Abstract
A green, efficient, and short route for recovering metal lead from spent lead-acid batteries has a great advantage in both environmental protection and sustainable development of lead industry. This paper developed a new scheme to recover metal lead by direct electrolysis in (NH4)2SO4 solution with desulfurized lead paste. Cyclic voltammetry showed that lead compounds of desulfurized lead paste could be reduced to metal lead. The effects of current density, electrolyte concentration, paste amount, and temperature on lead recovery ratio, energy consumption, electrolysis efficiency, and phase change in the whole electrolysis process were systematically investigated. The process presented an environment-friendly and economic strategy to obtain metal lead from desulfurized lead paste for the recycle of spent lead paste and lead resources.
Graphical Abstract
This paper proposed a green, clean, and efficient method to obtain metal lead by using desulfurized lead paste-coating plate with PVDF as binder. It could be clearly seen from the figure that Pb2+ gained electrons and reduced to metal Pb in the cathode and H2O molecule gained electrons and released H2, and CO32− entered the electrolyte and combined with NH4+ in the electrolyte to form (NH4)2CO3 while the H2O molecule lost its electrons and released O2 in the anode. The electrolyte after the direct electrolysis was mainly composed of a mixture of (NH4)2CO3 or NH4HCO3 and (NH4)2SO4. The generated (NH4)2CO3 or NH4HCO3 during the electrolysis of desulfurized lead paste can be reused for the next batch of desulfurization process for spent lead paste.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on the apparent advantages of low price, high stability, good safety, low working voltage, and wide application range of working temperature [1, 2], lead-acid batteries (LABs) are widely used in automobiles, electric vehicles, motorcycles, as well as in navigation, communications, power, and other fields [3, 4]. Recovery of secondary lead from spent LABs becomes an important research topic. Spent LABs contain waste lead paste, waste sulfuric acid electrolyte, lead alloy plate grid, and other parts [5]. Because lead paste contains not only a large amount of PbSO4, but only a small amount of PbO2, PbO, Pb, and other minor impurities (Sb, Ba, Fe, Si, Cu) [6, 7]; therefore, lead recovery from the lead paste is the most critical part in the whole lead-acid battery recycling [8]. Lead-acid batteries containing toxic elements can induce environmental problems and public health hazard without correct management and disposal [9, 10]. Developing an optimal route for the recovery of metal lead from spent lead-acid batteries with the characteristics of low pollution, low energy consumption, and high recovery efficiency of metal lead has attracted the significant attention of researchers.
Currently, pyrometallurgical process and hydrometallurgical process as dominant methods for treating the spent lead paste have been widely used industries worldwide. Unfortunately, there are a series of problems cannot be ignored in traditional pyrometallurgical process such as high decomposition temperature of PbSO4 (> 1000 °C) causing significant resources consumption and environmental hazard caused by lead vapor and SOX emission into the atmosphere [11]. In recent years, researchers proposed pre-desulfurization of spent lead paste using carbonate and other desulfurizers to obtain lead carbonate with lower decomposition temperature, greatly reducing energy consumption [5, 12, 13]. However, the process requires the consumption of chemical reagents in relatively large quantities and the thermal decomposition of PbCO3 produces greenhouse gas CO2, and the process of treating ammonium sulfate desulfurization mother liquor is quite complicated, so green, economical and efficient recovery is an obstacle to be overcome for pyrometallurgy process. Thus, hydrometallurgical technology as an alternative recycling method has been gradually developed [14].
A traditional hydrometallurgical process involves three main steps, respectively desulfurizing PbSO4 and reducing PbO2 to Pb2+, lead-containing compounds dissolving in HBF4 or H2SiF6 leaching reagents and then electrowinning high-purity metallic lead [15,16,17]. Nevertheless, owing to the high toxicity of HBF4 or H2SiF6 and corrosion of experimental equipment, direct electrochemical solid-phase electrolysis of spent lead paste to recover metal lead has been developed to offer a short process, cleanliness and high-efficiency recycling solution [18,19,20]. However, there are three disadvantages of solid-phase electrolysis process.
-
(1)
Lead paste gradually comes off the plate due to the production of hydrogen from the cathode during electrolysis, reducing lead recovery ratio.
-
(2)
The spongy lead with porous structure formed by electrolysis is easily oxidized by air and the purity of lead is reduced.
-
(3)
The addition of barium sulfate, sodium lignosulfonate, humic acid, and staple fiber in the process of cathode plate preparation leads to the introduction of impurity ions and increase the cost.
In this paper, a new scheme for recovering lead from waste lead paste is proposed as shown in Fig. 1, which includes three processes as described below.
-
(1) Desulfurization process
First, lead sulfate of spent lead paste was converted into lead carbonate with lower solubility via reacting with ammonium carbonate or ammonium bicarbonate [21]. Theoretically, lead carbonate will obtain more metallic lead per mass than lead sulfate in the electrolysis process. The reaction can be described by reaction (1).
$$ {\text{PbSO}}_{{4}} + {\text{ 2NH}}_{{4}} {\text{HCO}}_{{3}} = {\text{ PbCO}}_{{3}} + ({\text{NH}}_{{4}} )_{{2}} {\text{SO}}_{{4}} + {\text{ 2H}}_{{2}} {\text{O }} + {\text{ CO}}_{{2}}. $$(1) -
(2) Cathode plate manufacturing process
Then, an appropriate amount of acetylene black and PVDF were added to the desulfurized lead paste and grounded evenly in the mortar to form paste slurry to coat on the lead substrate grid, obtaining cathode plate after drying in vacuum oven.
-
(3) Electrolysis process
In this process, ruthenium-titanium net are used as the anode, and metallic lead was obtained from the ammonium sulfate solution through the electrochemical reduction process. The spent electrolyte was reused for the pre-desulfurization process of spent lead paste in the next batch after only adding ammonia providing NH4+ to realize a closed-loop process of (NH4)2CO3 as leaching reagent. When a small amount of ammonia was added to the electrolyte, the solution became weakly basic at first, but quickly became weakly acidic. Because ammonium sulfate is a strong acid and a weak base salt, the aqueous solution is acidic. It should be noted that NMP volatilized during high-temperature curing, so there was no NMP in the electrolyte. The reaction can be described by reactions (2)–(6).
$$ {\text{PbCO}}_{{3}} + {\text{2e}}^{ - } \to {\text{ Pb}} + {\text{CO}}_{{3}}^{{{2} - }}. $$(2)$$ {\text{PbO }} + {\text{ 2e}}^{ - } + {\text{ 2H}}^{ + } \to {\text{Pb }} + {\text{H}}_{{2}} {\text{O}}. $$(3)$$ {\text{PbO}}_{{2}} + {\text{ 2e}}^{ - } + {\text{ 4H}}^{ + } \to {\text{ Pb}}^{{{2} + }} + {\text{2H}}_{{2}} {\text{O}}. $$(4)$$ {\text{Pb}}^{{{2} + }} + {\text{ 2e}}^{ - } \to {\text{ Pb}}. $$(5)$$ {\text{CO}}_{{3}}^{{{2} - }} + {\text{ 2NH}}_{{4}}^{ + } \to \left( {{\text{NH}}_{{4}} } \right)_{{2}} {\text{CO}}_{{3}}. $$(6)
In this study, considering that the waste lead paste has poor electrical conductivity and low solubility in acidic solution because its main component is lead sulfate, the recovery of metallic lead from desulfurized lead paste by solid-phase electrolysis was initially proposed in ammonium sulfate solution. PVDF was used as an intermediary to improve the adhesion of desulfurized lead paste, preventing lead paste from falling off during electrolysis, improving the purity and quality of lead products and reducing the use of chemical reagents and costs. Cyclic voltammetry (CV) was used to investigate the electrochemical behavior of desulfurized lead paste. The factors (current density, electrolyte concentration, working temperature, and paste amount) affecting the electrolysis of desulfurized lead paste in terms of lead recovery ratio, cathode electrolytic efficiency, and energy consumption were optimized. Cathode product phase composition and morphology were investigated by XRD and SEM, respectively. The products of desulfurized waste lead paste with waste electrolyte were characterized by XRD. This approach of lead recovery can provide a new way for the resource recycling of waste lead paste.
Experimental Section
Preparation of Experimental Materials and Cathode Plate
Spent lead paste was provided by Hunan Jiangye Electromechanical Technology Company. Figure 2 displays the XRD pattern of the spent lead paste sample. The main compositions of spent lead paste were analyzed by ethylenediamine tetraacetic acid (EDTA) complexometric method, and the results are present in Table 1.
First, the spent lead paste reacted with NH4HCO3 in a flask, and the solution was filtered to obtain desulfurization of lead paste, in which the major phase was PbCO3 [22, 23]. The yield of lead carbonate was obtained by chemical analysis method as 96.25%. Figure 3 presents X-ray diffraction (XRD) pattern(a) of desulfurized lead paste and XRD pattern(b) of the product of desulfurized lead paste which was calcined at 600 °C for 2 h in muffle furnace. Apparently, it can be seen from the figure that the main component of desulfurized lead paste and calcined product is PbCO3 and PbO, respectively. The lead content of desulfurized lead paste was obtained by calculating the lead content of calcined products.
The preparation method of the cathode plate was as follows: acetylene black and polyvinylidene fluoride (PVDF), as the polymer binder widely used for lithium-ion batteries and usually dispersed in nitromethyl pyrrole (NMP) [24, 25], were added to desulfurized lead paste and ground in a mortar [26]. The mass ratio of desulfurized lead paste, acetylene black, and PVDF was 470:2:1 to mix. The appropriate amount of PVDF was evenly dispersed in NMP and stirred for 2 h. Then, the desulfurized lead paste and acetylene black were mixed and grounded in a mortar, and the dispersed PVDF was added and continuously grounded for a few minutes. Finally, the paste slurry was uniformly coated on the lead substrate grid (34 mm × 25 mm × 1.5 mm), and the prepared plates were dried in the in a vacuum oven at 120 °C overnight to get the cathode plates.
Cyclic Voltammetry
Cyclic voltammetry test of the desulfurized lead paste was performed at an electrochemical workstation (CS350H, China). A three-electrode cell was used with Pt electrode as counter electrode and Hg/Hg2SO4 (saturated K2SO4 solution) electrode as reference electrode. The electrolyte concentration was 200 g/L (NH4)2SO4 solution, the scanning rate was 10 mV/s, and the scanning range was from − 1.5 to − 0.2 V.
Electrochemical Reduction
The reduction experiment of metal lead in desulfurized lead paste was carried out in BH-Hull cell tester (10 A) DC electrolytic cell. Here, ammonium sulfate solution was used as the electrolyte with ammonia to provide ammonium ions, and ruthenium-titanium mesh was used as the anode in the direct electrolysis process. Accordingly, the reaction between anode and cathode is as follows:
Moreover, the influences of current density, electrolyte concentration, paste amount, and operating temperature on electrochemical reduction were studied systematically to get the appropriate parameters in terms of lead recovery ratio, electrolysis efficiency, and energy consumption. After electrolysis, the cathode was taken out of the cell and washed repeatedly with deionized water several times to remove the electrolyte on the surface, and dried at 65 °C in a vacuum oven. Then, the cathode with deposited lead was determined by EDTA titration method.
The lead recovery ratio, electrolysis efficiency, and specific energy consumption were used as the main evaluation parameters during the whole electrolysis process. The corresponding calculation formula was expressed as follows:
where m1 (g): mass of original desulfurized lead paste, w1 (wt%): mass fraction of lead in original desulfurized lead paste, m2 (g): the quantity of cathodic lead after electrolysis, U (V): cell voltage, I (A): electric current, T (h): the consumed time to reach the electrolysis end-point, and S (m2): cathode plate area.
Morphological and Structural Characterizations
Desulfurized lead paste, calcined products, and electrolytic products were analyzed by X-ray diffractometer (XRD) of Bruker D8-Advance in Germany with scanning angle of the test is 5–90°. The apparent morphology of the electrolytic products was observed by JSM-6610LV scanning electron microscope (SEM) in Japan. The lead content obtained by electrolysis was analyzed by an inductively coupled plasma (ICP) analyzer (Agilent 720ES).
Results and Discussion
The Preparation of Cathode Plate
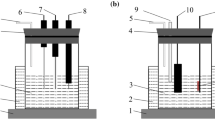
Figure 4 shows the phenomenon of desulfurized lead paste-coating plate obtained with (a) and without (b) PVDF in (NH4)2SO4 solution in the electrolysis process. As shown in Fig. 4a, part of the paste without PVDF was separated from the cathode plate after only 10 min of electrolysis, whereas, in the presence of PVDF of desulfurized lead paste, there was no paste to peel the cathode plate until electrolysis was completed (Fig. 4b). Moreover, Pb product on the cathode plate was dense, and the surface was smooth and flat after the electrolysis was completed. Unlike fluffy and porous sponge lead, this dense Pb would reduce the risk of being oxidized by air and further improved the purity of the product.
Cyclic Voltammetry
In order to investigate the reduction of lead in desulfurized lead paste by direct electrolysis, CV was used in this work, as shown in Fig. 5. The scanning potential of desulfurized lead paste range was from − 0.2 to − 1.5 V at a scanning speed of 10 mV/s. The potential was scanned from an initial X (− 0.2 V), and cathode current increased rapidly with negative scanning. There were obvious reduction peaks at A1 (− 0.48 V) and A2 (− 0.64), corresponding to the reduction of PbO2 to PbO and PbCO3 to metal lead by direct electrolytic, respectively [27, 28]. After that, the scanning potential reached Y (− 1.5 V) and began to scan in the positive direction, converting to an anode current and the anode current increased gradually. The anode peak current reached its maximum at B1 (− 1.2 V) and then continued to scan in the positive direction until the current reached zero. The oxidation peak was observed during the positive-going scanning process, which might be due to the oxidative dissolution of metal lead. Figure S1 in the online supplementary material shows the LSV curve of test cell at the scanning rate of 10 mV/s. From the figure, it was observed that the phenomenon of hydrogen evolution inevitably exists in the process of electrochemical reduction of lead paste under desulfurization. These results indicated that lead compounds in desulfurized lead paste could be further reduced to lead metal.
Electrolysis Process
The Effect of Current Density
In this paper, the current density is the ratio of the current to the area of the cathode plate. The effects of current density from 150 to 350 Am−2 on lead recovery ratio, electrolysis efficiency, and energy consumption of desulfurized lead paste were studied in 200 g/L (NH4)2SO4 solution under the electrolysis temperature of 30 °C, paste amount of 5 g, and an electrode spacing of 2 cm. Figure 6a displays the variation tendency of the lead recovery ratio with the change of current density. The lead recovery ratio rapidly decreased to 83.65% at 200 Am−2, after which the variation fluctuated in a small range with increasing current density, which could be ascribed to the excessive current density providing the possibility of hydrogen ion discharging to form hydrogen gas at the cathode. In addition, it could be observed from Figure S2 in the online supplementary material that the cell voltage rose from 2.58 to 3.02 V due to the increasing current density.
The corresponding change of electrolytic efficiency and energy consumption with the current density is presented in Fig. 6b. As the current density increased, the energy consumption began to significantly accelerate after reaching 200 Am−2, because the increase of the whole electrolytic cell voltage led to the increase of energy consumption. It could be observed that the electrolytic efficiency increased from 53.47 to 90.50% when the current density increased from 150 to 350 Am−2. Compared with the current density of 200 Am−2, both working voltage and the current were relatively low at 150 Am−2, and energy consumption was high although the lead recovery ratio was high. However, the result was reasonable due to the time required for the completion of electrolysis at this current density was prolonged. Therefore, in order to ensure a satisfactory lead recovery ratio and lower energy consumption, 200 Am−2 was chosen as the optimal current density.
The Effect of Electrolyte Concentration
The influence of electrolyte concentration on the direct electrolysis of desulfurized lead paste was carried out in the range between 150 and 350 g/L with the current density of 200 Am−2, and other parameters the same as “The Effect of Current Density” section, as shown in Fig. 7. As depicted in Fig. 7a, the lead recovery ratio first increased rapidly to the maximum value of 88.20% at the electrolyte concentration of 250 g/L and then decreased slowly with increasing electrolyte concentration. That is to say that an excessively high concentration of ammonium sulfate reduced lead recovery ratio because the solution would be in a saturation state, leading to the migration rate of ions in the solution moving slowly and immensely affecting the reduction process of lead.
The relationship between electrolytic efficiency, energy consumption, and electrolyte concentration is reflected in Fig. 7b. The figure showed the electrolytic efficiency first increased and then decreased from 200 g/L (NH4)2SO4 to 350 g/L (NH4)2SO4 with an increase of electrolyte concentration, while the corresponding energy consumption was the opposite. When the concentration of the (NH4)2SO4 solution was 150 g/L, the weak conductivity of the solution resulted in the time required to complete electrolysis increasing, causing low electrolysis efficiency and great energy consumption. Finally, the concentration of (NH4)2SO4 solution was selected as 250 g/L as the concentration of the next electrolysis texts.
The Effect of Paste Amount
As shown in Fig. 8, the effect of paste amount from 4 to 8 g on the direct electrolysis of desulfurized lead paste was investigated using 250 g/L (NH4)2SO4 solution and other parameters the same as “The Effect of Electrolyte Concentration” section. The results are shown in Fig. 8a that the lead recovery ratio increased from 85.17% to 93.41% with increasing paste amount from 4 to 8 g, indicating that the appropriate paste amount was beneficial to accelerate conversion process of metal lead, but excessive paste amount increased the thickness of the cathode plate, hindering current entering inside of the paste.
Furthermore, the paste amount had a specific effect on electrolytic efficiency and energy consumption in Fig. 8b, where the energy consumption increased slowly and the electrolytic efficiency followed the opposite trend with increasing of paste amount from 4 to 8 g. As increasing paste amount, the cell voltage increased because of the thickness of the paste making it difficult for the current to enter and the electrolysis time prolonged, leading to the energy consumption increasing and electrolytic efficiency reducing. Taking energy saving consumption and lead recovery ratio into consideration, it was satisfactory to employ paste amount at 7 g and to obtain the lead recovery ratio as high as 93%.
The Effect of Temperature
To investigate lead recovery ratio, electrolysis efficiency, and energy consumption of desulfurized lead paste for different temperatures from 20 to 60 °C, a study was carried out under the paste amount of 7 g and other parameters same as “The Effect of Paste Amount” section, with the results presented in Fig. 9. As seen in Fig. 9a, the lead recovery ratio of the electrolytic product gradually reached up to 95.64% with temperature increasing 60 °C, implying that the higher temperature was conducive to form metal lead due to the increase in working temperature accelerated ion diffusion and reduced ion hydration of desulfurized lead paste, resulting in the accelerated of the conversion process.
Accordingly, the influence of temperature on electrolytic efficiency and energy consumption is displayed in Fig. 9b, the results demonstrated that it had a lower energy consumption reaching 626.9 kWh per ton desulfurized lead paste and higher electrolytic efficiency with the increasing of temperature, benefiting from the ions moving quickly and the conductivity of the solution increasing as the temperature rising and promoting voltage reduction [29]. As was seen, temperature had played a crucial role in lead recovery ratio, electrolysis efficiency, and energy consumption, reflecting that an appropriate temperature was beneficial to the whole electrolysis process. Therefore, the temperature was set at 60 °C.
Direct Electrolytic Analysis of Desulfurized Lead Paste
Figure 10a displays the XRD patterns of electrolytic products of desulfurized lead paste in (NH4)2SO4 solution at different electrolytic periods, exhibiting the diffraction peaks of PbCO3 gradually converting to the diffraction peaks of Pb with the prolonging of electrolysis time. In the absence of the electrolysis, the desulfurized lead paste was mainly composed of PbCO3, corresponding to the initial time. With the prolonging of electrolysis time, the diffraction peaks of PbCO3 and PbO continued to weaken, while the characteristic diffraction peaks of metal lead strengthened gradually, demonstrating the reduction process of lead compounds.
a XRD pattern of the desulfurization lead paste after electrolysis in (NH4)2SO4 solution with different reaction periods, b SEM image of lead after electrolysis, c XRD pattern of the electrolyte using to desulfurize pure PbSO4 after electrolysis (current density: 200 Am−2; (NH4)2SO4 concentration: 250 g/L; paste amount: 7 g; temperature: 60 °C)
When the reaction time was 640 min, the diffraction peaks of lead compounds were hardly found, indicating that desulfurized lead paste had been entirely reduced to metal lead after the termination of electrolysis process. In addition, the weaker characteristic diffraction peaks of lead oxide could be identified owing to the rapid oxidation of metal lead in the air atmosphere. In order to further analyze the purity of the recovered metal lead, the obtained sample was analyzed by ICP method and the purity of metal lead is 90.725%.
The SEM image of lead obtained by electrolysis is presented Fig. 10b. As shown in the figure, the morphology of lead obtained by electrochemical reduction presented spongy structure and had a more porous microstructure. The XRD patterns of the electrolyte reapplied to pure PbSO4 desulfurization after electrochemical reduction is shown in Fig. 10c. The characteristic diffraction peaks of PbCO3 were extremely obvious, so the results clarified clearly that the electrolyte after electrolysis could be reused for desulfurization of spent lead paste. Therefore, the electrolyte was recycled, which had played a great role in saving resources.
Direct Electrolysis Mechanism of Desulfurized Lead Paste
The possible mechanism of electrochemical reduction process of desulfurized lead paste in (NH4)2SO4 solution was proposed, and the mechanism diagram is shown in Fig. 11. There are many advantages of PVDF as a binder including good electrochemical stability, strong mechanical properties, and high absorption rate of electrolyte. The addition of PVDF into desulfurized lead paste during the slurry process prevented the paste from falling off since PVDF was in linear contact with the paste, and the paste was tightly fixed on the collector fluid and PVDF played a binding role with the active substance through van der Waals force.
Although the effect of van der Waals force was weak, the active substance and conductive agent could be tightly attached to the collector to form a complete electrode and prevent the paste from falling off in the process of electrolysis. Furthermore, PVDF has the ability to evenly disperse paste and conductive carbon black, indicating that it can form a good ion penetration network and realize efficient electron transmission. Thus, it is feasible to consider PVDF as the optimal binder in the slurry process of desulfurized lead paste.
Experiments showed that the metal lead obtained by adding PVDF was dense and had a smooth surface, which showed that PVDF could affect the crystal growth of lead during the electrolysis process, and overcame the shortcomings of the spongy lead produced by electrolysis, which led to easy oxidation of products and the reduction of product quality caused by electrolyte entrainment. Although PVDF may remain in the lead product on the plate, it is easy to separate from the lead product in the subsequent process in the process of lead melting and ingot casting, because its density is much smaller than that of liquid lead.
It could be clearly seen from Fig. 11 that Pb2+ gained electrons and reduced to metal Pb in the cathode and H2O molecule gained electrons and released H2, and CO32− entered the electrolyte and combined with NH4+ in the electrolyte to form (NH4)2CO3 while the H2O molecule lost its electrons and released O2 in the anode. Based on the above analysis, the electrolyte after the direct electrolysis was mainly composed of a mixture of (NH4)2CO3 or NH4HCO3 and (NH4)2SO4.
On the other hand, the generated (NH4)2CO3 or NH4HCO3 during the electrolysis of desulfurized lead paste can be reused for the next batch of desulfurization process for spent lead paste, and hence, the process is considered as a typical recyclable procedure. The results show that the proposed new metal lead recovery method on the direct electrolysis of desulfurized lead paste has a higher lead recovery efficiency, less recovery steps which are greatly reduced the metal lead recycling cost.
Conclusion
In this paper, we have proposed a green, clean, and efficient method to obtain metal lead by using desulfurized lead paste-coating plate with PVDF as binder. Compared with traditional methods, PVDF as a more appropriate binder was applied to lead recovery of desulfurized lead paste, solving the problem of paste shedding, increasing the purity of lead because the lead produced was smoother and reducing the use of chemical reagents and costs.
The electrolysis conditions of desulfurized lead paste in (NH4)2SO4 electrolyte were optimized, and lead recovery reached up to 95.64% with a purity of 90.7264% and energy consumption was 626.9 KW h/t under the optimal operating conditions (current density of 200 Am−2, electrolyte concentration of 250 g/L, paste amount of 7 g, and temperature of 60 °C). Besides, the electrolyte after the completion of electrolysis could be reused for desulfurization of spent lead paste, and the XRD results showed the existence of desulfurization products, indicating that carbonate could be recycled in the recycling process of spent lead paste, saving resources, and reducing carbon emissions. Thus, this work provided a more economical and cleaner solution for recovering lead from the spent lead paste of old lead-acid batteries.
Data Availability
All data included in this study are available upon request by contact with the corresponding author.
References
Chang Y, Mao XX, Zhao YF, Feng SL, Chen HY, Finlow D (2009) Lead-acid battery use in the development of renewable energy systems in China. J Power Sources 191(1):176–183. https://doi.org/10.1016/j.jpowsour.2009.02.030
Tian X, Wu YF, Qu S, Liang S, Xu M, Zuo TY (2018) Modeling domestic geographical transfers of toxic substances in WEEE: a case study of spent lead-acid batteries in China. J Clean Prod 198:1559–1566. https://doi.org/10.1016/j.jclepro.2018.07.089
Ciro E, Lupi C, Mondal A, Pilone D (2021) Novel lead battery recycling process combining pyrometallurgical anode preparation and electrorefining. J Sustain Metall 7(4):1727–1735. https://doi.org/10.1007/s40831-021-00447-y
Cooper A (2004) Development of a lead-acid battery for a hybrid electric vehicle. J Power Sources 133(1):116–125. https://doi.org/10.1016/j.jpowsour.2003.11.069
Lyakov NK, Atanasova DA, Vassilev VS, Haralampiev GA (2007) Desulphurization of damped battery paste by sodium carbonate and sodium hydroxide. J Power Sources 171(2):960–965. https://doi.org/10.1016/j.jpowsour.2007.06.014
Chen TT, Dutrizac JE (1996) The mineralogical characterization of lead-acid battery paste. Hydrometallurgy 40(1):223–245. https://doi.org/10.1016/0304-386X(94)00081-D
Ma Y, Huang PZ, Cao J, Zhang JF, Huang Y, Chen B (2022) Analysis of a more sustainable method for recycling waste lead batteries: surface renewal promotes desulfurization agent regeneration. Waste Manage 137:319–328. https://doi.org/10.1016/j.wasman.2021.11.011
Ma YJ, Qiu KQ (2015) Recovery of lead from lead paste in spent lead acid battery by hydrometallurgical desulfurization and vacuum thermal reduction. Waste Manage 40:151–156. https://doi.org/10.1016/j.wasman.2015.03.010
Gottesfeld P, Cherry CR (2011) Lead emissions from solar photovoltaic energy systems in China and India. Energy Policy 39(9):4939–4946. https://doi.org/10.1016/j.enpol.2011.06.021
Pan HY, Geng Y, Dong HJ, Ali M, Xiao SJ (2019) Sustainability evaluation of secondary lead production from spent lead acid batteries recycling. Resour Conserv Recycl 140:13–22. https://doi.org/10.1016/j.resconrec.2018.09.012
Deng XB, Liu WF, Zhang DC, Lin C, Liu ZH, Yang TZ (2021) Hydrothermal desulfurization of spent lead paste based on comproportionation reaction. Sep Purif Technol 259:118115. https://doi.org/10.1016/j.seppur.2020.118115
Pan JQ, Zhang C, Sun YZ, Wang ZH, Yang YS (2012) A new process of lead recovery from waste lead-acid batteries by electrolysis of alkaline lead oxide solution. Electrochem Commun 19:70–72. https://doi.org/10.1016/j.elecom.2012.03.028
Pan JQ, Sun YZ, Li W, Knight J, Manthiram A (2013) A green lead hydrometallurgical process based on a hydrogen-lead oxide fuel cell. Nat Commun 4(1):1–6. https://doi.org/10.1038/ncomms3178
Andrews D, Raychaudhuri A, Frias C (2000) Environmentally sound technologies for recycling secondary lead. J Power Sources 88(1):124–129. https://doi.org/10.1016/S0378-7753(99)00520-0
Li L, Zhu XF, Yang DN, Gao LX, Liu JW, Kumar RV, Yang JK (2012) Preparation and characterization of nano-structured lead oxide from spent lead acid battery paste. J Hazard Mater 203–204:274–282. https://doi.org/10.1016/j.jhazmat.2011.12.021
Tan SY, Payne DJ, Hallett JP, Kelsall GH (2019) Developments in electrochemical processes for recycling lead-acid batteries. Curr Opin Electrochem 16:83–89. https://doi.org/10.1016/j.coelec.2019.04.023
Wu ZH, Dreisinger DB, Urch H, Fassbender S (2014) Fundamental study of lead recovery from cerussite concentrate with methanesulfonic acid (MSA). Hydrometallurgy 142:23–35. https://doi.org/10.1016/j.hydromet.2013.10.018
Dai FS, Huang H, Chen BM, Zhang PP, He YP, Guo ZC (2019) Recovery of high purity lead from spent lead paste via direct electrolysis and process evaluation. Sep Purif Technol 224:237–246. https://doi.org/10.1016/j.seppur.2019.05.023
Fan YY, Liu Y, Niu LP, Zhang WG, Zhang T (2021) High purity metal lead recovery from zinc direct leaching residue via chloride leaching and direct electrolysis. Sep Purif Technol 263:118329. https://doi.org/10.1016/j.seppur.2021.118329
Yang TZ, Xie BY, Liu WF, Zhang DC, Chen L (2020) An environment-friendly process of lead recovery from spent lead paste. Sep Purifi Technol 233:116035. https://doi.org/10.1016/j.seppur.2019.116035
Liu W, Ma BB, Fu Y, Zhang K, Mezaal MA, Li FJ, Zhao XY, Lei LX (2017) Electrochemical property of α-PbO prepared from the spent negative powders of lead acid batteries. J Solid State Electrochem 21(1):35–46. https://doi.org/10.1007/s10008-016-3333-1
Sun XJ, Yang JK, Zhang W, Zhu XF, Hu YC, Yang DN, Yuan XQ, Yu WH, Dong JX, Wang HF, Li L, Vasant KR, Liang S (2014) Lead acetate trihydrate precursor route to synthesize novel ultrafine lead oxide from spent lead acid battery pastes. J Power Sources 269:565–576. https://doi.org/10.1016/j.jpowsour.2014.07.007
Zhu XF, Yang JK, Gao LX, Liu JW, Yang DN, Sun XJ, Zhang W, Wang Q, Li L, He DS, Kumar RV (2013) Preparation of lead carbonate from spent lead paste via chemical conversion. Hydrometallurgy 134:47–53. https://doi.org/10.1016/j.hydromet.2013.01.018
Han BH, Piernas-Munoz MJ, Dogan F, Kubal J, Trask SE, Bloom ID, Vaughey JT, Key B (2019) Probing the reaction between PVDF and LiPAA vs Li7Si3: investigation of binder stability for Si anodes. J Electrochem Soc 166(12):A2396–A2402. https://doi.org/10.1149/2.0241912jes
Huang WB, Wang W, Wang Y, Qu QT, Jin CC, Zheng HH (2021) Overcoming the fundamental challenge of PVDF binder use with silicon anodes with a super-molecular nano-layer. J Mater Chem A 9(3):1541–1551. https://doi.org/10.1039/d0ta10301b
Lestriez B (2010) Functions of polymers in composite electrodes of lithium ion batteries. C R Chim 13(11):1341–1350. https://doi.org/10.1016/j.crci.2010.01.018
Fan YY, Liu Y, Niu LP, Pan XJ, Zhang WG, Zhang T (2019) Preparation of metal lead from waste lead paste by direct electrochemical reduction in NH3-NH4Cl solution. JOM 71(12):4518–4527. https://doi.org/10.1007/s11837-019-03797-x
Xie S, Zhao LX, Zhang M, Xie YH, Feng X, Qin SY (2022) Transformation process of cathode lead structure in the recovery of waste lead paste by suspension electrolysis. Electrochem Commun 141:107361. https://doi.org/10.1016/j.elecom.2022.107361
Zhang X, Sun YZ, Pan JQ (2017) A clean and highly efficient leaching-electrodeposition lead recovery route in HClO4 solution. Int J Electrochem Sci 12(8):6966–6979. https://doi.org/10.20964/2017.08.26
Acknowledgments
This research is financially supported by the National Natural Science Foundation of China (Grant No. 52070159). This work is partly supported by Hunan Province College students’ innovation and entrepreneurship training program (2021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Hongmin Zhu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, J., Cao, J., Zhou, S. et al. Directly Recovering Lead and Recycling Electrolyte via Electrolyzing Desulfurized Lead Paste with PVDF as Binder. J. Sustain. Metall. 9, 172–182 (2023). https://doi.org/10.1007/s40831-022-00635-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00635-4