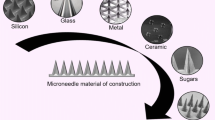
Abstract
Biopolymeric microneedles have emerged as an efficient tool for transdermal drug delivery system. Being operational alternative to hypodermic needles and non-biopolymeric microneedles, they offer plethora of advantages and ease in drug administration over conventional tools. Needles/microneedles used in medical practise can be disposable devices but may not necessarily be biodegradable, whereas biopolymeric microneedles completely serves for the green technology approach at medical/pharmaceutical outlet. Over a decade, investigation has been engrossed on leveraging biopolymers for microneedle synthesis, however, it seems to be growing more towards synthetic biopolymers than those of non-synthetic/natural biopolymers, may be due to their inherent physico-chemical properties. Nonetheless the outcomes bestowed from the investigations on natural biopolymeric microneedles are surely pertinent to the future practical applications. Henceforth, with the purpose of having insight, illuminating their potential and encouraging their utilisation for developing drug delivery devices, this review summarizes them from then to now with their implicational advancement and clinical potential.

Adapted by permission from [57]. Copyright 2009 Elsevier


Adapted by permission from [109]. Copyright 2018 Elsevier

Adapted by permission from [23]. Copyright 2009 Taylor & Francis

Adapted by permission from [87]. Copyright 2010 John Wiley and Sons

Adapted by permission from [47]. Copyright 2015 Springer Nature


Adapted by permission from [38]. Copyright 2015 RSC Publishing

Adapted by permission from [58]. Copyright 2013 Acta Materialia Inc. Published by Elsevier


Adapted by permission from [33]. Copyright 2012 Elsevier


Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Akers MJ (2002) Excipient-drug interactions in parenteral formulations. J Pharm Sci 91(11):2283–2300
Alarcón-Payán DA, Koyani RD, Vazquez-Duhalt R (2017) Chitosan-based biocatalytic nanoparticles for pollutant removal from waste water. Enzyme Microb Technol 100:71–78
Apollo NV, Jiang J, Cheung W, Baquier S, Lai A, Mirebedini A, Foroughi J, Wallace GG, Shivdasani MN, Prawer S, Chen S, Williams R, Cook MJ, Nayagam DAX, Garrett DJ (2018) Development and characterization of a sucrose microneedle neural electrode delivery system. Adv Biosys 2:1700187
Arya JM, Dewitt K, Scott-Garrard M, Chiang YW, Prausnitz MR (2016) Rabies vaccination in dogs using a dissolving microneedle patch. J Control Release 239:19–26
Bachy V, Hervouet C, Becker PD, Chorro L, Carlin LM, Herath S et al (2013) Langerin negative dendritic cells promote potent CD8+ T cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Nat Acad Sci USA 110(8):3041–3046
Bariya SH, Gohel MC, Mehta TA, Sharma OP (2012) Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol 64:11–29
Brogden NK, Milewski M, Ghosh P, Hardi L, Crofford LJ, Stinchcomb AL (2012) Diclofenac delays micropore closure following microneedle treatment in human subjects. J Control Release 163:220–229
Camović M, Biščević A, Brčić I, Borčak K, Bušatlić S, Ćenanović N, Dedović A, Mulalić A, Osmanlić M, Sirbubalo M, Tucak A, Vranić E (2020) Coated 3D printed PLA microneedles as transdermal drug delivery systems. In: Badnjevic A, Škrbić R, Gurbeta Pokvić L (eds) CMBEBIH 2019. CMBEBIH 2019. IFMBE Proceedings, vol 73, pp 735–742, Springer
Chen MC, Lai KY, Ling MH, Lin CW (2018) Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta Biomater 65:66–75
Chen BZ, Ashfaq M, Zhang XP, Zhang JN, Guo XN (2018) Invitro and invivo assessment of polymer microneedles for controlled transdermal drug delivery. J Drug Target 26(8):720–729
Chen Y, Chen BZ, Wang QL, Jin X, Guo XD (2017) Fabrication of coated polymer microneedles for transdermal drug delivery. J Control Release 265:14–21
Chen MC, Huang SF, Lai KY, Ling MH (2013) Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials 34:3077–3086
Chen MC, Ling MH, Lai KY, Pramudityo E (2012) Chitosan microneedle patches for sustained transdermal delivery of macromolecules. Biomacromolecules 13(12):4022–4031
Choi HJ, Yoo DG, Bondy BJ, Quan FS, Compans RW, Kang SM, Prausnitz MR (2012) Stability of influenza vaccine coated onto microneedles. Biomaterials 33:3756–3769
Choi SY, Kwon HJ, Ahn GR, Ko EJ, Yoo KH, Kim BJ, Lee C, Kim D (2017) Hyaluronic acid microneedle patch for the improvement of crow's feet wrinkles. Dermatol Ther 30:e12546
Chiu YH, Chen MC, Wan SW (2018) Sodium Hyaluronate/chitosan composite microneedles as a single-dose intradermal immunization system. Biomacromolecules 19(6):2278–2285
Choi JT, Park S, Park JH (2018) Microneedles containing cross-linked hyaluronic acid particulates for control of degradation and swelling behaviour after administration into skin. J Drug Target 26:884–894
Chu LY, Prausnitz MR (2011) Separable arrowhead microneedles. J Control Release 149(3):242–249
Coppola S (2016) Doctoral Thesis, ISASI CNR—Institute of Applied Sciences and Intelligent Systems of Naples, in Manipulation of multiphase materials for touch-less nanobiotechnology: a pyrofluidic platform, Pozzuoli, Italy, Springer Switzerland, Ch. 6, pp 85–97
Demir YK, Akan Z, Kerimoglu O (2013) Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS One 8(10):e77289
Demir YK, Akan Z, Kerimoglu O (2013) Sodium alginate microneedle arrays mediate the transdermal delivery of bovine serum albumin. PLoS One 8(5):e63819
Donnelly RF, Douroumis D (2015) Microneedles for drug and vaccine delivery and patient monitoring. Drug Deliv Trans Res 5:311–312
Donnelley RF, Morrowm DI, Singh TR, Migalska K, McCarron PA, O’Mahony C, Woolfson AD (2009) Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev Ind Pharm 35:1242–1254
Donnelly RF, Singh TRR, Woolfson AD (2010) Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv 17:187–207
Duverger E, Carpentier V, Roche AC, Monsigny M (1993) Sugar-dependent nuclear import of glycoconjugates from the cytosol. Exp Cell Res 207:197–201
Edens C, Collins ML, Goodson JL, Rota PA, Prausnitz MR (2015) A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine 33(37):4712–4718
Edens C, Dybdahl-Sissoko NC, Weldon WC, Oberste MS, Prausnitz MR (2015) Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine 33(37):4683–4690
Gaines AR, Pierce LR, Bernhardt PA (2008) Fatal iatrogenic hypoglycemia: falsely elevated blood glucose readings with a point-of-care meter due to a maltose-containing intravenous immune globulin product, 2008-US Food and Drug Adminstration, 2015
Gerstel MS, Place VA (1976) US Patent No. 3,964,482. Washington, DC: US Patent and Trademark Office
Gill HS, Prausnitz MR (2007) Coated microneedles for transdermal delivery. J Control Release 117(2):227–237
González-Vázquez P, Larrañeta E, McCrudden MTC, Jarrahian C, Rein-Weston A, Quintanar-Solares M, Zehrung D, McCarthy H, Courtenay AJ, Donnelly RF (2017) Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J Control Release 265:30–40
Hiraishi Y, Nandakumar S, Choi SO, Lee JW, Kim YC, Posey JE, Sable SB, Prausnitz MR (2011) Bacillus Calmette-Guerin vaccination using a microneedle patch. Vaccine 29:2626–2636
Hiraishi Y, Nakagawa T, Quan Y, Kamiyama F, Hirobe S, Okada N, Nakagawa S (2013) Performance and characteristics evaluation of a sodium hyaluronate based microneedle patch for a transcutaneous drug delivery system. Int J Pharm 441:570–579
Hreczuk-Hirst D, Chicco D, German L, Duncan R (2001) Dextrins as potential carriers for drug targeting: tailored rates of dextrin degradation by introduction of pendant groups. Int J Pharm 230:57–66
Hwa KY, Chang VHS, Cheng YY, Wang YD, Jan PS, Subramani BW, Wu MJ, Wang BK (2017) Analyzing polymeric matrix for fabrication of a biodegradable microneedle array to enhance transdermal delivery. Biomed Microdevices 19(4):84 (1–13)
Indermun S, Luttge R, Choonara YE, Kumar P, du Toit LC, Modi G, Pillay V (2014) Current advances in the fabrication of microneedles for transdermal delivery. J Control Release 185:130–138
Ito Y, Yoshimitsu JI, Shiroyama K, Sugioka N, Takada K (2006) Self-dissolving microneedles for the percutaneous absorption of EPO in mice. J Drug Target 14(5):255–261
Justin R, Roman S, Chen D, Tao K, Geng X, Grant RT, MacNeil S, Sunb K, Chen B (2015) Biodegradable and conductive chitosan–grapheme quantum dot nanocomposite microneedles for delivery of both small and large molecular weight therapeutics. RSC Adv 5:51934–51946
Kim S, Lee J, Shayan FL, Kim S, Huh I, Ma Y, Yang H, Kang G, Jung H (2018) Physicochemical study of ascorbic acid 2-glucoside loaded hyaluronic acid dissolving microneedles irradiated by electron beam and gamma ray. Carbohydr Polym 180:297–303
Kim HK, Lee SH, Lee BY, Kim SJ, Sung CY, Jang NK, Kim JD, Jeong DH, Ryuc HY, Lee S (2018) A comparative study of dissolving hyaluronic acid microneedles with trehalose and poly(vinyl pyrrolidone) for efficient peptide drug delivery. Biomater Sci 6(10):2566–2570
Kim YC, Park JH, Prausnitz MR (2012) Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 64:1547–1568
Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR (2010) Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release 142:187–195
Kolli CS, Banga AK (2008) Characterization of solid maltose microneedles and their use for transdermal delivery. Pharma Res 25(1):104–113
Kommareddy S, Baudner BC, Oh S, Kwon SY, Singh M, Ohagan DT (2012) Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J Pharma Sci 101:1021–1027
Koutsonanos DG, Esser ES, McMaster SR, Kalluri P, Lee JW, Prausnitz MR et al (2015) Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine 33(37):4675–4682
Koyani RD, Andrade M, Quester K, Gaytan P, Huerta-Sequero Vazquez-Duhalt R (2018) Surface modification of protein enhances encapsulation in chitosan nanoparticles. Appl Nanosci 8:1197–1203
Lahiji SF, Dangol M, Jung H (2015) A patchless dissolving microneedle delivery system enabling rapid and efficient transdermal drug delivery. Sci Rep 5:7914
Langer R (2004) Transdermal drug delivery: past progress, current status, and future prospects. Adv Drug Deliv Rev 56:557–558
Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF (2016) Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng Rep 104:1–32
Lee IC, Wu YC, Tsai SW, Chen CH, Wu MH (2017) Fabrication of two-layer dissolving polyvinylpyrrolidone microneedles with different molecular weights for in vivo insulin transdermal delivery. RSC Adv 7(9):5067–5075
Lee IC, Lin WM, Shu JC, Tsai SW, Chen CH, Tsai MT (2017) Formulation of two-layer dissolving polymeric microneedle patches for insulin transdermal delivery in diabetic mice. J Biomed Mater Res 105A:84–93
Lee H, Song C, Hong YS, Kim MS, Cho HR, Kang T, Shin K, Choi SH, Hyeon T, Kim DH (2017) Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Science 3:e1601314
Lee SG, Jeong JH, Lee KM, Jeong KH, Yang H, Kim M, Jung H, Lee S, Choi YW (2014) Nanostructured lipid carrier-loaded hyaluronic acid microneedles for controlled dermal delivery of a lipophilic molecule. Int J Nanomed 9:289–299
Lee K, Lee CY, Jung H (2011) Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose. Biomaterials 32(11):3134–3140
Lee JW, Choi SO, Felner EI, Prausnitz MR (2011) Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 7(4):531–539
Lee W, Park JH, Prausnitz MR (2008) Dissolving microneedles for transdermal drug delivery. Biomaterials 29:2113–2124
Li G, Badkar A, Nema S, Kolli CS, Banga AK (2009) In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharma 368:109–115
Ling MH, Chen MC (2013) Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater 9:8952–8961
Littauer EQ, Mills LK, Brock N et al (2018) Stable incorporation of GM-CSF into dissolvable microneedle patch improves skin vaccination against influenza. J Control Release 276:1–16
Liu S, Jin MN, Quan YS, Kamiyama F, Katsumi H, Sakane T, Yamamoto A (2012) The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J Control Release 161(3):933
Liu D, Mori A, Huang L (1992) Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposome. Biochem Biophys Acta 1104:95–101
Loizidou EZ, Williams NA, Barrow DA, Eaton MJ, McCrory J, Evans SL, Allender CJ (2015) Structural characterisation and transdermal delivery studies on sugar microneedles: experimental and finite element modelling analyses. Eur J Pharma Biopharma 89:224–231
Luo Z, Sun W, Fang J, Lee K, Li S, Gu Z, Dokmeci MR, Khademhosseini A (2019) Biodegradable gelatin methacryloyl microneedles for transdermal drug delivery. Adv Healthc Mater 8:1801054
Luzuriaga MA, Berry DR, Reagan JC, Smaldone RA, Gassensmith JJ (2018) Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 18:1223–1230
Ma G, Wu C (2017) Microneedle, bio-microneedle and bio-inspired microneedle: a review. J Control Release 251:11–23
Marin A, Andrianov AK (2011) Carboxymethylcellulose–Chitosan-coated microneedles with modulated hydration properties. J Appl Polym Sci 12:395
Martin CJ, Allender CJ, Brain KR, Morrissey A, Birchall JC (2012) Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications. J Control Release 158(1):93–101
Matsuo K, Yokota Y, Zhai Y, Quan YS, Kamiyama F, Mukai Y, Okada N, Nakagawa S (2012) A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J Control Release 161:10–17
Matsuo K, Hirobe S, Yokota Y, Ayabe Y, Seto M, Quan YS (2012) Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J Control Release 160:495–501
McGrath MG, Vucen S, Vrdoljak A, Kelly A, O’Mahony C, Crean Anne Moore AM (2014) Production of dissolvable microneedles using an atomised spray process: effect of microneedle composition on skin penetration. Eur J Pharm Biopharm 86:200–211
Mistilis MJ, Bommarius AS, Prausnitz MR (2015) Development of a thermostable microneedle patch for influenza vaccination. J Pharma Sci 104:740–749
Miyano T, Tobinaga Y, Kanno T, Matsuzaki Y, Takeda H, Wakui M, Hanada K (2005) Sugar micro needles as transdermic drug delivery system. Biomed Microdevices 7(3):185–188
Mönkäre J, Pontier M, van Kampen EEM, Du G, Leone M, Romeijn S, Reza Nejadnik M, O’Mahony C, Slütter B, Jiskoot W, Bouwstra JA (2018) Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur J Pharm Biopharm 129:111–121
Moreira S, Da Costa RMG, Guardao L, Gartne F, Vilanova M, Gama M (2010) In vivo biocompatibility and biodegradability of dextrin-based hydrogels. J Bioact Compat Polym 25:141–153
Morrow D, McCarron P, Gallagher M, Migalska K, Thakur RS, Morrissey R, Woolfson D, Donnelly R (2008) Assessment of galactose microneedles for enhanced transdermal drug delivery. J Pharma Pharma 60:A38–A39
Nguyen HX, Banga AK (2017) Fabrication, characterization and application of sugar microneedles for transdermal drug delivery. Ther Deliv 8(5):249–264
Ono A, Ito S, Sakagami S, Asada H, Saito M, Quan YS, Kamiyama F, Hirobe S, Okada N (2017) Development of novel faster-dissolving microneedle patches for transcutaneous vaccine delivery. Pharmaceutics 9(3):E27
Park Y, Kim B (2017) Skin permeability of compounds loaded within dissolving microneedles dependent on composition of sodium hyaluronate and carboxymethyl cellulose. Korean J Chem Eng 34(1):133–138
Park Y, Kim K, Chung M, Sung JH, Kim B (2016) Fabrication and characterization of dissolving microneedle arrays for improving skin permeability of cosmetic ingredients. J Ind Eng Chem 39:121–126
Park YH, Ha SK, Choi I, Kim KS, Park J, Choi N, Kim B, Sung JH (2016) Fabrication of degradable carboxymethyl cellulose (CMC) microneedle with laser writing and replica molding process for enhancement of transdermal drug delivery. Biotechnol Bioprocess Eng 21:110
Park JH, Allen MG, Praunitz MR (2005) Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Relaease 104(1):51–66
Pissinato Pere CP, Sophia NE, Lall G, Ziraud C, Boateng JS, Alexander BD, Lamprou DA, Douroumis D (2018) 3D printed microneedles for insulin skin delivery. Int J Pharm 544(2):425–432
Prausnitz MR, Langer R (2008) Transdermal drug delivery. Nat Biotechnol 26:1261–1268
Prausnitz MR, Mitragotri S, Langer R (2004) Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3:115–124
Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM (2009) Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One 4:e7152
Raphael AP, Crichton ML, Falconer RJ, Chen SMX, Fernando GJP, Huang H, Kendall MAF (2016) Formulations for microprojection/microneedle vaccine delivery: structure, strength and release profiles. J Control Release 225:40–52
Raphael AP, Prow TW, Crichton ML, Chen X, Fernando GJ, Kendall MA (2010) Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small 6:1785
Ryota N, Hirofumi M, Shigeki T (2017) A novel microneedle device having flexible substrate and strong needles using PVA and gelatin. Internat. In: Symposium micro-nano mechatronics human science (MHS), Nagoya, pp 1–6
Sachiko H, Risa O, Hiroshi I, Ying Q, Fumio K, Hideo A, Naoki O, Shinsaku N (2017) Clinical study of a retinoic acid-loaded microneedle patch for seborrheic keratosis or senile lentigo. Life Sci 168:24–27
Schipper P, van der Maaden K, Groeneveld V, Ruigrok M, Romeijn S, Uleman S, Omens CO, Kersten G, Jiskoot W, Bouwstra J (2017) Diphtheria toxoid and N-trimethyl chitosan layer-by-layer coated pH-sensitive microneedles induce potent immune responses upon dermal vaccination in mice. J Control Release 262:28–36
Sullivan SP, Koutsonanos DG, Martin MP, Lee JW, Zarnitsyn V, Murthy N, Compans RW, Skountzou I, Prausnitz MR (2010) Dissolving polymer microneedle patches for influenza vaccination. Nat Med 16(8):915–920
Sun W, Araci Z, Inayathullah M, Manickam S, Zhang X, Bruce MA, Marinkovich MP, Lane AT, Milla C, Rajadas J, Butte M (2013) Polyvinylpyrrolidone microneedles enable delivery of intact proteins for diagnostic and therapeutic applications. Acta Biomater 9(8):7767–7774
Takakura Y, Hashida M (1995) Macromolecular drug carrier systems in cancer chemotherapy: macromolecular prodrugs. Crit Rev Oncol Hematol 18:207–231
Tsai YS, Chen MY, Lan SK, Tsai HT, Chen MC, Tzai TS (2017) Transdermal delivery of leuprolide acetate with chitosan microneedles: a promising tool for androgen deprivation therapy. Eur Urol Suppl 16(3):e130
Vassilieva EV, Kalluri H, McAllister D, Taherbhai MT, Esser ES, Pewin WP et al (2015) Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug Deliv Transl Re 5(4):360–371
Wang QL, Zhu DD, Liu XB, Chen BZ, Guo XD (2016) Microneedles with controlled bubble sizes and drug distributions for efficient transdermal drug delivery. Sci Rep 6:38755
Wang C, Yangi Y, Gabrielle MH, Sadeghifar H, Gu Z (2016) Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett 16(4):2334–2340
Wang QL, Zhu DD, Chen Y, Guo XD (2016) Fabrication method of microneedle molds with controlled microstructures. Mater Sci Eng C 65(1):135–142
Weldon WC, Martin MP, Zarnitsyn V, Wang B, Koutsonanos D, Skountzou I, Prausnitz MR, Compans RW (2011) Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin Vaccine Immunol 18:647–654
Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, Cormier M (2006) Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine 24:1653–1664
Wu X, Chen Y, Gui S, Wu X, Chen L, Cao Y, Yin D, Ping M (2016) Sinomenine hydrochloride-loaded dissolving microneedles enhanced its absorption in rabbits. Pharm Dev Technol 21(7):787–793
Wu D, Quan YS, Kamiyama F, Kusamori K, Katsumi H, Sakane T, Yamamoto A (2015) Improvement of transdermal delivery of sumatriptan succinate using a novel self-dissolving microneedle array fabricated from sodium hyaluronate in rats. Biol Pharm Bull 38:365–373
Xie Y, Xu B, Gao Y (2005) Controlled transdermal delivery of model drug compounds by MEMS microneedle array. Nanomed Nanotechnol Biol Med 1:184–190
Yu W, Jiang G, Zhang Y, Liu D, Xu B, Zhou J (2017) Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater Sci Eng C 80:1987–1996
Yu W, Jiang G, Liu D, Li L, Chen H, Liu Y, Huang Q, Tong Z, Yao J, Kong X (2017) Fabrication of biodegradable composite microneedles based on calcium sulfate and gelatin for transdermal delivery of insulin. Mater Sci Eng C 71:725–734
Yu W, Jiang G, Liu D, Li L, Tong Z, Yao J, Kong X (2017) Transdermal delivery of insulin with bioceramic composite microneedles fabricated by gelatin and hydroxyapatite. Mater Sci Eng C 73:425–428
Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Busec JB, Gu Z (2015) Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. PNAS 112(27):8260–8265
Yu J, Qian C, Zhang Y, Cui Z, Zhu Y, Shen Q, Ligler FS, Buse JB, Gu Z (2017) Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucoseresponsive insulin delivery. Nano Lett 17:733–739
Zhang Y, Jiang G, Yu W, Liu D, Xua B (2018) Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats. Mater Sci Eng C 85:18–26
Zhang JN, Chen BZ, Ashfaq M, Zhang XP, Guo XD (2018) Development of a BDDE-crosslinked hyaluronic acid based microneedles patch as a dermal filler for anti-ageing treatment. J Ind Eng Chem 65:363
Zhong H, Chan G, Hu Y, Hu H, Ouyang D (2018) A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics 10:263
Zhu Z, Ye X, Ku Z, Liu Q, Shen C, Luo H (2016) Transcutaneous immunization via rapidly dissolvable microneedles protects against hand-foot-and-mouth disease caused by enterovirus 71. J Control Release 243:291–302
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koyani, R.D. Biopolymers for microneedle synthesis: from then to now. Biomanuf Rev 4, 1 (2019). https://doi.org/10.1007/s40898-019-0006-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40898-019-0006-8