Abstract
Lead acid batteries are processed mainly by using pyrometallurgical operations with problems related to SO2 evolution. Many efforts have been devoted to solving this concern. In this work, where only the anode preparation was a pyrometallurgical process, this problem has been overcome by limiting the process temperature. Several tests have been carried out in order to determine the starting mix composition that allows to reduce the process temperature and then SO2 emissions. Three different anode types were cast and tested. Independently on the anode type, the complex composition of the anode requires to design a special electrolytic cell composed by two different compartments. Preliminary electrorefining tests highlighted that the best results were obtainable by using the anode cast in a titanium holder that allowed to obtain high-purity lead, high anode durability, and low quantity of anodic mud. By using this anode, the specific energy consumption varied over the 0.04–0.17 kWh/kg range.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the oldest types of rechargeable batteries are lead acid batteries (LABs). Ever since its invention the use of LABs has been increasing rapidly every year and are used in automobile, chemical, energy, transportation and telecommunication [1]. In order to fulfill the demand, lead is either sourced from its ore or from recycling of the spent LABs [1]. Since there is only 85 million tons of known lead ore on the earth, it has become highly necessary to recycle lead. With the increasing demand and utilization, the quantity of spent LABs is also increasing rapidly. Currently about 85% of the total LABs are recycled. In the last few decades, the technologies related to recycling and refining of lead have reached very high efficiencies. The two traditional ways of lead recycling and recovering are pyrometallurgy [2,3,4] and hydrometallurgy [4,5,6,7]. This paper deals with lead recycling from spent LABs with a procedure involving both techniques.
Historically, the consumption of lead for the production of LABs increased from 0.6 metric tons in 1965 to 11.9 thousand metric tons in 2019. The huge increase in the consumption of LABs is basically due to the increase in the number of automobiles in the emerging countries, which account for 75% of the LABs and ‘deep cycle’ LABs which are popular for standby and emergency power supplies. Apart from its widespread applications, the use of lead is not without problems and limitations. But with the great success of the recycling industry, up to 85% of the LABs are now recycled. Industrial lead recycling is dominated by pyrometallurgical processing. Despite all its success, there are still several operational and environmental drawbacks related to pyrometallurgical Pb recycling. Because of these drawbacks, pyrometallurgical operations are slowly being replaced by the hydrometallurgical ones. These processes have many potential benefits as they are performed at low temperature and significantly reduce operational safety concerns.
In the last 30 years, hydrometallurgy has been developed as an eco-friendly alternative to pyrometallurgy and used in growing cases for Pb recovery.
In the recent past, a lot of research has been conducted in the field of hydrometallurgical extraction. The most recent research has been conducted to eliminate SO2 emission during the extraction process [8, 9]. One of the most difficult challenges in the development of the electrowinning process is to find an electrolyte that has high electrical conductivity and that is able to keep in solution high quantities of lead [8,9,10,11,12].
In the hydrometallurgical process, battery dismantling followed by leaching and then electrowinning has become the fixed blueprint for the recovery process. In particular, PLACID process, which uses an acidic electrolyte, is considered one of the most efficient processes in the industry with the 99.995% average purity of lead obtained and with a recovery efficiency of 99.5%. Conventional hydrometallurgical processes are characterized by an initial Pb paste desulfurization carried out by using Na2CO3, NaOH, (NH4)2CO3, or NaC2O4 solutions. The insoluble Pb product obtained is then leached with an acidic solution such as HCl or HBF4 and metallic lead is recovered by means of electrowinning [13]. Many industrial processes have been set up over the last decades [14,15,16]. Because of the difficulties related to the dendrite growth on the cathodic lead and to the high specific energy consumption attributed to the anodic reaction, many laboratory investigations have been carried out both to improve the deposit morphology and to lower specific energy consumptions [17,18,19].
A different approach available in literature is the one described by Owais [20]. This research studied the direct electrorefining of anode particles obtained by crushing. These particles were fed into a titanium basket that works as anode in an electrorefining cell. The electrorefining process was carried out with a current efficiency up to 64.69% and specific energy consumption varying over the range 2.598–3.827 kWh/kg Pb.
This paper explores the possibility of using a process where the pyrometallurgical preparation of the anode is followed by its electrorefining. It should be highlighted that, as opposed to a process involving only pyrometallurgical operations, the proposed process has very limited gaseous emissions.
Material and Methods
Anode Preparation
The recovery of the exhausted battery starts with the dismantling process. The battery system is constituted by a Pb cathode, a PbO2 anode, and PbSO4 paste. A mix of these components has then been used for preparing the anode. The right mix has been selected after testing different ratio Pb:PbO2:PbSO4 in order to obtain lower melting temperature with higher melt homogeneity. Once the mix composition was selected, several tests have been performed to optimize the process temperature. This selection was necessary to avoid SO2 evolution during melting and to reach a good quality of the anode in terms of compactness and homogeneity to guarantee an almost complete consumption.
An analysis of the mix was necessary in order to verify its composition. This was done by carrying out X-ray diffractions and SEM/EDS analyses.
The mix was then melted and cast in order to create an anode to be used in the subsequent electrorefining stage with the aim of depositing pure lead at the cathode. Once melted, it was poured into a permanent mold having dimension 2 × 3 × 0.5 cm.
Three different anode types were prepared:
-
1.
Anode with copper wire cast in a steel mold (Fig. 1a) that was removed after the anode solidification.
-
2.
Anode with titanium holder (Fig. 1b)
-
3.
Anode with copper holder (Fig. 1b)
The copper holder was then coated with an insulating varnish to avoid its dissolution during the electrorefining process.
The anode with copper wire cast in a steel mold that self-sustains, could easily crumble. In the case of titanium and copper anode holders, the molten material was directly poured into the holders, which constituted an anode integral part. This method allowed to reduce the anode cracking and crumbling during electrorefining and to increase the percentage of anode utilization. Moreover, this configuration provided better electrical conductivity of the anodic structure with the current that is distributed more homogeneously throughout the anode. This homogeneous current distribution cannot be obtained by simply inserting a copper wire in the anode that contains lead compounds.
Electrodes and Cell Configuration
Electrorefining tests were performed using a Plexiglas laboratory cell. This cell, having a volume of 500 mL, was divided in two compartments by a polypropylene membrane (Fig. 1c and d). This membrane is necessary because the composition of the anode is complex and some compounds could migrate towards the cathode, where they could be embedded in the lead deposit, so decreasing its purity. An AISI 316L cathode, with a surface area of 7.2 cm2, and the prepared lead anode were spaced 30 mm apart. Prior to each experiment, the cathode was washed with distilled water and cleaned with acetone. The electrolytic solution used in the cell was prepared by dissolving 200 g/L HBF4, 1.2 g/L H3PO4, 10 g/L H3BO3, and 80 g/L PbO in distilled water. The constant current density needed for the electrorefining was provided by an Amel Galvanostat/Potentiostat equipped with a function generator and driven by a computer. The anolyte was withdrawn, purified, and fed in the cathodic compartment. This operation was necessary to avoid those impurities present in the anodic compartment from reaching the cathode and decrease the lead purity. Impurities could either co-deposit or be physically embedded in the cathode. For every electrorefining test, current efficiency (CE) and specific energy consumption (SEC) have been calculated by using the following equations:
where G is the product actual mass (kg), Q is the actual electricity consumed (Ah), and K is the electrochemical equivalent for lead (kg/Ah).
where V is the cell voltage (Volt).
Anode Characterization
Characterization by means of X-ray diffraction (XRD) was performed on battery paste and on both skin and core of the cast anode. These analyses were carried out by using a CuKα source.
The mix samples were subjected to thermogravimetric analysis (TG–DTA). The tests were performed under a nitrogen atmosphere at a heating rate of 10 °C/min up to 1000 °C.
Cathode Characterization
Lead deposits have been analyzed by means of SEM/EDS in order to determine their purity and morphology.
Results and Discussion
Thermogravimetric Analysis of the Anode Material
By using thermogravimetric analysis (TG–DTA), we investigated the behavior of the studied mix by detecting the chemical and physical transformations.
From Fig. 2, it can be noticed that at 101 °C, water is evaporated. The reaction that takes place at 329 °C is due to the melting of lead present in the mix. By analyzing the data available in the literature, the mass decrease that is recorded up to 600 °C is due to the removal of constitutional water and to the reduction of PbO2 to PbO that implies several steps [21]. Considering that our material is a mix of different compounds, there could be concurring reactions that affect the reaction temperature. Data available in the literature highlight that at temperature higher than 600 °C, the reduction of PbO2 to PbO and the decomposition of PbSO4 to PbOPbSO4 occur [8]. At 878 and 972 °C, the two endothermic peaks are related to the decomposition of PbSO4 to PbOPbSO4 and at 878 °C to PbO melting. A simulation of the reactions, performed by using the HSC chemistry software, confirmed that PbO melts at this temperature and highlighted that as the temperature approaches 1000 °C, metallic lead is formed with SO2 evolution. For this reason, in the proposed process, a temperature of 960 °C was selected for the anode production. This is possible by using the following mix composition, which is very close to the spent battery composition: 50%Pb–33%PbSO4–17%PbO2.
Analysis of the Anodic Material
After disassembling the batteries, the ground mix was subjected to X-Ray diffraction in order to identify the phases. Figure 3 shows the X-Ray diffraction pattern obtained.
The main phases observed (Fig. 3) were lead sulfate (PbSO4), lead oxide (PbO2), and metallic lead.
After that, the mix was melted to obtain a solid compact anode. The presence of lead sulfate that melts at 1170 °C, increased the global melting temperature. Several attempts were done to identify the most suitable temperature to obtain the anode by changing the mix composition. It was finally melted and cast at 960 ℃.
By observing the section of the cast anode (Fig. 4), it is evident that it has a compact skin and an irregular core. The analyses revealed that its composition is not homogeneous and that there are areas with higher Pb content and, therefore, with higher electrical conductivity.
The surface skin is formed during cooling, and it is mainly made up of Pb3O2SO4 and Pb5O4SO4 which are revealed by XRD (Fig. 5a). The core contains PbO, PbSO4, PbOPbSO4, and PbSO4(PbO)2 (Fig. 5b).
Preliminary Electrorefining Tests
Electrorefining is the final stage of the proposed process that allows the recovery of metallic lead from exhausted batteries. Some preliminary electrorefining tests have been performed galvanostatically to verify the effectiveness of the proposed process in terms of recovered lead and percentage of recoverable anode before its remelting for a new anode preparation. The three different anode types have been tested for choosing the one with the best performance. Those tests have been carried out by using an acidic electrolyte containing 200 g/L HBF4, 1.2 g/L H3PO4, 10 g/L H3BO3 and 80 g/L PbO. The used process parameters and the results of these tests are reported in Table 1.
In the tests performed by using the anode with the copper wire, high anode overvoltage and, therefore, high average cell voltage were obtained, even for low current densities. This is due to less homogeneous current distribution determined by the absence of a metallic holder. For this reason, the use of this anode has been discarded. Moreover, this anode easily crumbles producing the detachment of large quantities of anode material.
The second anode tested was the one with the copper holder. Even if the copper holder has been externally covered with an insulating varnish layer, then small quantities of copper dissolve in the electrolyte and subsequently deposit on the cathode, so lowering the lead purity. For that reason, it was discarded even if it presented the lowest SEC. The anode with the titanium holder represents the best choice in terms of specific energy consumption, lead purity, and percentage of anode utilization (Table 1).
By comparing the three deposits obtained by using the three different anodes in the same operative conditions (Fig. 6), it is evident that those obtained by using the anodes with copper and titanium supports (Fig. 6b and c) show similar morphology, although the one obtained by using the titanium holder shows slightly bigger grains. The deposit obtained with the anode without holder (Fig. 6a) is the most heterogeneous and presents large cavities. By analyzing the results reported in Table 1, it is evident that by using the titanium holder, it is possible to obtain a very high lead purity: for this reason, this anode is the one selected for the proposed process.
Preliminary Electrorefining Tests Performed by Using the Anode with Titanium Holder
By using anodes with titanium holder, experiments were performed by varying current density and temperature. Particularly current density and temperature were changed in the range 50–200 A/m2 and 25–60 °C, respectively, in order to evaluate the best compromise between CE and SEC. Figure 7a and b shows these parameters as a function of current density at different temperatures. The highest CEs, always higher than 98%, were obtained at 25 °C probably due to the lower hydrogen ion diffusion from the bulk solution to the cathode. However, at that temperature, the SEC is higher than that at 40 °C because of the minor ion mobility and, thus, of the lower conductivity that produces higher cell voltage. The effect of cell voltage was demonstrated to be higher than CE on SEC. That is highlighted in Fig. 7b where SEC at 40 °C is always lower than that obtained at 25 °C, and it is always the lowest excluding the test performed at 50 A/m2. SEC at 40 °C varies from 0.04 to 0.12 kWh/kg, and it is lower than the one of the tests carried out at 25 °C: the difference between the two is 0.03 kWh/kg.
On the ground of these results, the best operative temperature seems to be 40 °C. Deposits obtained at 40 °C, by varying current density from 50 to 200 A/m2, are shown in Fig. 8. These three deposits show similar morphologies characterized by a lamellar growth that is more evident at 50 A/m2 (Fig. 8a). By increasing the current density, the grains, which are characterized by sharp edges at 50 A/m2, become more rounded and at 200 A/m2 cavities along the grain boundaries are visible (Fig. 8c). The most compact deposit is obtained at 100 A/m2 (Fig. 8b).
Proposed LABs Recycling Process
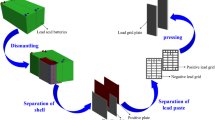
The simplified flowsheet of the proposed process is reported in Fig. 9. Exhausted lead batteries, after storage and draining to eliminate and recover the sulfuric acid, are ground and screened in order to separate coarse and fine particles.
Coarse particles are mainly constituted by plastics and lead particles coming from grids and poles: these two materials are easily separated by gravimetric methods, due to their very different density. Fine particles are constituted by lead paste. The two lead components are combined and sent to the furnace for the anode preparation. The molten material is poured into the titanium anode holder and used in the electrolytic cell for lead electrorefining. Lead is deposited on an AISI 316L cathode. Initially a pure electrolytic solution, obtained by dissolving 200 g/L HBF4, 1.2 g/L H3PO4, 10 g/L H3BO3, and 100 g/L PbO in distilled water, is fed into the cell. When about 90% of the anodic lead is consumed, the residue is sent back to the furnace after removing the titanium holder. Pure lead is periodically stripped from the cathode, while the electrolyte is continuously filtered before being fed into the cathodic compartment of the cell. This filtration is necessary to remove from the anolyte small particles that can detach from the anode surface during electrorefining.
Conclusions
Tests carried out on spent lead acid batteries allowed to set up an environmentally friendly recycling process that includes five different steps: (a) dismantling and sulfuric acid separation, (b) shredding, (c) screening for separating polymeric from metallic materials, (d) anode preparation via pyrometallurgical operation and (e) lead recovery by electrorefining.
Anode preparation and electrorefining steps have been studied to find the best operative conditions in order to optimize the recovery process avoiding SO2 formation. Compositional and thermogravimetric analyses of the mix used for producing the anode allowed to select the composition that minimizes the melting temperature. Different anodes have been characterized and tested in an electrorefining cell where the anolyte after purification is recirculated to the cathodic compartment. The best performing anode was the one having the titanium holder: it allowed to obtain a higher lead purity and a higher percentage of anode utilization.
Preliminary electrorefining tests carried out by using anodes with titanium holder gave for all the tested operative conditions good results in terms of CE, SEC, and deposit morphology, particularly by working at 40 °C and 100 A/m2.
References
Zhang W, Yang J, Wu X et al (2016) A critical review on secondary lead recycling technology and its prospect. Renew Sustain Energy Rev 61:108–122. https://doi.org/10.1016/j.rser.2016.03.046
Li M, Liu J, Han W (2016) Recycling and management of waste lead-acid batteries: a mini-review. Waste Manag Res 34:298–306. https://doi.org/10.1177/0734242X16633773
Ballantyne AD, Hallett JP, Riley DJ et al (2018) Lead acid battery recycling for the twenty-first century. R Soc Open Sci. https://doi.org/10.1098/rsos.171368
Ellis TW, Mirza AH (2010) The refining of secondary lead for use in advanced lead-acid batteries. J Power Sources 195:4525–4529. https://doi.org/10.1016/j.jpowsour.2009.12.118
Dai F, Huang H, Chen B et al (2019) Recovery of high purity lead from spent lead paste via direct electrolysis and process evaluation. Sep Purif Technol 224:237–246. https://doi.org/10.1016/j.seppur.2019.05.023
Brandon NP, Pilone D, Kelsall GH, Yin Q (2003) Simultaneous recovery of Pb and PbO2 from battery plant effluents. Part II. J Appl Electrochem 33:853–862. https://doi.org/10.1023/A:1025824130320
Lupi C, Pescetelli A (2008) Treatment of some liquid waste associated with lead battery recycling. In: Proceedings of the 2008 global symposium on recycling, waste treatment and clean technology, REWAS 2008. Cancun, Mexico, pp 807–813
Li Y, Yang S, Taskinen P et al (2019) Novel recycling process for lead-acid battery paste without SO 2 generation - reaction mechanism and industrial pilot campaign. J Clean Prod 217:162–171. https://doi.org/10.1016/j.jclepro.2019.01.197
Li Y, Yang S, Taskinen P et al (2020) Recycling of spent lead-acid battery for lead extraction with sulfur conservation. JOM 72:3186–3194. https://doi.org/10.1007/s11837-019-03885-y
Andrews D, Raychaudhuri A, Frias C (2000) Environmentally sound technologies for recycling secondary lead. J Power Sources 88:124–129. https://doi.org/10.1016/S0378-7753(99)00520-0
Liu N, Senthil RA, Zhang X et al (2020) A green and cost-effective process for recovery of high purity α-PbO from spent lead acid batteries. J Clean Prod 267:122107. https://doi.org/10.1016/j.jclepro.2020.122107
Li M, Yang J, Liang S et al (2019) Review on clean recovery of discarded/spent lead-acid battery and trends of recycled products. J Power Sources 436:226853. https://doi.org/10.1016/j.jpowsour.2019.226853
Tan yin S et al (2019) Developments in electrochemical processes for recycling lead–acid batteries. Curr Opin Electrochem 16:83–89. https://doi.org/10.1016/j.coelec.2019.04.023
Stevenson M (2009) Recycling|lead-acid batteries: overview. Encycl Electrochem Power Sources. https://doi.org/10.1016/B978-044452745-5.00402-0
Ojebuoboh F, Wang S, Maccagni M (2003) Refining primary lead by granulation-leaching-electrowinning. JOM 55:19–23. https://doi.org/10.1007/s11837-003-0082-2
Frias C, Ocaña N, Diaz G et al (2006) A clean-lead factory is available for lead-acid batteries recycling by means of the “cleanlead process.” The Minerals, Metals & Materials Society 2006 TMS Annual Meeting & Exhibition. San Antonio, Texas, pp 943–954
He W, Liu A, Guan J et al (2017) Pb electrodeposition from PbO in the urea/1-ethyl-3-methylimidazolium chloride at room temperature. RSC Adv 7:6902–6910. https://doi.org/10.1039/c6ra27383a
Yeh HW, Chang CJ, Huang GG, Chen PY (2019) Electrochemical conversion of ionic liquid-lead sulfate paste into metallic lead or lead(IV) oxide: Extracting lead from water-insoluble lead salt and formation of cobalt oxide electrocatalyst via galvanic displacement. J Electroanal Chem 834:64–70. https://doi.org/10.1016/j.jelechem.2018.12.055
Maccagni M (2014) New approaches on non ferrous metals electrolysis. Chem Eng Trans 41:61–66. https://doi.org/10.3303/CET1441011
Owais A (2015) Direct electrolytic refining of lead acid battery sSludgeDirekte elektrolytische Raffination von Bleibatterie-Schlamm. BHM Berg- Huettenmaenn Monatsh 160:134–144. https://doi.org/10.1007/s00501-014-0293-6
Rahmani L, Fitas R, Messai A, Ayesh AI (2019) Investigation of proton diffusion coefficient for PbO2 prepared from intermediate oxides. Russ J Electrochem 55:643–650. https://doi.org/10.1134/S1023193519070103
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Hojong Kim.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ciro, E., Lupi, C., Mondal, A. et al. Novel Lead Battery Recycling Process Combining Pyrometallurgical Anode Preparation and Electrorefining. J. Sustain. Metall. 7, 1727–1735 (2021). https://doi.org/10.1007/s40831-021-00447-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00447-y