Abstract
Purpose
Production of a tissue-engineered scaffold for replacement of colon would be a breakthrough affecting the clinical approach to several conditions, and it would alter the fate of patients who need total colectomy with ileoanal anastomosis. In the current study, we aimed to determine the histological aspects of a decellularized colon graft for further colon recellularization using omentum as a natural bioreactor in healthy rats.
Material and Methods
Colon tissues were decellularized by a 24-h detergent perfusion procedure. The scaffolds were examined by several investigations to verify the efficacy of the decellularization procedure. For recellularization, scaffolds were implanted in omental wraps and biopsies were taken from implanted scaffolds 2 and 4 months post-operatively to examine the extent of cell infiltration and cellular differentiation of regenerated tissues.
Results
Implanted scaffolds of 2 months were not completely recellularized and were mostly populated by undifferentiated cells and some inflammatory cells. Immunohistochemical evaluation of the implanted scaffolds after 4 months demonstrated sufficient cellularity and appropriate cell differentiation into epithelial and muscular cells, as well as similar structure to the normal colon.
Conclusion
The present study displays the efficiency of omentum as a natural bioreactor for recellularization of decellularized colon scaffolds.
Lay summary
Previously, we evaluated the histological aspects of a decellularized colon scaffold for bladder augmentation in rat model. In this study, we try to assess the efficacy of a perfusion-based decellularization protocol of colon organ for further colon tissue engineering. We also aim to evaluate the effectiveness of omentum as a natural bioreactor for recellularization of decellularized colon scaffolds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon acts as a reservoir for microbial flora of intestine and is basically the location for absorption of water and electrolytes from the stool [1]. Several conditions including colon cancer, diverticular disease/repeated and complicated diverticulitis, abdominal trauma, inflammatory bowel disease, intestinal ischemia, and familial syndromes such as Lynch syndrome may result in removal of colon (total colectomy). Ileal pouch construction following colectomy has complications including leakage from anastomosis (1%), obstruction (1%), and sepsis and pelvic abscess (5–20%). The mentioned procedure bears a failure rate of 30% and a mortality rate of 3% [2]. Severe pouchitis is another grave complication which affects 50% of patients in the first decade after surgery [3, 4]. The impact of complications of an ileal pouch on patient’s quality of life as well as their mortality and prognosis has encouraged researchers to investigate alternative approaches for the creation of colon; one of these alternative approaches is tissue engineering.
The successful regeneration of intestinal mucosa in rodents indicates the therapeutic potential and the possibility of engineering the human intestinal tissue [5, 6]. Cells can be preserved from the large intestinal biopsy specimens and used to rebuild the colon [7]. These techniques will reduce the problems associated with implant including tissue deficiency and complications of immunosuppressive drugs [8]. There are still several limitations in these techniques that need to be considered prior to their application in the clinic [9].
Several investigations have demonstrated the potential of decellularized colon grafts for repairing and regeneration of the colon tissue in different injuries and diseases [10]. In addition, rat’s colon has incomparable resistance with suitable mechanical properties and steady thickness which is associated with mucus producing capability. This background provoked our interest in performing in vivo colon regeneration using this type of decellularized tissue graft in omentum. Subsequently, we studied the efficacy of rich vasculature of omentum versus the colon as natural bioreactors to recellularize rat colon scaffolds, which have not been evaluated previously.
In this study, we aimed to use a decellularized colon scaffold for further colon regeneration in a rat model using omentum as a natural bioreactor and also investigated the histological characteristics of this tissue in different follow-up periods. Furthermore, we intend to discover the appropriate protocol for the decellularization of colon tissue.
Materials and Methods
Perfusion-based colon decellularization
Colon tissues were obtained from healthy male adult Sprague Dawley rats by harvesting the whole length of the colon and completely dissecting the surrounding connective tissue. Afterward, the harvested colon tissues were thoroughly rinsed by a solution with a pH of 7.4, containing phosphate-buffered saline (PBS) to discard any residues from its lumen. The harvested tissues were preserved in a solution of PBS and antibiotics including 10% penicillin/streptomycin, 2% gentamicin, and 4% amphotericin B before starting the decellularization process. The colon tissue was cannulated with a 22-gauge plastic intravenous catheter (Terumo) via mesenteric artery and ligated to the catheter by 6-0 Prolene ligatures (Ethicon) and then connected to a perfusion system with a peristaltic pump set to a speed of 6 mL/min. The tissues were then cleansed by repeated irrigations by distilled water and Gentamycin at 4 °C for 48 h, changing the solution every 6 h. Different concentrations of solutions were used to reach the appropriate decellularization protocol: 0.5% sodium dodecyl sulfate (SDS) in deionized water (24 h); deionized water (DI H2O, 1 h); 1% Triton-X in deionized water (30 min); phosphate-buffered saline (20 h). To remove any residual materials, tissues were preserved in PBS and 1% antibiotics solution prior to implantation, for a minimum of 24 h. The decellularized tissues were evaluated by several examinations to ensure complete cell removal as well as the preservation of extracellular structures. In order to assess extent of decellularization, DNA quantification was also performed on decellularized segments of colon (1 mg). Afterward, DNA concentrations of decellularized and native scaffolds were measured on a spectrophotometer (Thermo scientific Nanodrop 1000, USA) at 230 nm.
DAPI staining
For visualization of dsDNA (Sigma, St Louis, MO, USA), rat normal tissues and decellularized scaffolds were evaluated, using 0.5 mg/mL blue-fluorescent 4′, 6-diamidino-2-phenylindole (DAPI). The solution was diluted to 30 nM with PBS, and 300 μL of the obtained solution was pipeted directly on each slide and incubated in a dark room for 30 min. Afterward, slides were rinsed in dH2O and evaluated with a Nikon Diaphot 200 inverted fluorescence microscope. Images were taken by a specialist who was blind to the study.
Histological examinations
Samples from both decellularized colon scaffolds and natural tissues were sectioned into pieces of 5 mm × 4 mm and fixed in neutral buffered formalin 10% solution for 24 h. After getting washed with distilled water, dehydration in graded alcohol was performed and samples were then embedded in paraffin. Paraffin blocks were sliced into sections with 5-μm thickness prior to staining. Specimens were stained with hematoxylin and eosin (H&E) to investigate cellularity in decellularized scaffolds and to compare with normal tissues. To better evaluate the architectural integrity of scaffolds and extracellular matrix collagen content, Sirius Red and Movat Pentachrome staining were performed on decellularized scaffolds. As DNA is directly associated with adverse host reactions, the content of DNA was quantified in the dry scaffolds before implantation. The amount of DNA in decellularized tissue was calculated and expressed as the amount of DNA per dry tissue weight (μg/mg).
Photoshop v10.0 (Adobe Systems Inc., Mountain View, CA, USA) and Image Pro (Image Pro Inc., Boston, MA, USA) software were used for image analyses. For scoring the images, five photomicrographs (× 100) were used and the mean scores were used as final values for analysis.
Scanning Electron Microscopy Analysis
Several scanning electron microscopy (SEM) images were taken from both natural and decellularized colons to evaluate the efficacy of the decellularization process and estimate preservation of the ECM. For SEM evaluation, the samples were dehydrated in graded ethanol, critical point-dried in CO2. Eventually, they were coated with 3.5-nm-thick chromium layer by using a Gatan ion beam coater in order to obtain electrical conductivity. Samples were examined using field emission SEM (FE-SEM; JSM-6340F, JEOL, Tokyo, Japan). A working distance of 8 mm and an acceleration voltage at 10 kV were adjusted.
Biochemical Analysis
Sulfated glycosaminoglycan (sGAG) and collagen and content of the native and decellularized colon tissues were quantified using the Blyscan and Sircol assay kits (Biocolor Ltd., UK), respectively.
For GAG quantification, 50 mg of minced wet tissue was added to a micro-centrifuge tube including 1 mL of Papain digestion buffer. Afterward, the incubation was performed in a water bath for the next 18 h. Then, we used 1,9-dimethyl-methylene blue dye and reagents from the GAG assay kit to combine the aliquots of each sample. A Tecan Infinite microplate reader (Tecan, Austria) was applied to calculate the absorbance at 595 nm.
For quantifying the collagen content, after homogenizing the samples the collagen was solubilized in 0.5 M acetic acid. Then, Sirius Red dye was applied to incubate the extracts and a Tecan Infinite microplate reader (Tecan, Austria) demonstrated the absorbance at 555 nm.
Surgical technique
Sixteen healthy male adult Sprague Dawley rats, with a weight of 250–300 g, were selected for implantation of decellularized scaffolds with the aim of in vivo recellularization. All experiments on animals including the selection of animals, surgical procedures, and pre-op and post-op care were approved by The Animal Ethics Committee of the Tehran University of Medical Sciences, School of Medicine and Education Section of Basic Sciences. The animal experiment was carried out in accordance with the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000). All animals were housed in communal cages and they had unlimited access to standard laboratory diet and water prior to the surgery. All animals were anesthetized prior to surgery by an intramuscular administration of ketamine (100 mg/kg) and xylazine (15 mg/kg). Abdominal area of all rats was shaved and sterilized by isopropyl alcohol.
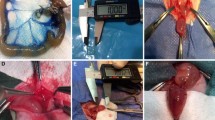
We aseptically prepared 3-cm segments of the decellularized tissues for further implantation. In order to implant these scaffolds, a sub-xiphoid abdominal midline incision was made and omentum was reached through this incision. Then, the omentum has been mobilized and wrapped around the graft to develop supportive blood supply. Scaffold alongside omentum was placed and fixed by PBS5.0 USP sutures between rectus abdominal muscles and subcutaneous fascia. Scaffolds were extracted at 2 and 4 months post-implantation to investigate the extent of cell infiltration and tissue regeneration (Fig. 1). This implies a separation of intra-abdominal adhesions and careful preparation of the colon graft, and the graft wall was completely explanted.
H&E staining was performed in implanted tissues to evaluate extents of cell differentiation, angiogenesis, and presence of epithelial cells, ganglionic cells, and muscular propria. Immunohistochemical (IHC) investigations were also performed on recellularized scaffolds by antibodies for α-SMA, CD31, pancytokeratin, and vimentin. Evaluation and interpretation of histological and IHC examinations were done by an experts pathologist who was blind to specimen’s categories.
Statistical analysis
Statistical analysis was performed using SPSS®, v19 (IBM SPSS Statistics, IBM Corporation, Chicago, IL, USA). Data are presented as mean ± SE. An independent-sample t test was used to compare the results of IHC staining. Statistical testing for biochemical analysis was performed using a one-way analysis of variance (ANOVA) and Duncan tests. p values <0.05 were considered statistically significant.
Results
H&E staining of decellularized specimen indicated complete elimination of nuclei and preservation of structural integrity of scaffolds. Examination of the decellularized colon using SEM showed that the micro-structural features of the natural colon and the cryptic appearance were well preserved with intact ECM structure and without collagen degradation (Fig. 2 A–D). DAPI staining confirmed optimal decellularization of all the scaffolds with the persistence of ECM and total cell removal (Fig. 2 E–F). Sirius Red and Movat Pentachrome staining of decellularized scaffolds revealed that ECM integrity was preserved and collagenous, elastic, and reticular fibers were intact following decellularization process, compared to normal colon (Fig. 2 G–J). DNA quantification demonstrated that decellularization has removed almost all of the DNA contents in the scaffolds (193.3 ng/μL, for native scaffold vs 7.4 ng/μL for decellularized). Total collagen and sGAG of decellularized and native colon tissues were quantified and expressed as μg/mg wet corneal tissue. The concentration of collagen in decellularized colon (1.39 ± 0.07 μg/mg) was significantly higher than that of the native tissue (1.03 ± 0.03 μg/mg). Additionally, mild decrease (p < 0.05) was observed in sGAG content of the decellularized colon (0.25± 0.06 μg/mg), compared with native one (0.39 ± 0.2 μg/mg). These results are in accordance with histopathologic evaluations of decellularized and natural colon tissues.
H&E staining of (A) normal and (B) decellularized colons. SEM analysis of (C) normal and (D) decellularized scaffolds. DAPI staining of (E) normal and (F) decellularized scaffolds. (G) Picrosirius red and (H) Pentachrome staining in decellularized scaffolds. (I) Picrosirius red and (J) Pentachrome staining in normal colons
All rats survived the entire period of the study. All of the implanted scaffolds persisted at the same location without noticeable shrinkage in the macroscopic view. Histological and IHC assays were performed for implanted specimens in different periods by an expert pathologist. First H&E staining was performed to evaluate the extent of cell infiltration on implants and to estimate cellularity and differentiation of implanted scaffolds. Implanted scaffolds of 2 months were not completely recellularized and were mostly populated by undifferentiated cells and inflammatory cells. H&E assays of the extracted implants of 4 months old showed moderate regeneration of submucosa and repopulation of smooth muscle cells as well as mild regeneration of epithelium and submucosal glands (Fig. 3 A–B).
Implanted decellularized colon: (A) α-SMA staining, (B) DAPI staining (C) merged in short- and long-term follow-ups; (D) CD31 staining, (E) DAPI staining (F) merged in short- and long-term follow-ups; (G) pancytokeratin (AE1/AE3) staining, (H) DAPI staining (I) merged in short- and long-term follow-ups; (J) vimentin staining, (K) DAPI staining (L) merged in short- and long-term follow-ups
Figures 3 (C–D)–7 show the Trichrome, and IHC staining for αSMA, CD31, vimentin, and pancytokeratin of natural and implanted scaffolds after 2 and 4 months of operation, the results of which proved successful cell infiltration. Histopathological evaluations of the samples showed a colon regenerative process in all scaffolds, with numerous cellular constituents which were noticeable in all layers of the scaffolds following the implantation. Trichrome staining revealed a normal collagen rich blue submucosa with a basement membrane and a large number of muscle fiber tissues, at 2 and 4 months post-transplantation. IHC evaluation and of 4-month-old specimens revealed positive staining by αSMA, CD31, pancytokeratin which indicates the presence of smooth muscle cells, and endothelial and epithelial cells, respectively.
IHC staining for α-SMA revealed a significantly (p < 0.05) higher amount of α-SMA-positive cells in long-term follow-up. Moreover, IHC assessment of endothelial and epithelial cells demonstrated the presence of pancytokeratin and CD31-positive cells in the recellularized scaffolds indicative of an angiogenesis process following the implantation, which showed significant difference between the 2 and 4 months of implantation (Figs. 3 and 4). Histological assessments were suggestive for colon recellularization and infiltration of the epithelial cells on the inner surface of the tissue which were more significant in long-term follow-up (p < 0.05). The results of IHC analysis demonstrated a significant enhancement in expression of IHC markers 4 months after surgery compared to biopsies obtained after 2 months of surgery (Table 1).
Discussion
The histological results of this preliminary study suggested that grafting decellularized colon matrix in omentum causes minimal host immune response and tissue reaction in a rat model, with satisfactory cell infiltration process.
Pouchitis is a common morbidity of ileal pouch after total proctocolectomy, and there are post-colectomy complications related to lack of large intestinal physiologic function. The symptoms associated with pouchitis including stool frequency, abdominal cramping, fever, and extra-intestinal manifestations are very critical [11]. In spite of the fact that pouchitis is definitely related to host factors, which causes an increased incidence of pouchitis in ulcerative colitis as compared with familial adenomatous polyposis [12], the adaptation of small intestine to colon function is a crucial factor. The proposed approach to the preparation of tissue engineered colon (TEC) in this study could lead in avoiding implantation of non-autologous tissues or alternative autologous tissues with different functions [13]. The need for TEC is undeniable due to the high prevalence of diseases that would necessitate colectomy. Yet, the location and function of this tissue make its implantation and bio-fabrication quite distinctive [14].
The anatomical location of colon tissue and the tension on it during defecation requires the use of a high tensile tissue. On the other hand, tissue structure is important regarding the existence of specific cells such as the goblet cells. Another point which should be taken into account is the need for tissue neural and circulatory supplies, the lack of which would cause collapse and obstruction of the colon as well as ischemia and perforation [15]. In one study in 2016, the outcomes showed that decellularized colon may also provide a viable material for bladder augmentation in rats with satisfactory outcomes [16]. It has been also verified by several examinations that colon decellularization can provide three-dimensional biologic scaffold with preserved ECM and complete cell removal to enable further tissue augmentation [17]. In one study in 2012, a rat decellularized small bowel scaffold was produced, using detergent-enzymatic treatment. The results of the investigations demonstrated the preservation of villus-cryptarchitecture for intestinal regeneration [18].
In the present study, we intended to evaluate in vivo recellularization of colon tissue using omentum as a natural bioreactor. The first phase of the study involved reaching a decellularization protocol with complete cellular cleansing along with the preservation of extracellular matrix and structural integrity of the tissue. This goal was reached, using 0.5% SDS and 1% Triton-X. Previous studies have verified that the usage of competently decellularized scaffolds could be associated with minimum immunologic reactions due to complete cellular removal. The remaining detergents within the scaffolds could also cause an immune reaction and rejection after the implantation. The perfusion-based decellularization technique in this study was in accordance with our previously published paper concerning the decellularization process of rat colon scaffold with satisfactory results [16]. We believe that this efficient decellularization method, including a final step of 24-h PBS rinsing, could offer bio-scaffolds with the least immunogenicity and adequately diminished detergents.
Most studies used synthetic polymers instead of decellularized natural scaffolds for creating tissue-engineered colon, which provided them with the possibility of determining physical and mechanical properties of the scaffolds and in some cases enhancing cell attachment by some biomechanical modifications; however, these scaffolds can result in the inflammatory response [19]. On the other hand, studies on decellularized scaffolds indicated that these biodegradable scaffolds possessed their native structure and growth factors, leading to better angiogenesis and regeneration of tissue with minimal inflammatory reactions [20].
In the second phase of our study, we attempted to recellularize the colon scaffolds by implanting these scaffolds in the omentum, which has been used as a natural bioreactor. In vitro cell seeding process can also be used instead; however, there are complexities regarding the recellularization of scaffolds in the laboratory [21]. One of the issues in in vitro recellularization of colon tissue would be attaining an appropriate source of stem cells [22]. Several studies have suggested that organoids extracted from mouse primary fetal intestinal epithelial cells express markers of neonatal development [23, 24]. In the clinical setting, the extraction of organoids from the intestines of human newborns is difficult and sometimes impossible, especially in autologous stem cell transplantation [25].
Several aspects should be considered when performing in vivo recellularization of scaffolds including the amount of time needed for recellularization, the technique of surgery, and adequate care of the animal. Insufficient blood supply for tissue or inappropriate sterilization during surgery would prevent proper recellularization, or would cause complications and infections for the animals.
Another issue regarding in vitro and in vivo recellularized scaffolds is the restoration of the intestinal peristaltic movements [26, 27]. Previous studies have shown that recellularized tissue might gain mucus formation function, but they lacked functional muscles and neural networks. Lack of functional neural networks and in turn lack of normal peristalsis would lead to obstruction and megacolon as seen in mucosal aganglionosis or Hirschsprung disease [2]. Grikscheit et al. have investigated the regeneration of colon tissue by seeding intestinal-derived organoids on a polymer scaffold and then culturing the cell-seeded scaffold in an omental wrap in vivo [14]. The implanted tissues had a similar mucosal structure to native colon, and they possessed appropriate lamina propria and ganglionic network with electrophysiological activity comparable to native colon neural network [1]. Collagen sponge scaffolds were also seeded with autologous smooth muscle cells and implanted as a patch graft, using a canine model. The results showed regeneration of the mucosal and intestinal epithelial layers as well as the villi structures. However, shrinkage was the main problem related to these grafts [28].
In this study we tested our hypothesis that the adult/somatic mesenchymal stem cells in the fibro-adipose and vascular tissues of the omentum have the ability to differentiate into endodermal, mesodermal, and ectodermal tissues. It should be also taken into consideration that there are difficulties in vascularizing and innervating an engineered colon. One of the limitations of the current technique in the clinical setting would be insufficient omentum for wrapping the scaffold, given the fact that patients with total colectomy have manipulated omentum and caused adhesions.
Regarding the strength, to the best of our knowledge, this is the first study in which the decellularized colon was used for colon regeneration in a rat model with satisfactory postoperative results and without major complications and also there is an advantage in using omentum. A perfusion-based decellularization technique was used that allowed the tissue to maintain its physical properties and ECM structure. Altogether, our data confirmed the hypothesis that the omentum and colon scaffold could perform as proficient natural bioreactors. Also, this study emphasized the role of the body as a natural bioreactor in cellular proliferation, differentiation, and recellularization of decellularized colon tissues which could be broadly used in the field of regenerative medicine. However, further studies are needed to regenerate tissue-engineered colon tissue with the functional neural network.
Conclusion
The outcomes showed regeneration of decellularized colon by the aid of omentum; however, there is a long way until this technique can be performed in a clinical setting. Omentum as a natural bioreactor provides implanted scaffolds with stem cells and growth factors due to its high vascularity and stimulates neovascularization in implanted scaffolds; however, these recellularized colon scaffolds do not own functional submucosal neural networks.
References
Grikscheit TC, Ogilvie JB, Ochoa ER, Alsberg E, Mooney D, Vacanti JP. Tissue-engineered colon exhibits function in vivo. Surg. 2002;132(2):200–4. https://doi.org/10.1067/msy.2002.125310.
Beyer-Berjot L, Joly F, Dokmak S, Bretagnol F, Corcos O, Bouhnik Y, et al. Intestinal transplantation: indications and prospects. J Visc Surg. 2012;149(6):380–4. https://doi.org/10.1016/j.jviscsurg.2012.10.008.
Meagher AP, Farouk R, Dozois RR, Kelly KA, Pemberton JH. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. British J Surg. 1998;85(6):800–3. https://doi.org/10.1046/j.1365-2168.1998.00689.x.
Nguyen N, Zhang B, Holubar SD, Pardi DS, Singh S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2019;5:CD001176. https://doi.org/10.1002/14651858.CD001176.pub4.
Wales PW, de Silva N, Kim J, Lecce L, To T, Moore A. Neonatal short bowel syndrome: population-based estimates of incidence and mortality rates. J Pediatr Surg. 2004;39(5):690–5. https://doi.org/10.1016/j.jpedsurg.2004.01.036.
Andoh A, Bamba S, Fujiyama Y, Brittan M, Wright NA. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol. 2005;40(12):1089–99. https://doi.org/10.1007/s00535-005-1727-4.
Keane TJ, Badylak SF. Biomaterials for tissue engineering applications. Sem Pediatr Surg. 2014;23(3):112–8. https://doi.org/10.1053/j.sempedsurg.2014.06.010.
Cushman GK, Stolz MG, Blount RL, Reed B. Executive Functioning in pediatric solid organ transplant recipients: a meta-analytic review. Transplant. 2019. https://doi.org/10.1097/tp.0000000000002954.
Patil PB, Chougule PB, Kumar VK, Almstrom S, Backdahl H, Banerjee D, et al. Recellularization of acellular human small intestine using bone marrow stem cells. Stem Cell Trans Med. 2013;2(4):307–15. https://doi.org/10.5966/sctm.2012-0108.
Kajbafzadeh AM, Fendereski K, Khorramirouz R, Daryabari SS, Masoomi A, Moosavi S, et al. In vivo application of decellularized rat colon and evaluation of the engineered scaffolds following 9 months of follow-up. Cell Biol Int. 2020;44(11):2253–62.
Hata K, Ishihara S, Nozawa H, Kawai K, Kiyomatsu T, Tanaka T, et al. Pouchitis after ileal pouch-anal anastomosis in ulcerative colitis: Diagnosis, management, risk factors, and incidence. Dig Endosc. 2017;29(1):26–34. https://doi.org/10.1111/den.12744.
Gao XH, Li JQ, Khan F, Chouhan H, Yu GY, Remer E, et al. Difference in the frequency of pouchitis between ulcerative colitis and familial adenomatous polyposis: is the explanation in peripouch fat? Colorectal Dis. 2019;21(9):1032–44. https://doi.org/10.1111/codi.14651.
Grikscheit TC, Vacanti JP. The history and current status of tissue engineering: the future of pediatric surgery. J Pediatr Surg. 2002;37(3):277–88. https://doi.org/10.1053/jpsu.2002.30802.
Grikscheit TC, Ochoa ER, Ramsanahie A, Alsberg E, Mooney D, Whang EE, et al. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Ann Surg. 2003;238(1):35–41. https://doi.org/10.1097/01.SLA.0000074964.77367.4a.
Tsai YH, Murakami N, Gariepy CE. Postnatal intestinal engraftment of prospectively selected enteric neural crest stem cells in a rat model of Hirschsprung disease. Neurogastroenterol Motil. 2011;23(4):362–9. https://doi.org/10.1111/j.1365-2982.2010.01656.x.
Kajbafzadeh AM, Khorramirouz R, Sabetkish S, Ataei Talebi M, Akbarzadeh A, Keihani S. In vivo regeneration of bladder muscular wall using decellularized colon matrix: an experimental study. Pediatr Surg Int. 2016;32(6):615–22. https://doi.org/10.1007/s00383-016-3871-8.
Kajbafzadeh A-M, Masoumi A, Hosseini M, Borjian MA, Akbarzadeh A, Mohseni MJ. Sheep colon acellular matrix: immunohistologic, biomechanical, scanning electron microscopic evaluation and collagen quantification. J Biosci Bioeng. 2014;117(2):236–41.
Totonelli G, Maghsoudlou P, Garriboli M, Riegler J, Orlando G, Burns AJ, et al. a rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomater. 2012;33(12):3401–10.
Cardoso L, Cleto MC, Barbo MLP, Esposito AR, Orgaes FS, Duek EAR. Bioresorbable scaffold as a dermal substitute. Int J Burn Trauma. 2017;7(4):34–46.
Penkala RA, Kim SS. Gastrointestinal tissue engineering. Exp Rev Med Dev. 2007;4(1):65–72. https://doi.org/10.1586/17434440.4.1.65.
Portner R, Nagel-Heyer S, Goepfert C, Adamietz P, Meenen NM. Bioreactor design for tissue engineering. J Biosci Bioeng. 2005;100(3):235–45. https://doi.org/10.1263/jbb.100.235.
Barthel ER, Levin DE, Speer AL, Sala FG, Torashima Y, Hou X, et al. Human tissue-engineered colon forms from postnatal progenitor cells: an in vivo murine model. Reg Med. 2012;7(6):807–18. https://doi.org/10.2217/rme.12.91.
Wieck MM, El-Nachef WN, Hou X, Spurrier RG, Holoyda KA, Schall KA, et al. Human and murine tissue-engineered colon exhibit diverse neuronal subtypes and can be populated by enteric nervous system progenitor cells when donor colon is aganglionic. Tissue Eng A. 2016;22(1-2):53–64. https://doi.org/10.1089/ten.TEA.2015.0120.
Sala FG, Matthews JA, Speer AL, Torashima Y, Barthel ER, Grikscheit TC. A multicellular approach forms a significant amount of tissue-engineered small intestine in the mouse. Tissue Eng A. 2011;17(13-14):1841–50. https://doi.org/10.1089/ten.TEA.2010.0564.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nat. 2011;470(7332):105–9. https://doi.org/10.1038/nature09691.
Hotta R, Stamp LA, Foong JP, McConnell SN, Bergner AJ, Anderson RB, et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest. 2013;123(3):1182–91. https://doi.org/10.1172/jci65963.
Pan WK, Zheng BJ, Gao Y, Qin H, Liu Y. Transplantation of neonatal gut neural crest progenitors reconstructs ganglionic function in benzalkonium chloride-treated homogenic rat colon. J Surg Res. 2011;167(2):e221–30. https://doi.org/10.1016/j.jss.2011.01.016.
Nakase Y, Hagiwara A, Nakamura T, Kin S, Nakashima S, Yoshikawa T, et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue Eng. 2006;12(2):403–12.
Funding
The authors would like to thank Tehran University of Medical Sciences for funding this study (Grant Number 35805).
Author information
Authors and Affiliations
Contributions
Concept/design: Abdol-Mohammad Kajbafzadeh*
Data analysis/interpretation: Amir Hossein Zabolian, Minoo Rostami, Sahar Eftekharzadeh
Drafting article: Shabnam Sabetkish
Critical revision of article, approval of article: Abdol-Mohammad Kajbafzadeh*
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zabolian, A.H., Rostami, M., Eftekharzadeh, S. et al. In Vivo Colon Regeneration: from Decellularization to In Vivo Implantation in a Rat Model Using the Body as a Natural Bioreactor. Regen. Eng. Transl. Med. 8, 106–116 (2022). https://doi.org/10.1007/s40883-021-00195-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-021-00195-1