Abstract
The hydrochemical study was conducted to outline the suitability of groundwater for drinking and irrigational purposes of two distinctly located geographical areas, Koprivnica-Križevci County, Croatia and district Allahabad, India. A total 160 groundwater samples were collected from sources (in hot and cold seasons) of both countries during 2 years (2011–2012) to evaluate the recent hydrochemical status of groundwater. Samples were analyzed for 12 various physico-chemical parameters.The Koprivnica-Križevci County has Ca–HCO3 water type, whereas in Allahabad it prevails as Mg–HCO3. All the parameters were below the maximum acceptable value (MAV) of standards (WHO, US EPA, Indian and Croatian Standards). The usage and consumption of groundwater should be of no concern with regard to inorganic pollutants. Irrigational indices (residual sodium carbonate content, magnesium hazard, permeability index, exchangeable sodium percentage, and salinity hazard) showed that groundwater is suitable for irrigational purposes. Hierarchical clustering analysis (HCA) has resulted into four groups due to the processes on natural and anthropogenic factors. The principal component analysis (PCA) has shown that first factor (all physico-chemical analyzed variables) explains 27.75% of the total variability and another factor (geographical distribution) accounts only for 10.46%. We found that local environmental conditions are more important predictor than geographical distribution. The present study may be helpful in dealing for further studies concerning groundwater quality issues in the distinctly located geographical areas. The groundwater quality is essential for management and sustainability of water resources, economic development, and human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is a regional resource, but water scarcity and pollution are becoming a global issue due to increasing population, economic growth, and climate change. Development of new sources of water beside its efficient use, together with conservation measures, should be an important component of any (developed and developing) country’s national water plan. In many parts of the world watersheds, river, ponds, aquifers and the associated ecosystems have undergone significant modifications; hence the vitality, quality, and availability of the water resources have been facing further human threats (Singh et al. 2015a). The causes of groundwater pollution are natural and human-induced. In recent years, human-induced causes have increased and could be easily linked to unprecedented population growth; a changing climate, rapid urbanization, expansion of infrastructure, migration; land conversion; extensive agricultural activities and over exploitation (Singh et al. 2010, 2013a, b, 2015a, b; Srivastava et al. 2013; Nemčić-Jurec et al. 2013; Nemčić-Jurec and Jazbec 2017; Jacintha et al. 2016; Rawat et al. 2017a, b; Singh et al. 2017). To understand the impact of human activities, scientists have studied chemical speciation, saturation, transport of pollutant and decay, dissolution, precipitation, ion-exchange, sorption, and desorption, together with the residence time occurring along the flow path and change in land use control the variation in chemical of groundwater (Belkhiri et al. 2011; Singh et al. 2012; Thakur et al. 2015). These studies help in developing and defining the feasible strategies for the protection of groundwater resources from pollution (Chidambaram et al. 2013a, b).
The hydrogeological setting is important, the alluvial aquifers constitute a hydrological unit formed by the alluvial deposits and are characterized by a linear and shallow feature as they are spread along the fluvial valleys of the basin (Helena et al. 1999). The shallow aquifers have high permeability, hence are vulnerable to pollution (Ravikumar and Somashekar 2012). Hence, it is very important to monitor the quality of groundwater to suppress the contamination and to minimize the pollution. According to literature (Singh et al. 2009, 2015; Tziritis et al. 2016), groundwater chemistry is generally influenced by the characteristics of catchment area, such as natural (geogenic) factors like climatic conditions, bedrock geology, soil properties, and transport or flow of water through environment. Hydrogeochemical characteristics are dependent on the chemistry of rock-forming minerals and on the physical process of erosion, respectively, mineral dissolution. As a result, groundwater is enriched in ions and other elements (Robinson and Ayotte 2006). Anthropogenic impact is mainly related with extensive agricultural practices which favor nitrate enrichment and salinization (Nemčić-Jurec and Jazbec 2017) or related to industry and waste water sources (Gautam et al. 2013).
Groundwater quality has been investigated by many researchers (Mikac et al. 1998; Ahel et al. 1998; Stambuk-Giljanovic 2005; Yidana et al. 2008; Ravikumar and Somashekar 2012; Singh et al. 2012, 2013a, b; Tiwari and Singh 2014; Chandra et al. 2015; Thakur et al. 2015; Gautam et al. 2016). Multivariate statistical techniques (MST) help in effective management of large and complex groundwater data. Principal component analysis (PCA) and cluster analysis (CA) are widely used statistical techniques for the characterization and evaluation of groundwater quality (Singh et al. 2004, 2009). Researchers have used MST to characterize and evaluate surface and groundwater quality and have found them very useful for studying the variations caused by different factors (Vega et al. 1998; Singh et al. 2005, 2009, 2012, 2013a, b, 2015a, b; Shrestha and Kazama 2007; Belkhiri et al. 2011; Tziritis et al. 2016). Astel et al. (2006) have applied MST for the determination of chloro/bromo disinfection by-products in drinking water at 12 locations in the Gdańsk area (Poland). Ujevic et al. (2012) have used cluster, factor, and discriminant analyses to study the groundwater chemistry of Eastern Croatia and to identify the main geochemical processes responsible for high arsenic concentrations in analyzed groundwater.
Despite the importance of groundwater in Allahabad (India) and Koprivnica-Križevci County (Croatia), it is known about natural and anthropogenic influences but only in some areas of countries. The importance of the groundwater resources in the area should not be underestimated. Groundwater quality indices provide a simple and easily understandable tool for managers on the water quality and possible uses for irrigation water. An individual quality factor alone is not enough to evaluate the drinking or irrigational water quality because it could be restrictive and can give unfavorable qualification. The groundwater hydrochemistry of Koprivnica-Križevci County and Allahabad district, especially for irrigation purposes, is poorly understood. Increased knowledge of hydrochemical water quality of Allahabad, India and Koprivnica-Križevci County of Croatia will be leading to sustainable development of water resources. In some parts of India, irrigation is applied without control and supervision, and in Croatia irrigation is rarely for now. During the past two decades, irrigational uses in India have been rapidly increased and started in Croatia too but often without adequate planning. The aim of this paper was: (1) to study groundwater quality on intense agricultural area of Koprivnica-Križevci County (Croatia) and on agricultural and urban area of Allahabad (India) to assess the influences on groundwater quality and in the same way estimate the suitability of groundwater for drinking and irrigational uses, and (2) to assess whether local conditions are more important predictor for groundwater quality than geographical distribution. Assessing of groundwater quality could be applied in such a way in other unexplored areas of the two countries and provide better water management and human health.
Study area
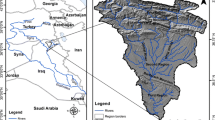
In this paper, there are two study areas that were targeted to assess the groundwater quality. The first is Koprivnica-Križevci County located between 46°10′N and 16°50′E and covers an area of 1748 km2 (Fig. 1a). The study area belongs to the catchment area of the River Drava where prevailing quaternary gravel-sand aquifer (Mayer et al. 1996). On the north-western hydrogeological area, dominate carcasses of mountain Kalnik. In the deeper layers laying reservoir of groundwater, lime dolomite aquifer. Some parts on north-west hydrogeological area stretch on the Pleistocene terrace prevailing gravel and sand aquifer.
Map showing the water sampling locations of the two study area. a Koprivnica-Križevci County of country Croatia (C1–C5); b Allahabad district, state Uttar Pradesh of country India (I1–I30) [see “Appendix 1” Station code and sampling locations in Croatia (n = 5) and India (n = 30)]
The climate is characterized by hot and dry season which includes spring and summer and cold season which includes autumn and winter. Average annual amount of rainfall on study area is 803 mm. The amount of rainfall is similar in the whole area. July and September are the 2 months of maximum rainfall. Average annual temperature is 10.9–11.2 °C and the climate is moderately warm; July usually being the hottest month year with mean daily maximum temperature at 38.2 °C and minimum temperature 7 °C; January being the coldest month of the year with mean daily maximum temperature 5 °C and minimum − 22.6 °C. The relative humidity in study area was 80–96% during the rainy and cold season and about 15–20% during the dry and hot season. Average annual wind speed for the study area is 2.5–3 m/s and maximum wind speed is about 14 m/s.
The research area (Fig. 1a) is located on three different hydrogeological units (Fig. 2a). The first area of sources C1, C4, and C5 is located in the water area of central Podravina. This water area extends from line-Legrad Koprivnica, in the east, to the line of Podravska Slatina-Sopje, in the west. From the north area, Podravina is bounded by the state border with Hungary, and in the south area, it is bounded by northern slopes of Bilogora and Papuk. The area of the old alluvial terraces composed of layers of the Drava River and its tributaries, and terraced sediments. Hydrogeological characteristics were caused by geological structure of the area. To the hydrogeological features, the most important is Quaternary gravel-sand aquifer, with a thickness of 30 m to the south (C4 and C5 locations) and in some parts of the river basin district reaches up to 50 m (C1 location). Top part—the roof of the aquifer is composed of dust, sand, and clay with a significant occurrence of live sands and marsh loess mainly in southern and eastern part of the river basin district. The thickness of this layer and its texture strongly influence on the permeability of the substances from the surface layers to the aquifer. On the west side from Virovitica, the thickness of the roof is about 10 m. In the vicinity of the Virovitica, the roof is thicker and further to the east regularly it is thicker than 20 m. There is no unique hydrogeological regime on the water area. The impact of the Drava River on the direction of groundwater flow is clearly visible in the zone width of 2–3 km along the Drava River and the area from Legrad to Pitomača (C1, C4, and C5). In this zone, groundwater imitates the regime of the Drava River during a year. In the rest part of the area, the influence of the Drava is not clear and unambiguous. The aquifer is filled by the infiltration of rainwater through the low permeable cover and by seepage from the bed of the Drava River in the upstream part of the territory. Natural water quality varies from site to site (Mayer et al. 1996). The second and third hydrogeological unit covers an area of Prigorje region (C2 and C3 locations). The C2 landscape area dominate the carcasses of mountain Kalnik. Morphologically most prominent parts were built from the Paleogene and Neogene rocks while the northern area of the Kalnik was built from the Mesozoic deposits. The massif of mountain Kalnik is built from porous lime dolomite breccia and permeable to poor permeable Tortonian deposits. Deposits in the surface area represent collector of rainwater and in the deeper layers is reservoir of underground water, lime dolomite aquifer (Šimunic 1984). Area of C3 location, which also belongs to the Prigorje, stretches on the Pleistocene terrace. After 7–9 m of cover clay, lies aquifer to 9 m thick build from various grit of sand and gravel. Beneath aquifer, lies a thin layer of coal, clay and marl clay. The roof layers build from soil and Pleistocene clay are poorly permeable materials that protect the aquifer (Sebastian and Britvic 1989).
The second study area is Allahabad district located (24°47′N–25°47′N latitudes and 81°19′E–82°21′E longitudes) by covering an area of 5246 km2 in India. Geologically and hydrogeologically, it is an interesting area; the two major rivers Ganga and Yamuna flow through the district (Fig. 1b). The whole Trans-Ganga tracts, the greater portion of doab (interfluve region), are composed of gangetic alluvium (Fig. 2b). The alluvial detritus of the Vindhyans is found in the southern part of the doab. The Trans-Yamuna tract and the Vindhyan detritus merge in the gangetic sand and silt. The gangetic alluvium consists of alterations of fluvial deposition of sand, silt, and clay. The thickness of alluvium decreases from north to south. Glass sand deposits are found in the neighborhood of Shankargarh.
The climate of region is characterized by a long and hot summer (March–middle of June), monsoon (June–September), and cold seasons (mid-November–February). The rainfall generally decreases from the south-east to the north-west. About 88% of the annual rainfall is received during the monsoon season. The average annual rainfall is 975.4 mm with 48 rainy days in a year. From about the middle of November, the temperature begins to fall rapidly and in January (the coldest month) the mean daily maximum is 23.7 °C. The heat in the summer season, particularly, in May and the early part of June is intense; May usually being the hottest month of the year with the mean daily maximum temperature at 41.8 °C and the mean daily minimum at 26.8 °C.
The study area of Allahabad district is a part of Quaternary alluvium and Vindhyan Plateau and ranges from Proterozoic to recent geological origin. The basement in the area is formed by Quartzite of Kaimur group and is unconformably overlain by Quaternary alluvium. The distribution of groundwater is dependent on the type of formation; in study area, it ranges from unconsolidated to alluvial formation and groundwater occurs under unconfined to confined conditions. The groundwater is shallow (northern part of the district) to deep aquifers (southern part of the district) and depth ranges from 2 to 20 m during pre-monsoon period, and in post-monsoon period the groundwater recharge and hence the shallow water tables recorded as 1–18 m. In the Vindhyans formation (consolidated), the water tables are much dynamic in nature and it ranges from 3 to 10 m below ground level during pre-monsoon period and 2–8 mgbl in post-monsoon period. The seasonal fluctuations were ranging from 1 to 4 m. The lithologs in Trans-Ganga and Tran-Yamuna area suggest that these areas have unique geological and hydrogeological characteristics. Further, the alluvial plain is also classified as younger and older alluvial plain; in the study area, older plain have two subdivisions (Fig. 2b) as Banda older alluvium and Varanasi alluvium (Singh et al. 2015b). The groundwater exploration studies have revealed a three-tier aquifer systems in the alluvial area that have unique granular zones as follows: (1) I aquifer group (shallow) ranging from 0.0 to 110 mbgl; (2) II aquifer group (middle aquifer) ranging from 120 to 250 mbgl; and (3) III aquifer group (deeper aquifer) lying below 260 down to depth 400 mbgl. The aquifer material is medium-to coarse-grained sand admixed with gravel at places. The tube well which is located in the alluvium plains have high yield 2000–3000 lpm (liter per minute), whereas the of Vindhyans regions have limited fractured zones, and sustain less water and exists down to 125 m only and yield is low 500–1000 lpm; within this sandstone domain, a silica sand horizon exists having a thickness of 5–40 m which also contains groundwater (Singh et al. 2015b).
Materials and methods
Water sampling and analysis
During 2011–2012, a total of 40 samples of groundwater were taken during hot and dry season which includes spring and summer and cold season which includes autumn and winter (4 times per year). The groundwater quality has been assessed systematically for all samples and from all the five locations of sources of public water supply (Fig. 1a). Samples were collected (according to HRN ISO 56667-5 2000) in clean polyethylene bottles (1 l volume) for laboratory analysis. The water sampling from wells of sources was done after releasing the water for 2–3 min on the outlet of a water supply system. The samples were stored in the portable fridge, in the dark at 1–5 °C for transportation. After delivery to the laboratory, the samples were stored at the same temperature until the analysis (within 24 h).
pH, electrical conductivity (EC), and total dissolved solids (TDS) were measured using pH meter/conducto-meter system (Mettler Toledo) employing standard methods (Clesceri et al. 1998; Rice et al. 2012). Anions (Cl−, SO42−, F−, and NO3−) were measured by ion chromatography (Dionex-ICS 3000, Ion pack AS15, using a Na2CO3/NaHCO3-Eluent with a flow rate of 1 ml/min). ASRS ULTRA II-4 mm performed chemical suppression. The analysis was done according to HRN EN ISO 10304-1 (2009)/Ispr.1:2012. Cations (Na+, K+, Mg2+, and Ca2+) were measured by ion chromatography (Dionex-ICS 3000, Ion pack CS16, using a 30 mM MSA-Eluent with a flow rate of 1 ml/min). CSRS 300 4-mm has been used to perform chemical suppression. The analysis of cations was done according to HRN EN ISO 14911 (2001). Total alkalinity (as HCO3−) were measured by a method prescribed in standard methods (Clesceri et al. 1998; Rice et al. 2012), by titration with HCl. Precision judged (% RSD) from the duplicates is on an average < 10% for all parameters.
Collection and analysis of Indian samples (N = 120) during hot (pre-monsoon) and cold season (post-monsoon) have been performed as specified in the standard international methods (APHA 1996). Total alkalinity (as HCO3−) and physical parameters, such as EC, total dissolved solids (TDS), and pH, were measured in situ using digital probe. Alkalinity is measured using a Hath field titration kit (through titration with 0.1 M HCl). The major cations (Mg2+, Ca2+, Na+, and K+) were analyzed using an atomic absorption spectrometer (Varian, 280 FS), and major anions (F−, Cl−, SO42− and NO3−) were analyzed using an ion chromatography (Dionex Dx-120) using anion (AS12A/AG12) columns coupled to an anion self-regenerating suppressor (ASRS) in recycle mode. Bicarbonate (HCO3−) was determined by titration method as described in the standard methods for the examination of water and wastewater (APHA 1996). The precision and accuracy of the analyses are within 5% (evaluated through repeated analyses of standards and samples).
Data analysis
Descriptive statistic of physicochemical parameters of groundwater samples (N = 160) collected from studied areas. Afterwards, the normalized concentrations anions and cations were plotted as Piper plot, it comprises three pieces: a ternary diagram (in the lower left representing the cations, in the lower right representing the anions, and a diamond plot in the middle representing a combination of the two). The intersected third point on diamond plot represents a sample.
Groundwater quality datasets were further subjected to two MST: HCA and PCA. HCA was designed to perform classification by assigning observations to groups in more-or-less homogeneous and distinct forms. Its method of analysis was Euclidean distance as distance of measure and complete linkage as linkage algorithm; however, helps in creation and identification of natural groupings for samples. Moreover, PCA was carried out to decipher the sources of ions in the groundwater samples and hydrogeochemical interpretation (Singh et al. 2009, 2015a, b). PCA we calculated with respect to their annual average concentration of parameters which has given the eigen value and percentage of the variance explained by each factor. It also gives a communality of unity for each component. Communality is the variance in observed variables accounted for by common factors. The results were compared to the maximum acceptable level (MAV) of the Regulation (2013) and to other standards (WHO 2011; Indian Standard 2012) for drinking water purposes. The suitability of water for irrigation purposes was identified by the following irrigation indices: residual sodium carbonate content (RSC), magnesium hazard (MH), permeability index (PI), salinity hazard (SH), and exchangeable sodium percentage (ESP). The statistical analysis was carried out through statistical software packages as: descriptive statistic (SAS 9.2 2008), HCA and PCA (STATISTICA 8.0 2011), and graphical presentations through both statistical packages. The Piper was plotted with the help of AqQA (version 1.1.1) software.
Results and discussions
Hydrochemistry
The analytical results are shown in Table 1. Hydrogeochemistry of groundwater is characterized by the prevalence of calcium and bicarbonate as dominant cation and anion in Koprivnica-Križevci County of samples. The descending order of cations is as follows: (Ca2+ > Mg2+ > Na+ > K+) at sampling locations. The Na/Cl molar ratio greater than 1 typically suggests that the Na+ ion was released in water from silicate by weathering and dissolution of silicate minerals, and it contributes to Ca2+ and Mg2+ ions in the groundwater (Gautam et al. 2015). With respect to anion descending order, it is as follows: HCO3− > NO3− > SO42− > Cl− > F− or HCO3− > SO42− > NO3− > Cl− > F−. The HCO3− may be derived from soil zone carbon dioxide, parent rock materials (carbonates and silicates) through weathering, and dissolution (Singh et al. 2012, 2013a, b, Gautam et al. 2015). NO3− is normally considered as a minor anion in non-polluted waters hence their elevated concentration are indicator for the ongoing agricultural impact from the excessive use of manure or mineral fertilizers (Nemčić-Jurec et al. 2013; Nemčić-Jurec and Jazbec 2017). The maps for the spatial distributions of the hydro-chemical parameters are shown on Fig. 3a. Interactions between groundwater and surrounding rocks are mostly the main processes responsible for the observed characteristics of groundwaters on the locations (Belfar et al. 2017). Figure 3a shows an anthropogenic factor (higher concentrations of nitrate and chloride) controlling the groundwater composition on C1 location (Nemčić-Jurec et al. 2013; Nemčić-Jurec and Jazbec 2017).
Mg2+ and HCO3− are dominant ions at most of the sampling locations at district Allahabad. Cations are in the descending order of concentrations as follows: Mg2+ > Ca2+ > Na+ > K+, while anions are highly variable and the descending order depends on location. At most sites, anions have been following the descending order of concentrations as HCO3− > Cl− > SO42− > NO3− > F−. Nitrate concentration was found low in groundwater samples at many locations of Allahabad which suggests low agricultural activities compared to Koprivnica-Križevci County of samples. The areas have more trees combined cultivating crops which may decrease the probability of groundwater contamination by nitrate. The studies suggest that even in areas with high nitrogen input despite the extensive use of fertilizers, and even well-drained soils (Nolan et al. 1998) have devoid of nitrate in the groundwater. Hubbard and Sheridan (1989) have suggested explanations for low nitrate in shallow groundwater that include dilution from precipitation, denitrification, and uptake by plants. Lasagna and De Luca (2016) and Eiche et al. (2016) have suggested the influence of oxido-reductive process on groundwater which results in low nitrate concentration. In Table 1, it was shown that standard deviation of nitrate is higher in India in comparison with Koprivnica-Križevci County. Generally, our results indicate higher variability of ion concentrations in Allahabad samples. The water chemistry of Allahabad water samples are controlled primarily by rock–water interaction along distinct subsurface flow paths. The Allahabad water samples are located lying in the thick alluvial plain, thin alluvial plain, and marginalized alluvial plain (hard rock region in southern part). The temporal variation is controlled by mixing-primarily dilution during rainfall periods that replenishes the groundwater of the area. It may be possible that seasonal variations of pCO2 have played a role in temporal variation. Calcium, bicarbonate, and magnesium are derived from carbonate dissolution. The relatively high concentrations of magnesium indicate dissolution of very magnesium-rich calcite mineral (Meyer 1999). The contour maps of the spatial distributions of the hydro-chemical parameters at district Allahabad are shown in Fig. 3b. The interaction of soil–rock–water with human (weathering, geochemical reactions, alkaline nature of water, ion-exchange, and high variability in temperature range) mostly controls processes of elevated concentration of ions in the groundwaters of the study area (Singh et al. 2013a, b). The anthropogenic contribution of nitrate and chloride is high at few locations. The average elevated concentration of F− was found at Koraon (or Koran), Bahariya and Karchhana. Weathering of limestone, dolomite, gypsum and anhydrite, and cation-exchange processes release Ca2+ and Mg2+ ions into groundwater (Garrels 1976). The possible sources of Na+ in groundwater are the dissolution of rock salts and weathering of sodium-bearing minerals. The HCO3 concentration in groundwater is derived from carbonate weathering as well as dissolution of carbonic acid in the aquifers (Jeevanandam et al. 2007; Singh et al. 2012, 2013a, b).
Hydrochemical facies
The Piper’s tri-linear diagram (Piper 1944) includes three triangles. Back and Hanshaw (1965) have defined the subdivisions of the diamond field that have represented water-type or categories that form the basis for one common classification scheme for natural waters. Lithology, solution kinetics, and flow patterns of the aquifer control hydrochemistry of any facies. Based on the Fig. 4a, b, it is evident that the dominant water type of Indian sample is Mg–HCO3 and at few location water type is Ca–HCO3 or shallow fresh groundwater and Na–HCO3 or deep groundwater influenced by ion exchange, whereas of Koprivnica-Križevci County it is Ca–HCO3 or shallow fresh groundwater in all the season. The seasonal variations of few types are visible in Allahabad samples, whereas it is absent in Koprivnica-Križevci County samples. During summer and winter season of 2012, the sample of I1c and I8d was Ca–HCO3, whereas the samples of I9a (summer) and I9b and I10b (winter season) of 2011 was Ca–HCO3 and I14c, I26c, I27c, I28c, I29c (summer), I14d, I21d, I23d, I24d (winter) of 2012 was reported as Ca–HCO3. The Na–HCO3 water type was reported from I19a (summer 2011), and I21c (summer 2012), it is attributed to drop of water table, whereas I11b, I12b, I19b, I20b, I22b, I27b, and I28b in winter season of 2011 and I19d, I28d in winter season of 2012, this may be due to less infiltration of rain water. The chemical composition of Ca–HCO3 water type may be evolved from silicate hydrolysis, cation exchange or a combination of both (Toran and Saunders 1999; Singh et al. 2013a, b). Few authors have also reported the involvement of irrigation return flow and anthropogenic activities (Jeevanandam et al. 2007; Gautam et al. 2015; Derby and Casey 2009).
Gibbs (1970, 1972) has suggested a graphical representation based on these illustrations; there are three main mechanisms, which govern the chemical composition or chemistry of any waters. These are as follows: (1) evaporation dominance, (2) rock–water interaction or weathering dominance, and (3) precipitation dominance that control the chemistry of waters. The distribution of Koprivnica-Križevci County and Allahabad water sample points demonstrated that the major ion chemistry of the groundwater of two regions seems to be largely controlled through chemical weathering of rock-forming minerals and anthropogenic activities.
Gibbs diagram has illustrated (Fig. 5) that the samples of both the locations in different seasons of year 2011 and 2012 are weathering dominance. The water–rock/soil interaction is responsible for the chemical composition of the groundwater in both the region. The chemical composition of samples does not originate from evaporation-crystalline and precipitation dominance.
Water quality for drinking water purposes
The chemical parameters of the groundwater were compared to the standard guideline values as recommended by the Regulation (2013) for the drinking and public health purposes (Table 2). Concentrations of all the measured parameters (N = 480) in the research area of Koprivnica-Križevci County in groundwater from wells of public water supply were below the maximum acceptable value (MAV) of Regulation (2013) and other standards (WHO 2011), indicating that the usage and consumption of groundwater should be of no concern with regard to inorganic pollutants. Water quality is safe for drinking water purposes. However, nitrate concentration in some wells was at or above the recommended level value (RLV) of Nitrate Directive (Council Directive 1991). It was shown (Nemčić-Jurec et al. 2013, Nemčić-Jurec and Jazbec 2017) that nitrate pollution is present in research area particularly in groundwater from shallow wells. Scientists have different opinions about the concentrations considered as pollution (Hallberg 1989; Jalali 2005; Kazemi 2004; Amano et al. 2016). We assume that the contamination is present in the research area and after an increase in the intensity of use of agricultural land, an increase in nitrate concentration above MAV followed. Eiche et al. (2016) have shown the influence of these processes. Nitrate concentration was higher in some of the investigated wells which are mainly located on the densely populated and agriculturally dominated areas compared to most other water sources.
Groundwater as a source of water supply has been utilized in India from ancient time, mainly for domestic needs and as well as for irrigation. Exploitation of groundwater has exceeded 80% in Gujarat, Haryana, and Punjab; 70% in Rajasthan, Tamil Nadu, and Uttar Pradesh; while in Andhra Pradesh and Maharashtra it is 49 and 55%, respectively, and in the remaining states, it is below 40%, touching a low mark of 2% in Manipur (ARDC 1979). Over the few decades, shallow hand pumps are installed in large numbers in the rural and suburban region of the Gangetic Plains to over withdrawal and improperly used for the disposal of domestic and industrial wastes which adversely affected the quality of groundwater. In India, Allahabad, concentrations of all the measured parameters (N = 1440) from all groundwater samples were below MAV of standards WHO (2011) and Indian Standard (2012) (Table 2). In researched part of India in this paper, groundwater is safe for drinking water purposes with regard to inorganic parameters. Since water is not hygienic in all areas of Allahabad, the deterioration of groundwater quality is due to overexploitation (Kamra et al. 2002; Singh et al. 2002; Négrel et al. 2007) resulting in the higher salinity, fluoride (Vaishya et al. 2014), nitrate, iron, and other heavy metals in groundwater. Many researchers have assessed the groundwater quality in various parts of India (Prasad 1998; Kaul et al. 1999; Abbasi et al. 2002; Jagdap et al. 2002; Gupta and Deshpande 2004; Khaiwal and Garg 2006; Prakash and Somashekar 2006; Singh and Chandel 2006; Shivran et al. 2006; Bishnoi and Arora 2007; Gupta et al. 2008; Srinivasamoorthy et al. 2011; Singh et al. 2009, 2012, 2013a, b, 2015b; Kumar et al. 2014). It is important to examine similar researches in other areas of India. We consider that it is important to examine other parameters and indicators of water safety prescribed by the laws and regulations related to drinking water that are not examined in this paper.
Water quality for irrigation purposes
Residual sodium carbonate content (RSC)
The suitability of water for irrigation is often affected by the available quantity of bicarbonate and carbonate in the excess of alkaline earths (Ca2+ + Mg2+) (Singh et al. 2013a). When the total carbonates and bicarbonates are in excess of total of Ca2+ and Mg2+, there may be possibility of complete precipitation of these ions in water. RSC index has been used to quantify the effects of carbonate and bicarbonate by the following Eq. (1).
Higher value of RSC in water has led to fast adsorption of sodium in soil (Singh et al. 2013a). If the RSC values are > 2.5 meq/l, these water sources are considered to be harmful for the growth of plants, and < 1.25 are safe, whereas with RSC values > 1.5–2.5 meq/l water is not suitable for irrigation purposes (Table 3). All the water samples of Koprivnica-Križevci County have shown that these are suitable for irrigation, but for Indian samples very few are suitable for irrigation uses.
Magnesium hazard (MH)
The presence of high concentrations of Mg2+ in water has affected the soil quality by converting it into alkaline soil, which means low crop yield (Gowd 2005). Szabolcs and Darab (1964) have proposed MH index to know identify whether the water sample is suitable for irrigation applications as defined by the following Eq. (2)
The MH > 50 means that water is unsafe for irrigation use, whereas < 50 suggests that water is safe for irrigation uses (Table 3). As per this index, all the water samples of Koprivnica-Križevci County samples fall under safe category, whereas for Indian samples the majority of sample does not fall under the safe category. Only few samples fall in the safe category.
Permeability index (PI)
Porosity and permeability are the important physical properties of soils; the permeability is defined as the ability of the soil to transmit water and air. An impermeable soil is good for aquaculture as the water loss through seepage or infiltration is low. The soil permeability is generally affected by the long-term applications of irrigation water as it is influenced by the groundwater which contains ions, such as Na+, Ca2+, Mg2+ and HCO3− content of the soil (Singh et al. 2013a, b). Excess concentration of Na+ leads to the development of an alkaline soil that can change the physical properties of soil by reducing the permeability (Raju 2007). We applied Doneen (1964) method of classification of irrigation water based on the PI. The expression of PI is given by following Eq. (3)
where, concentrations are given in meq/l. PI values > 75, 25–75, and < 25 which fall in class I (safe), class II (marginally safe), and class III (unsafe), respectively, are shown in Table 3.
Based on Fig. 6, it is evident that all the Koprivnica-Križevci County samples fall under the category of marginally safe, whereas the majority of Indian samples fall under marginally and safe category in the Doneen’s chart; it implies that the water which has higher value of PI is safe for irrigation purposes.
Exchangeable sodium percentage (ESP)
The percentage of Na has been calculated using the Eq. (4)
where all the concentration units are in meq/l. The sodium reacts with soil to reduce its permeability; higher amount of sodium in water has reduced crop yield. Wilcox (1955) has used ESP and EC (Fig. 7) to classify groundwater and divided into five categories. It is recommended that the ESP should not exceed 60% in water which is used for irrigation purposes. The ESP was lowest (1.95%) and highest (14.13%) in Koprivnica-Križevci County water sample. All the Koprivnica-Križevci County water samples have low concentration of Na% and fall in excellent to good category. The Indian water samples have lowest (3.14%) and highest (61.45%) ESP. Majority of Indian samples falls under the excellent to good category and few samples lie in the category of good to permissible. Hence, generally water is suitable for agriculture application.
Salinity hazard (SH)
Total concentration of soluble salts or salinity is an important parameter. The higher concentration of salts in groundwater will greatly affect the growth of plants by changing the physical properties of soil (soil structure, affecting soil permeability, aeration, texture, and makes soil hard) (Trivedy and Goel 1984; Ramesh and Elango 2012). The United States Salinity Laboratory (USSL) (Richards 1954) diagram demonstrates the combined effect of EC and SH, and categorizes the water samples for irrigation uses. According to Singh et al. (2015b), total concentration of soluble salts in irrigation water can be expressed for the purpose of classification of irrigation water as low (EC ≤ 250 μS/cm), medium (250–750 μS/cm), high (750–2250 μS/cm), and very high (2.250–5.000 μS/cm) in salinity zones (Richards 1954).
The calculated value of SH in the groundwater ranges from 0.06 to 0.5 in case of Koprivnica-Križevci County water samples, whereas in Allahabad water samples it ranges from 4.19 to 61.45. The resultant plot of groundwater sample of Koprivnica-Križevci County and Allahabad on US salinity diagram shows that the groundwater majority of samples fall in the category C2S1 (medium salinity hazard and low sodium hazard), and rest samples fall C3S1 (high salinity hazard and low sodium hazard) region (Fig. 8). The groundwater in the world is very often contaminated with one or more pollutants (Derby and Casey 2009; Amano and Nakagawa 2016; Liu et al. 2003; Sharma et al. 2016). Some pollutants degree with time and some remain unchanged. Researchers usually investigate within a country or within the neighboring countries (Ujevic et al. 2010, 2013; Noshadi and Ghafourian 2000), but we examined whether there is a similarity in the groundwater quality in some locations within two geographically different and distant countries (different geographical distribution). We estimate whether influences of local environment are more important predictor for groundwater quality than geographical distribution.
Cluster analysis
The groundwater in the world is very often contaminated with one or more pollutants (Derby and Casey 2009; Amano and Nakagawa 2016; Liu et al. 2003; Sharma et al. 2016). Some pollutants degree with time and some remain unchanged. Researchers usually investigate within a country or within the neighboring countries (Ujevic et al. 2010; Ujević et al. 2013; Noshadi and Ghafourian 2000), but we examined whether there is a similarity in the groundwater quality in some locations within two geographically different and distant countries (different geographical distribution). We estimate whether influences of local environment are more important predictor for groundwater quality than geographical distribution.
Based on Fig. 9, four clusters (groups) with different hydrogeochemical characteristic can be distinguished. The first cluster includes the locations of Allahabad (India) (I9, I10, I15, I16, I17 and I18). In this cluster, groundwater conductivity ranges from 1007 to 1805 μS/cm. Bicarbonates dominate but as anions chlorides and sulfates are also present. As cations dominate magnesium but natrium and potassium are also present. Groundwater from these locations is rich in salts and the highest conductivity was reported. Kura et al. (2013) found that some ions HCO3, K+, and Mg2+ and other major ions strongly influence the TDS and EC of groundwater. These locations (I9 and I10) come under the agricultural influences, whereas locations (I15, I16, I17 and I18) are mainly influenced by geological setting and hence anthropogenic and geogenic influences are contributing the elevated concentrations of these ions (Singh et al. 2013a; Nakagawa et al. 2016; Amano et al. 2016).
The second cluster consists of Allahabad locations (I1, I2, I7, I8, I11, I12, I21, I22, I23, I24, I25, I26, I28, and I29). Groundwater conductivity ranged from 484 to 1167 μS/cm. This monitoring groundwater sampling sites have alkaline pH, higher values of TDS, EC, NO3−, F−, Cl−, Ca2+, Mg2+, Na+, and low value of K+ and SO42−. The sites are at urban–rural fringe (I1, I2), under agricultural activities (I7, I8), urban residential area (I11, I12), area under agriculture and industrial dominance (I21, I22, I23, I24), and area under extensive agriculture (I25, I26, I28, and I29) (Singh et al. 2013a). Anthropogenic activities and geological settings have major influences on the groundwater quality but minor than in the first cluster (Hingston and Gailitis 1976).
Isolated third cluster of I20 forms due to EC, and it ranges from 330 to 951 μS/cm. The pH and F− at this location is higher and the concentration of sulfates, calcium, and magnesium is low. This may be attributed to local geological settings and may be due to regional differences in rainwater salt composition (Hingston and Gailitis 1976).
The fourth cluster consists of both Indian and Koprivnica-Križevci County locations (C1, C2, C3, C4, C5, I3, I4, I5, I6, I13, I14, I19, and I27). Groundwater conductivity in this cluster ranged from 327 to 814 μS/cm. This group explains weathering or leaching of ions from sewage (Chidambaram et al. 2013a) and agricultural areas (Aris et al. 2007), anthropogenic pollution (Sundaray 2010), respectively. In this cluster, groundwater sample has similar mineralization on an average over all the seasons for both Indian and Koprivnica-Križevci County locations. Some other researchers (Eiche et al. 2016) have found that the mineralization as well as ion concentrations in some locations have shown a dependence on the season with decreasing values during rainy season. In comparison with Allahabad locations, nitrate concentrations are considerably higher in Koprivnica-Križevci County and ranges from 1.97 to 26.7 mg/l (Nemčić-Jurec et al. 2013; Nemčić-Jurec and Jazbec 2017). Allahabad samples of groundwater have shown a specific hydrochemistry with low nitrate of < 1 mg/l. In Koprivnica-Križevci County locations, C2 (hard rock) and C4 (alluvial) has shown low nitrate in groundwater. On C2 location, source of groundwater is 400 m deep (Mayer et al. 1996) and C4 location lies on the area rich in iron (Ujević et al. 2010, 2013). Since the groundwater in some parts of Koprivnica-Križevci County are generally anoxic and under reducing conditions (Ujević et al. 2010, 2013), reductive dissolution of iron (hydr) oxides can result in the release of iron or arsenic. Nitrate concentration of the groundwater gets also clearly influenced by the oxido-reductive process which is indicated by the low nitrate concentration (Lasagna and De Luca 2016; Eiche et al. 2016; McMahon and Chapelle 2008). In Indian case, sampling sites are located in the thick alluvial plain and few on marginally alluvial terrain. In study area, direct dumping of organic waste like untreated sewage is applied. Further, continuous use of groundwater causes soil salinization in parts of the aquifer(s) which is caused by the irrigation water return flow due to the mismanagement of the irrigation scheme and the overexploitation of groundwater resources. Over-pumping introduces excess dissolved oxygen that oxidizes immobile minerals, releases some metals by reductive dissolution of (hydr) oxides, and increases metals in water. We assume that by oxido-reductive processes nitrate is reduced indicating low nitrate concentration on agricultural areas of Allahabad (Liu et al. 2003; Lasagna and De Luca 2016; Eiche et al. 2016; McMahon and Chapelle 2008). Dilution from precipitation, denitrification, and uptake by plants contributes to low nitrate in groundwater in Allahabad (Amano et al. 2016; Nakagawa et al. 2016). We presented all the processes on research areas but we have not revealed which processes prevail.
Principal component analysis (PCA)
To compare and confirm similarity or differences of groundwater quality from both countries with respect to average annual concentration of all analyzed variables (2011–2012), we used PCA (Fig. 10). PCA includes correlated variables with the purpose of reducing the numbers of variables and explaining the same amount of variance with fewer variables (principal components). The new variables created, the principal components scores (PCS), are orthogonal and uncorrelated to each other, being linear combinations of the original variables. They are obtained in such a way that the first PC explains the largest fraction of the original data variability, the second PC explains a smaller fraction of the data variance than the first one and so forth (Ielpo et al. 2012; Singh et al. 2015a, 2017). Our results show that first factor (all analyzed variables) explains 27.75% of the total variability and another factor (geographical distribution) accounts only for 10.46%. It means that the location inside country and environment nearby source of groundwater is more important predictor of groundwater quality than the geographical distribution. Love et al. (2004) in the two case studies determined that the difference in groundwater quality is due to all analyzed variables too. Except difference in groundwater quality, they showed contaminated and uncontaminated areas that are in accordance with our results in case of Koprivnica-Križevci County and India. The hydrogeochemical conditions of aquifer units are mainly controlled by the agricultural practices in Koprivnica-Križevci County case indicating high nitrate concentration in some locations. In Indian case, anthropogenic influences in some areas and influence on variability of groundwater quality and high mineralization and conductivity are determined.
Fig. 8 also shows how locations are grouped based on the average annual concentration of all analyzed variables indicating two groups: Koprivnica-Križevci County (C1–C5) and India (I1–I30) suggested that geographical distribution does not reveal the sources of the constituents and pollution (Liu et al. 2003; Kim et al. 2005) but contribute to the total variability of groundwater quality (Sharma et al. 2016).
Conclusion
Based on groundwater analysis in case of Koprivnica-Križevci County (Croatia), the water facies was Ca–HCO3 type while in Allahabad (India) case mostly Mg–HCO3 and few waters type have reported Ca–HCO3 and Na–HCO3. The results suggests decreasing values of cation as follows Ca2+ > Mg2+ > Na+ > K+ in Koprivnica-Križevci County and Mg2+ > Ca2+ > Na+ > K+ in Allahabad. Anions follow decreasing order HCO3− > NO3− > SO42− > Cl− > F− or HCO3− > SO42− > NO3− > Cl− > F− in Koprivnica-Križevci County and HCO3− > Cl− > SO42− > NO3− > F in the most of locations in Allahabad. Groundwater resources of both the regions are controlled by different physical, chemical, and biological factors, whose contribution is oriented in the form of land use activities, bedrock geology and hydrogeological setting, climate of the area, and depth of the water tables. The suitability of groundwater quality for drinking water purposes is evaluated based on comparison with the standard guideline values as recommended by the Regulation (2013) and other standards (WHO 2011; Indian Standard 2012) in Koprivnica-Križevci County case. In Allahabad case, values were evaluated based on comparison with WHO 2011. Concentrations of all the measured parameters are indicating that the usage and consumption of groundwater should be of no concern with regard to inorganic pollutants. Water quality is safe for drinking water purposes in both countries.
The suitability of groundwater for irrigation is evaluated based on residual sodium carbonate, magnesium hazard, permeability index, salinity hazard, and sodium absorption ratio. Based on RSC, MH, PI, and SH, all the water samples of Koprivnica-Križevci County have shown suitability for irrigation. In Indian case, most of the sample fall in the suitable range for irrigation purpose but few samples are exceeding the permissible limits. Based on sodium absorption ratio, all the samples in Koprivnica-Križevci County and Allahabad are falling under the excellent to good category and few samples in India lies in the category of good to permissible. Generally, water is suitable for agriculture application in the research area of Koprivnica-Križevci County and in the most locations of the research area in India. Hierarchical clustering analysis (HCA) resulted into four groups which difference is based on natural and anthropogenic factors. It has revealed that Allahabad samples are more influenced by weathering, rainfall, and anthropogenic impact generally that contribute to higher variability of groundwater. Elevated NO3− concentrations at few locations are reported from Koprivnica-Križevci County samples due to impact of ongoing agricultural practices. Although direct dumping of organic waste like untreated sewage is determined in Indian case, the values of nitrate are low. Dilution from precipitation, denitrification, oxido-reductive processes and uptake by plants contribute to low nitrate. Principal component analysis (PCA) showed that all analyzed variables have explained 27.75% of the total variability. Another factor (geographical distribution) accounts only for 10.46%. In case of comparison with Koprivnica-Križevci County and Allahabad in groundwater quality, we confirmed that the local environmental conditions are more important factor for groundwater quality than geographical distribution. In general, the application of rational irrigation practices and the control of aquifer overexploitation in India and good agricultural practice in Koprivnica-Križevci County are significant for a strategic management plan of sustainable groundwater resources management. The present study may be helpful for further similar studies concerning groundwater quality issues in these countries and in other distinctly located geographical areas. This is a very important prerequisite for the reduced risk for human health.
References
Abbasi SA, Khan FI, Sentilvelan K, Shabudeen A (2002) Modelling of Buckingham Canal water quality, India. J Environ Health 44(4):290–297
Ahel M, Mikac N, Cosovic B, Prohic E, Soukup V (1998) The impact of contamination from a municipal solid waste landfill (Zagreb, Croatia) on underlying soil. Water Sci Technol 37(8):203–210
Amano H, Nakagawa K, Berndtsson R (2016) Groundwater geochemistry of a nitrate-contaminated agricultural site. Environ Earth Sci 75:1145. doi:10.1007/s12665-016-5968-8
APHA (1996) Standard methods for examination of water and waste water, 19th edn. Washington DC
ARDC (1979) Report of the groundwater over exploitation committee. Agriculture Refinance and Development Corporation, Bombay
Aris AZ, Abdullah MH, Ahmed A, Woong KK (2007) Controlling factors of groundwater hydrochemistry in a small Island’s aquifer. Int J Environ Sci Technol 4:441–450
Astel M, Bizuik A, Prazyjazny J, Nameisink J (2006) Chemometrics in monitoring spatial and temporal variations in drinking water quality. Water Res 40:1706–1716
Ayers RS, Westcot DW (1994) FAO irrigation and drainage paper. Water Quality Agriculture 29, revision 1, pp 1–130
Back W, Hanshaw B (1965) Chemical geohydrology. In: Chow VT (ed) Advances in hydroscience, vol 2. Academic Press Inc, New York, pp 49–109
Belfar D, Fehdi C, Baali F, Salameh E (2017) Results of a hydrogeological and hydrogeochemical study of a semi-arid karst aquifer in Tezbent plateau, Tebessa region, northeast of Algeria. Appl Water Sci 7:1099–1105
Belkhiri L, Boudoukha A, Mouni L (2011) A multivariate statistical analysis of groundwater chemistry data. Belkhiri Int J Environ Res 5(2):537–544
Bishnoi MS, Arora S (2007) Potable groundwater quality in some villages of Haryana, India: focus on fluoride. J Environ Biol 28:291–294
Chandra S, Singh PK, Tiwari AK, Panigraphy BP, Kumar A (2015) Evaluation of hydrogeological factors and their relationship with seasonal water table fluctuation in Dhanbad district, Jharkhand, India. ISH J Hydraul Eng 21(2):193–2006. doi:10.1080/09715010.2014.1002542
Chidambaram S, Prasad MBK, Manivannan R, Karmegam U, Singaraja C, Anandhan P, Prasanna MV, Manikandan S (2013a) Environmental hydrogeochemistry and genesis of fluoride in groundwaters of Dindigul district, Tamilnadu (India). Environ Earth Sci 68(2):333–342. doi:10.1007/s12665-012-1741-9
Chidambaram S, Anandhan P, Prasanna MV, Srinivasamoorthy K, Vasanthavigar M (2013b) Major ion chemistry and identification of hydrogeochemical processes controlling groundwater in and around Neyveli Lignite Mines, Tamil Nadu, South India. Arab J Geosci 6(9):3451–3467. doi:10.1007/s12517-012-0589-3
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard Methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Derby N, Casey FX (2009) Lomg-term observations of vadose zone and groundwater nitrate concentrations under irrigated agriculture. Vadose Zone J 8:290–300
Doneen LD (1964) Notes on water quality in agriculture. Department of Water Science and Engineering. University of California, Davis
Eiche E, Hochschild M, Haryono E, Neumann T (2016) Characterization of recharge and flow behaviour of different water sources in Gunung Kidul and its impact on water quality based on hydrochemical and physico-chemical monitoring. Appl Water Sci 6:293–307
European Union (EU) (1991) Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off J L 375:0001–0008
Garrels RM (1976) A survey of low temperature water mineral relations. Interpretation of environmental isotope and hydrogeochemical data in groundwater hydrology. International Atomic Energy Agency, Vienna, pp 65–84
Gautam SK, Sharma D, Tripathi JK, Ahirwar S (2013) A study of the effectiveness of sewage treatment plants in Delhi region. Appl Water Sci 3:57–65
Gautam SK, Maharana C, Sharma D, Singh AK, Tripathi JK, Singh SK (2015) Evaluation of groundwater quality in the Chotanagpur plateau region of the Subarnarekha river basin, Jharkhand State, India. Sustain Water Qual Ecol 6:57–74. doi:10.1016/j.swaqe.2015.06.001
Gautam SK, Singh AK, Tripathi JK, Singh SK, Srivastava PK, Narsimlu B, Singh P (2016) Appraisal of surface and groundwater of the Subarnarekha River Basin, Jharkhand, India: using remote sensing, irrigation indices, and statistical technique. In: Srivastava PK et al (eds) Geospatial technology for water resource development. CRC Press, Taylor and Francis, USA, pp 144–169
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science (New Series) 170(3962):1088–1090
Gibbs RJ (1972) Water chemistry of the Amazon River. Geochim Cosmochim Acta 36(9):1061–1066
Gowd SS (2005) Assessment of groundwater quality for drinking and irrigation purposes: a case study of Peddavanka watershed, Anantapur District, Andhra Pradesh, India. Environ Geol 48(6):702–712
Gupta SK, Deshpande RD (2004) Water for India in 2050: first-order assessment of available options. Curr Sci 86:1216–1223
Gupta S, Mahato A, Roy P, Datta JK, Saha RN (2008) Geochemistry of groundwater, Burdwan district, West Bengal, India. Environ Geol 53:1271–1282
Hallberg GR (1989) Nitrates in groundwater in the United States of America. In: Follet RF (ed) Nitrogen management and groundwater protection: developments in agriculture and managed-forest ecology, vol 21. Elsevier, Dordrecht
Heisig PM (2000) Relation of stream baseflow chloride and nitrate concentrations to intensity of unsewered land use in the Croton watershed, southeastern New York [abs.]. In: National Ground Water Association, focus conference on eastern regional ground water issues, Newburgh, NY, 4–6 Oct 2000, p 20
Helena BA, Vega M, Barrado E, Pardo R, Fernandez L (1999) A case of hydrochemical characterization of an alluvial aquifer influenced by human activities. Water Air Soil Pollut 112:365–387
Helena B, Pardo R, Vega M, Barrado E, Fernandes JM, Fernandes L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res 34(3):807–816
Hingston FJ, Gailitis V (1976) The geographic variation of salt precipitated over Western Australia. Aust J Soil Res 14(3):319–335
HRN ISO 10304-1 (2009) Water quality—determination of dissolved anions by liquid chromatography of ions-part 1: determination of bromide, chloride, fluoride, nitrate, nitrite, phosphate and sulfate. 2009/Ispr.1:2012
HRN EN ISO 14911 (2001) Water quality—determination of dissolved Li+, Na+, NH4 +, K+, Mg2+, Ca2+, Sr2+, and Ba2+ using ion chromatography—method for water and waste water
Hubbard RK, Sheridan JM (1989) Nitrate movement to groundwater in the southeastern coastal plain. J Soil Water Conserv 44:20–27
Ielpo P, Cassano D, Lopez A, Pappagallo P, Uricchio VF, De Napoli PA (2012) Source apportionment of groundwater in Apulian agricultural sites using multivariate statistical analyses: case study of Foggia province. Chem Cent J 6(Suppl 2):S/5
Indian Standard (2012) Bureau of Indian Standards drinking water specifications, BIS 10500:2012, New Delhi, India
Jacintha TGA, Rawat KS, Mishra A, Singh SK (2016) Hydrogeochemical characterization of groundwater of peninsular Indian region using multivariate statistical techniques. Appl Water Sci 1–13
Jagdap J, Kachawe B, Deshpande L, Kelkar P (2002) Water quality assessment of the Purna River for irrigation purpose in Buldana district, Maharastra. India. J Environ Health 44(3):247–257
Jalali M (2005) Nitrates leaching from agricultural land in Hamadan, western Iran. Agric Ecosyst Environ 110:210–218
Jeevanandam M, Kannan R, Srinivasalu S, Rammohan V (2007) Hydrogeochemistry and groundwater quality assessment of lower part of the Ponnaiyar River Basin, Cuddalore district, South India. Environ Monit Assess 132(1–3):263–274
Kamra SK, Lal K, Singh OP, Boonstra J (2002) Effect of pumping on temporal changes in groundwater quality. Agric Water Manag 56:169–178
Kaul SN, Mahajan AU, Nandy T (1999) Water and waste water treatment, recycle and reuse. J Ind Assoc Environ Manag 26:74–90
Kazemi GA (2004) Temporal changes in the physical properties and chemical composition of the municipal water supply of Shahrood, northeastern Iran. Hydrogeol J 12:723–734
Khaiwal R, Garg VK (2006) Distribution of fluoride in groundwater and its suitability assessment for drinking purpose. Int J Environ Health Res 16:163–166
Kim JH, Kim RH, Lee J, Cheong TJ, Yum BW, Chang HW (2005) Multivariate statistical analysis to identify the major factors governing groundwater quality in the coastal area of Kimje, South Korea. Hydrol Process 19:1261–1276
Kumar RP, Ranjan RK, Ramanathan AL, Singh SK, Srivastava PK (2014) Geochemical modeling to evaluate the mangrove forest water. Arab J Geosci 8(7):4687–4702. doi:10.1007/s12517-014-1539-z
Kura NU, Ramli FM, Sulaiman WNA, Ibrahim S, Aris AZ, Mutapha A (2013) Evaluation of factors influencing the groundwater chemistry in a small tropical Island of Malaysia. Int J Environ Res Publ Health 10:1861–1881. doi:10.3390/ijerph10051861
Lasagna M, De Luca DA (2016) The use of multilevel sampling techniques for determining shallow aquifer nitrate profiles. Environ Sci Pollut Res 23(20):20431–20448. doi:10.1007/s11356-016-7264-2
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Total Environ 313:77–89
Love D, Hallbayer D, Amos A, Haranova R (2004) Factor analysis as a tool in groundwater quality management: two southern African case studies. Phys Chem Earth 29:1135–1143
Mayer D, Urumović K, Hlavnjak B, Gold H (1996) Hydrogeological study of the development of water source Ivanščak. Faculty of Mining, Geology and Petroleum Engineering, Zagreb
McMahon PB, Chapelle FH (2008) Redox processes and water quality of selected principal aquifer systems. Groundwater 46(2):259–271
Meyer J (1999) Spatial and temporal variation of groundwater chemistry in pettyjohns cave, northwest Georgia, USA. J Cave Karst Stud 61(3):131–138
Mikac N, Cosovic B, Ahel M, Andreis S, Tonzic Z (1998) Assessment of groundwater contamination in the vicinity of a municipal solid waste landfill (Zagreb, Croatia). Water Sci Technol 37:37–44
Motzer WE (2006) Nitrate forensics, hydrovisions newsletter, pp 1–8
Nakagawa K, Amano H, Kawamura A, Berndtsson R (2016) Classification of groundwater chemistry in Shimabara, using self-organizing maps. Hydrol Res. doi:10.2166/nh.2016.072
National Newspaper 125 (2013) Regulation about the parameters of assessment and methods of analysis of water for human consumption, Zagreb, Croatia
Négrel P, Lemière B, de Grammont HM, Billaud P, Sengupta B (2007) Hydrogeochemical processes, mixing and isotope tracing in hard rockaquifers and surface waters from the Subarnarekha River Basin, (East Singhbhum District, Jharkhand State, India). Hydrogeol J 15:1535–1552
Nemčić-Jurec J, Jazbec A (2017) Point source pollution and variability of nitrate concentrations in water from shallow aquifers. Appl Water Sci 7(3):1337–1348. doi:10.1007/s13201-015-0369-9
Nemčić-Jurec J, Konjačić M, Jazbec J (2013) Monitoring of nitrates in drinking water from agricultural and residential areas of Podravina and Prigorje (Croatia). Environ Monit Assess 185:9509–9520
Nolan BT, Ruddy BC, Hitt KJ, Dennis R, Helsel DR (1998) A national look at nitrate contamination of ground water. Water Cond Purif 39(12):76–79
Noshadi M, Ghafourian A (2000) Arsenic in groundwater of the United States: occurrence and geochemistry. Groundwater 38:589–604
Piper A (1944) A graphical procedure in the geochemical interpretation of water analysis. Am Geophys Union Trans 25:914–928 (25, 914)
Prakash KL, Somashekar RK (2006) Groundwater quality-Assessment on Anekal Taluk, Bangalore Urban district, India. J Env Biol 27:633–637
Prasad R (1998) Fertilizer urea, food security, health and the environments. Curr Sci 75:667–683
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah district, Andhra Pradesh, South India. Environ Geol 52(6):1067–1074
Ramesam V, Rajagopalan K (1985) Fluoride ingestion into the natural water of hardrock areas, peninsular India. J Geol Soc India 26:125–132
Ramesh K, Elango L (2012) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. Environ Monit Assess 184(6):3887–3899
Ravikumar P, Somashekar RK (2012) Assessment and modelling of groundwater quality data and evaluation of their corrosiveness and scaling potential using environmetric methods in Bangalore South Taluk, Karnataka State, India. Water Resour 39(4):446–473
Rawat KS, Mishra AK, Singh SK (2017a) Mapping of groundwater quality using normalized difference dispersal index of Dwarka sub-city at Delhi national capital of India. ISH J Hydraul Eng 23(3):229–240. doi:10.1080/09715010.2016.1277795
Rawat KS, Tripathi VK, Singh SK (2017b) Groundwater quality evaluation using numerical indices: a case study (Delhi, India). Sustain Water Resour Manag. doi:10.1007/s40899-017-0181-9
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) Standard Methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Soil Sci 78(2):154
Robinson GR Jr, Ayotte JD (2006) The influence of geology and land use on arsenic in stream sediments and ground waters in New England, USA. Appl Geochem 21:1482–1497
SAS 9.2 (2008) http://support.sas.com/documentation/92/index.html. Accessed 25 April 2008
Sebastian DM, Britvic V (1989) Feasibility study for obtaining a water management permission of Trstenik source. Faculty of Mining, Geology and Petroleum Engineering, Zagreb
Sharma S, Kaur J, Nagpal AK, Kaur I (2016) Quantitative assessment of possible human health risk associated with consumption of arsenic contaminated groundwater and wheat grains from Ropar Wetand and its environs. Environ Monit Assess 188:506
Shivran HS, Kumar D, Singh RV (2006) Improvement of water quality though biological denitrification. J Environ Sci Eng 48(1):57–60
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Softw 22(4):464–475
Silva-Filho EV, Pedlowski MA, Paiva RP, Wasserman JC, Lacerda LD (1999) Atmospheric inputs to Ecosystems to the East Fluminense coast, State of Rio de Janeiro, Brazil. In: Knoppers BA, Bidone ED, Abrão JJ (eds) Environmental geochemistry of coastal lagoon systems of Rio de Janeiro, Brazil. UFF/Programa de Geoquímicaambiental, Niterói, FINEP, Rio de Janeiro pp 71–87
Silva-Filho EV, Sobral Barcellos RGS, Emblanch C, Blavoux B, Sella SM, Daniel M, Simler R, Wasserman JC (2009) Groundwater chemical characterization of a Rio de Janeiro coastal aquifer, SE—Brazil. J South Am Earth Sci 27:100–108
Šimunić A (1984) Hydrogeology of Kalnik. Faculty of Mining, Geology and Petroleum Engineering, Zagreb
Singh V, Chandel CPS (2006) Analysis of wastewater of Jaipur City for agricultural use. Res J Chem Environ 10(1):30–33
Singh RV, Sinha RM, Bisht BS, Banerjee DC (2002) Hydro-geochemical exploration for unconformity-related uranium mineralization: example from Palnadu sub-basin, Cuddapah Basin, Andhra Pradesh, India. J Geochem Explor 76:71–92
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Res 38(18):3980–3992
Singh KP, Malik A, Sinha S (2005) Water quality assessment and apportionment of pollution sources of Gomti river (India) using multivariate statistical techniques—a case study. Anal Chim Acta 538(1):355–374
Singh S, Singh C, Kumar K, Gupta R, Mukherjee S (2009) Spatial-temporal monitoring of groundwater using multivariate statistical techniques in Bareilly district of Uttar Pradesh, India. J Hydrol Hydromech 57:45–54
Singh SK, Singh CK, Mukherjee S (2010) Impact of land-use and land-cover change on groundwater quality in the Lower Shiwalik hills: a remote sensing and GIS based approach. Cent Eur J Geosci 2:124–131
Singh SK, Srivastava PK, Gupta M, Mukherjee S (2012) Modeling mineral phase change chemistry of groundwater in a rural-urban fringe. Water Sci Technol 66:1502–1510
Singh SK, Srivastava PK, Pandey AC, Gautam SK (2013a) Integrated assessment of groundwater influenced by a confluence river system: concurrence with remote sensing and geochemical modelling. Water Resour Manag 27:4291–4313
Singh SK, Srivastava PK, Pandey AC (2013b) Fluoride contamination mapping of groundwater in Northern India integrated with geochemical indicators and GIS. Water Sci Technol Water Supply 13:1513–1523
Singh SK, Mustak S, Srivastava PK, Szabó S, Islam T (2015a) Predicting spatial and decadal LULC changes through cellular automata Markov chain models using earth observation datasets and geo-information. Environ Process 2:61–78
Singh SK, Srivastava PK, Singh D, Han D, Gautam SK, Pandey AC (2015b) Modeling groundwater quality over a humid subtropical region using numerical indices, earth observation datasets, and X-ray diffraction technique: a case study of Allahabad district, India. Environ Geochem Health 37:157–180
Singh SK, Basommi BP, Mustak SK, Srivastava PK, Szabo S (2017) Modelling of land use land cover change using earth observation data-sets of Tons River Basin, Madhya Pradesh, India. Geocarto Int. doi:10.1080/10106049.2017.1343390
Srinivasamoorthy K, Nanthakumar C, Vasanthavigar M, Vijayaragavan K, Ganthi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2011) Groundwater quality assessment from a hard rock terrain, Salem district of Tamilnadu, India. Arab J Geosci 4:91–102
Srivastava PK, Singh SK, Gupta M, Thakur JK, Mukherjee S (2013) Modeling impact of land use change trajectories on groundwater quality using remote sensing and gis. Environ Eng Manag J 12:2343–2355
Stambuk-Giljanović N (2005) The quality of water resources in Dalmatia. Environ Monit Assess 104(1–3):235–268
Statistica 8.0 (Electronic version): StatSoft, Inc (2011) Electronic statistics textbook. StatSoft, Tulsa. http://www.statsoft.com/textbook/. Accessed 1 Oct 2011
Sundaray SK (2010) Application of multivariate statistical techniques in hydrogeological studies—a case study of Brahmani-Koel River (India). Environ Monit Assess 164:297–310
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content on soils. In: Szabolics I (ed) Proceedings of the 8th international congress soil science sodics soils, Research Institute for Soil Science and Agricultural Chemistry of the Hungarian Academy of Sciences, ISSS Trans II, pp 802–812
Thakur J, Singh P, Singh SK, Baghel B (2013) Geochemical modelling of fluoride concentration in hard rock terrain of Madhya Pradesh, India. Acta Geol Sin (Engl Ed) 87:1421–1433
Thakur JK, Diwakar J, Singh SK (2015) Hydrogeochemical evaluation of groundwater of Bhaktapur municipality, Nepal. Environ Earth Sci 74(6):4973–4988. doi:10.1007/s12665-015-4514-4
Tiwari A, Singh AK (2014) Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. J Geol Soc India 83:329–343
Toran LE, Saunders JA (1999) Modeling alternative paths of chemical evolution of Na–HCO3-type groundwater near Oak Ridge, Tennessee, USA. Hydrogeol J 7(4):355–364
Trivedy R, Goel P (1984) Chemical and biological methods for water pollution studies, vol 215. Environmental Publications, Karad
Tziritis E, Skordas K, Kelepertsis A (2016) The use of hydrogeochemical analyses and multivariate statistics for the characterization of groundwater resources in a complex aquifer system. A case study in Amyros River basin, Thessaly, central Greece. Environ. Earth Sci 75:339. doi:10.1007/s12665-015-5204-y
Ujević M, Duić Ž, Casiot C, Sipos L, Santo V, Dadić Ž, Halamić J (2010) Occurrence and geochemistry of arsenic in the groundwater of Eastern Croatia. Appl Geochem 25:1017–1029
Ujević BM, Capak K, Jazbec A, Casiot C, Sipo L, Poljak V, Dadić Z (2012) Hydrochemical characterization of Arsenic contaminated alluvial aquifers in eastern Croatia using multivariate statistical techniques and arsenic risk assessment. Sci Total Environ 420:100–110. doi:10.1016/j.scitotenv.2012.01.021
Ujević M, Casiot C, Duić Ž, Fazinić S, Halamić J, Sipos L, Santo V, Dadić Ž (2013) Sediment characterization and its implications for arsenic mobilization in deep aquifers of eastern Croatia. J Geochem Explor 126:55–66
Vaishya RC, Srivastava D, Agarwal IC (2014) Assessment of groundwater quality of rural areas of Allahabad district, India. Int J Environ Sustain 3(2):8–14
Vega M, Pardo R, Barrado E, Debán L (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32(12):3581–3592
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization (WHO), Geneva
Wilcox L (19550 Classification and use of irrigation waters. US Department of Agriculture (USDA) Circular No. 969, USA, p 19
Yidana SM, Ophori D, Banoeng YB (2008) Hydrogeological and hydrochemical characterization of the voltaian basin: the Afram Plains area, Ghana. Environ Geol 55:1213–1223
Acknowledgements
Author SKS express his thanks to University Grants Commission, New Delhi, India for providing the financial support Project Grant number (F. no. 42-74/2013(SR)). All the authors express their gratitude to the Editor in Chief of Journal.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
See Table 4.
Rights and permissions
About this article
Cite this article
Nemčić-Jurec, J., Singh, S.K., Jazbec, A. et al. Hydrochemical investigations of groundwater quality for drinking and irrigational purposes: two case studies of Koprivnica-Križevci County (Croatia) and district Allahabad (India). Sustain. Water Resour. Manag. 5, 467–490 (2019). https://doi.org/10.1007/s40899-017-0200-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-017-0200-x