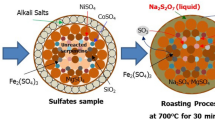
Abstract
In the present study, the leaching behavior of scandium from limonitic laterites under sulfation roasting–water leaching (SAL) was explored. The mineralogical analysis of limonitic laterites showed that scandium was associated with the iron phase. The roasting temperature played an important role in the iron phase conversion during the sulfation roasting process. With an increase in the roasting temperature from 100 to 800 °C, the iron phase (goethite, magnetite, and hematite) gradually converted to monoclinic Fe2(SO4)3 (400 °C), then to rhombohedral Fe2(SO4)3 (600 °C), and finally to hematite (800 °C). Due to the iron phase conversion mechanism, the leaching efficiency of scandium gradually increased first and then decreased with the increase of roasting temperature from 100 to 800 °C. When limonitic laterites roasted at temperature of 600 °C for 2 h with sulfuric acid/laterite ratio of 1/4 (mL/g), a total of 81.15% of scandium and only 0.37% of iron were extracted into leaching solution at 30 °C with the liquid-to-solid ratio of 4:1 (mL/g) for 1 h with agitation. The SAL process enables the selective and efficient enrichment of scandium from low-grade limonitic laterites with lower costs for equipment and operation compared to high-pressure acid leaching (HPAL), lower acid consumption, and lower dissolution of iron compared to atmospheric pressure acid leaching (AL).
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laterite nickel ore is a promising scandium resource with economically interesting concentrations and large amounts [1,2,3,4,5,6]. It can be simply divided into two types, limonitic and saprolitic laterite [7, 8]. Typical chemical composition and metallurgical process of two different types of laterite nickel ore is shown in Table 1 [1,2,3,4,5,6, 9, 10]. The limonitic laterite is rich in iron in the form of goethite, while the content of valuable metals such as nickel, cobalt, and scandium is relatively low [11, 12]. Goethite is the main Sc-bearing mineral in limonitic laterite [13, 14], and scandium is mainly presented in the following two states: (a) weakly adsorbed to the crystalline goethite surface and (b) as lattice substituents [15, 16]. These results indicate that the extraction of scandium is highly dependent upon the degree of the decomposition of the Sc-bearing minerals [17]. Scandium is usually extracted by the high-pressure acid leaching (HPAL) process, followed by the concentration and separation of scandium from the pregnant leach solution by using the pH-controlled precipitation [18] or solvent extraction [19]. The HPAL process provides high recoveries of scandium, allows acceptable acid consumption, and produces low residual iron in solution, but requires expensive autoclaves and has high maintenance costs and more neutralization of acid [20, 21]. Processes with a lower-cost alternative to the HPAL process especially for lower-grade ores have been widely studied due to growing interest from the industry. Among them, hydro-pyro integration in the processing of laterites could potentially be the way to process laterite with the complexion of their own merits [21,22,23]. The sulfation roasting–leaching route is shown to be quite promising [24]. Although corrosive gases are released during the above process, and the roasting temperature is relatively high. The whole procedure is conducted in atmosphere air, and so the requirement for equipment and facilities is not high. Conventional equipment with cheaper materials of construction and lower maintenance costs are acceptable. For example, rotary kilns can be used for roasting and non-pressure vessels in water leaching [25]. SAL has been investigated for the extraction of nickel and cobalt from laterites in which iron rejection is performed by the thermal decomposition of ferric sulfate at ~ 700 °C, while nickel and cobalt are selectively converted into water-leachable sulfates [17, 20, 21]. A summary of previous studies on the extraction of nickel and cobalt from laterites utilizing SAL is provided in Table 2(a). However, the leaching behavior of scandium in the SAL process is seldom studied in literatures. Anawati et al. [26] have previously explored the recovery of scandium from laterite nickel ore by acid roasting–water leaching. Nevertheless, the acid ore–water mixtures need to be heated at 50 °C for 24 h to ensure sulfation. Moreover, the leaching efficiency of iron is very high (~ 15%), and the selective extraction of scandium is not good enough. Most of the research in the selective sulfation roasting–water leaching field focused on the extraction of scandium from bauxite residue [27,28,29,30,31]. A summary of previous studies on the extraction of valuable metals from bauxite residue utilizing acid roasting–water leaching is provided in Table 2(b). However, the properties of bauxite residue and laterites are quite different [32,33,34,35]. Bauxite residue is the hydrometallurgical slag produced in the production process of the Bayer process, which is less difficult to deal with than the primary mineral resources [36,37,38,39,40,41,42,43,44]. Scandium is usually hosted in bauxite residue in the form of surface adsorption and is easy to extract [36, 45], while scandium in laterites usually exists in refractory minerals such as goethite and silicate in the form of isomorphism [1, 13]. As the demand for Al-Sc alloys and solid fuel cells increases year by year, there is an urgent need for a scandium supply chain with sufficient resources, stable processes, and reasonable prices.
In this work, the feasibility of selective extracting scandium from limonitic laterite by traditional sulfation roasting–water leaching was evaluated. The effects of various parameters on the leaching behavior of metals in the SAL process have been systematically investigated. Although the high-temperature transformation mechanism and water-leaching behavior of nickel and cobalt have been mentioned in this work, the extraction of these metals from limonitic laterite by SAL has been reported in the literature, so it is not the focus of this study. This work focuses on the migration and transformation mechanism of scandium in this process and optimizes the process parameters for efficient and selective recovery of scandium, to meet the growing demand for this rare and expensive metal in the world.
Experimental
Materials
Limonitic laterite used in the experiments was obtained from Sulawesi, Indonesia. The results for particle size, XRF, ICP, SEM–EDX, FTIR, XPS, and XRD analysis of the limonitic laterite material have already been reported elsewhere [13]. The composition of the raw material is listed in Table 3. Thermal gravimetric analysis and differential scanning calorimetry (TG-DSC, STA 449 C, Netzsch, German) were conducted on the samples from 30 to 800 °C with a linear heating rate of 10 °C/min using an air atmosphere and a flow rate of 20 mL/min. The concentrated sulfuric acid (98%, Sinopharm) used in this study was all the reagent grades, and all aqueous solutions were prepared by using deionized water.
Methods
Sulfation roasting of the limonitic laterite was carried out in a tubular atmosphere furnace (Brand: Hefei Kejing Material Technology Co., Ltd., Model: OTF-1200X). As an experimental procedure, 10.00 g ore was intensively mixed with 98 wt% H2SO4 with a predetermined acid to ore ratio in a porcelain crucible and then roasted in the furnace for a preset time at a desired temperature. The gas produced during the sulfation roasting process was absorbed with 2 mol/L NaOH solution. The acid-roasted samples were cooled and then leached with deionized water using a glass reaction vessel equipped with a magnetic stirrer. Subsequently, liquid/solid separation was performed with a Büchner funnel. The pregnant leach solution produced by each experiment was analyzed using an atomic emission spectrometer (ICP-AES, IRIS Intrepid II XSP, Thermo Electron Corporation, USA). The leaching efficiency of the metal was calculated according to Eq. (1).
where ηi is the leaching efficiency of metal i (%), Ci is the concentration of metal i in the leaching solution (g/L), V is the volume of leaching solution (L), m is the mass of raw material (g), and ωi is the mass fraction of metal i in raw material (wt%).
Results and Discussion
Effect of Roasting Temperature on Leaching
The TG-DSC curves of the limonitic laterite (Fig. 1) show that there are two obvious endothermic peaks at 72.4 °C and 329.6 °C. The endothermic peak was at 72.4 °C with a weight loss of 0.85% due to free water removal. Another major endothermic peak at 329.6 °C was due to the dehydroxylation of goethite (FeO(OH)) to hematite (Fe2O3) [16, 46]. For different laterites, the dehydroxylation temperature depended on the properties of the ore, such as crystallinity and the degree of cation substitution in the ore. The dehydroxylation temperature of goethite with poor crystallinity is lower than that with good crystallinity [16, 46]. Trivalent ions can substitute directly for iron, and due to differing bonding energies with the hydroxyl group, they can inhibit the dehydroxylation process [48].
In the sulfation roasting process, the roasting temperature is crucial for evaluating the sulfation effect, leaching efficiency, and energy consumption. The main driving mechanism for this selective process is the differences in thermal decomposition temperatures of different water-soluble metal sulfates [26]. The onset of Fe2(SO4)3 decomposition is reported to be 545 °C, and the onset of Sc2(SO4)3 decomposition is 700 °C [26, 44]. Some reports indicate that the initial decomposition temperatures of pure nickel and cobalt sulfates are in the ranges of 640 °C to 676 °C, and 644 °C to 690 °C, respectively, depending upon the composition of the atmosphere [20, 49, 50]. In this study, HSC Chemistry software (version 6.0) was utilized to establish the diagram of Gibbs free energy for the chemical reactions that are relevant to sulfation roasting (Fig. 2). First, the ore is mixed with concentrated H2SO4 to convert all the iron and scandium species to the corresponding sulfate forms (Eq. 2, 3, 4, and 5). Finally, the sulfated solids are roasted at 600 ~ 800 °C to convert the soluble contaminant sulfates (Fe2(SO4)3), which have lower thermal stability to water-insoluble oxide forms, while driving off the sulfate groups as SO3 vapors (Eq. 6). However, when the temperature is higher than 338 °C, the concentrated sulfuric acid will decompose into sulfur trioxide (Eq. 7), which will inhibit the conversion of metal to the corresponding sulfate.
Experiments to investigate the sulfation roasting temperature effect were carried out in the temperature range of 100 ~ 800 °C. Samples mixed with 98 wt% H2SO4 with an acid to ore ratio of 1:1 (mL/g) were roasted for 1 h and then water leached at 80 °C and liquid-to-solid ratio of 2:1 for 1 h. The results, shown in Fig. 3, show that the roasting temperature has a significant effect on metal conversion. With an increase in roasting temperature from 100 to 400 °C, the leaching efficiency of nickel, cobalt, scandium, and iron gradually increased. When the temperature increased to 400 °C, the leaching efficiency of valuable metals reached a maximum. When the temperature increases to 400 ~ 800 °C, the leaching of iron dramatically decreases due to the occurrence of the decomposition reaction of the corresponding sulfates. Meanwhile, the leaching efficiency of nickel, cobalt, and scandium decreased slightly and then decreased greatly. Consequently, further tests were carried out by keeping the roasting temperature fixed at 600 °C.
To investigate the mineralogical changes of the iron phases during the sulfation roasting process, samples roasted and water-leaching residue at 400, 600, and 800 °C were characterized with XRD (Figs. 4 and 5) and SEM–EDX (Fig. 6 and Table 4). It was observed from the XRD results (Fig. 4) that the major iron phase in the laterite was goethite, magnetite, and hematite, indicating that the iron phase was gradually digested and replaced with sulfate phases at 400 and 600 °C. However, the crystal structures of the obtained sulfate phases are different. It is monoclinic Fe2(SO4)3 (iron(III) sulfate: PDF#75-1767) [51, 52] at 400 °C and becomes rhombohedral Fe2(SO4)3 (iron sulfate: PDF#42-0229)[53] as the temperature increases to 600 °C. As the temperature further increased to 800 °C, the hematite peaks became stronger due to the thermal decomposition of the iron sulfate. In addition, it can also be found from Fig. 4 that there is obvious quartz phase in the roasted sample. As shown in Fig. 5, the major phase of the water-leaching residue at different roasting temperatures is hematite, and the minor phase is quartz. Because scandium is associated with iron, the leaching of scandium is determined by the phase transformation from iron phases (goethite, magnetite, and hematite) to monoclinic Fe2(SO4)3 (400 °C), then to rhombohedral Fe2(SO4)3 (600 °C), and finally to hematite (800 °C). Backscattered SEM images of samples roasted and water-leaching residue are presented in Fig. 6. EDX analysis was used to identify the composition of different regions in SEM images. The EDX results associated with the 12 main regions marked in Fig. 6 are presented in Table 4. The results of SEM–EDX analysis corroborate the observed XRD spectra, confirming that the resulting iron phase after sulfating roasting depends on the roasting temperature.
Effect of Sulfuric Acid Amount on Leaching
A series of experiments were carried out by varying the ratio of sulfuric acid to ore from 0.25 (1:4 ratio) to 1.25 (5:4 ratio). The samples were roasted for 1 h at a fixed temperature of 600 °C. After roasting, the samples were leached with water at 80 °C and a liquid-to-solid ratio of 2:1 (mL/g) for 1 h with agitation. Figure 7 shows the extraction of the scandium and major elements in the leachate with an increasing initial acid to ore ratio. As seen in Fig. 7, the scandium, nickel, and cobalt extraction gradually decreases with increasing acid to ore ratio. However, iron extraction dramatically increases with increasing acid-to-ore ratio. Probably, the excess acid leads to more iron sulfate formation, which to some extent hinders the subsequent gas–solid sulfation reaction of nickel, cobalt, and scandium in the iron phase [54]. Furthermore, the excess acid can decompose into SO3 with time (Eq. 7), hindering the decomposition of soluble iron sulfate into insoluble compounds (Eq. 6) [25].
Effect of Roasting Duration on Leaching
Roasting experiments were conducted for different durations to understand the effect of roasting time on the dissolution of scandium and major elements. In these experiments, samples were prepared with a constant sulfuric acid to ore ratio of 1:4 (mL/g). Samples were roasted at 600 °C for different durations. Finally, the samples were leached with water at 80 °C and a liquid-to-solid ratio of 2:1 (mL/g) for 1 h with agitation. Figure 8 shows the effect of roasting duration on the leaching of scandium and major elements. The scandium extraction slightly decreases with roasting time up to 2 h. A further increase in roasting time has a negative effect on scandium dissolution due to the increasing amount of sulfates that are decomposed to oxides that are insoluble in water. The extraction of iron decreases with an increase in roasting time, again because of the increased amount of sulfates that are decomposed. Therefore, roasting is performed at a higher temperature, and the roasting time needed for selective leaching will be shorter. The optimal roasting time is determined considering two main factors: scandium dissolution and iron dissolution. Based on the results, the optimal roasting time at 600 °C is 2 h. At this temperature, scandium dissolution is still high, while iron dissolution is low.
Effect of Water Immersion Parameters on Leaching
Leaching experiments were conducted with agitation at different leaching durations, liquid-to-solid ratios, and leaching temperatures for samples roasted at 600 °C and acid-to-ore ratios of 1:4 (mL/g) for 2 h. Leaching experiments were conducted at different L/S ratios to determine the effect of agitation on the dissolution of scandium and major elements (Fig. 9a). Figure 9a shows that the leaching efficiency of scandium and cobalt increased gradually with a liquid-to-solid ratio up to 4:1 (mL/g), beyond which they tended to be stable with a further increase in the liquid-to-solid ratio. This shows that the effect of the L/S ratio on nickel dissolution is insignificant. Additionally, no change in the dissolution of iron was observed. iron dissolution is less than 1% at all L/S ratios. From the results, it can be concluded that scandium can be selectively dissolved even at L/S ratios of 4:1 (mL/g). The leaching temperature has an insignificant effect on the leaching of all metals. Therefore, the optimal leaching temperature was 30 °C. To study the extraction of metals at different leaching times, a series of experiments were conducted while other conditions were kept constant, including 4:1 (mL/g) L/S ratios and 30 °C of leaching temperature. The results are shown in Fig. 9c. Figure 9c indicates that leaching time has a similar effect on scandium, nickel, and cobalt. When the leaching time is 1 h, the leaching efficiency of scandium, nickel, cobalt, and iron reaches 80.47%, 61.37%, 80.06%, and 0.35%, respectively. After 1 h, the leaching of scandium, nickel, and cobalt remained constant. Therefore, the optimal leaching time was 1 h.
Through the above single-factor experimental results, determine the following suitable process conditions: the roasting temperature is 600 °C, the roasting acid to ore ratio is 1/4 (mL/g), and the roasting time is 2 h. After roasting, the samples were leached with water at 30 °C and liquid-to-solid ratio of 4:1 (mL/g) for 1 h with agitation. As shown in Fig. 9d, under these conditions, the leaching efficiency of nickel, cobalt, scandium, and iron are 62.53%, 80.83%, 81.15%, and 0.37%, respectively. The main chemical analysis of the leaching solution is given in Table 5. According to the analysis results, compared with the conventional high-pressure acid leaching (HPAL) process, the SAL process proposed in this research has a lower iron content in the leaching solution. Therefore, the existing pH-controlled precipitation method [18, 55,56,57] or solvent extraction method [19] can be used to separate and purify scandium in the leaching solution.
Preliminary Economic Analysis
Based on the mechanism described above and the optimized experimental results, SAL process is proposed (Fig. 10). Rather than HPAL, SAL process for laterites has not been commercially proven. Therefore, a preliminary economic analysis of the process is shown in Table 6, including the estimated costs of consumed reagents, values of the generated products, equipment and operating costs, energy consumption, and gas exhaust treatments. While the calculations are based on the 10.00 g scale experiments, the results are given for 1 ton of laterites. Comparing the SAL process with AL and HPAL process, it can be seen that the proposed process has some advantages. First, the reagents consumption is reasonable. Based on the data of the study, about 460 kg of sulfuric acid is used for sulfating one ton of ore. Acid consumption is much lower than AL process. Moreover, gas exhaust (SO2/SO3) can be recovered in the associated sulfur burning acid plant (generally, metallurgical plants will be equipped with acid-making system). Thus, the acid depletion is much lower and it will close or equal to that in HPAL under an ideal condition. Second, scandium leaching is high and the main impurity iron leaching is very low. About 81.15% of scandium can be extracted and the co-leaching of iron can be controlled to about 0.37%, under the optimal conditions in the study. Although AL process providing similar or better leaching performances than SAL process, the separation of iron and scandium is complicated due to the chemical similarity. Actually, some complicated processes including removal of the iron by precipitation are necessary to be used in industry. Thus, both the material cost and operating cost are high. Third, the investment costs are relatively low and easy to be achieved in engineering. Since the proposed process mainly relies on two conventional equipments (i.e., corrosion-resistant rotary kiln/mixing equipment for the sulfation roasting, an atmospheric stirred tank for water leaching), the investment, and operating costs are relatively low.
However, the SAL process also has some shortcomings. The energy consumption of this process is higher, mainly including the energy consumption of bituminous coal, electricity, water, and gas, among which electricity consumption accounts for the largest proportion [22, 58]. Moreover, there are still numerous low content SO2 streams needing recovery and disposal, which will also increase the technical and production costs. Similarly, the AL process also produces a large amount of acid-containing wastewater that needs to be disposal. In addition, the physical characteristics of residue obtained by AL are unstable and difficult to handle. From the economic perspective, the SAL process is feasible as long as low content SO2 and waste heat can be reasonably utilized.
Conclusions
In the present study, the leaching behavior of scandium from limonitic laterite ores under SAL was explored. The mineralogical analysis of limonitic laterites showed that scandium was associated with the iron phase. The roasting temperature played an important role in the iron phase conversion during the sulfation roasting process. With an increase in the roasting temperature from 100 to 800 °C, the iron phase (goethite, magnetite, and hematite) is gradually converted to monoclinic Fe2(SO4)3 (400 °C), then to rhombohedral Fe2(SO4)3 (600 °C), and finally to hematite (800 °C). The iron phase conversion mechanism could determine the leaching of scandium. An increase in the sulfuric acid amount decreases nickel, cobalt, and scandium dissolution and increases the leaching efficiency of iron. Most likely, the excess acid can decompose into SO3, which will hinder the decomposition of soluble iron sulfate into insoluble compounds and the conversion of scandium, nickel, and cobalt in the iron phase to the corresponding sulfate. When limonitic laterites roasted at temperature of 600 °C for 2 h with sulfuric acid/laterite ratio of 1/4 (mL/g), a total of 81.15% of scandium and only 0.37% of iron were extracted into leaching solution at 30 ℃ with the liquid-to-solid ratio of 4:1 (mL/g) for 1 h with agitation. The existing pH-controlled precipitation method or solvent extraction method can be used to separate and purify scandium in the leaching solution. The SAL process enables the selective and efficient enrichment of scandium from low-grade limonitic laterite with lower costs for equipment and operation compared to HPAL, lower acid consumption, and lower dissolution of iron compared to AL.
References
Chasse M, Griffin WL, O’Reilly SY, Calas G (2017) Scandium speciation in a world-class lateritic deposit. Geochem Perspect Lett 3(2):105–113. https://doi.org/10.7185/geochemlet.1711
Kim J, Azimi G (2020) An innovative process for extracting scandium from nickeliferous laterite ore: carbothermic reduction followed by NaOH cracking. Hydrometallurgy 191:1–11. https://doi.org/10.1016/j.hydromet.2019.105194
Luo J, Li G, Rao M, Peng Z, Zhang Y, Jiang T (2015) Atmospheric leaching characteristics of nickel and iron in limonitic laterite with sulfuric acid in the presence of sodium sulfite. Miner Eng 78:38–44. https://doi.org/10.1016/j.mineng.2015.03.030
Meshram P, Abhilash PBD (2019) Advanced review on extraction of nickel from primary and secondary sources. Miner Process Extr Metall Rev 40(3):157–193. https://doi.org/10.1080/08827508.2018.1514300
Van der Ent A, Baker AJM, van Balgooy MMJ, Tjoa A (2013) Ultramafic nickel laterites in Indonesia (Sulawesi, Halmahera): Mining, nickel hyperaccumulators and opportunities for phytomining. J Geochem Explor 128:72–79. https://doi.org/10.1016/j.gexplo.2013.01.009
Yan K, Liu L, Zhao H, Tian L, Xu Z, Wang R (2021) Study on extraction separation of thioarsenite acid in alkaline solution by CO32-type Tri-n-octylmethyl-ammonium chloride. Front Chem 8:1–13. https://doi.org/10.3389/fchem.2020.592837
Garces-Granda A, Lapidus GT, Restrepo-Baena OJ (2018) The effect of calcination as pre treatment to enhance the nickel extraction from low-grade laterites. Miner Eng 120:127–131. https://doi.org/10.1016/j.mineng.2018.02.019
Xiao J, Xiong W, Zou K, Chen T, Wang Z (2021) Extraction of nickel from magnesia-nickel silicate ore. J Sustain Metall 7:642–652. https://doi.org/10.1007/s40831-021-00364-0
Guo X, Zhang C, Tian Q, Yu D (2021) Liquid metals dealloying as a general approach for the selective extraction of metals and the fabrication of nanoporous metals: a review. Mater Today Commun 26(4):102007. https://doi.org/10.1016/j.mtcomm.2020.102007
Makuza B, Tian Q, Guo X, Chattopadhyay K, Yu D (2021) Pyrometallurgical options for recycling spent lithium-ion batteries: a comprehensive review. J Power Sources 491:229–622. https://doi.org/10.1016/j.jpowsour.2021.229622
Chang Y, Zhai X, Li B, Yan F (2010) Removal of iron from acidic leach liquor of lateritic nickel ore by goethite precipitate. Hydrometallurgy 101(1–2):84–87. https://doi.org/10.1016/j.hydromet.2009.11.014
Zhao D, Ma B, Shi B, Zhou Z, Wang C (2020) Mineralogical characterization of limonitic laterite from Africa and its proposed processing route. J Sustain Metall 6(3):491–503. https://doi.org/10.1007/s40831-020-00290-7
Tian Q, Dong B, Guo X, Xu Z, Wang Q, Li D, Yu D (2021) Comparative atmospheric leaching characteristics of scandium in two different types of laterite nickel ore from Indonesia. Miner Eng 173:107212. https://doi.org/10.1016/j.mineng.2021.107212
Pintowantoro S, Widyartha AB, Setiyorini Y, Abdul F (2021) Sodium thiosulfate and natural sulfur: novel potential additives for selective reduction of limonitic laterite ore. J Sustain Metall 7(2):481–494. https://doi.org/10.1007/s40831-021-00352-4
Liu K, Chen Q, Hu H (2009) Comparative leaching of minerals by sulphuric acid in a Chinese ferruginous nickel laterite ore. Hydrometallurgy 98(3–4):281–286. https://doi.org/10.1016/j.hydromet.2009.05.015
Schwertmann U, Schulze DG, Murad E (1982) Identification of ferrihydrite in soils by dissolution kinetics, differential X-ray diffraction, and Mossbauer spectroscopy. J Soil Sci Soc Am 46(4):869–875. https://doi.org/10.2136/sssaj1982.03615995004600040040x
Fan C, Zhai X, Yan F, Chang Y, Li B, Zhang TA (2010) Extraction of nickel and cobalt from reduced limonitic laterite using a selective chlorination–water leaching process. Hydrometallurgy 105(1–2):191–194. https://doi.org/10.1007/s40831-021-00352-4
Kaya S, Dittrich C, Stopic S, Friedrich B (2017) Concentration and separation of Sc from Ni laterite ore processing streams. Metals 7(12):557. https://doi.org/10.3390/met7120557
Ferizoglu E, Kaya S, Topkaya YA (2018) Solvent extraction behaviour of scandium from lateritic nickel-cobalt ores using different organic reagents. Physicochem Probl Miner Process 54(2):538–545. https://doi.org/10.5277/ppmp1855
Guo X, Li D, Park KH, Tian Q, Wu Z (2009) Leaching behavior of metals from a limonitic nickel laterite using a sulfation–roasting–leaching process. Hydrometallurgy 99(3–4):144–150. https://doi.org/10.1016/j.hydromet.2009.07.012
Li J, Chen Z, Shen B, Xu Z, Zhang Y (2017) The extraction of valuable metals and phase transformation and formation mechanism in roasting-water leaching process of laterite with ammonium sulfate. J Clean Prod 140:1148–1155. https://doi.org/10.1016/j.jclepro.2016.10.050
Oxley A, Barcza N (2013) Hydro–pyro integration in the processing of nickel laterites. Miner Eng 54:2–13. https://doi.org/10.1016/j.mineng.2013.02.012
Zhang L, Guo X, Tian Q, Li D, Zhong S, Qin H (2022) Improved thiourea leaching of gold with additives from calcine by mechanical activation and its mechanism. Miner Eng 178:107403. https://doi.org/10.1016/j.mineng.2022.107403
Ribeiro P, Neumann R, Santos I, Rezende MC, Radino-Rouse P, Dutra A (2019) Nickel carriers in laterite ores and their influence on the mechanism of nickel extraction by sulfation-roasting-leaching process. Miner Eng 131:90–97. https://doi.org/10.1016/j.mineng.2018.10.022
Wang W, Du S, Guo L, Tang J, Lu Y, Dong L, Wang W, Du S, Guo L, Tang J (2017) Extraction of nickel from Ramu laterite by sulphation roasting-water leaching. In: 3rd International Conference on Chemical Materials and Process (ICCMP 2017) 1879(1):050004. https://doi.org/10.1063/1.5000474
Anawati J, Yuan R, Kim J, Azimi G (2020) Selective recovery of scandium from nickel laterite ore by acid roasting–water leaching. In: Anawati J, Yuan R, Kim J, Azimi G (eds.) Rare metal technology 2020, Springer, Cham, pp 77–90. https://doi.org/10.1007/978-3-030-36758-9_8
Reid S, Tam J, Yang M, Azimi G (2017) Technospheric mining of rare earth elements from bauxite residue (red mud): process optimization, kinetic investigation, and microwave pretreatment. Sci Rep 7(1):15252. https://doi.org/10.1038/s41598-017-15457-8
Rivera RM, Ulenaers B, Ounoughene G, Binnemans K, Van Gerven T (2018) Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner Eng 119:82–92. https://doi.org/10.1016/j.mineng.2018.01.023
Liu Z, Zong Y, Hongxu LI, Jia D, Zhao Z (2017) Selectively recovering scandium from high alkali Bayer red mud without impurities of iron, titanium and gallium. J Rare Earths 9(35):844–941. https://doi.org/10.1016/S1002-0721(17)60992-X
Narayanan RPN, Kazantzis NK, Emmert MH (2018) Selective process steps for the recovery of scandium from jamaican bauxite residue (red mud). ACS Sustain Chem Eng 6(1):1478–1488. https://doi.org/10.1021/acssuschemeng.7b03968
Onghena B, Borra CR, Gerven TV, Binnemans K (2017) Recovery of scandium from sulfation-roasted leachates of bauxite residue by solvent extraction with the ionic liquid betainium bis(trifluoromethylsulfonyl)imide. Sep Purif Technol 176:208–219. https://doi.org/10.1016/j.seppur.2016.12.009
Gamaletsos PN, Godelitsas A, Filippidis A, Pontikes Y (2018) The rare earth elements potential of Greek bauxite active mines in the light of a sustainable REE demand. J Sustain Metall 5:20–47. https://doi.org/10.1007/s40831-018-0192-2
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Gerven TV (2016) Recovery of rare earths and other valuable metals from bauxite residue (red mud): a review. J Sustain Metall 2:365–386. https://doi.org/10.1007/s40831-016-0068-2
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Gerven TV (2016) Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J Sustain Metall 2(1):28–37. https://doi.org/10.1007/s40831-015-0026-4
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Gerven TV (2016) Recovery of rare earths and major metals from bauxite residue (red mud) by alkali roasting, smelting, and leaching. J Sustain Metall 3(2):393–404. https://doi.org/10.1007/s40831-016-0103-3
Gentzmann MC, Schraut K, Vogel C, Gbler HE, Adam C (2021) Investigation of scandium in bauxite residues of different origin. Appl Geochem 126:104898. https://doi.org/10.1016/j.apgeochem.2021.104898
Zhang XK, Zhou KG, Chen W, Lei QY, Huang Y, Peng CH (2019) Recovery of iron and rare earth elements from red mud through an acid leaching-stepwise extraction approach. J Cent South Univ 26(2):458–466. https://doi.org/10.1007/s11771-019-4018-6
Zhou KG, Teng CY, Zhang XK, Peng CH, Chen W (2018) Enhanced selective leaching of scandium from red mud. Hydrometallurgy 182:57–63. https://doi.org/10.1016/j.hydromet.2018.10.011
Ding W, Bao SX, Zhang YM, Xiao JH (2022) Efficient selective extraction of scandium from red mud. Miner Process Extr Metall Rev. https://doi.org/10.1080/08827508.2022.2047044
Li G, Ye Q, Deng B, Luo J, Rao M, Peng Z, Jiang T (2018) Extraction of scandium from sc-rich material derived from bauxite ore residues. Hydrometallurgy 176:62–68. https://doi.org/10.1016/j.hydromet.2018.01.007
Ochsenkuhnpetropulu M, Lyberopulu T, Parissakis G (1995) Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal Chim Acta 315:231–237. https://doi.org/10.1016/0003-2670(95)00309-N
Abhilash HS, Schippers A (2021) Distribution of scandium in red mud and extraction using Gluconobacter oxydans. Hydrometallurgy 202:105621. https://doi.org/10.1016/j.hydromet.2021.105621
Zhang D, Chen H, Nie Z, Xia J, Li E, Fan X, Zheng L (2020) Extraction of Al and rare earths (Ce, Gd, Sc, Y) from red mud by aerobic and anaerobic bi-stage bioleaching. Chem Eng J 401:125914. https://doi.org/10.1016/j.cej.2020.125914
Borra CR, Mermans J, Blanpain B, Pontikes Y, Binnemans K, Gerven TV (2016) Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner Eng 92:151–159. https://doi.org/10.1016/j.mineng.2016.03.002
Botelho Junior AB, Romano Espinosa DC, Soares Tenorio JA (2021) Extraction of scandium from critical elements-bearing mining waste: silica gel avoiding in leaching reaction of bauxite residue. J Sustain Metall 7:1627–1642. https://doi.org/10.1007/s40831-021-00434-3
Swamy YV, Kar BB, Mohanty JK (2003) Physico-chemical characterization and sulphatization roasting of low-grade nickeliferous laterites. Hydrometallurgy 69(1):89–98. https://doi.org/10.1016/S0304-386X(03)00027-6
Anawati J, Azimi G (2019) Recovery of scandium from Canadian bauxite residue utilizing acid baking followed by water leaching. Waste Manage 95:549–559. https://doi.org/10.1016/j.wasman.2019.06.044
Harris CT, Peacey JG, Pickles CA (2011) Selective sulphidation of a nickeliferous lateritic ore. Miner Eng 24(7):651–660. https://doi.org/10.1016/j.mineng.2010.10.008
Tagawa H (1984) Thermal decomposition temperatures of metal sulfates. Thermochim Acta 80(1):23–33. https://doi.org/10.1016/0040-6031(84)87181-6
Siriwardane RV, Poston J Jr, Fisher EP, Shen MS, Miltz AL (1999) Decomposition of the sulfates of copper, iron (II), iron (III), nickel, and zinc: XPS, SEM, DRIFTS, XRD, and TGA study. Appl Surf Sci 152(3–4):219–236. https://doi.org/10.1016/S0169-4332(99)00319-0
Long GJ, Longworth G, Battle P, Cheetham AK, Thundathil RV, Beveridge D (1979) A study of anhydrous iron (III) sulfate by magnetic susceptibility, Moessbauer, and neutron diffraction techniques. Inorg Chem 18(3):624–632. https://doi.org/10.1021/ic50193a021
Coombs P, Munir Z (1989) The decomposition of iron (III) sulfate in air. J Therm Anal 35(3):967–976. https://doi.org/10.1007/BF02057253
Mason CW, Gocheva I, Hoster HE, Denis Y (2014) Iron (III) sulfate: a stable, cost effective electrode material for sodium ion batteries. Chem Commun 50(18):2249–2251. https://doi.org/10.1039/c3cc47557c
Yu D, Utigard TA, Barati M (2014) Fluidized bed selective oxidation-sulfation roasting of nickel sulfide concentrate: Part II. Sulfation roasting. Metall Mater Trans B 45(2):662–674. https://doi.org/10.1007/s11663-013-9959-9
Yagmurlu B, Dittrich C, Friedrich B (2016) Precipitation trends of scandium in synthetic red mud solutions with different precipitation agents. J Sustain Metall 3(1):90–98. https://doi.org/10.1007/s40831-016-0098-9
Sadri F, Kim R, Ghahreman A (2021) Behavior of light and heavy rare-earth elements in a two-step Fe and Al removal process from rare-earth pregnant leach solutions. J Sustain Metall 7(3):1327–1342. https://doi.org/10.1007/s40831-021-00423-6
Peters EM, Kaya S, Dittrich C, Forsberg K (2019) Recovery of scandium by crystallization techniques. J Sustain Metall 5(1):48–56. https://doi.org/10.1007/s40831-019-00210-4
McDonald RG, Whittington BI (2008) Atmospheric acid leaching of nickel laterites review Part I. Sulphuric acid technologies. Hydrometallurgy 91(1–4):35–55. https://doi.org/10.1016/j.hydromet.2007.11.009
Wan X, Taskinen P, Shi J, Jokilaakso A (2021) A potential industrial waste–waste co-treatment process of utilizing waste SO2 gas and residue heat to recover Co, Ni, and Cu from copper smelting slag. J Hazard Mater 414:125541. https://doi.org/10.1016/j.jhazmat.2021.125541
Acknowledgements
The authors gratefully acknowledge the financial support from the National Key R&D Program of China (Grant Nos. 2019YFC1907402 and 2018YFC1902501) and National Natural Science Foundation of China (Grant Nos. 51922108, 52074363, and 52104355).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
The contributing editor for this article was Zhi Sun.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, B., Tian, Q., Guo, X. et al. Leaching Behavior of Scandium from Limonitic Laterite Ores Under Sulfation Roasting–Water Leaching. J. Sustain. Metall. 8, 1078–1089 (2022). https://doi.org/10.1007/s40831-022-00551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00551-7