Abstract
The long-term storage of bauxite residue (red mud) is harmful to the environment and the tailing ponds also cover large areas. At the moment there is no large-scale utilization of bauxite residue. However, some bauxite residues contain considerable concentrations of rare-earth elements (REEs) and the recovery of these REEs together with(out) other metals and utilization of the generated residue in other applications (e.g., building materials) can solve the storage problem of bauxite residue. This paper reviews the recovery of REEs, possibly alongside other valuable metals, from bauxite residue. REEs can be recovered from bauxite residue by direct leaching or by smelting followed by leaching. The main disadvantages of direct acid leaching are the consumption of large amounts of acid for neutralization, the handling of large volumes of effluents, and the difficulty in using the bauxite residue after leaching. Recovery of iron prior to leaching can improve the economics of the process. However, high alumina in the bauxite residue increases the flux and acid consumption. Therefore, alumina needs to be removed by alkali roasting prior to smelting in order to decrease the flux and acid consumption. The alkali roasting–smelting–leaching process allows recovery of aluminum, iron, titanium, and REEs from bauxite residue. The residue generated in this process can be used in building materials and cementitious binders. Other processes with commercial potential are the Orbite, the pressure leaching, and the acid baking processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bauxite Residue
Bauxite residue (red mud) is a reddish-brown solid waste generated during the production of purified aluminum (oxy) hydroxides from bauxite. Bauxite is the main aluminum ore and it is a mixture of the aluminum minerals such as gibbsite (Al(OH)3), boehmite (γ-AlO(OH)), and diaspore (α-AlO(OH)), as well as other minerals, such as hematite (Fe2O3), goethite (FeO(OH)), quartz (SiO2), rutile/anatase (TiO2), and kaolinite (Al2Si2O5(OH)4). Alumina must be purified before it can be used as a feed for the production of aluminum metal by electrolysis in the Hall–Héroult process. This purification is performed by the Bayer process, where bauxite is digested in a concentrated sodium hydroxide solution at temperatures between 150 and 250 °C, in an autoclave, at pressures up to 40 atm (Fig. 1). Under these conditions, aluminum hydroxide dissolves in the aqueous solution due to the amphoteric character of aluminum. The other components of bauxite do not dissolve. The solution is then clarified for removing the solid impurities. The waste slurry is called red mud, while the solid fraction is called bauxite residue. Although red mud and bauxite residue are often used as synonyms, there is in fact a difference between these terms. About 1–1.5 metric tons of bauxite residue is generated for each ton of alumina produced [1].
Flow sheet for the Bayer process. Reproduced from Ref. [2]
Alumina can also be produced from silica-rich bauxite ores, low grade ores, and diasporic ores by alkali sintering/roasting process as the Bayer process consumes a large amount of soda or requires high temperatures for these kinds of ores [3]. This technology is only used in a few plants due to the high energy consumption. This process is explained in detail in “Aluminium recovery from bauxite residue in view of iron, titanium and/or REEs recovery” section.
Annually, about 140 million tons of bauxite residue are produced [4], while more than 2.7 × 109 tons [5] were already stockpiled by 2011. The cumulative amount of bauxite residue generated by 2015 is estimated to be close to 4 × 109 tons [6]. The pH of the wet bauxite residue slurry is about 12, due to the presence of residual NaOH. The bauxite residue is stored in disposal sites typically stretching over many square kilometers and this poses a significant hazard [2]. This disposal site also helps in dewatering of bauxite residue. Currently, the preferred disposal method is lagooning as marine dumping has been ceased, as dumping of bauxite residue in the sea may adversely affect the marine ecological balance. The long-term storage of bauxite residue is a major issue, since the bauxite residue disposal sites not only occupy vast areas of land, which could otherwise be used for agriculture, but the bauxite residue also can lead to serious pollution of the surrounding soil, air, and groundwater. The dike breach of a bauxite residue stockpiling yard at the Ajkai Timfoldgyar Zrt alumina plant in Hungary on October 4, 2010, released between 600,000 and 700,000 m3 of red mud and caused serious environmental pollution [7]. This incident shows that there is a need for remediation of the bauxite residue stockpiles and a utilization solution of the stored (legacy sites) and fresh bauxite residue. However, there are currently no applications of bauxite residue besides minor use in cement and ceramic production [1, 5, 8]. Bauxite residue has been reported to have several possible applications [4, 5, 9] in the field of pollution control (wastewater treatment, adsorption, and purification of acid waste gases), as a coagulant, adsorbent, and catalyst, in pigments and paints, in ceramic production, for soil amendment, metal recovery, and in building materials. However, none of these applications has been commercially exploited on an industrial scale yet [10].
Bauxite residue is a polymetallic material and a potential source of several metals [11]. Extraction of metals from bauxite residue could become economically feasible provided that suitable extraction processes are available. Iron oxides are the main constituent of bauxite residue and it can make up to 60 % of the mass of the bauxite residue. In fact, the red color of bauxite residue is caused by iron(III) oxides (mostly hematite, Fe2O3). Bauxite residue also contains oxides of aluminum, titanium, silicon, and some valuable metals, such as rare-earth elements (REEs). The chemical compositions of the bauxite residue of different origins are summarized in Table 1. The actual composition of bauxite residue depends on the type of bauxite, the mining location, and the process parameters of the Bayer process. During the processing of bauxite by the Bayer process, the REEs report to the bauxite residue. The REEs are enriched in bauxite residue compared to bauxite by a factor of approximately two [12]. The REEs are considered to be worth recovering from bauxite residue, since they are currently considered as critical raw materials by many countries [10].
REEs in Bauxite Residue
The REEs are the fifteen metallic elements of the lanthanide series, together with yttrium and scandium [14]. These elements are subdivided into light rare-earth elements (LREEs) which include lanthanum to europium, and heavy rare-earth elements (HREEs), which include the remaining lanthanide elements, gadolinium to lutetium, as well as yttrium [14, 15]. Scandium is not included in either the LREE or HREE classifications, although it is considered to be a rare-earth element [14].
The consumption of REEs is increasing due to an increase in demand for green technologies. The REEs are used in the production of permanent magnets, lamp phosphors, rechargeable NiMH batteries, catalysts and other applications [16]. REEs are classified under the most critical raw materials group, with the highest supply risk [17]. REEs are not rare, however, it is difficult to find their minerals in economic concentrations [14, 18]. There are approximately 100 million tons of proven rare-earth oxide reserves in the world, spread over more than 30 countries [19]. However, 90 % of the world’s supply currently originates in China [20, 21]. Increased demand in China has prompted the Chinese government to limit yearly export quotas to approximately 35,000 tons of rare-earth oxides, whereas non-Chinese annual demand was expected to reach 80,000 tons by the year 2015 [16, 19]. However, in August 2014, the World Trade Organization (WTO) ruled against China. Hence, China abolished export quota on REEs on December 31, 2014. However, analysts suggest that the abolition of the REEs export quotas will not prevent China from significantly influencing the global market or from finding other ways to give preferential treatment to domestic companies [22]. In the meantime REEs consumption is increasing rapidly [23]. The restricted supply is being met by the development of new rare-earth mining projects, each having its own unique mining and processing challenges [19]. Therefore, rare-earth recovery from secondary sources like bauxite residue can be considered as one of the options to meet the demand.
One of the factors that affect the composition of the bauxite residue is the type of the ore. Bauxite ores can be classified into three categories based on host-rock lithology. They are the lateritic bauxites (88 %), the karst bauxites (11.5 %), and the Tikhvin-type bauxites (0.5 %) [24, 25]. Bauxite deposits lying on carbonates are classified as karst bauxites. Bauxite deposits lying on aluminosilicate rocks can be further subdivided in lateritic bauxites and Tikhvin-type bauxites [26]. The world-wide geographic distribution of bauxite deposits is shown in Fig. 2. From this figure, it becomes evident that karst bauxite reserves are mainly located in Europe, Jamaica, Russia, and China. Karst bauxites contain higher REEs concentrations compared to lateritic bauxites [27]. REEs are present in the bauxite ore as ions adsorbed on the surface of the minerals, replacing similar ions in some minerals, and in REE minerals [26]. The REEs present in a bauxite ore end up in its bauxite residue during the Bayer process [8]. The REE concentrations in the Jamaican bauxite residue is 1500–2500 ppm [28]. The average concentration of REEs in Greek karst bauxite residue is ca. 1000 ppm [12]. A detailed analysis of the rare-earth content of Greek bauxite residue is given in Table 2.
World distribution of bauxite deposits. Reproduced from Ref. [29]
Scandium in Bauxite Residue
Scandium is the most valuable element among the REEs that are present in the bauxite residue. It represents more than 95 % of the economic value of REEs in bauxite residue [8]. The current price of scandium oxide (99.99 % purity) is around 5000 US$/kg [30]. Scandium is mainly used in advanced solid oxide fuel cells (SOFC) and as an alloying element (<2 wt%) in aluminum alloys [31]. Scandium is used as a stabilizing agent for zirconia in SOFCs. It helps in lowering the operation temperatures, extending the life, and increasing the power density of the SOFC unit. As an alloying element, scandium increases the strength of aluminum alloys by modifying the grain structure. It also helps in increasing weldability. Aluminum–scandium alloys are mainly used in the aerospace industry, bicycle frames, and baseball bats. Other uses for scandium include electronics, lasers, high intensity lighting, and radioactive isotopes.
The average crustal abundance of scandium is about 22 ppm [32], which is more than that of better-known metals such as lead and tin. However, scandium is rarely concentrated in nature because it lacks affinity to combine with the common ore-forming anions [30]. Hence, time and geologic forces only rarely form scandium concentrations over 100 ppm. The scandium minerals with a high scandium content, e.g., thortveitite ((Sc,Y)2Si2O7) and kolbeckite (ScPO4·2H2O)), are rare [8, 32]. In general, scandium is obtained by mining of thortveitite deposits and as a byproduct during the processing of uranium, titanium, tin, tantalum, and tungsten ores as well as from phosphate rock [33]. Scandium is mainly produced in China (from titanium and REE ores), Kazakhstan (from uranium ore), Russia (from apatite ore), and Ukraine (from uranium ore). The total scandium oxide production in 2014 was between 10 and 15 tons [30, 31].
Materials with a scandium content between 20 and 50 ppm can be considered as an ore [34]. Some of the bauxite residues contain a higher scandium concentration so they can be treated as scandium resources. According to Laverenchuk, bauxites contain 70 % of total scandium in forecast reserves [35]. In Table 3, scandium concentrations in bauxite residues from different origins are shown. All the bauxite residues shown in the table contain more than 50 ppm of scandium. Therefore, bauxite residue can be considered as a potential scandium resource. Large quantities of scandium could be produced from bauxite residue, which could decrease the price of scandium and increase the availability of scandium for many applications. For example, RUSAL’s alumina refineries annually stockpile about 2 million tons of bauxite residue, which contain in total about 480 tons of scandium [35], whereas Aluminum of Greece generates 0.7 million tons of bauxite residue every year that contain in total about 90 tons of scandium.
Recovery of REEs From Bauxite Residue
It is difficult to leach the REEs from bauxite ore when compared to bauxite residue due to differences in their mineralogy and morphology [41, 42]. REEs from bauxite residue can be extracted either by hydrometallurgical processes or combined pyrometallurgical and hydrometallurgical processes [32]. Some researchers have tried to increase the concentration of scandium in the bauxite residue by physical beneficiation before leaching [43]. The operating costs can be minimized during leaching by increasing the scandium concentration, which minimizes the acid consumption and waste for disposal (acidic effluent, leaching cake, etc.). However, the recovery of scandium is relatively low in the concentrate (less than 20 %) during physical beneficiation and the rest will be lost in tailings. On the other hand, the fine particle size of bauxite residue limits the performance of the magnetic separation (i.e., a specific form of physical beneficiation).
Direct Leaching of Bauxite Residue
Table 4 summarizes the different kinds of reagents used, the conditions applied, and the achieved recovery of REEs during leaching of bauxite residue. It also gives details on the different extraction processes that were used during the recovery of REEs from the leach solution.
Leaching with Mineral and Organic Acids
Leachates containing large concentrations of iron require a large number of processing steps and consume large amounts of chemicals in the subsequent downstream processes, e.g., solvent extraction [42]. Therefore, selective leaching of REEs, leaving behind iron oxide in the residue, simplifies further the extraction processes. Hence, leaching at higher pH values (1.8–3) was conducted to selectively dissolve REEs while leaving iron and titania substantially undissolved from the bauxite residue [42, 49]. Selectivity values of REEs in this leaching were higher compared to the values at lower pH, but the recoveries were low, especially for scandium. For example, recovery of scandium was always less than 42 % except in one case (56 %) where the leaching was carried out at 150 °C [49]. During leaching, even at these pH values, sodium, aluminum, and silicon that are present under the form of sodalite were brought into solution. However, the REEs could be selectively recovered by solvent extraction. Borra et al. found a striking relation between iron and scandium dissolution [37]. Scandium can be selectively dissolved up to about 50 % with very low iron (<5 %) dissolution. Further increase in scandium dissolution caused dissolution of large amount of iron into the solution. These authors also found that sodium and calcium were almost completely dissolved during leaching and the dissolution of aluminum, silicon, and titanium was between 30 and 50 % [37]. According to Ochsenkühn-Petropulu, both the temperature and pressure have no marked influence on REEs leaching [41]. However, other studies show that higher temperatures enhance the recovery of REEs from bauxite residue [49, 50]. This difference in results may be due to the difference in origin of bauxite residue or due to difference in testing conditions (e.g. acid concentration).
Nitric acid was found to be selective for leaching of REEs over iron [41]. On the other hand, iron is more readily dissolved during HCl leaching compared to HNO3 leaching. Therefore, the leaching process with dilute HNO3 has been performed and optimized at a pilot scale for the recovery of scandium from bauxite residue [48]. According to Petrakova et al., leaching with nitric acid could be a problem due to the absorption of nitrate ions on the residue, so that large volumes of water are needed for their removal [43]. The leaching rates depend on the type of acid used. According to Ochsenkühn-Petropulu et al., leaching rates are as follows: HNO3 > HCl > H2SO4 [41], although Wang et al. concluded that H2SO4 is the best leaching agent [50]. Nevertheless, one has to keep in mind that the H2SO4 contains twice the number of protons compared to HCl or HNO3 at a given molarity. According to Binnemans et al. the leaching efficiency could also depend on the type (minerology) of bauxite residue [8]. Borra et al. found that the recovery yields are higher in HCl at high acid concentrations compared to other acids but the selectivities are low [37]. Recovery yields were very low when organic acids such as acetic acid or citric acid were used for leaching [37, 38, 40, 41]. However, the efficiency could be improved by leaching at higher temperatures [37, 38].
REEs are present in bauxite as mineral phases or as ions that are either adsorbed on the surface of minerals or replacing similar ions in the lattice of some matrix minerals [26]. Ochsenkühn-Petropulu et al. found lanthanides in hydroxyl-bastnasite phase in bauxite ore. However, it is difficult to find the mineralogy of REEs in bauxite residue due to their concentration and fineness of the bauxite residue [41]. Nonetheless, Gamaletsos et al., and Sugita et al., proposed that cerium and other lanthanides are present in the perovskite (CaTiO3) mineral in bauxite residue [49, 54]. Borra et al., proposed that scandium could be in an iron oxide phase because chemical similarity was found between iron and scandium and the subsequent relation between iron and scandium dissolution during leaching [37].
Recently, Orbite Aluminae Inc. filed a patent for a multistep process for recovery of all elements, including REEs, from bauxite residue [45]. The conceptual process flow sheet is shown in Fig. 3. The first stage of the process comprises treatment of bauxite residue by an HCl solution in an autoclave at 150–170 °C. All components except titania and silica are dissolved. After solid–liquid separation, the leach liquor is treated with HCl gas to increase the chloride concentration, which helps in AlCl3·6H2O precipitation. AlCl3·6H2O is filtered off from the iron-rich liquor. The AlCl3·6H2O precipitate is transformed to Al2O3 by calcination at 900–950 °C. The calcination stage generates HCl gas that can be reused in the leaching and aluminum precipitation stages. The leachate after aluminum removal contains FeCl3 together with other elements. Fe2O3 is produced from the leachate by hydrolysis of the FeCl3 at 180 °C. After iron removal the solution is rich in Mg, Ca, Na, Ga, and REEs. Magnesium is recovered from the solution by treating it with HCl to precipitate MgCl2 followed by calcination. The REEs are separated from the leach solution by conventional solvent extraction. The alkaline metals are recovered as hydroxides by electrolysis and the HCl acid can be regenerated. Recovery yields in this process are about 93 % for aluminum and more than 90 % for the other metals, including the REEs.
Conceptual process flow sheet of Orbite process. Adapted from Ref. [45]
Leaching with Alkali (Hydrogen) Carbonates
The solubility of scandium is high in a NaHCO3 solution (16.7 g L−1) when compared to a Na2CO3 solution (0.43 L−1) at 25 °C and 100 L−1 of respective carbonate solution [40]. Yatsenko et al. (2010) developed a process based on the high solubility of scandium in a NaHCO3 solution by treating bauxite residue with NaHCO3 to recover scandium. During the process, some of the NaHCO3 is converted to Na2CO3, which decreases the recovery of scandium due to the above-described lower solubility in Na2CO3. Therefore, treating the bauxite residue slurry with CO2 increases the NaHCO3 content that helps increasing the recovery. The process flow sheet is shown in Fig. 4. About 26 % of scandium could be recovered at 60 °C with pCO2 being 6 atm and a liquid-to-solid ratio of 4 [35, 55]. Due to the absence of silica gel formation and other metal dissolution, such a low liquid-to-solid ratio is feasible. About 30 % of sodium, 68 % of zirconium, and 6 % titanium could also be recovered at the same conditions. However, the major drawback of this process is the poor recovery of scandium (<30 %). This low recovery of scandium is maybe due to the presence of scandium in the iron oxide lattice. The recovery of other REEs is even lower. The main advantage of this process is that part of the scandium can be recovered without any acid consumption for neutralization. The pH of the bauxite residue after this process is around eight, which allows to store bauxite residue in a less harmful way [40].
Conceptual process flow sheet of the carbonate leaching process. Adapted from Ref. [35]
Bioleaching
In bioleaching, metals are extracted with the aid of microorganisms. Two different kinds of microorganisms are commonly used for bioleaching in extractive metallurgy: bacteria and fungi [10]. Bacteria are not suitable for bioleaching of bauxite residue due to the high pH and absence of energy sources (sulfur or reduced iron). However, fungi can survive in high pH and they excrete metabolites such as organic acids, amino acids, and proteins in the presence of an organic medium to form complexes with metal ions in bauxite residue. REEs and radioactive elements were leached from bauxite residue with the filamentous fungus RM-10 in the presence of a sucrose medium [10]. The authors found that two-step leaching (preculturing the fungus followed by leaching) was superior to one-step leaching (culturing in the presence of bauxite residue). The fungus Aspergillus niger was used in another study for bioleaching on the same material used in the previous study [53]. Leaching was conducted both in batch and continuous mode. In the batch-mode leaching was carried out in three ways: one-step leaching, two-step leaching, and spent medium (cell-free spent medium after 10-day incubation of fungi) leaching. The results show that the spent medium process is best at 2 % pulp density with 44 % scandium recovery. The pulp density can be increased to 10 % in the continuous leaching. However, the scandium leaching was decreased to 30 %.
Leaching with Ionic Liquids
Ionic liquids are selective to certain metals and are stable even at high temperatures. The [EMIM][HSO4] ionic liquid was used for high-temperature leaching of bauxite residue [51, 56]. Recovery of REEs was 70 % at 190 °C except for La, which is 100 %. In those conditions, iron and titanium dissolution was 100 % and for aluminum it was about 35 %. Low-temperature leaching (90 °C) with [EMIM][HSO4] decreased the recovery of REEs (Sc < 40 %), however, it improved the selectivity of REEs over major elements [52, 56].
Comparison of Different Approaches to Direct Leaching
Mineral acids are superior to organic acids for leaching of REEs from bauxite residue [38, 40]. However, the recovery yields achieved with some of the organic acids are comparable with those of mineral acids during high-temperature leaching. According to Petrakova et al., H2SO4 is the best and cheapest option for leaching when compared to other mineral acids [43]. The main disadvantages of acid leaching are the consumption of large amounts of acid during neutralization of the alkaline bauxite residue, the handling of large volumes of effluents, and the difficulty in using the bauxite residue after leaching [43]. Furthermore, direct acid leaching dissolves large amounts of iron, titanium, and aluminum [37]. The coadsorption of these elements to the ion-exchange resins decrease the efficiency of the recovery process. Furthermore, the elution of these impurities requires large amounts of acids; this increases the process cost and it also requires large amount of bases for neutralization further on in the process [32]. On the other hand, alkali leaching looks promising due to no acid consumption and less harmful waste generation, but unfortunately the recovery of scandium is low. Preliminary experiments on high-pressure leaching show selectivity for scandium over iron, but further studies are required. The process developed by Orbite Aluminae Inc. is promising if the economics work out. It recovers all the metals and most of the acid used in the process, which makes it a near-zero-waste process. Nevertheless, handling of corrosive HCl is a major concern in the process. Glass-lined reactors and valves and pipes made with high-performance chemically resistant polymers are used in this process, which increases the capital cost [57]. Economic analysis based on the chemical cost shows that leaching with citric acid is two times more costly than bioleaching, while leaching with H2SO4 is much cheaper than bioleaching [53]. Bioleaching of the bauxite residue may be a greener option, but the recovery of LREEs is low and the dissolution of major elements is also unknown. Use of ionic liquids does not improve the selectivity for REEs during leaching, however, new ionic liquids may be developed in future for selective leaching.
Pyrometallurgical Pretreatment of Bauxite Residue
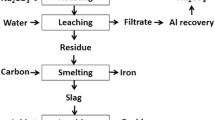
Karl Bayer stated: “The red, iron-containing residue that occurs after digestion settles well and, with sufficient practice, can be filtered and washed. Due to its high iron and low aluminum oxide content, it can, in an appropriate manner, be treated or with other iron ores be smelted to iron” [58]. However, the recovery of iron alone from bauxite residue may not be economical. Therefore, iron recovery by a pyrometallurgical step followed by leaching of REEs and/or titanium from the oxide slag [59–61] or tailings after magnetic separation [62–64] could be an economical and low- (or even zero-) residue option. During smelting, REEs are concentrated in the slag phase due to their thermodynamic stability [65, 66], after which they can be recovered by leaching.
If prices of iron ores are high, bauxite residue can be a potential raw material for the production of pig iron provided that an efficient technology for its processing would exist [67]. Iron recovery studies from bauxite residue were initiated as early as in the 1950s and may be classified into two major approaches: (1) reductive smelting and (2) solid-state reduction. In a smelting process, bauxite residue is treated in a blast furnace with prior sintering in the presence of a reducing agent so that the iron oxides are reduced, generating pig iron and a titanium-rich slag. Simple methods of utilizing bauxite residue in a blast furnace process are limited by impurities (Na, K, S, P, As) and low concentration of iron. Furthermore, the large sodium content may damage the refractory and also leads to the formation of so-called “sodium nests” [8]. Therefore, direct (solid-state) reduction was considered as an alternative process for the production of iron from bauxite residue. In the solid-state reduction, bauxite residue is reduced with a solid or gaseous reductant [68–70]. The reduced mass after magnetic separation can be used for steel making or as a charge to the blast furnace [71]. So far, no process has been a commercial success [1, 71], because of specific problems associated with the bauxite residue such as low iron content, high alkali content, fineness of the particles, and moisture.
Magnetic separation of iron from the bauxite residue with(out) prereduction was studied by some researchers [72–76]. Conventional magnetic separation and high-gradient superconducting magnetic separation (HGSMS) were used in direct magnetic separation [72, 73]. However, the recoveries and grade were poor, except for the coarse-grained bauxite residue obtained after size separation. It is due to the fineness and mineralogy (goethite has low magnetic susceptibility over hematite) of the bauxite residue. The recoveries can be improved by prereduction, which converts the hematite into magnetite or iron metal. Addition of Na2CO3 salt during prereduction further improved the reduction, which in turn improved the metal separation [74]. It should be noted that the bauxite residue used in that study was coarse and contained a high concentration of iron, which may be the reason for the high recoveries reported. The tailings generated after magnetic separation could be leached with aqueous Na2CO3 solution at ambient pressures for alumina recovery [75]. Magnetic tailings after alumina removal could be used for the recovery of titanium and REEs, however, they still contain a large amount of iron (about 10 %), which is easily soluble during leaching. Furthermore, scandium, being the most valuable element in bauxite residue, may report to the iron-rich phase (concentrate) as iron oxide is a host phase for scandium. Iron together with aluminum can be recovered from bauxite residue by reduction roasting (sintering) with sodium carbonate and carbon, followed by water leaching and magnetic separation. This process is explained in “Aluminium Recovery From Bauxite Residue in View of Iron, Titanium and/or REEs Recovery” section.
Liu et al. carried out reduction at 1300 °C for 110 min with a charge containing bauxite residue, carbon, and flux (100:18:6) [76]. They studied the magnetic separation after milling the reduced sample. The grade and recovery of iron was ca. 89 and 81 %, respectively. Zhu et al. reduced the bauxite residue with a soft coal in the presence of 8 % sodium carbonate at 1050 °C for 80 min [74]. After grinding, the reduced mass (90 % < 74 µm) was subjected to magnetic separation by a magnetic field of 0.08 Tesla. Addition of sodium carbonate helped improve the recovery yield and the grade of iron produced. A recovery of 96 % of iron was achieved with a grade of 91 %.
Reductive Smelting of Bauxite Residue and Recovery of Metals From Slag
Alternative liquid-phase reduction (smelting) processes like Corex, Finex, Hismelt, Romelt, AusIron, and EAF smelting can be considered for smelting of the bauxite residue [67]. So far, two processes have been tested for bauxite residue smelting: the Romelt process [77], and the Electric Arc Furnace (EAF) smelting [60, 62, 68, 78–86]. The Moscow Institute of Steel and Alloys (MISA), together with NALCO and RSIL (India), studied processing of bauxite residue by the Romelt process [77]. The advantage of this process is that it can handle raw materials with moisture level up to 10 %. Its main disadvantage is the high energy consumption and the low grade of the produced pig iron (high sulfur and phosphorus) [67]. In the EAF process, a mixture of bauxite residue, flux, and carbonaceous material was smelted in an electric arc furnace at 1500–1800 °C to form an iron alloy with more than 90 % iron recovery [62, 78, 81]. The iron recovery can be further improved by subsequent magnetic separation of ground slag to recover the remaining interlocked iron droplets in the slag [68, 87, 88]. The slag generated after smelting can be used for the production of slag wool [78] and building materials [89], and for the extraction of titanium [60, 61, 81, 82, 87, 88], other non-ferrous metals, or REEs [8, 36, 60, 88, 89]. REEs from the slag can be leached by a H2SO4 solution [34, 60, 61]. However, HNO3 and HCl solutions were studied by Borra et al. and similar or even better recoveries were obtained when compared to H2SO4 [88]. The lower recovery yields in H2SO4 were due to the formation of a solid calcium sulfate layer.
Table 5 lists the different studies on smelting of bauxite residue to recover iron. Smelting studies were generally carried out above 1500 °C. However, smelting was also studied below 1500 °C in two cases. This may be due to the low amount of alumina present in these samples. High amount of fluxes were added during the smelting of bauxite residue due to the high alumina content. The fluxes used during smelting are limestone, lime, silica, and dolomite. Different types of reducing agents were tested for bauxite residue smelting: coal, coke, graphite, wood, etc. The sulfur content of the pig iron depends on the slag basicity and can be minimized by increasing the basicity [60]. The phosphorus content of the pig iron can be minimized by choosing a reducing agent with a low phosphorus content. The pig iron produced from bauxite residue can be used in steel making [90].
A fundamental study on the smelting of bauxite residue at a laboratory scale was carried out by Kaußen and Friedrich [92]. They modeled the process using FactSage® and compared the modeling and experimental results. They found a good agreement between both. They also found that prolonging the reduction time decreases the sodium content in the slag. Increase in the carbon content of the charge beyond a certain limit reduces the silica, which increases the slag melting point drastically. Logomerac found that a carbon charge above a certain limit also reduced TiO2 and created problems during the tapping of the slag [60]. Reduction of silica and titania during smelting was also observed by Borra et al. when high amounts of carbon was used [88]. They also optimized the flux amount to decrease the energy and acid consumptions.
The conceptual semiquantitative flow sheet of the process suggested by Logomerac is shown in Fig. 5 [60]. The flow sheet shows the complexity of the process and the huge quantities of reagents and water consumed by such a type of process. The flux requirement in the process is very high, being as much as the quantity of bauxite residue processed. Therefore, the slag-to-metal ratio was also high. The energy consumption of the process was 3600 kWh per ton of pig iron produced, which is very high compared to conventional pig iron production (~1500 kWh/ton). A similar kind of a complex process was used by Ercag et al. to recover a small quantity of TiO2 from bauxite residue [87].
Conceptual semiquantitative flow sheet for complex processing of bauxite residue. Adapted from Ref. [60]
In a pilot-scale process 350 kg of dry bauxite residue, 77 kg of coke fines, 70 kg of silica, and 53 kg of burnt lime were smelted in an EAF for producing 120 kg of metal and 280 kg of slag [78, 90]. In another study, the authors proposed to recover scandium by selective leaching before smelting [96]. However, it is questionable whether acidified bauxite residue can be used in EAF due to the formation of hazardous gases (Cl2, SO2/SO3, NOx etc.). In addition, most of the calcium is dissolved during leaching and calcium needs to be added again during smelting. Nevertheless, the authors claimed that this process can be economical and part of a zero-waste process.
Generally, the slag is ground or granulated before leaching to increase its reactivity. After leaching, the valuable elements can be recovered from the leachate by solvent extraction, precipitation, hydrolysis, or neutralization. For example, Udy recovered aluminum sulfate from a leach solution by evaporation crystallization and TiOSO4 by hydrolysis [82]. These sulfates were calcined for acid regeneration and oxide production. Aluminum from the slag can also be recovered by sodium carbonate leaching [62]. However, the slag chemistry needs to be adjusted for maximum alumina recovery. Kaußen and Friedrich leached the almost entire aluminum from slag with 500 g/L NaOH aqueous solution at 250 °C under pressure [91]. However, high amount of sodium and high temperatures increase the wear of leaching equipment. During acid leaching of slag, silica in the slag creates filtration problems due to gel formation. Therefore, large volumes of water are required to overcome these filtration problems [60, 61, 88].
Iron can be removed from bauxite residue by smelting at temperatures from 1200 to 1800 °C, depending on the slag composition. Iron removal followed by slag leaching for the recovery of titanium, aluminum, and REEs can be a zero-waste process. However, smelting of bauxite residue requires large amounts of flux, which increase the energy consumption and decrease the bauxite residue throughput in the furnace. Furthermore, large volumes of acids are consumed during slag leaching as the basic elements from the flux consume acid. Decrease in flux consumption decreases the energy consumption as well as acid consumption during metal recovery. Silica dissolution is the main problem during slag leaching. It creates filtration problems by silica gel formation. However, this problem might be solved by carrying out the leaching above 100 °C to hydrolyze silica.
Aluminum Recovery From Bauxite Residue in View of Iron, Titanium, and/or REEs Recovery
High concentrations of alumina in the bauxite residue increase the melting point as well as the viscosity of the slag during reductive smelting [92]. To compensate this alumina effect, large amounts of flux and/or high temperatures are required. Both increase in temperature and the use of a flux increase the energy consumption. Therefore, it is beneficial to decrease the alumina content before the reductive smelting of bauxite residue. Alumina can be removed from bauxite residue by two processes: (1) high-temperature hydrometallurgy and (2) alkali roasting (sintering) [75, 97]. In high-temperature hydrometallurgy, bauxite residue is leached in autoclaves at high temperatures (>260 °C) and high alkalinity (molar ratio of Na2O to Al2O3 > 10). This process has not been industrialized yet due to corrosion problems of the equipment at high temperatures and the low alumina concentration in the leach solution. In alkali roasting, the bauxite residue is mixed with sodium carbonate (Na2CO3) and the mixture is then roasted at temperatures around 800–1200 °C to convert alumina to sodium aluminate. The main reactions involved in the process are:
The roasted mass is leached with water to dissolve the formed sodium aluminate. Aluminum can be recovered from the leach solution by precipitation with CO2 (Eq. 6) or by hydrothermal precipitation (Eq. 7), along with the green liquor of the Bayer process.
Alkali (sodium carbonate) roasting of the bauxite ore for recovering alumina was developed in 1854 by Louis de Le Châtelier (Fig. 6). This was the sole industrial process for alumina recovery until the Bayer process was developed in 1892 [98]. The alumina plants in Eastern Europe (Russia, Ukraine, Turkey) and China are still using alkali roasting technology for the production of alumina from silica-rich bauxite ores because a high amount of soda will be lost in sodalite in the Bayer process [99]. The energy consumption of the alkali roasting process is 2–3 times higher than that of the Bayer process.
Le Châtelier process for alumina purification. Adapted from Ref. [98]
Table 6 lists the different studies on alkali roasting of bauxite residue to recover alumina. The main variables studied in the process are roasting temperature, roasting time, alkali ratio, CaO/MgO/BaO ratio, leaching temperature, and leaching time. The table summarizes the experimental conditions and alumina recovery.
The Le Châtelier process was applied by Fursman et al. for recovering alumina from bauxite residue [62]. They modified the process by adding carbon during sintering for converting Fe2O3 into metallic iron, which helps in further iron recovery by magnetic separation. Although the iron recovery was high (about 80 %), the magnetic product was finely divided and difficult to separate from the gangue and tended to be reoxidized. The researchers did not study the recovery of REEs from the leach solution. A similar type of process was applied by Li et al. [97]. They observed that when the sintering temperature is higher than 1323 K, the alumina recovery rate decreases rapidly because of complex phase formation. They also observed that too long a sintering time (about 120 min) has a negative effect on the alumina recovery rate. Liu et al. found that leaching at higher temperatures results in higher recovery of aluminum and sodium, but with increase in time above 15 min the recovery decreases [100]. They suggested that this may be due to the precipitation of hydrated aluminosilicates.
It was reported in the literature that the roasting temperatures are lower when carbon is added. This may be due to the formation of CO by reaction between CO2 generated from the reaction of alumina with sodium carbonate and carbon, which favors the reaction of alumina with sodium carbonate to form sodium aluminate [97].
Raghavan et al. observed that the high amount of sodium carbonate makes the sinter hygroscopic and this creates problems in the dry grinding step [101]. They also found that an increase in lime content (>0.1 ratio) decreases the alumina recovery. This may be due to the complex phase formation of alumina with lime. They suggested performing an extra desilication step for the removal of silicon or mixing the leach solution with the green liquor from the Bayer process to decrease the silicon content. Generally CaO is used for fixing the silica by forming calcium silicate during roasting, which lowers the silica dissolution during leaching. Other basic oxides like BaO [104] and MgO [106] were also studied for fixation of silica.
Different authors suggested that the residue generated after alkali roasting can be used for different applications. According to Meher and Padhi, the residue is environment friendly due to the low sodium content and can be suitable for making cement and refractory bricks [107]. Raghavan et al. proposed that the leach residue can be used for recovering titania and iron(III) sulfate by sulfuric acid leaching [101]. Fursman et al. recovered a titania product, assaying 96 % of TiO2, from the non-magnetic tailings of Suriname's bauxite residue sinter by leaching with H2SO4. Rayzman and Filipovich suggested to use the leach residue after alkali roasting for recovering iron, titanium, and REEs [108]. They also suggested a desilication step after water leaching to remove soluble silica. A similar process was proposed by Hammond et al. [64] (Fig. 7). These researchers expressed that it is difficult to separate iron from the remaining oxides by physical means due to the mineralogy of the bauxite residue where fine iron oxides are intimately mixed with other oxides, which does not allow the separation of reduced iron in a concentrated form.
Conceptual flow sheet for bauxite residue utilization. Adapted from Ref. [64]
Alkali roasting process can be applied for the recovery of alumina from bauxite residue. This process can be divided into two parts: (1) without carbon, and (2) with carbon. Carbon was added during roasting to convert iron oxides into magnetite or metallic iron to help in magnetic separation. It is difficult to achieve higher recoveries of iron during magnetic separation after roasting because of the fineness and complex mineralogy of the bauxite residue. After reduction roasting, if iron is removed by magnetic separation, some of the soluble alumina will be lost together with the iron fraction. If alumina is leached before the magnetic separation step, iron metal will be oxidized. Most of the researchers converted iron oxides to magnetite, which is a stable phase during water leaching and wet magnetic separation. The silica content in the leach solution can be decreased by adding MgO, CaO, and BaO during roasting or it can be removed by lime treatment of the leach solution [109]. Higher temperatures and increased residence times during sintering adversely affect the recovery of alumina due to complex phase formation. Roasting temperatures were studied from 500 to 1100 °C. The temperature for maximum recovery was not consistent in the literature as it varied between 900 and 1100 °C. Water leaching studies were carried out from room temperature to 105 °C. Increase in leaching temperature enhances the recovery of sodium and aluminum. However, leaching for longer times at high temperatures decreases the alumina recovery by complex phase formation.
Complex Processing of Bauxite Residue
Bauxite residue is a polymetallic material containing valuable minor metals like REEs [5]. The recovery of only iron and/or alumina from bauxite residue cannot make the process economically viable. Therefore, extraction of several metals in a single integrated process is needed to make the process economically viable and to minimize the generated residues [5, 71]. Several authors proposed different complex processes to recover aluminum, iron, titanium [36, 60, 62, 63, 64, 82, 108, 110, 111]. It should be noted that most of these studies are only proposals. These complex processes can be divided into three types: (1) smelting of bauxite residue followed by slag leaching, (2) reduction roasting of bauxite residue followed by magnetic separation and water leaching followed by residue leaching, and (3) alumina removal from bauxite residue by leaching or roasting, followed by reduction or smelting and slag leaching (Figs. 8, 9, 10).
Rayzman et al. beneficiated the bauxite residue by NaOH leaching at 285–300 °C to decrease (recover) the alumina content and increase the scandium content in the residue [36]. As mentioned previously, the low aluminum concentration in the leach solution and the corrosion problems of the autoclave due to high temperatures are hurdles for industrialization of this process. After alumina removal, the residue was smelted to remove iron. Reduction smelting was studied on a bench scale as well as in pilot scale. They proposed to recover scandium from the slag by leaching.
Energy and flux consumption are high in the direct smelting process, therefore, reduction roasting followed by magnetic separation could be a better option. However, the iron recovery is low during magnetic separation. Iron in the tailings dissolve during the leaching, which requires large amount of reagents during further processing (e.g., solvent extraction). Alumina removal by roasting followed by smelting and slag leaching appears a promising approach [36]. However, this process has not been studied in detail yet. Furthermore, there is no open literature available about the process details.
Other Processes
Other processes that are worth considering for REEs recovery from bauxite residue are high-pressure acid leaching (HPAL) and acid bake leaching. Currently, scandium is recovered only as a byproduct and there is no primary production from a specific ore. However, a company called Scandium International found a scandium-rich lateritic nickel ore in Australia and developed two above-mentioned processes for recovering scandium from that ore [112].
The scandium-rich bauxite residue and the lateritic ores have similar chemical compositions. Both are rich in scandium, iron, and aluminum. Therefore, the process developed for scandium ore can be applied to bauxite residue. Scandium International (earlier known as EMC Metals Corporation) patented the acid bake process [113], which is shown in Fig. 11. This process includes an acid-mixing stage followed by a baking (roasting) stage. The gas (SO3) generated during baking is used for the production of acid that can be used back in mixing stage. After the baking step, scandium is recovered from the residue by leaching. The pH of the solution during leaching was maintained at pH > 2.5, which might be chosen to keep iron levels low in the solution. The redox potential of the solution was maintained between +0.7 V and +1.2 V to suppress the formation of jarosite. Suppression of jarosite formation improves the scandium recovery. Scandium recovery during leaching was about 75 %. Scandium can be extracted from the leach solution by processes like ion exchange, solvent extraction, selective precipitation, etc. However, the amount of acid, the baking temperature, and other conditions were not mentioned in the patent. In the HPAL process, hydrolysis of titanium, aluminum, and iron takes place above 180 °C. Therefore, the acid consumption will be low. Hence, REEs can be selectively recovered from the solution. Kaya and Topkaya studied leaching of scandium from a refractory nickel lateritic ore by the HPAL process [114]. They were able to recover about 80 % of scandium and >85 % of nickel and cobalt at 255 °C and 260 kg of sulfuric acid per ton of ore. In contrast to the earlier hypothesis, they found that change in redox potential did not enhance the recovery of scandium. The residues generated in the HPAL and acid bake process of bauxite residue are rich in calcium sulfate and poor in sodium. Therefore, these residues can be considered for applications in cementitious binders.
Acid bake process for recovering scandium. Adapted from Ref. [113]
Borra et al. studied the sulfation–roasting–leaching process for recovering REEs from bauxite residue, which is similar to the acid baking process [115]. They were able to recover about 60 % of Sc and >80 % other REEs. The dissolution of other major elements are Na > 90 %, Al~20 %, Fe < 1 %, Ti < 1 %, and Ca~5 % at L/S ratio 5:1. The residue generated in the process is poor in sodium and rich in calcium sulfate, therefore, the residues can be considered for using in cementitious binders.
Conclusions
The long-term storage of bauxite residue is an environmental threat and poses a space concern. Currently this material is not being used in bulk applications, except for small quantities in cements and ceramics. Recovery of REEs and other valuable metals, in combination with the application of the leached residues in building materials can be an economical and a (nearly) zero-waste option. Direct acid leaching of bauxite residue for recovering REEs dissolves large amounts of major elements (iron, aluminum,..), which creates issues in further recovery process steps such as solvent extraction, ion exchange, etc. The generation of large volumes of effluents is also a major concern. Furthermore, direct acid leaching hardly reduces the volume of solid residue to be stored, because the valuable metals are present only in small concentrations. Recovery of iron by smelting followed by slag leaching could be a good option for recovering iron, alumina, titanium dioxide, and REEs. However, this process requires large amounts of flux due to the high alumina content of the bauxite residue. The use of large amounts of flux not only increases energy consumption and process costs but also increases the acid consumption during leaching. Alumina and iron can be recovered together from bauxite residue by a reduction roasting process. However, it is difficult to achieve high recoveries of iron during the subsequent magnetic separation step because of the fineness and morphology. Furthermore, residual iron in the magnetic separation tailings dissolves easily during the leaching process, which creates problems in the further recovery of titanium and REEs from the leachates. Therefore, the complex or near-zero-waste processes are the most promising ones.
References
Kumar S, Kumar R, Bandopadhyay A (2006) Innovative methodologies for the utilisation of wastes from metallurgical and allied industries. Resour Conserv Recycl 48:301–314. doi:10.1016/j.resconrec.2006.03.003
Power G, Gräfe M, Klauber C (2011) Bauxite residue issues: I current management, disposal and storage practices. Hydrometallurgy 108:33–45. doi:10.1016/j.hydromet.2011.02.006
Smith P (2009) The processing of high silica bauxites: review of existing and potential processes. Hydrometallurgy 98:162–176. doi:10.1016/j.hydromet.2009.04.015
Evans K (2016) The history, challenges, and new developments in the management and use of bauxite residue. J Sustain Metall. doi:10.1007/s40831-016-0060-x
Klauber C, Gräfe M, Power G (2011) Bauxite residue issues: II options for residue utilization. Hydrometallurgy 108:11–32. doi:10.1016/j.hydromet.2011.02.007
Wang W, Pranolo Y, Cheng CY (2013) Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep Purif Technol 108:96–102. doi:10.1016/j.seppur.2013.02.001
Reeves HJ, Wealthall G, Younger PL (2011) Advisory visit to the bauxite processing tailings dam near Ajka, Vesprem County, western Hungary. Br. Geol. Surv. Keyworth, UK, Open Report OR/11/006
Binnemans K, Jones PT, Blanpain B, Van Gerven T, Pontikes Y (2015) Towards zero-waste valorisation of rare-earth-containing industrial process residues: a critical review. J Clean Prod 99:17–38. doi:10.1016/j.jclepro.2015.02.089
Wang S, Ang HM, Tadé MO (2008) Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 72:1621–1635. doi:10.1016/j.chemosphere.2008.05.013
Qu Y, Lian B (2013) Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour Technol 136:16–23. doi:10.1016/j.biortech.2013.03.070
Smirnov DI, Molchanova TV (1997) The investigation of sulphuric acid sorption recovery of scandium and uranium from the red mud of alumina production. Hydrometallurgy 45:249–259. doi:10.1016/S0304-386X(96)00070-9
Ochsenkühn-Petropulu M, Tyberopulu T, Parissakis G (1994) Direct determination of lanthanides, yttrium and scandium in bauxites and red mud from alumina production. Anal Chim Acta 296:305–313. doi:10.1016/0003-2670(94)80250-5
Gräfe M, Power G, Klauber C (2011) Bauxite residue issues: III alkalinity and associated chemistry. Hydrometallurgy 108:60–79. doi:10.1016/j.hydromet.2011.02.004
Krishnamurthy N, Gupta CK (2004) Extractive metallurgy of rare earths. CRC Press, Boca Raton
Trifonov D (1963) The rare-earth elements. Pergamon, London
Binnemans K, Jones PT, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22. doi:10.1016/j.jclepro.2012.12.037
European Commission (2014) Report on critical raw materials for the EU: report of the Ad hoc working group on defining critical raw materials. European Commission, Enterprise and Industry, Brussels
Vijayan S, Melnyk AJ, Singh RD, Nuttall K (1989) Rare earths: their mining, processing, and growing industrial usage. Min Eng 41:13–18
Zhanheng C (2011) Global rare earth resources and scenarios of future rare earth industry. J Rare Earths 29:1–6
Wübbeke J (2013) Rare earth elements in China: policies and narratives of reinventing an industry. Resour Policy 38:384–394. doi:10.1016/j.resourpol.2013.05.005
Gambogi J (2015) Rare earths, USGS Mineral Commodity Summaries. U.S. Geological Survey, Reston. http://minerals.usgs.gov/minerals/pubs/commodity/rare_earths/mcs-2015-raree.pdf. Accessed 22 July 2016
Anonymous (2015) Chinese government abolishes rare earth export quotas. Bridg Wkly 19(1):10–11. http://www.ictsd.org/bridges-news/bridges/news/chinese-government-abolishes-rare-earth-export-quotas. Accessed 22 July 2016
Anonymous (2015) With rare earth oxides estimated to reach 207,205 tons in 2019. http://www.bccresearch.com/pressroom/avm/with-rare-earth-oxides-estimated-to-reach-207,205-tons-in-2019. Accessed 22 July 2016
Bardossy G (1982) Karst bauxites (bauxite deposits on carbonate rocks). Elsevier, Hungary
Bárdossy G, Aleva GJJ (1990) Lateritic bauxites. Elsevier Science Ltd, Amsterdam
Li Z, Din J, Xu J, Liao C, Yin F, Lu T, Cheng L, Li J (2013) Discovery of the REE minerals in the Wulong-Nanchuan bauxite deposits, Chongqing, China: insights on conditions of formation and processes. J Geochem Explor 133:88–102. doi:10.1016/j.gexplo.2013.06.016
Mordberg LE (1993) Patterns of distribution and behavior of trace elements in bauxites. Chem Geol 107:241–244. doi:10.1016/0009-2541(93)90183-J
Wagh AS, Pinnock WR (1987) Occurrence of scandium and rare earth elements in Jamaican bauxite waste. Econ Geol 82:757–761. doi:10.2113/gsecongeo.82.3.757
Meyer FM (2004) Availability of bauxite reserves. Nat Resour Res 13:161–172. doi:10.1023/B:NARR.0000046918.50121.2e
Gambogi J (2014) Scandium, USGS Mineral Commodity Summaries. http://minerals.usgs.gov/minerals/pubs/commodity/scandium/mcs-2015-scand.pdf
Duyvesteyn W, Putnam G (2014) White paper: scandium. Sparks, Nevada. http://www.scandiummining.com/i/pdf/Scandium-White-PaperEMC-Website-June-2014-.pdf
Wang W, Pranolo Y, Cheng CY (2011) Metallurgical processes for scandium recovery from various resources: a review. Hydrometallurgy 108:100–108. doi:10.1016/j.hydromet.2011.03.001
Ochsenkühn-Petropulu M, Lyberopulu T, Parissakis G (1995) Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal Chim Acta 315:231–237. doi:10.1016/0003-2670(95)00309-N
Shaoquan X, Suqing L (1996) Review of the extractive metallurgy of scandium in China (1978-1991). Hydrometallurgy 42:337–343. doi:10.1016/0304-386X(95)00086-V
Petrakova OV, Panov AV, Gorbachev SN, Klimentenok GN, Perestoronin AV, Vishnyakov SE, Anashkin VS (2015) Improved efficiency of red mud processing through scandium oxide recovery. In: Hyland M (ed) Light Metals 2015. Wiley, New York, pp 91–96
Rayzman VL (1998) Red mud revisited-special paper on scandium potential. Alum Today 10:64–68
Borra CR, Pontikes Y, Binnemans K, Van Gerven T (2015) Leaching of rare earths from bauxite residue (red mud). Miner Eng 76:20–27. doi:10.1016/j.mineng.2015.01.005
Ujaczki É, Zimmermann Y, Feigl V, Lenz M (2015) Recovery of rare earth elements from hungarian red mud with combined acid leaching and liquid–liquid extraction. In: Pontikes Y (ed) Proceedings of Bauxite Residue Valorisation and Best Practices Conference, Leuven (Belgium), 5–7 Oct 2015, pp 339–346
Abhilash Sinha S, Sinha MK, Pandey BD (2014) Extraction of lanthanum and cerium from Indian red mud. Int J Miner Process 127:70–73. doi:10.1016/j.minpro.2013.12.009
Yatsenko SP, Pyagai IN (2010) Red mud pulp carbonization with scandium extraction during alumina production. Theor Found Chem Eng 44:563–568. doi:10.1134/S0040579510040366
Ochsenkühn-Petropulu M, Lyberopulu T, Ochsenkühn KM, Parissakis G (1996) Recovery of lanthanides and yttrium from red mud by selective leaching. Anal Chim Acta 319:249–254. doi:10.1016/0003-2670(95)00486-6
Fulford GD, Lever G, Sato T (1991) Recovery of rare earth elements from Bayer process red mud. US Patent US5030424
Petrakova O, Klimentenok G, Panov A, Gorbachev S (2014) Application of modern methods for red mud processing to produce rare earth elements. In: 1st conference on European rare earth resources (ERES 2014), Milos (Greece), 5–6 Sept 2014, pp 221–229
Wang KQ, Yu YB, Wang H, Chen C (2010) Study of hydrochloric acid leaching scandium from red mud. Chin Rare Earths 31:95–98
Boudreault R, Fournier J, Primeau D, Labrecque-Gilbert M-M (2015) Processes for treating red mud. US Patent US20150275330
Xue A, Chen X, Tang Y (2010) The technological study and leaching kinetics of scandium from red mud. Nonferr Met Extr Metall 2:51–53
Zhang J, Deng Z, Xu T (2005) Experimental investigation on leaching metals from red mud. Light Met 2:13–15
Ochsenkuhn-Petropoulou MT, Hatzilyberis KS, Mendrinos LN, Salmas CE (2002) Pilot-plant investigation of the leaching process for the recovery of scandium from red mud. Ind Eng Chem Res 41:5794–5801. doi:10.1021/ie011047b
Sugita K, Kobayashi Y, Taguchi Y, Takeda S, Ota Y, Ojiri M, Oda K, Sano H (2015) Method of recovering rare-earth elements. US Patent US20150086449
Wang W, Pranolo Y, Cheng CY (2013) Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep Purif Technol 108:96–102. doi:10.1016/j.seppur.2013.02.001
Davris P, Balomenos E, Panias D, Paspliaris I (2014) Leaching of rare earths from bauxite residues using imidazolium based ionic liquids. In: 1st Eur. Rare Earth Resour. Conf. (ERES 2014), Milos (Greece), 4–7 Sept 2014, pp 241–252
Davris P, Balomenos E, Panias D, Paspaliaris I (2015) The use of ionic liquids for rare earth element extraction from bauxite residue. In: Pontikes Y (ed) Proceedings of Bauxite Residue Valorisation and Best Practices Conference, Leuven (Belgium) 5–7 Oct 2015, pp 323–329
Qu Y, Li H, Tian W, Wang X, Wang X, Jia X, Shi B, Song G, Tang Y (2015) Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Miner Eng 81:1–4. doi:10.1016/j.mineng.2015.07.022
Gamaletsos PN, Godelitsas A, Kasama T et al (2016) The role of nano-perovskite in the negligible thorium release in seawater from Greek bauxite residue (red mud). Sci Rep 6:21737. doi:10.1038/srep21737
Petrakova OV, Panov AV, Gorbachev SN, Milshin ON (2015) Improved efficiency of red mud process through scandium oxide recovery. In: Pontikes Y (ed) Proceedings of Bauxite Residue Valorisation and Best Practices Conference, Leuven (Belgium), 5–7 Oct 2015, pp 355–362
Davris P, Balomenos E, Panias D, Paspaliaris I (2016) Leaching rare earth elements from bauxite residue using brønsted acidic ionic liquids. In: Borges De Lima I, Leal Filho W (eds) Rare earths industry. Elsevier, Amsterdam, pp 183–197
Sibanda V, Ndlovu S, Dombo G, Shemi A, Rampou M (2016) Towards the utilization of fly ash as a feedstock for smelter grade alumina production: a review of the developments. J Sustain Metall. doi:10.1007/s40831-016-0048-6
Bayer KJ (1892) Verfahren zur darstellung von thonerhydrat und alkalialuminat (Process for the production of gamma aluminum (oxy)hydroxide and alkali aluminate). German patent, DE65604
Liu W, Yang J, Xiao B (2009) Review on treatment and utilization of bauxite residues in China. Int J Miner Process 93:220–231. doi:10.1016/j.minpro.2009.08.005
Logomerac VG (1979) Complex utilisation of red mud by smelting and solvent extraction. Trav Com Int Etude Bauxites, Alum Alum 15:279–285
Sargic V, Logomerac V (1974) Leaching and extraction in the complex processing of red mud. Trav Com Int Etude Bauxites Alum Alum 11:71–78
Fursman OC, Mauser JE, Butler MO, Stickney WA (1970) Utilization of red mud residues from alumina production: U.S. Bureau of Mines Report of Investigations. The National Archives and Records Administration, College Park
Piga L, Pochetti F, Stoppa L (1993) Recovering metals from red mud generated during alumina production. JOM 45(11):54–59. doi:10.1007/BF03222490
Hammond K, Mishra B, Apelian D, Blanpain B (2013) CR3 Communication: red mud: a resource or a waste? JOM 65:340–341. doi:10.1007/s11837-013-0560-0
Ding Y, Wang J, Wang G, Xue Q (2012) Innovative methodology for separating of rare earth and iron from Bayan Obo complex iron ore. ISIJ Int 52:1772–1777. doi:10.2355/isijinternational.52.1772
Ding Y, Xue Q, Wang G, Wang J (2013) Recovery behavior of rare earth from Bayan Obo complex iron ore. Metall Mater Trans B 44:28–36. doi:10.1007/s11663-012-9762-z
Panov A, Klimentenok G, Podgorodetskiy G, Gorbunov V (2012) Directions for large scale utilization of bauxite residue. In: Suarez CE (ed) Light Metals 2012. Wiley, Hoboken, pp 93–98
Guccione E (1971) Red mud, a solid waste, can now be converted to high-quality steel. Eng Min J 9:136–138
Mishra B, Slavik M (2000) Pyrometallurgical extraction of alumina and iron from red mud. In: EPD Congress 2000. TMS, Warrendale, pp 369–382
Peiwang L, Zhengquan H, Songqing G, Jiayu D, Jinyong Z, Gaoxing L (1995) Magnetic dressing iron mineral concentrate from Bayer red mud. In: Evans JW (ed) Light Metals 1995. TMS, Warrendale, pp 149–153
Paramguru RK, Rath PC, Misra VN (2005) Trends in red mud utilisation-a review. Miner Process Extr Met Rev 26:1–29. doi:10.1080/08827500490477603
Liu Z, Li H (2015) Metallurgical process for valuable elements recovery from red mud: A review. Hydrometallurgy 155:29–43. doi:10.1016/j.hydromet.2015.03.018
Liu Y, Naidu R (2014) Hidden values in bauxite residue (red mud): recovery of metals. Waste Manag 34:2662–2673. doi:10.1016/j.wasman.2014.09.003
Zhu DQ, Chun TJ, Pan J, He Z (2012) Recovery of iron from high-iron red mud by reduction roasting with adding sodium salt. J Iron Steel Res Int 19:1–5. doi:10.1016/S1006-706X(12)60131-9
Chun T, Zhu D, Pan J, He Z (2014) Recovery of alumina from magnetic separation tailings of red mud by Na2CO3 solution leaching. Metall Mater Trans B 45:827–832. doi:10.1007/s11663-014-0023-1
Liu W, Yang J, Xiao B (2009) Application of Bayer red mud for iron recovery and building material production from alumosilicate residues. J Hazard Mater 161:474–478. doi:10.1016/j.jhazmat.2008.03.122
Mishra S, Bagchi M (2002) Mud to metal: Romelt is an answer. In: Jouhari AK (ed) Smelting reduction for iron making. Allied publishers, New Delhi, pp 167–170
Balomenos E, Gianopoulou I, Panias D, Paspaliaris I (2011) A novel red mud treatment process. Trav ICSOBA 36:255–266
Ni LP (1960) Izv Akad Nauk Kaz SSR. Ser Met 1:21
Zalesskaya SV (1968) Izv Vyss Uchebn Zaved Chern Met 11:40
Szépvölgyi J, Bertóti I, Tóth A, Székely T (1988) Chlorination of a slag produced from red mud. React Solids 5:139–153. doi:10.1016/0168-7336(88)80083-4
Udy MJ (1958) Process for the separation and recovery of Fe, Ti, and Al values from ores and waste materials containing same. US patent US2830892 A
Dobos G, Horvath G, Felfoldi Z (1974) Complex utilization of red mud including the production of pig iron. Trav Com Etude Bauxites Alum Alum 12:151–159
Grzymek J, Derdacka-Grzymek A, Konik Z, Grzymek W (1982) Methods for obtaining iron, alumina, titania and binders from metallurgical slags and from“red mud” remaining in the Bayer method. In: Andersen JE (ed) Light Metals 1982. The Metallurgical Society, Warrendale, pp 143–155
Hareter M (1974) Possibilities to dispose of red mud economically. Trav Com Etude Bauxites Alum Alum 12:135–150
Ziegenbalg S, Rudolf M, Lowewe D, Ufer H, Jeschke D, Felfoeldi Z, Gerenczi T, Horvath G, Siklosi P (1983) Recovering alkali content esp. of red mud-by redn. smelting with coke, lime and sand. German patent DD200896-A
Erçag E, Apak R (1997) Furnace smelting and extractive metallurgy of red mud: recovery of TiO2, Al2O3 and pig iron. J Chem Technol Biotechnol 70:241–246
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T (2015) Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J Sustain Metall 2:28–37. doi:10.1007/s40831-015-0026-4
Raspopov NA, Korneev VP, Averin VV, Lainer YA, Zinoveev DV, Dyubanov VG (2013) Reduction of iron oxides during the pyrometallurgical processing of red mud. Russ Metall 1:33–37
Balomenos E, Panias D (2013) Iron recovery and production of high added value products from the metallurgical by-products of primary aluminium and. In: Proceedings of 3rd International Slag Valorisation Symposium, Leuven (Belgium), 19–20 March 2013, pp 161–172
Kaußen FM, Friedrich B (2016) Methods for alkaline recovery of aluminum from bauxite residue. J Sustain Metall. doi:10.1007/s40831-016-0059-3
Kaußen F, Friedrich B (2015) Reductive smelting of red mud for iron recovery. Chemie Ing Tech 87:1535–1542. doi:10.1002/cite.201500067
Guo Y, Gao J, Xu H, Kai Z, Shi X (2013) Nuggets production by direct reduction of high iron red mud. J Iron Steel Res Int 20:24–27
Jayasankar K, Ray PK, Chaubey AK, Padhi A, Satapathy BK, Mukherjee PS (2012) Production of pig iron from red mud waste fines using thermal plasma technology. Int J Miner Metall Mater 19:679–684. doi:10.1007/s12613-012-0613-3
Srikanth S, Alex TC, Bandopadhyay A, Jha A (2000) Utilisation of red mud for the recovery of metallic values. In: Chakraborty D, Jana R, Kumar V, Pandey BD, Goswami NG (eds) Proceedings of International Seminar on non-ferrous Metals, Jamshedpur, pp 205–209
Balomenos E, Panias D, Pontikes Y (2015) Mud2metal: A holistic flow sheet for the bauxite residue valorisation. In: Pontikes Y (ed) Proceedings of Bauxite Residue Valorisation and Best Practices Conference, Leuven (Belgium), 5–7 Oct 2015, pp 129–136
Li XB, Xiao W, Liu W, Liu GH, Peng ZH, Zhou QS, Qi TG (2009) Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering. Trans Nonferrous Met Soc China 19:1342–1347. doi:10.1016/S1003-6326(08)60447-1
Habashi F (2004) Karl Josef Bayer and his time: part 1. CIM Bull 97:61–64
Smith P (2009) The processing of high silica bauxites: review of existing and potential processes. Hydrometallurgy 98:162–176. doi:10.1016/j.hydromet.2009.04.015
Liu W, Sun S, Zhang L, Jahanshahi S, Yang J (2012) Experimental and simulative study on phase transformation in Bayer red mud soda-lime roasting system and recovery of Al, Na and Fe. Miner Eng 39:213–218. doi:10.1016/j.mineng.2012.05.021
Raghavan PKN, Kshatriya NK, Wawrynink K (2011) Recovery of metal values from red mud. In: Lindsay SJ (ed) Light Metals 2011. Wiley, Hoboken, pp 103–106
Meher SN, Padhi BK (2011) During thermo chemical leaching for extraction of alumina from red mud by lime soda ash sinter process. Br J Appl Sci Technol 1:41–52
Meher S, Rout A, Padhi B (2010) Extraction of Al and Na from red mud by magnesium oxide sodium carbonate sinter process. Afr J Environ Sci 4:897–902
Meher SN, Rout AK, Padhi BK (2011) Recovery of Al and Na values from red mud by BaO-Na2CO3 sinter process. E J Chem 8:1387–1393. doi:10.1155/2011/528134
Alp A, Goral MS (2003) The effects of the additives, calcination and leach conditions for alumina production from red mud. Scand J Metall 32:301–305. doi:10.1111/j.1600-0692.2003.00656.x
Meher SN, Padhi BK (2012) Effects of MgO and Na2CO3 Additives, sintering temperature and leaching conditions for extraction of alumina from Bayer’s process waste residue (red mud). Chem Sci Trans 1:456–462. doi:10.7598/cst2012.180
Meher SN, Padhi BK (2011) During thermo chemical leaching for extraction of alumina from red mud by lime soda ash sinter process. Br J Appl Sci Technol 1:41–52
Rayzman VL, Filipovich IK (1999) Integrating coal combustion and red mud sintering at an alumina refinery. JOM 51(8):16–18. doi:10.1007/s11837-999-0235-z
Rayzman V, Aturin A, Pevzner I, Sizyakov V, Ni L, Filipovich I (2003) Extracting silica and alumina from low-grade bauxite. JOM 55(8):47–50. doi:10.1007/s11837-003-0105-z
Mishra B, Stanely A, Kirkpatrick D (2001) Recovery and utilization of iron from red-mud. In: Anjier JL (ed) Light metals. Minerals, Metals and Materials Society, New Orleans, pp 149–156
Kumar R, Srivastava J (1998) Utilization of iron values of red mud for metallurgical applications. In: Bandopadhyay A, Goswami NG, Rao PR (eds) Environmental and Waste Management. NML, Jamshedpur, pp 108–119
Anonymous (2016) Nyngan Scandium Project. http://www.scandiummining.com/s/nyngan.asp?ReportID=749853. Accessed 22 July 2016
Duyvesteyn WPC (2012) System and Method for Recovery of Scandium Values from Scandium-Containing Ores. US patent application no. US20120207656
Kaya Ş, Topkaya YA (2016) Extraction behavior of scandium from a refractory nickel laterite ore during the pressure acid leaching process. In: Borges De Lima I, Leal Filho W (eds) Rare earths industry. Elsevier, Amsterdam, pp 171–182
Borra CR, Mermans J, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T (2016) Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner Eng 92:151–159. doi:10.1016/j.mineng.2016.03.002
Acknowledgments
The authors acknowledge KU Leuven for financial support (DBOF PhD grant to CRB and IOF-KP RARE3).
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Dimitrios Panias.
Rights and permissions
About this article
Cite this article
Borra, C.R., Blanpain, B., Pontikes, Y. et al. Recovery of Rare Earths and Other Valuable Metals From Bauxite Residue (Red Mud): A Review. J. Sustain. Metall. 2, 365–386 (2016). https://doi.org/10.1007/s40831-016-0068-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-016-0068-2