundwater to flow under phreatic conditions. Aquifers deeper in the ground contain sand and gravel of medium to coarse grains (CGWB 2007; Fig.S1). A groundwater reservoir formed in Patna, which is a part of the Indo-Gangetic Plains, is made up of ancient alluvial sediments varying in clay, sand, and silt grades from the Quaternary period. At a depth of 45–70 m, an aquitard layer separates the upper shallow and lower deeper aquifers. During the pre-monsoon season, the depth of wells varied from 2 to 5 mbgl in the southwest part, while in the eastern part, it varied from > 10 mbgl. Meanwhile, in the post-monsoon season, the water level is > 10 mbgl in the southern, central, and eastern parts of the area.
Collection of samples and analysis
In total, 20 water samples post-flood event from different locations (Rajendra Nagar; Bahadurpur; Ramkrishna Nagar; Rampur; Patliputra; Bhagwat Nagar; Jaganpura Pipra; Kankarbagh; Bhoothnath Road; Sairam place and Saidpur Nandnagar) and from different sources, inclusive of bore well, deep pumphouse, deep bore, supply water, hand pump, submersible, and floodwater, were gathered from different sampling locations of Patna district, Bihar, India, enlisted in Table 1. The composite sampling technique was employed by the different challenges faced during the collection process, ensuring a representative sample from different flood- affected areas. The samples were collected in duplicates. The sampling site's coordinates were recorded using GPS (Garmin Montana 650) and detail is provided in Fig. 1. The colour, odour and effect on the human health around the sampling site were noted during the sampling period. Samples were collected in cleaned plastic bottles following American Public Health Association (APHA 2005) standards.
Water was run for ten minutes before the collection of samples. After which the water was collected in high-density polyethylene (HDPE) thoroughly cleaned bottles followed by filtration with membrane filters of pore size 0.45 and closed tightly to prevent air exposure (Vig et al. 2023a). Sample’s pH, EC and TDS were determined by applying an electronic meter (Waterproof pen tester 7200 pH/Conductivity/TDS/Salt of KUSAM-MECO) in each sample and then the reading was recorded instantaneously. After collecting samples, bottles were stored in an icebox to maintain the temperature below 4 °C and immediately transferred to the laboratory for physicochemical and elemental examination. After every reading, pH, EC, TDS meter was rinsed with distilled water before recording the value of another sample.
The other parameters, such as total Hardness (TH), total alkalinity (TA), calcium (Ca2+), chloride (Cl−) and magnesium (Mg2+), were analyzed using the titrimetric method. Fluoride (F−) and nitrate (NO3−) were analyzed using the spectrophotometry method. Sodium (Na+) and potassium (K+) were analyzed using a Flame photometer. Different heavy and trace elements such as silver (Ag), aluminium (Al), arsenic (As), boron (B), barium (Ba), bismuth (Bi), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), gallium (Ga), indium (In), potassium (K), lithium (Li), manganese (Mn), molybdenum (Mo), phosphorus (P), lead (Pb), mercury (Hg), sulphur (S), scandium (Sc), strontium (Sr), titanium (Ti) and zinc (Zn) were also determined with inductively coupled plasma mass spectrometry (ICP-MS). For cation analysis samples were fixed with concentrated HNO3. Before elemental analysis, water samples were filtered with Whatman no. 42 and then fixed with 1% analytical grade (AR) nitric acid (HNO3). After that, samples were run on ICP-MS by preparing the standard curves of standards solutions.
Water quality and pollution indices
Water quality index (WQI), heavy pollution index (HPI) and metal index (MI) were computed. Ten parameters were considered, such as pH, EC, TDS, TH, Cl−, NO3−, Ca2+, Mg2+, F−, and Fe for WQI analysis. The estimation was done by following mathematical expression using the relative weightage method as used in previously conducted studies (Vig et al. 2023a; Sadat-Noori et al. 2014; Sethy et al. 2017; Vasanthavigar et al. 2010) as shown in Table 2.
WQI was computed in three major steps (Noori et al. 2014; Sethy et al. 2017; Vasanthavigar et al. 2010). First, a weight (wi) between 1 and 5 was assigned to calculate the relative weight (Wi). The weighting given to chemical parameters, reflect their relative value in the overall evaluation of water quality, and largely determines the significance of the overall assessment of water quality. Wi was computed by following Eq. 1:
where Wi represents the relative weight, wi denotes the weight assigned to individual parameters, and n signifies the total number of parameters involved.
The second step showed the quality rating scale (qi) for particular parameters, divided by its concentration in the tested samples (ci) with its corresponding standard (si) of drinking water quality prescribed by WHO in mg/L, then multiplied by 100 as shown in Eq. 2.
The third step included calculating the SIi for an individual chemical parameter that depicted the sub-index of ith parameter, following Eq. 3.
Lastly the WQI was calculated concerning the submission of SIi by following Eq. 4
After the calculation of WQI, the water can be grouped into 5 classes as per WQI (Brown et al. 1972): excellent (0–25), good (25–50), poor (50–75), very poor (75–100) and unsuitable for drinking (> 100). In addition, the pollution extent of particular metals HPI and MI were computed by following the methodology as detailed in a previous study by Ravindra and Mor (2019). For HPI calculations, the metal rating system ranges of metal is ranged between 0 and 1 according to their importance in drinking water. A unit weightage (Wi) was taken as a value in the assessment that is reciprocal to the standard permissible limits (Si) for metals identified in this study. The HPI was estimated following Eq. (5):
Wi is considered the unit weightage of ith heavy metal; Qi is the ith metal sub-index; n signifies the evaluation of metal's number. To determine the unit weightage (Wi) Eq. (6) was applied.
where K represents the proportionality constant and is calculated by using Eq. (7)
Si signifies the standard recommended value for the ith heavy metal (mg/L). Finally, the sub-index (Qi), as given in Eq. (5) of each metal, was assessed employing Eq. (8):
Ii as an ideal value of ith heavy metal unified from Indian BIS (2012) drinking water standards limits; Mi is the metal's monitored value. The Si and Ii for each metal are given in Table S1.
Human health risk assessment
Following USEPA guidelines, the health risks due to heavy metals (As, Cd, Co, Cr, Cu, Ni, Pb and Zn) intake via ingestion and dermal pathways as explained below:
Non-carcinogenic
This was calculated by following equations Eqs. (9) and (10):
The intake of metal was also determined with the dermal pathway, which was calculated using Eq. (10):
C is the concentration (mg/kg) of metal. BW is the body weight (kg), which is defined as 60 for adults and 15 for children according to USEPA (1989) guidelines. EF is the exposure frequency of 365 (days/year) (USDoE 2011); ED depicts the exposure duration (years) and is defined as 24 years for adults and 6 years for children (UNEP 1999). LT demonstrates the lifetime (years), which is 65 (MoHFW 2010). AT is the averaging time (days) which is (ED × 365 days) for non-carcinogenic and (LT × 365 days) for carcinogenic (USDoE 2011). IngR (ingestion rate mg/day) is 2 L for adults (Valtin 2002).
SA (exposed area of skin 2800 cm2), SL (Skin adherence factor 0.2 mg/cm2/day) (USEPA, 2011), ABS (dermal absorption factor 0.001) for all except As and it is 0.03 (USDoE 2005).
In addition, the ingested and dermal amounts of heavy metals computed above and used to calculate the hazard quotient (HQ) and hazard index (HI), respectively (USEPA 1989) by Eqs. (11) and (12):
where 'ex' is an exposure pathway such as ingestion and dermal is considered as an exposure mode. The values for RfD were obtained from Ravindra and Mor (2019). Lastly, HI was calculated by summating all the HQ results of both (ingestion and dermal) exposure pathways. According to USEPA guidelines, HQ and HI values of less than one are considered to be safe for human health and more than one is considered unsafe.
Carcinogenic health risk assessment
Intake of metals can also pause the carcinogenic health risks. This was calculated by multiplying the individual metal’s HQ values with that specific metal’s cancer potency factor of that specific metal. The values > 10−6 are thought to potentially cause cancer (USEPA 1989).
Data analysis
The Pearson correlation analysis p < 0.01, 0.05 and principal component analysis (PCA) between multiple physicochemical parameters and heavy metals were discussed. Origin Pro 21 data analysis and graphing software were used to compute the results and plot the graphs.
Results and discussion
Physico-chemical analysis
The information regarding the colour, odour and health effects of the population living around the sampling area was noted and listed in Table 3. It was observed that most of the samples (50%) were clear and without smell, while 15% of the samples were clear with smell, 20% of the samples were yellowish without smell and the remaining 5% of the samples were yellowish with a smell. The inhabitants around the sampling sites reported itching, diarrhoea, cold cough, fever, and low blood pressure during the sampling.
Figures 2, 3 and 4 present the results of all physicochemical parameters. The pH of water samples collected from different sources ranged from 6.73 to 7.88, with a mean of 7.28. The pH values showed a slightly alkaline trend. The acceptable pH limit, as per BIS standards is 6.5–8.5. Flooding water generally has a lower pH than river water as it increases the solubility of metals and nutrients (Saha et al. 2020). Further, this is also explained by higher amounts of bicarbonate ions produced by rainwater seeping through the soil. The pH of the research area may be influenced by the rock’s weathering and subsequent interactions with water resources (Ravindra et al. 2024; Zafar et al. 2022; Ram et al. 2021). The observed level of pH poses no major adverse effects on human health. However, pH can modify the reaction mechanism of various ions in water as reported by Muhammad et al. (2010).
The presence of inorganic dissolved solids in water affects the EC. Increased conductance indicates a rise in dissolved solids content, and vice versa, and serves as a primary approach for determining the suitability of water for various applications. The EC values for the collected water samples were found to be in the range of 325–1550 μS/m and the average value of 662.5 μS/m. According to WHO (2017) and BIS (2012) EC has no permissible limit. However, the highest EC was found for sample 2 taken from a well. This may be due to the enhanced salinity and minerals in the water sample during the flood. Consuming water with a high EC can cause gastrointestinal discomfort and an unpleasant smell (Zhang et al. 2018).
The TDS of water is denoted by the cations and anions level in the water. A high TDS value in water may negatively affect the consumer’s health (Mor et al. 2006; WHO 2006). TDS levels of more than 500 mg/L are not considered safe for drinking water supply. The value of TDS ranges from 228 to 1120 mg/L with a mean of 418.55 mg/L. Out of 20 water samples, only 4 samples including 1 of flood water, 2 of hand pumps, and 1 from a well, were found to have TDS values higher than the permissible limits of BIS (2012) and WHO (2017). This may be attributed to the high concentration of cations, anions, organic matter, salt and other dissolved impurities in water. Anthropogenically, it increases primarily by waste sewage, soap and detergents.
In the different sources of water samples of Patna, TH values were 107–575 mg/L with a mean of 403.3 mg/L. According to the BIS standards 2012, the acceptable limit for TH is 200 mg/L. The results revealed that the majority of the samples, i.e. 65%, have TH higher than the prescribed limits due to the higher 'cation's (Ca+ and Mg+) and 'anion's concentration (HCO3−, CO3−, SO4− and Cl−). TA of the water samples varied from 911.25 to 3516.75 mg L−1 with a mean of 1664.55 mg L−1. In all the samples, alkalinity was observed to be relatively higher than desirable limits. Bicarbonate and a weak acid like carbonic acid contribute to the alkalinity of water samples. High alkaline water is highly corrosive and can irritate the eyes.
Increased hardness of freshwater is typically attributed to Ca2+ and Mg2+ salts, which can limit its usage for cleaning, washing, and other industrial purposes. Ca2+ in the collected water samples ranged from 12.97 mg/L to a maximum of 47.35 mg/L with a mean of 21.78 mg/L. All the samples observed Ca2+ below the permissible limit of BIS (75 mg/L). Mg2+ is an enzyme activator in many living things and a lack of it can cause structural and functional alterations. The elevated Mg2+ ion content in the waterbodies may be caused by weathering and reverse ion exchange processes dissolving silicate and dolomite rocks (Gaikwad et al. 2020; Panneerselvam et al. 2021). This high level, can be poisonous and have a cathartic and diuretic impact. Mg2+ concentration in the collected samples ranged from a minimum of 4.37 mg/L to a maximum of 23.81 mg/L with a mean of 18.23 mg/L. The amount of Mg2+ observed in all the samples was noted below acceptable limits, i.e., 30 mg/L, as per BIS 2012 standards.
Na+ and K+ ions are vital for human health, and they must be consumed regularly to keep the blood salt level stable. As a result, it aids in regulating the human body's water balance. From the figures, it could be noted that the concentration of Na+ ranged from 4.732 to 30.77 mg/L with a mean of 11.60 mg/L. As per BIS standards for Na+, the prescribed limit is 200 mg/L and all the samples were found to be within the prescribed standard limits. K+ levels in drinking water are very low, so there are no health-based guidelines. However, some studies reported that K+ might be harmful to those within high-risk groups, such as those with kidney disease, cardiac problems, hypertension, diabetes, or older people and infants. The value of K+ ranged from 12.97 to 44.09 mg/L with a mean value of 5.73 mg/L.
The presence of major anions in the water samples was observed in the order: Cl− > SO42− > F− > NO3−. Cl− is found in the water by natural processes such as dissolution of salt deposits, road salting, and chemical industries effluent. As per BIS standards, the permissible limit of Cl− is 250 mg/L. Cl− varied from 130 to 2380 mg/L with a mean of 745.5 mg/L in the water samples. Most of the samples (60%) were observed to exceed the prescribed limit, including at least one sample from each type of source with the highest Cl− content in floodwater. The high concentration of Cl− in the samples may be due to the landfill leaching, effluent discharge, and septic tanks (Mor et al. 2006). High Cl− content may affect metallic pipes and plants' growth.
NO3− results from nitrogenous material conversion and the final product of aerobic stabilization of organic nitrogen. Also, NO3− proficient solubility in water, which can seep through the soil and contaminate water when combined with precipitation (Alessandro et al. 2008). In the water samples of Patna, the value of NO3− obtained varied from 0.139 to 2.33 mg/L with a mean of 0.33 mg/L and are shown in Fig. 3. As per drinking water standards of BIS and WHO, the results were found within the limits of 45 mg/L. F− is widely distributed in the natural environment and a significant trace element in groundwater, soil, food, air, rocks, flora, fauna and the human body (Singh et al. 2015; Patel et al. 2016; Enalou et al. 2018; Li et al. 2021). It is an essential micronutrient as it strengthens teeth and skeletal structure (Rashid et al. 2018). However, the excess level of F− in water leads to various health concerns in which fluorosis and dental caries are well recognized (Singh and Kumar 2017; Nayak et al. 2009; WHO 2006). This is particularly important in the plains that experienced flood event. In this study, the F− values in water samples ranged from 0.259 to 0.749 mg/L, with a mean of 0.558 mg/L, showing that all the samples were within limits (1 mg/L).
SO42− can cause biological corrosion and dysentery in infants if present in high concentrations in the water (Mazloomi et al. 2009). The consumption of drinking water containing high amounts of SO42− may result in intestinal disorder, dehydration and diarrhoea. If the concentration of SO42− in water surpasses 250 mg/L, it may have a bitter or medicinal flavour that makes it unpleasant to drink. The results obtained for Patna water samples showed that in the studied area, the concentration of SO42− ranged from 13.24 mg/L to 63.91 mg/L with a mean of 33.98 mg/L. No samples were found to be above the recommended limit (200 mg/L).
The physicochemical parameters were comparable with studies conducted latest before flooding of this area. Sukumaran et al. (2015) reported the pH (6.59–7.69); EC (229–2010); TDS (mg/L) (174–1283); TA (mg/L) (140–572); TH (mg/L) (156–760); Cl−(mg/L) (2–247.9); Ca2+ (mg/L) (44.2–212.6); NO3− (mg/L) (0.06–13.71); SO42− (mg/L) (2.27–78.4); Mg2+ (mg/L) (10.2–68.8) in groundwater of Patna. Further Majhee et al. (2019) done groundwater analysis in different blocks of Patna during Pre-monsoon and Post-monsoon season and resulted with pH (7.62–8.97); EC (220–1050); TDS (mg/L) (174–1283); TA (mg/L) (27.52–250.2); TH (mg/L) (25–465); Cl− (mg/L) (21.5–390); Ca2+ (mg/L) (8.02–148.3); NO3− (mg/L) (0.008–0.624); SO42− (mg/L) (0.314–15.3); Mg2+ (mg/L) (0–66.8) during Pre-Monsoon and pH (7.94–8.92); EC (232–995); TDS (mg/L) (150–639); TA (mg/L) (40–247.7); TH (mg/L) (25–1777.7); Cl− (mg/L) (22.5–400); Ca2+ (mg/L) (6–104.2); NO3− (mg/L) (0.001–0.138); SO42− (mg/L) (0.01–67.75); Mg2+ (mg/L) (1.8–37.1) during Post-monsoon season.
Elemental analysis of similar water samples was done using ICP-MS. The minimum and maximum values of the detected elements along with their prescribed limits (BIS 10500: 2012), are tabulated in Table 4. Based on the findings, it was observed that (Al, As, Ba, Mn, Mo and Zn) were found above the prescribed limits. However, the remaining elements were identified as below detection limits (BDL). Only floodwater samples with higher values than other samples were noticed. One sample showed the highest value of Mn at 2.041 mg/L, resulting in a black to brown colour of water, making the water taste metallic and bitter (USEPA, 2004). Out of 20 samples, 14 samples were found with a high concentration of Al. Only 3 samples showed a high value of As, 2 samples showed a high value of Ba and 4 samples showed a high value of Mo with a maximum value of 0.098 mg/L. The cause of these heavy metals was intended to be the erosion of former contaminated soils and sediments. Moreover, a high level of heavy metals contamination in water may also affected by flow velocity, huge overland flow, natural properties of sediment matrix and adsorbed compounds during the flood (Lim et al. 2021).
Water quality index and pollution index
The results of WQI are presented in Fig. 5. The outer layer shows the quality of water and inner layer shows the respective water source. WQI varied from 41 to 161. As a result, 7 samples (35%) were in the good category, 5 samples (25%) demonstrated of poor category, 4 samples (20%) indicated very poor water quality and further remaining 4% samples were found to be unfit for human consumption.
The poor quality of water samples may be attributed to effluent discharge as many colonies have close proximity to water and sewage pipes, introducing the leachate in the water and further raising the risk of water contamination (Fuentes-Rivas et al. 2020; Sawyer et al. 2016). Additionally, the surface run-off during flood can transport microbial contaminants into the water body, particularly Escherichia coli (E. coli), in pit latrines and serves as a strong indicator of water quality degradation. The discharge from pit latrines introduces contaminants into water sources, increasing the risk of waterborne diseases. Several previous studies argued that flood events significantly affect the prevalence of diarrhoea (Mellor et al. 2016; Chowdhury et al. 2018; Zhang et al. 2019). This is also noted that flood-affected areas have a higher risk of water and vector-borne illnesses and prevalence of diarrhoea rises during the period of floods (Pant et al. 2023; Kikuchi 2023). In contrast, HPI results revealed that all the samples were below the critical limits (100), demonstrating an insignificant pollution level.
Health risks from different (As, Cd, Pb, Zn, Co, Cr and Co) metals through the oral and dermal intake to people were assessed. The calculated health risk were categorized into adult and children risks in the context of carcinogenic and non-carcinogenic. HI values were varied according to the different exposure roots, as shown in Fig. 6 and Table S2. HQoral and HQdermal for metals in adults ranged from − 0.1286 to 11.333 and − 0.11 to 51.41. Similarly, the values observed for children ranged from − 0.61 to 52.20 for oral and − 0.228 to 149.1058 for dermal, respectively. HQ value for dermal is more than oral intake, suggesting that dermal exposure is the most prominent pathway for non-carcinogenic health risks. It was observed highest for arsenic (As) with HQ dermal (201.43) and HQ oral (66.03).
On the basis of HI values, it was noticed that As, Pb and Zn metals are more vulnerable to non-carcinogenic health risks. The value for HI was observed to be the highest for As, followed by Pb. On the other hand, children were shown to be more vulnerable to non-carcinogenic health risks than adults in the area under investigation. Moreover, similar results were observed in the case of carcinogenic risk assessment. The highest HI values were found for As (HIoral, 69.4) and (HIdermal, 211). The values were higher for adults (214.09) than children (52.6). Arsenic (As) is a naturally existing element present in soil, air, rocks, natural streams, and living organisms. The majority of the environmental problems linked to arsenic are caused by natural events like floods but human activity also plays a role in the release of As into hydrologic systems. In geochemical settings, arsenic forms a stable association with sediments, and its dissolved levels typically remain minimal until released by the parent rock systems (Vig et al. 2023b; Reddy and Sunitha 2023). During floods, the disturbance of sediment layers can lead to the release of As into the water, potentially increasing dissolved levels and posing risks to water quality. Moreover, the other source of As in waterbodies is As bound to iron oxides in flood plain sediments (Khan and Rai 2023). The study results were coherent with previous studies conducted in the similar region (Thakur and Gupta 2019; Chakraborti et al. 2018; Rajmohan and Prathapar 2016). Consumption of As contaminated water results in various health issues mainly skin cancer are well and various other forms of cancer in different parts of the human body (Vig et al. 2023c). Skin lesions can even occur at a value of 50 µg/L of As in drinking water with the people over 40 years of age (Chakraborti et al. 2018). Peripheral neuropathy and skin hyper- and hypopigmentation are the results of long-term arsenic exposure (Reddy and Sunitha 2023). Children show more severe arsenical skin lesions than adults and are more vulnerable to the toxic effects of As than adults as the daily water intake per unit of infant’s body weight is three to four times greater than that of an adult (Wasserman et al. 2007).
Several previous studies reported the high level of arsenic in groundwater and associated health risk problems in various districts of Bihar, threatening more than 10 million people in the state (Chakraborti et al. 2003; Saha 2009; Chakraborti et al. 2016; Nath et al. 2013; Kumar et al. 2019; Dubey et al. 2018; Abhinav et al. 2019). These studies reported a high concentration of As in this area in the hand pump-tube wells and bore well water along with the associated health risks among the population of this area. Therefore, measures must be taken to address multiple health risks in the study area.
Data analysis
In correlation analysis, the parameters of the post-flood samples were found to be weakly and moderately correlated to each other at the p (0.05) and p (0.01) levels as shown in Fig. 7. The heavy metals pair Zn-Mo (0.652) showed a substantial positive correlation, indicating that their sources of origin are geogenic. Similarly, a strong positive correlation was also noticed between physicochemical parameters. EC showed a positive correlation with TDS (0.982), TA (0.801), TH (0.857), Cl− (0.828), Na+ (0.90), Ca+ (0.848), Mg+ (0.904) and moderate positive correlation with NO3− (0.766) and K+ (0.690). Similarly, TDS was found to be positively correlated with TA, TH, Cl−, Na+, Ca+ and Mg+. NO3− was found to be positively correlated with Mg2+ (0.621). A positive correlation was also observed between cations and anions (NO3−–Ca+–K+). TH with Ca2+ (0.863) and Mg2+ (0.855). Na+–Ca2+–Mg2+ (0.743, 0.942, 0.801) showed positive correlation.
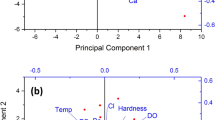
PCA is also regarded as an exemplary method for identifying sources (Islam et al. 2018). The PCA results are presented in Table 5 and Fig. 8. The components were identified using the varimax rotation approach based on the Eigenvalues. PCA revealed three components with eigenvalues greater than one. The first, second, and third components had a variance of 31.17%, 21.34%, and 11.23%. Components were found to have a cumulative frequency of 31.17%, 52.52%, and 63.75%. Component 1 showed that EC, TDS, TA, TH, Ca, Mg, Li, Mn, and Sr had high loading values of more than 0.6. The results were consistent with the correlation analysis, which revealed a high degree of association between these measures. TDS, nitrate, Na, K, and S were found in the second component, with the greatest loading of 0.941. The third component found high loadings of Al, Mo, Pb, and Zn, with Mo having the highest loading value of 0.801, indicating similar sources of these elements in the water samples, revealing geogenic and anthropogenic pollution causes.
Conclusion
The current study established a detailed water quality analysis of 20 samples obtained from various sources during the post-flood event. The consequences of heavy metal contamination on human health were explored. The water samples were found alkaline and most parameters such as TDS, TH, TA, Mg2+, and Cl− for a few flood samples, hand pump, and well samples were found above the BIS and WHO acceptable limits. The elevated water quality characteristics caused by rock weathering, mineral dissolution, and human activity such as sewage seepage. During flood, these factors include increased runoff, sediment transport, changed land use, and infrastructure disruption, ultimately raising the concentrations of contaminants, suspended particles, and dissolved minerals in water bodies. According to the WQI, 20% of samples from floods, hand pumps, and wells were found unsafe for drinking due to very poor water quality which may be due to the high bacteriological contamination. The HPI findings confirmed that all of the samples met the guidelines. On the other hand, health risk assessment revealed that adult and child population are vulnerable towards arsenic metal pollution. Additionally, the HQ values were observed higher for the dermal pathway than the ingestion pathway. A strong positive correlation between physicochemical parameters and heavy metals showed the significant correlation, implying a similar source of origin. Additionally, the PCA analysis showed that the majority of the heavy metal in water supply comes from anthropogenic inputs, and that geogenic activity during floods contributes to water contamination. The study results revealed a necessity for regular water quality monitoring after flooding and considerable upgrades on a priority basis.
Recommendations and future perspectives
For the comprehensive assessment of water quality, the water samples of this area should undergo further bacteriological examination. Some practical solutions include application of sodium hypochlorite bleach or calcium hypochlorite powder to ensure the safety of drinking water. To mitigate the spread of water borne infections among flood affected areas, implementing a water treatment approach is highly recommended. Chlorine spot treatment has also proven to be a particularly successful approach. Further floodwater harvesting techniques are a common and beneficial practice using floodwater to recharge various water resources in this area for irrigation.
The study makes it evident that the challenge for future research is to encourage the creation of dynamic vulnerability assessments that consider citizens' ability to cope with flood events. This is necessary to promote routes towards flood risk reduction and to improve our understanding of flood susceptibility (Moreira et al. 2021). Further policy documents of India have given valuable recommendations on different aspects of flood management such as flood damage assessment; performance evaluation of flood management schemes and works, requisite funds, master plans for flood management, future multi-purpose projects and emphasis on research, education and training on flood management. In addition, India must evaluate how climate change may affect rainfall and the effectiveness of flood-related infrastructure, and it must start making plans for the necessary climate adaptation. When it comes to fighting unending floods, advanced scientific and technological expertise, such as the use of remote sensing and geographic information systems, can be a very successful tactic (Singh and Kumar 2017). Further flood zoning and flood mapping are also beneficial for flood disaster preparedness. The community must be included in a basin-specific, holistic approach to increase the effectiveness of flood management efforts. It is necessary for everyone to participate in disaster response, flood prevention, and readiness. It is essential to educate the community, farmers, professional associations, businesses, and nonprofit organization on flood management.
Data availability
Not applicable.
References
Abhinav S, Navin S, Shankar P, Rahman MS, Kumar R, Niraj PK, Ghosh AK (2019) Health impact of chronic arsenic exposure in the population of Gyaspur-Majhi village, Patna, Bihar India. In: Environmental arsenic in a changing world: proceedings of the 7th international congress and exhibition on arsenic in the environment (AS 2018), July 1–6, 2018, Beijing, vol 13, no 2. CRC Press, p 369
Alessandro D, Bellomo W, Parello S, Brusca FL, Longo M (2008) Survey on fluoride, bromide and chloride contents in public drinking water supplies in Sicily (Italy). Environ Monit Assess 145(1):303–313
Alfieri L, Bisselink B, Dottori F, Naumann G, de Roo A, Salamon P, Feyen L (2017) Global projections of river flood risk in a warmer world. Earth’s Future 5(2):171–182
APHA A (2005) AEF. Stand. Methods Exam. Water Wastewater, 21st ed., Am. Public Heal. Assoc. Washington, DC
Bates B, Kundzewicz Z, Wu S (2008) Clim. Chang. Water. Intergovernmental Panel on Climate Change Secretariat
BIS ISDWS (2012) Bureau of Indian Standards. New Delhi, pp 2–3
Brown RM, McClelland NI, Deininger RA, O’Connor MF (1972) A water quality index—crashing the psychological barrier. In: Indicators of environmental quality. Springer, Boston, pp 173–182
Census of India (2011) Government of Bihar. https://dse.bihar.gov.in/Source/Provisional%20Population%20Totals%202011-Bihar.pdf
CGWB (2007) Ground Water Information Booklet Patna District, Bihar State, Central Ground Water Board, Ministry of Water Resources (Govt. of India), Mid-Eastern Region, Patna
Chakraborti D, Mukherjee SC, Pati S, Sengupta MK, Rahman MM, Chowdhury UK, Basu GK (2003) Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: a future danger? Environ Health Perspect 111(9):1194–1201
Chakraborti D, Rahman MM, Ahamed S, Dutta RN, Pati S, Mukherjee SC (2016) Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere 152:520–529
Chakraborti D, Singh SK, Rahman MM, Dutta RN, Mukherjee SC, Pati S, Kar PB (2018) Groundwater arsenic contamination in the Ganga River Basin: a future health danger. Int J Environ Res Public Health 15(2):180
Ching YC, Lee YH, Toriman ME, Abdullah M, Yatim BB (2015) Effect of the big flood events on the water quality of the Muar River, Malaysia. Sustain Water Resour Manag 1(2):97. https://doi.org/10.1007/s40899-015-0009-4
Chowdhury FR, Ibrahim QSU, Bari MS, Alam MJ, Dunachie SJ, Rodriguez-Morales AJ, Patwary MI (2018) The association between temperature, rainfall and humidity with common climate-sensitive infectious diseases in Bangladesh. PLoS ONE 13(6):e0199579
Dubey R, Upadhyaya A, Singh AK, Mondal S, Kumar A, Shukla RR (2018) Assessment of Arsenic content in water, soil and plant samples of Patna: arsenic content in water, soil and plant. J AgriSearch 5(4):254–259
Enalou HB, Moore F, Keshavarzi B, Zarei M (2018) Source apportionment and health risk assessment of fluoride in water resources, south of Fars province, Iran: stable isotopes (δ18O and δD) and geochemical modeling approaches. Appl Geochem 98:197–205
Ferrari A, Dazzi S, Vacondio R, Mignosa P (2020) Enhancing the resilience to flooding induced by levee breaches in lowland areas: a methodology based on numerical modelling. Nat Hazards Earth Syst Sci 20(1):59–72
Fida M, Hussain I, Rashid A, Shah SAA, Khan S (2021) Change detection in land use and land cover of District Charsadda Pakistan along River Kabul (2010 flood): taking advantage of geographic information system and remote sensing. Geol Behav (GBR) 5(2):35–41
Fuentes-Rivas RM, Martínez-Alva G, Ramos-Leal JA, de León GSC, Moran-Ramírez J, de Oca RMGFM (2020) Assessment of contamination by anthropogenic dissolved organic matter in the aquifer that underlies the agricultural area. Environ Sci Pollut Res 27(36):45859–45873
Gaikwad S, Gaikwad S, Meshram D, Wagh V, Kandekar A, Kadam A (2020) Geochemical mobility of ions in groundwater from the tropical western coast of Maharashtra, India: Implication to groundwater quality. Environ Dev Sustain 22(3):2591–2624
Islam MS, Hossain MB, Matin A, Sarker MSI (2018) Assessment of heavy metal pollution, distribution and source apportionment in the sediment from Feni River estuary, Bangladesh. Chemosphere 202:25–32
Jennerjahn TC, Ittekkot S, Klöpper S, Adi S, Nugroho SP, Sudiana N, Yusmal A, Gaye-Haake B (2004) Biogeochemistry of a tropical river affected by human activities in its catchment: Brantas River estuary and coastal waters of Madura Strait, Java, Indonesia. Estuar Coast Shelf Sci 60(3):503. https://doi.org/10.1016/j.ecss.2004.02.008
Khan MU, Rai N (2023) Distribution, geochemical behavior, and risk assessment of arsenic in different floodplain aquifers of middle Gangetic basin, India. Environ Geochem Health 45(5):2099–2115
Kikuchi M (2023) Influence of sanitation facilities on diarrhea prevalence among children aged below 5 years in flood-prone areas of Bangladesh: a multilevel analysis. Environ Sci Pollut Res 30(43):97925–97935
Krasner SW, Westerhoff P, Chen B, Amy G, Nam SN, Chowdhury ZK, Sinha S, Rittmann BE (2008) Contribution of wastewater to DBP formation Awwa. Res. krasner, Denver
Kumar A, Rahman MS, Kumar R, Ali M, Niraj PK, Srivastava A, Ghosh AK (2019) Arsenic contamination in groundwater causing impaired memory and intelligence in school children of Simri village of Buxar district of Bihar. J Mental Health Hum Behav 24(2):132–138
Kundzewicz ZW, Kanae S, Seneviratne SI, Handmer J, Nicholls N, Peduzzi P, Sherstyukov B (2014) Flood risk and climate change: global and regional perspectives. Hydrol Sci J 59(1):1–28
Li Y, Li P, Cui X, He S (2021) Groundwater quality, health risk, and major influencing factors in the lower Beiluo River watershed of northwest China. Hum Ecol Risk Assess Int J 27(7):1987–2013
Lim KY, Zakaria NA, Foo KY (2021) Geochemistry pollution status and ecotoxicological risk assessment of heavy metals in the Pahang River sediment after the high magnitude of flood event. Hydrol Res 52(1):107–124
Mahapatra R (2020) Floods cost India Rs 4.7 lakh crore in last 6 decades. DownToEarth
Majhee R, Mahanta N, Sahoo H, Goswami S (2019) Hydrogeological framework and groundwater assessment of Keonjhargarh and Patna Blocks, Keonjhar District, Odisha
Masson T, Bamberg S, Stricker M, Heidenreich A (2019) “We can help ourselves”: does community resilience buffer against the negative impact of flooding on mental health? Nat Hazard 19(11):2371–2384
Mazloomi S, Dehghani MH, Norouzi M, Davil MF, Amarluie A, Tardast A, Karamitabar Y (2009) Physical and chemical water quality of ilam water treatment plant. World Appl Sci J 6(12):1660–1664
Mellor JE, Levy K, Zimmerman J, Elliott M, Bartram J, Carlton E, Nelson K (2016) Planning for climate change: the need for mechanistic systems-based approaches to study climate change impacts on diarrheal diseases. Sci Total Environ 548:82–90
Mishra SK, Mishra N (2010) Vulnerability and adaptation analysis in flood affected areas of Orissa. Soc Change 40(2):175–193
Mohan D, Suresh P, Chackochan LK (2019) The influence of flood and the variation in water quality index in River Periyar, Kerala India. IOSR J Environ Sci Toxicol Food Technol (IOSR-JESTFT) 13(3):08–12
MoHFW I (2010) The assisted reproductive technology (regulation) bill (draft)
Mor S, Ravindra K, Dahiya RP, Chandra A (2006) Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ Monit Assess 118(1):435–456
Moreira LL, de Brito MM, Kobiyama M (2021) A systematic review and future prospects of flood vulnerability indices. Nat Hazard 21(5):1513–1530
Muhammad S, Shah MT, Khan S (2010) Arsenic health risk assessment in drinking water and source apportionment using multivariate statistical techniques in Kohistan region, northern Pakistan. Food Chem Toxicol 48(10):2855–2864
Nanditha JS, Mishra V (2021) On the need of ensemble flood forecast in India. Water Secur 12:100086
Nath A, Vendan PS, Kumar S, Kumar A, Singh JK (2013) Toxicity due to arsenic in Gangetic zone of Patna, India and its linkage with cancer. J Environ Anal Toxicol 3(192):2161–2525
Nayak B, Roy MM, Das B, Pal A, Sengupta MK, De Prasad S, Chakraborti D (2009) Health effects of groundwater fluoride contamination. Clin Toxicol 47(4):292–295
Panneerselvam B, Muniraj K, Thomas M, Ravichandran N, Bidorn B (2021) Identifying influencing groundwater parameter on human health associate with irrigation indices using the automatic linear model (ALM) in a semi-arid region in India. Environ Res 202:111778–111790
Pant M, Bhatla R, Ghosh S, Das S, Mall RK (2023) Will warming climate affect the characteristics of summer monsoon rainfall and associated extremes over the gangetic plains in India? Earth Space Sci 10(2):e2022EA002741. https://doi.org/10.1029/2022EA002741
Patankar A (2019) Impacts of natural disasters on households and small businesses in India. Asian Development Bank Economics Working Paper Series, (603)
Patel P, Raju NJ, Reddy BSR, Suresh U, Gossel W, Wycisk P (2016) Geochemical processes and multivariate statistical analysis for the assessment of groundwater quality in the Swarnamukhi River basin, Andhra Pradesh, India. Environ Earth Sci 75:1–24
Rajmohan N, Prathapar SA (2016) Arsenic distribution in ground water and soil in the Eastern Ganges basin. A review. In: Proceedings, Sixth international ground water conference, 11–13 February, 2016, Chennai, Paper ID TS4–05, pp TSIV-78–93
Ram A, Tiwari SK, Pandey HK, Chaurasia AK, Singh S, Singh YV (2021) Groundwater quality assessment using water quality index (WQI) under GIS framework. Appl Water Sci 11(2):1–20
Rammelt C, Masud ZM, Masud A (2018) The waterways of Tangail: failures to learn from flood control efforts in the Brahmaputra basin of Bangladesh. Water Altern 11(1):106
Rashid A, Guan DX, Farooqi A, Khan S, Zahir S, Jehan S, Khan R (2018) Fluoride prevalence in groundwater around a fluorite mining area in the flood plain of the River Swat, Pakistan. Sci Total Environ 635:203–215
Ravindra K, Mor S (2019) Distribution and health risk assessment of arsenic and selected heavy metals in Groundwater of Chandigarh, India. Environ Pollut 250:820–830
Ravindra K, Vig N, Agarwal N, Malik VS, Arumugam E, Rajkumar P, Mor S (2024) Metal contamination of different water categories and surface soil near thermal power plants at varied geographic sites of India and their health risk assessment. Water Air Soil Pollut 235(2):152
Reddy YS, Sunitha V (2023) Assessment of Heavy metal pollution and its health implications in groundwater for drinking purpose around inactive mines, SW region of Cuddapah Basin, South India. Total Environ Res Themes 8:100069
Reed R, Godfrey S, Kayaga S, Reed B, Rouse J, Fisher J, Vilholth K, Odhiambo F (2013) Technical notes on drinking-water, sanitation and hygiene in emergencies
Sadat-Noori SM, Ebrahimi K, Liaghat AM (2014) Groundwater quality assessment using the water quality index and GIS in Saveh-Nobaran aquifer, Iran. Environ Earth Sci 71:3827–3843
Saha D (2009) Arsenic groundwater contamination in parts of middle Ganga plain, Bihar. Curr Sci 97(6):753–755
Saha A, Salim SM, Sudheesan D, Suresh VR, Nag SK, Panikkar P, Das BK (2020) Impacts of a massive flood event on the physico-chemistry and water quality of river Pampa in Western Ghats of India. J Environ Anal Chem 102(19):7969–7987
Sam AS, Kumar R, Kächele H, Müller K (2017) Vulnerabilities to flood hazards among rural households in India. Nat Hazards 88:1133–1153
Sangu RPS, Shanker V, Sharma SK (1987) An assessment of water quality of River Ganga at Garhmukteshwar (Ghaziabad) U. P. Indian J Ecol 14(2):278–282
Sawyer CN, McCarty PL, Parkin GF (2016) Chem Environ Eng Sci
Sethy SN, Syed TH, Kumar A (2017) Evaluation of groundwater quality in parts of the Southern Gangetic Plain using water quality indices. Environ Earth Sci 76:116
Singh O, Kumar M (2017) Flood occurrences, damages, and management challenges in India: a geographical perspective. Arab J Geosci 10:1–19
Singh S, Raju NJ, Ramakrishna C (2015) Evaluation of groundwater quality and its suitability for domestic and irrigation use in parts of the Chandauli-Varanasi region, Uttar Pradesh, India. J Water Resour Prot 7(07):572
Slater LJ, Villarini G (2016) Recent trends in U.S. flood risk. Geophys Res Lett 43(24):12428–12436. https://doi.org/10.1002/2016GL071199
Srivastava PK, Mukherjee S, Gupta M, Singh SK (2011) Characterizing monsoonal variation on water quality index of River Mahi in India using geographical information system. Water Qual Exposure Health 2:193–203
Sukumaran D, Saha R, Saxena RC (2015) Ground water quality index of Patna, the capital city of Bihar, India. Am J Water Resour 3(1):17–21
Tapsell SM, Tunstall SM (2008) “I wish I’d never heard of Banbury”: the relationship between ‘place’ and the health impacts from flooding. Health Place 14(2):133–154
Thakur BK, Gupta V (2019) Valuing health damages due to groundwater arsenic contamination in Bihar, India. Econ Hum Biol 35:123–132
U.S. Department of Education (2005) A profile of the American high school sophomore in 2002: initial results of the base year of the Educational Longitudinal Study of 2002. National Center for Educational Statistics (NCES 20)
Udhayakumara R, Manivannanb P, Raghuc K, Vaidekid S (2016) Assessment of physico-chemical characteristics of water in Tamilnadu. Ecotoxicol Environ Saf 134(Part 2):474–477
UNEP UBTF (1999) The potential effects on human health and environment arising from possible use of depleted uranium during the Kosovo conflict: A preliminary assessment. DU Report
US EPA (U.S. Environmental Protection Agency), Office of Water (2004) (4304J) Health and Ecological Criteria Division Washington DC 20460 Drinking Water Health Advisory for Manganese. EPA-822- R-04-003
US Environmental Protection Agency (USEPA) (2011) Electronic Code of Federal Regulations. Title 40: part 180. Tolerances and exemptions for pesticide chemical residues in food. http://ecfr.gpoaccess.gov/
USDOE (2011) The Risk Assessment Information System (RAIS), U.S. Department of ' 'Energy's Oak Ridge Operations Office (ORO), Washington, DC
USEPA (1989) Risk assessment guidance for superfund. Human health evaluation manual (Part A)
Valtin H (2002) “Drink at least eight glasses of water a day”. Really? Is there scientific evidence for “8×8”? Am J Physiol Regul Integr Comp Physiol 28(5):R993–R1004
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi RR, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: Thirumanimutharu sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609
Vig N, Ravindra K, Mor S (2023a) Evaluation of groundwater for drinking and irrigation applications concerning physicochemical and ionic parameters through multiple indexing approach: a case study around the industrial zone, Punjab, India. Environ Geochem Health 45(5):2077–2097
Vig N, Ravindra K, Mor S (2023b) Heavy metal pollution assessment of groundwater and associated health risks around coal thermal power plant, Punjab, India. Int J Environ Sci Technol 20(6):6259–6274
Vig N, Ravindra K, Mor S (2023c) Environmental impacts of Indian coal thermal power plants and associated human health risk to the nearby residential communities: a potential review. Chemosphere 341:140103. https://doi.org/10.1016/j.chemosphere.2023.140103
Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline J, Graziano JH (2007) Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ Health Perspect 115(2):285–289
WHO (2006) Fluoride in drinking water. A series of World Health Organization monographs
WHO (2017) Guidelines for drinking water quality: training pack. 4th ed. Incorporating The First Addendum, Geneva
Zafar MM, Sulaiman MA, Prabhakar R, Kumari A (2022) Evaluation of the suitability of groundwater for irrigational purposes using irrigation water quality indices and geographical information systems (GIS) at Patna (Bihar), India. Int J Energy Water Resour 1–14
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci 77:273
Zhang N, Song D, Zhang J, Liao W, Miao K, Zhong S, Huang C (2019) The impact of the 2016 flood event in Anhui Province, China on infectious diarrhea disease: an interrupted time-series study. Environ Int 127:801–809
Acknowledgements
The authors are particularly grateful to the Health Care Without Harm for this work. We also acknowledge the Environment Studies Department of Panjab University, 160014, Chandigarh, for their kind support for the laboratory analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ravindra, K., Vig, N., Chhoden, K. et al. Impact of massive flood on drinking water quality and community health risk assessment in Patna, Bihar, India. Sustain. Water Resour. Manag. 10, 104 (2024). https://doi.org/10.1007/s40899-024-01052-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-024-01052-z