Abstract
The dengue disease epidemiological patterns of Nepal have become a geographical challenge. It is one of the emerging public health issues in the country. In Nepal, the disease has been reported since 2004 in both tropical and sub-tropical regions. In this work, the dengue disease epidemic model is formulated by using fractional-order derivatives. The least-squares method is used for estimation of the parameters with the help of the classical compartmental model of the dengue infections recorded in the year 2019, which is the largest-ever outbreak to occur in Nepal. The existence and uniqueness of a non-negative solution are discussed for the fractional-order model. An epidemiologically important dimensionless number, \(R_0\) is obtained using the next-generation method. Two equilibriums disease free and endemic points are obtained and their stability is studied. The Euler’s method is implemented to solve the fractional-order model numerically. The numerical results suggest that the fractional model fits better with an appropriate choice of the memory level, \(\alpha \) than the classical model based on the real data of Nepal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dengue is highly infectious mosquito-borne viral disease. It has scattered throughout the tropics with local divergence due to the seasonal impact (rainfall, temperature) and unplanned rapid civic [37]. Dengue virus imparts to humans through the bite of infected Aedes mosquito, especially called Aedes Aegypti. The infected mosquito remains with one of the four dengue virus serotypes (DEN-1, DEN-2, DEN-3 and DEN-4) for life which transmits the virus to humans during feed [45]. Dengue can affect almost all age groups (infant to adult) of humans. The epidemic of dengue-like illnesses was reported in the French West Indies in 1635 and Panama in 1699. In 1780, doctors in Philadelphia, Pennsylvania recorded an epidemic of the disease later known as dengue [22]. About 390 million dengue virus infections occurs every year, of which 96 million manifest the disease apparently with any level of severity [8]. According to WHO [70], about 3.9 billion people are at risk with dengue virus including 129 countries, 70% are from Asia. Nowadays, dengue disease is affecting Asian and Latin American countries and has become a leading cause of hospitalization and death among children and adults [22]. According to WHO, mosquito transmit malaria, dengue, yellow fever, filariasis, and several other infectious diseases. This is due to the gradually increasing urbanization, uncontrolled population and civilization.
In the context of Nepal, Peters et al. [47] were first to study on mosquito and reported the existence of the species of Anopheles in the Terai region. Peters and Dewar [46] recorded culicine species of mosquito existing in Nepal. Darsie and Pradhan [13] focused their study on biological distribution and indentification of the mosquito in Nepal. Aedes Aegypti species was first reported in Nepal in 2009 [21] although the first dengue outbreak was reported in 2004 [30], and other outbreaks of dengue have been recorded from both tropical and subtropical regions of Nepal including the capital city Kathmandu [44, 54]. During 2004–2013 the major outbreaks of dengue epidemic was recorded in 2010 and 2013. In the years 2014–2019, a yearly outbreaks of the dengue have been more frequent than in the years 2004–2013. The number of confirmed annual dengue incidence varied from 336 to 17,992 in 2014–2019 [57]. The largest ever outbreak of dengue diseases in Nepal was reported in 2019. In this year the infectious cases was more than 15,000 people during mid-summer from a tropical region and then it spread to hilly subtropical locations (the Sunsari district, the eastern region of Nepal, the Makwanpur district and the southwest district of Kathmandu). A total nineteen deaths were reported from 2006 to 2019 from dengue infection [3, 40]. According to EDCD [40] of Nepal, there are around 564 dengue infection cases reported in 2002, most of the affected districts are Myagdi, Kaski, Sindhuli, Kailali, Sunsari, and Rupandehi. Dengue virus infection has now become an emerging disease in Nepal. Subedi and Robinson [64] revealed that the problem in finding timely medical treatment, poor facilities for disease diagnosis and a scarcity of mosquito control programs are major issues to cause increment in dengue cases in Nepal. Kawada et al. [30] observed the insecticides’ resistance status and its impact on the transmission and the prevention of dengue in Nepal (Fig. 1).
Dengue cases in Nepal from 2006 to 2020 (Data source: Epidemiology Disease Control Division, Nepal [40])
Mathematical modeling of dengue has become one of the effective strategies for the prevention and control of the disease [37]. Kermack and McKendrick contributed on upgrading the mathematical modeling of infectious diseases by SIR (Susceptible, Infected and Removed of individuals) compartmental model [31, 32]. Esteva and Vargas [18, 19] modified this model to study transmission dynamics of dengue disease. In the transmission of dengue virus, there are two periods of incubation, one is the time when the mosquito takes viremic bloodmeal and it becomes infectious (5–33 days), another is time from infection due to the bite of infected mosquito to the appearance of symptoms in human (2–10 days) [11]. Pongsumpun [52] studied the length of time during incubation of dengue virus in humans and mosquito, and analyzed how to reduce the outbreak of the dengue virus. The SEIR model [61] is proposed to study dengue disease transmission of dengue disease taking exposed classes of both vector and host population. Prevention of mosquito bites is one of the ways to prevent dengue disease. The developing countries may not be able to provide treatment to each infected individual. The transmission model with treatment by considering logistic growth of mosquito is formulated and analyzed by [62]. The effect of temperature and rainfall on mosquito to spread the dengue disease in a community is one of natural problems [2, 66]. In epidemic and endemic areas, awareness about dengue will lessen the contact rate between host and mosquito. Most of the epidemic model [9, 12, 60, 63] address the impact of media coverage to the spread and control of infectious diseases for social awareness and activity of social disturbances. Nicholson’s blowflies model [20] can be used for the study of dynamic of differential system. In Nepal, Phaijoo and Gurung [49, 50] studied a vector host epidemic model of dengue disease by considering control measures of the disease and also suggested that the proper management of human movement between patches helps to reduce the burden of dengue disease.
The fractional models are useful concord for the disease epidemics which frame the memory and nonlocal affects [1, 5]. Though fractional calculus was introduced more than 300 years ago and is applied into many fields of science and engineering, the development of applications is still an important task in the area of fractional calculus [51]. Fractional calculus has real world applications in science and engineering [65]. Authors in [29, 56] has studied the stability analysis, uniqueness and existence of dynamical system of fractional Duffing problem and applied fixed point theory for the stability of epidemic model by using fractional calculus. Hajiseyedazizi et al. [24] established the existence of a solution for a novel class of singular q-integro-differential equations on a time scale. Under certain boundary conditions on the time scale, Samei and Rezapour [58] evaluated the possibility of a solution for an m-dimensional system of fractional q-differential inclusions using the sum of two multi-term functions. Fractional-order differential equations are naturally related to systems with memory which exists in most biological systems. It is applicable with initial value differential equations [16]. Pooseh et al. [53] studied the fractional-order derivative in the dengue epidemic and showed a better realistic result than show by the classical model. Diethelm [15] formalized the fractional-order model for the simulation of an outbreak of dengue fever using the SIR compartment model. Authors in [26, 27] developed a fractional-order dengue epidemic models representing the human and mosquito dynamics. The effects of memory index, \(\alpha \) is used in dengue disease transmission model [17, 59]. The fractional-order mathematical models in epidemic reflect the dynamical behaviors in real-world [65]. Transformation of a classical model into a fractional-order model with the order of memory level \(\alpha \) gives more accurate result [15, 53, 59].
In the present work, the SEIR-SEI compartmental model is used to study transmission dynamics of dengue disease in Nepal. The model parameters like: birth rate, death rate, vector incubation rate are determined from the real data [40]. The deterministic model is used to estimate remaining model parameters applying the method of the least square, which is based on cumulative data of dengue patients of the year 2019. The reproduction number is obtained and used to study the stability of model. Furthermore, we formulate the model with fractional-order derivative and use the Euler’s method for the numerical simulation of the model.
Model Formulation
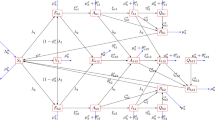
We represent the total mosquito population and the total human population by \(N_m(t)\) and \(H_h(t)\) respectively for \(t>0\). The total population divided into different compartment as: susceptible \((M_s)\), exposed \((M_e)\) infected \((M_i)\) groups of the vector population and susceptible \((H_s)\), exposed \((H_e)\), infected \((H_i)\) and recovered \((H_r)\) groups of host population. The schematic diagram is presented in Fig. 2.
The differential equation of the model is as given;
with initial conditions \(H_s(0)> 0\), \(H_e(0) \ge 0\), \(H_i(0)\ge 0\), \(H_r(0)\ge 0\), \(M_s(0)> 0\), \(M_e(0)\ge 0\), \(M_i(0)\ge 0\)
The biological meaning of state variables and the parameters used in the model are presented in Table 1; where, \(H_h = H_s+H_e+H_i+H_r\) and \(N_v = M_s+M_e+M_i\) and \(\displaystyle \frac{dN_v}{dt} = A-\mu _v N_v \).
Again introducing the relations, \(s_h=\displaystyle \frac{H_s}{H_h}\), \(e_h=\displaystyle \frac{H_e}{H_h}\), \(i_h=\displaystyle \frac{H_i}{H_h}\), \(r_h=\displaystyle \frac{H_r}{H_h}\), \(s_v=\displaystyle \frac{M_s}{\frac{A}{\mu _v}}\), \(e_v=\displaystyle \frac{M_e}{\frac{A}{\mu _v}}\), \(i_v=\displaystyle \frac{M_i}{\frac{A}{\mu _v}}\).
The Eq. (1) becomes
The system of Eq. (2) has the same qualitative behavior as the system of Eq. (1).
Parameter Estimations
The yearly dengue disease information is taken from Epidemiology and Diseases Control Division (EDCD) and Early Warning Reporting System (EWARS) which also includes death cases from the years 2006 to 2020 [40]. The study includes the new weekly dengue data available for Nepal in 2019. We used the disease cumulative weekly data for the model equations. For the prediction of new infected cases C(t) at time t is used for the solution of differential equation [55].
where, \(\psi \) is the proportion of the reported cases of dengue. We apply the Fourth order Runge–Kutta method for the numerical solution of Eq. (2). Non-linear least square regression method is used for parameter estimation, which minimizes the sum of the square residuals
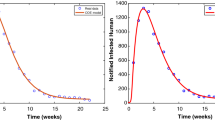
where, \(\theta = \{b, \beta _{h},\beta _{v}, \alpha _h,{\gamma _h}\}\) to be estimated and \(C(t_i)\) and \({\overline{C}}(t_i)\) are given available data and new cases of weekly recorded infectious cases predicted by the model respectively. In this study, we use “ode45” (ODE Solver) and “fmincon”(Optimization) tools for simplification (Fig. 3).
The data available for weekly cumulative dengue infectious cases is fitted to the model for the estimation of the parameters. Estimated parameters are presented in Table 2.
Preliminaries on Fractional Derivative
Definition 4.1
[14, 39, 51] The Riemann–Liouville’s fractional integral of order \(\alpha \) is defined by
where \(\alpha>0, \, t>0\), \(\Gamma {(\alpha )}\) is gamma function of \(\alpha \)
Definition 4.2
[14, 39, 51] The Caputo fractional derivative of the function f(t) is defined by
Definition 4.3
[14, 39, 51] The Laplace transform for the Caputo fractional derivative of the function f(t) is
Definition 4.4
[14, 39, 51] The Mittag–Leffler function is defined by,
and the Laplace transform formula for the Mittag–Leffler function is
Theorem 4.1
[14, 16] Consider the two-parameter Mittag–Leffler function \(E_{\alpha ,\beta }(z)\) for some \(\alpha ,\beta >0\). The power series defining \(E_{\alpha ,\beta }(z)\) is convergent for all \(z\in {\mathbb {C}}\). In other words, \(E_{\alpha ,\beta }(z)\) is an entire function.
Fractional Dengue Diseased Model
Pooseh et al. [53], Diethelm [15] and [17, 26, 27] introduced the fractional order derivative for different classical integer model. The memory effect plays a significant role in the transmission of the dengue disease from host to vector and vector to host which is taken as same in the model. Other parameters like birth, death, recovery rate do not have own memory. The Caputo fractional-order derivative model for the classical model Eq. (1) is,
where, \(0<\alpha \le 1\) and the initial conditions of the fractional model are \(H_s(0) > 0\), \(H_e(0) \ge 0\), \(H_i(0) \ge 0\), \(H_r(0) \ge 0\), \(M_s(0) > 0\), \(M_e(0) \ge 0\), \(M_i(0) \ge 0\), for all \(t\ge 0\). Here,
and for the mosquito population
Positiveness and Boundedness
Consider the dynamic of Caputo fractional model Eq. (7) in the feasible region
Theorem 6.1
(Generalized Mean Value Theorem) [42] Let \(0<\alpha \le 1\), \(f(t)\, \in \, C[a,b]\) and if \(D_{t}^{\alpha }f(t) \in C[a,b]\), then
Remark 6.1
Let \(f(t) \in C[a,b]\) and \(D_{t}^{\alpha }f(t)\in C[a,b] \) for \(0<\alpha \le 1\)
-
(i)
If \(D_{t}^{\alpha }f(t) \ge 0 ,\,\forall t\in (a,b)\), then f(t) is nondecreasing.
-
(ii)
If \(D_{t}^{\alpha }f(t) \le 0 ,\,\forall t\in (a,b) \), then f(t) is nonincreasing.
Lemma 6.1
The set \({\mathcal {W}}\) attracts all positive solutions of the model Eq. (7) for given initial conditions with \(t\ge 0\).
Proof
By applying theorem (6.1) and the Remark (6.1) for existence of the solution of the model Eq. (7) in \((0,\infty )\) with given initial conditions, we get the following relations,
Hence, the set \({\mathcal {W}}\) is non -negative orthant in \({\mathbb {R}}_{+}^7\) for all \(t\ge 0\). \(\square \)
Theorem 6.2
The solution y(t) of the model Eq. (7) uniformly bounded.
Proof
From the model Eq. (7) we get,
For Eq. (9), we get \(H_h(t) = k \) and \(H_h(0) =k\), where k is arbitrary constant. That means, \(H_h(t)\) is bounded.
From Eq. (10), we get
Taking Laplace Transform on both sides,
Taking Laplace inverse on both sides
Using the definition (4.4), we get
Here, \(M_1 = \biggl (\frac{A}{\mu _v}-M(0)\biggr )\). From theorem (4.1) \(E_{\alpha ,1}(-\mu _vt^{\alpha })\) is bounded for all \(t\ge 0\). Thus, the solution of model (7) is uniformly bounded and system will remain in \({\mathcal {W}}\) (from Lemma 6.1). \(\square \)
Uniqueness of Solution
Introducing the relations, \(s_h=\displaystyle \frac{H_s}{H_h}\), \(e_h=\displaystyle \frac{H_e}{H_h}\), \(i_h=\displaystyle \frac{H_i}{H_h}\), \(r_h=\displaystyle \frac{H_r}{H_h}\), \(s_v=\displaystyle \frac{M_s}{\frac{A}{\mu _v}}\), \(e_v=\displaystyle \frac{M_e}{\frac{A}{\mu _v}}\), \(i_v=\displaystyle \frac{M_i}{\frac{A}{\mu _v}}\), \(L = \displaystyle \frac{A}{\mu _vH_h}b^{\alpha } \beta _h\) and \(W= b^{\alpha }\beta _v\). The Eq. (7) becomes
The system of Eq. (11) also satisfy non-negative with uniformly boundedness and has a biologically feasible region. Let us defined a region as
where B is a finite positive value.
Lemma 7.1
[34] Consider the system
with initial condition \(y(t_0) = y_{t_0}\), where \(\alpha \in (0,1]\), \(f:[t_0,\infty ) \times {\mathcal {W}} \rightarrow {\mathbb {R}}^n, C \subseteq {\mathbb {R}}^n \), if local Lipschitz condition is satisfied by f(t, x) with respect to x, then there exists a solution of (12) on \([t_0,\infty ) \times {\mathcal {W}}\) which is unique.
Theorem 7.1
For the initial value of each \(D_0 = (s_h(0),e_h(0),i_h(0),e_v(0),i_v(0)) \in {\mathcal {W}}_1\) and there exists a unique solution of \(D(t)\in {\mathcal {W}}_1 \) of system of Eq. (11) for all \(t \ge 0\).
Proof
Using the approaches [34, 35], let us define a relation \(Z(D) =\{Z_1(D),Z_2(D),Z_3(D),Z_4(D),Z_5(D)\}\), where
For any \(D,{\overline{D}} \in {\mathcal {W}}_1 \),
Where, \(V= \hbox {max} \{ \mu _h+2LB, 2WB+W+\gamma _h+\mu _h, 2\alpha _h+\mu _h, \mu _v+2k_v+WB, 2LB+\mu _v+WB \}\). Thus, Z(D) satisfies the Lipschitz condition with respect to D. Hence, from Lemma (7.1), D(t) has a unique solution of Eq. (11) with initial condition \(D_0\). \(\square \)
Stability of Model
Basic Reproduction Number
Basic reproduction number [68] is the expected number of secondary infections caused by a single infectious individual during their entire infectious lifetime. Using next-generation operator method [67, 68], we find the expression for \(R_0\). For this, we find the matrices F (infection new cases term) and V (transition terms) of Eq. (11). Here, \(L = \frac{A}{\mu _vH_h}b^{\alpha } \beta _h\), \(\beta = (\alpha _h+\mu _h)\), \(k =\gamma _h+\mu _h\) and \(W= b ^{\alpha }\beta _v\).
Also,
The spectral radius of the next generation matrix is
which is threshold condition equivalent to reproduction number of the model.
The basic reproduction number in dimensionless form is
Equilibrium Points
For the evaluation of equilibrium points, the system of Eq. (11) is formed as
So, using \(L = \frac{A}{\mu _vH_h}b^{\alpha } \beta _h\), \(\beta = (\alpha _h+\mu _h)\), \(k =\gamma _h+\mu _h\) and \(W= b ^{\alpha }\beta _v\)
After solving, we get a diseases free equilibrium (DFE) point \(E_0 = (1,0,0,0,0)\) and the endemic equilibrium (EE) point \(E^*=(s_h^{*}, e_h^{*}, i_h^{*}, e_v^{*}, i_v^{*} )\) where,
Asymptotic Behaviour
Lemma 8.1
[36, 38, 48] Consider the fractional-order system
with initial condition \(y(t_0) = t_0\), where \(\alpha \in (0,1]\), \(f\in {\mathbb {R}}^n\). The system is said to be locally asymptotically stable iff \(|arg(\lambda _i)|>\frac{\alpha \pi }{2}\) for all eigenvalues \(\lambda _i\) where, \(i= 1,2,3,\ldots \) which is obtained from given Jacobian matrix.
Theorem 8.1
The disease free equilibrium \(E_0\) is locally asymptotically stable if \(R_0 <1\) and unstable if \(R_0 >1\).
Proof
The Jacobian matrix \(J(E_0)\) of the Eq. (11) about the diseases free equlibrium point is
The characteristics equations is;
where the eigenvalue is \(\lambda = - \mu _h\) and for remaining Jacobian matrix of order 4 is
The characteristic equation of \(J_1(E_0)\) is as
where
Here, the parameters \(p_1,p_2,p_3\) are non-negative and \(p_4\) is positive when \(R_0 < 1\).
The approaches followed by [6, 25, 36, 62] for the characteristic Eq. (19) and the eigenvalue is \(\lambda = - \mu _h\) and remaining are determined by polynomial Eq. (20). So,
where, \(\alpha \in (0,1]\). Similarly, the argument of roots of characteristic polynomial Eq. (20) are also greater than \(\frac{\alpha \pi }{2}\) in similar processes. Form the Lemma 8.1, we conclude that DFE is locally asymptotically stable for \(R_0 < 1\). \(\square \)
Theorem 8.2
The endemic equilibrium \(E^*=(s_h^{*}, e_h^{*}, i_h^{*}, e_v^{*}, i_v^{*} )\) of the system of Eq. (11) is locally asymptotically stable if \(R_0>1\) and unstable if \(R_0<1\).
Proof
The Jacobian matrix evaluated at endemic equlibrium point \(E^*\) in (17) of the system of Eq. (11) is
where
The characteristic equation of \(J(E^*)\) is as
where,
According to Routh–Hurwitz criterion followed in [4, 37, 38], we have the following associated conditions,
As followed by \(n_i >0\) for all \(i =1,2,3,4,5\) and the relation \(n_1>0,n_2>0,n_3>0,n_4>0,n_5>0\), \(n_1n_2n_3>n_3^2+n_1^2n_4\), \((n_1n_4-n_5)(n_1n_2n_3-n_3^2-n_1^2n_4) > n_5(n_1n_2-n_3)^2+n_1n_5^2\) is satisfied when \(R_0>1\). Hence, the equilibrium point \(E^*\) is locally asymptotically stable. Which complete the proof. \(\square \)
Basic Reproduction Number \(R_0\) With Model Parameters
Figures 4 and 5 are simulated to observe the impacts of model parameters on the basic reproduction number. Figure 4a describes the relation between \(R_0\), \(\alpha \), and \(b^\alpha \), which suggests that when level of memory increases \((\alpha \rightarrow 0)\), \(b^{\alpha }\) increases and \(R_0 \propto b^{\alpha }\). When biting rate increases, basic reproduction number \(R_0\) also increases while \(R_0\) decreases with the decrease in \(\alpha \) (Fig. 4b). The variation in the value of \(R_0\) versus the transmission rate of host \(\beta _h\) and recovery rate \(\gamma _h\) is shown in Fig. 5a. \(R_0\) increases with increase in \(\beta _h\) and \(R_0\) decreases with increase in \(\gamma _h\). From Fig. 5b the relation of \(R_0\), transmission rate of vector \(\beta _v\) and host incubation rate \(\alpha _h\) is observed. The figures show that \(R_0\) can be effectively reduced or increased with respective change in parameters \(\beta _v\) and \(\alpha _h\).
Numerical Results and Discussion
There are different numerical methods discussed in [28, 33, 41, 69] for the solution of the fractional order system of equation. The simulation technique used for the model (11) is the Euler’s method, which can be expressed as;
Consider the initial value problem
where, \(t_i = q+ih\), \(i = 0,1,2,3,\ldots ,n\) and \(h = \frac{r-q}{n}\). The Euler’s method for (22) is,
Applying (23) to the model Eq. (11) we can write the equations for all \(i = 1,2,\ldots ,n-1\), \(t>0\) as;
For the numerical simulation, We have taken a 32 week’s data of nepal from July 2019 to December 2019. In this year, monsoon started from March, so the dengue cases were reported to appear from May affecting over 68 districts out of 77 districts of Nepal [43]. Around 97% dengue cases in Nepal are from Terai and mid-mountain region [23, 57]. According to Central Bureau of Statistics 2011 [10], the population size of Nepal is 26.5 million and around 51% population are living in Terai region. So, 20 million peoples are considered as a susceptible population for dengue cases. \(\frac{1}{\mu _h}\) and \(\frac{1}{\mu _v}\) are respectively the maximum life span of host and vector population taken as \(\frac{1}{365 \times 70}\) and \(\frac{1}{ 50}\) per day respectively [7, 50]. The vector incubation rate \(k_v = \frac{1}{15}\) per day is taken for simulations [7, 55]. The parameters value taken from Table 2 and the initial values are taken according to available data of Nepal [40].
Numerical simulation for the model (11) by using the Caputo derivative for \((\alpha =1,0.98,0.95,0.92,0.9)\)
Figure 6a, presents the solution of classical model and the fractional-order model taking real infectious disease cases data of Nepal. This shows that the fractional order system fit better than the classical model system when the memory level \(\alpha = 0.92\). The numerical simulations of \(s_h,e_h, i_h, e_v,i_v\) are displayed in Fig. 6b for \(\alpha =0.92\). When the suspectible humans come in contact with the infectious mosquito, they become infected. Then the suspectible population size decreases and the infected (exposed) population size starts to increase. This infected population size decreases after attaining its peak as the infected (exposed) people become infectious showing the symptoms of diseases and some infected people may die due to the natural cause. The infectious population size initially increases and the population size decreases later, due to recovery and natural death. The similar dynamics can be observed in the mosquito population (Fig. 6b).
Figure 7a–e are simulated with different values of \(\alpha \) taking time in weeks and keeping other parameters constant. With the change in \(\alpha \) there is a significant change in the disease dynamics. Interestingly, it is observed that \(s_h\) drops rapidly in short period of time with the decrease in the values of \(\alpha \) (Fig. 7a). There is noticeable variation in disease transmission with the change in the different values of \(\alpha \). \(e_h\) and \(i_h\) also complete the chain of infection with increase value of the memory. Similar, dynamics of the populations can be observed with small change of memory \(\alpha \). It is observed that the variation of fractional order \(\alpha \) has a great influence on the infection level of dengue in both vector and host populations.
Conclusion
Fractional-order calculus has wide applications in real life problems. Nowadays, this calculus is being used in epidemic diseases modeling to obtain better results. Fractional-order derivative is used in the classical integer model (SEIR-SEI). The theoretical and epidemiological aspects of the dynamical behaviour of the diseases are studied in detail using the fractional-order system. The disease-free equilibrium is seen locally asymptotically stable whenever the associated basic reproduction number \(R_0<1\), and the endemic equilibrium is observed to be locally asymptotically stable whenever \(R_0>1\). We obtained feasible results for the dynamics of dengue infection with the variation in the memory index \(\alpha \). Present study suggests that index of the memory has a logical effects for the system. The fractional-order model can explore the dengue epidemic disease transmission more accurately rather than the integer-order model under an appropriate choice of memory level. The transformation in fractional-order model with a small change in \(\alpha \) produces a large change in the transmission dynamics. From the study of data structure of infectious cases, overall model dynamics shows that \(R_0 =3.26 \), and we observed that before July \(R_0\) is less than unity, August–September \(R_0\) is greater than unity where infections cases is in peak and after October \(R_0\) is less than unity. This is due to the lack of preventive measures in society and a seasonal behaviours (temperature, rainfall). So, these behaviours can be introduced in the future work for an appropriate study of dengue disease spread in Nepal.
Data Availability
Enquiries about data availability should be directed to the authors.
References
Aba Oud, M.A., Ali, A., Alrabaiah, H., Ullah, S., Khan, M.A., Islam, S.: A fractional order mathematical model for COVID-19 dynamics with quarantine, isolation, and environmental viral load. Adv. Differ. Equ. 2019, 1–19 (2021)
Abdelrazec, A., Gumel, A.B.: Mathematical assessment of the role of temperature and rainfall on mosquito population dynamics. J. Math. Biol. 74, 1351–1395 (2017)
Adhikari, N., Subedi, D.: The alarming outbreaks of dengue in Nepal. Trop. Med. Health (2020)
Ahmed, E., El-Sayed, A.M.A., Hala, A., El-Saka, A.E.: On some Routh–Hurwitz conditions for fractional order differential equations and their applications in Lorenz, Rossler, Chua and Chen systems. Phys. Lett. A 358, 1–4 (2006)
Ahmeda, E., Elgazzarb, A.S.: On fractional order differential equations model for nonlocal epidemics. Physica A 379, 607–614 (2007)
Al-Sulami, H., El-Shahed, M., Nieto, J.J., Shammakh, W.: On fractional order dengue epidemic model. Math. Probl. Eng. 1–6 (2014)
Andraud, M., Hens, N., Marais, C., Beutels, P.: Dynamic epidemiological models for dengue transmission: A systematic review of structural approaches. PLOS ONE 7, e49085 (2012)
Bhatt, S., et al.: The global distribution and burden of dengue. Nature 498(7446), 504–507 (2013)
Caetano, M.A.L., Yoneyama, T.: Optimal and sub-optimal control in dengue epidemics. Optim. Control Appl. Methods 22, 63–73 (2001)
Central Bureau of Statistics: National Planning Commission Secretariat. Government of Nepal www.cbs.gov.np (2011)
Chan, M., Johansson, M.A.: The incubation periods of dengue viruses. PLoS ONE 7, e50972 (2012)
Cui, J., Sun, Y., Zhu, H.: The impact of media on the control of infectious diseases. J. Dyn. Differ. Equ. 20(1), 31–53 (2008)
Darsie, R.F., Pradhan, S.P.: The mosquitoes of Nepal: their identification, distribution and biology. Mosquitoes Syst. 22, 69–130 (1990)
Diethelm, K.: The Analysis of Fractional Differential Equations. Springer, Berlin (2010)
Diethelm, K.: A fractional calculus based model for the simulation of an outbreak of dengue fever. Nonlinear Dyn. 71, 613–619 (2013)
Diethelm, K., Ford, N.J.: Analysis of fractional differential equations. J. Math. Anal. Appl. 260(2), 229–248 (2002)
Du, M., Wang, Z., Hu, H.: Measuring memory with the order of fractional derivative. Sci. Rep. 3, 3431 (2013)
Esteva, L., Vargas, C.: Analysis of a dengue disease transmission model. Math. Biosci. 150, 131–151 (1998)
Esteva, L., Vargas, C.: A model for dengue disease with variable human population. J. Math. Biol. 38, 220–240 (1999)
Eswari, R., Alzabut, J., Samei, M.E., Zhou, H.: On periodic solutions of a discrete Nicholson’s dual system with density-dependent mortality and harvesting terms. Adv. Differ. Equ. 2021, 1–19 (2021)
Gautam, I., Dhimal, M., Shrestha, S.R., Tamrakar, A.S.: First record of Aedes aegypti (L.) vector of dengue virus from Kathmandu, Nepal. J. Nat. Hist. Museum 24, 156–64 (2009)
Gubler, D.J.: Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11(3), 480–496 (1998)
Gupta, B.P., Tuladhar, R., Kurmi, R., Manandhar, K.D.: Dengue periodic outbreaks and epidemiological trends in Nepal. Ann. Clin. Microbiol. Antimicrob. 17, 6 (2018)
Hajiseyedazizi, S.N., Samei, M.E., Alzabut, J., Chu, Y.M.: On multi-step methods for singular fractional q-integro-differential equations. Open Math. 19, 1378–1405 (2021)
Hamdan, N., Kilicman, A.: A fractional order SIR epidemic model for dengue transmission. Chaos Solitons and Fractals 114, 55–62 (2018)
Hamdan, N., Kilicman, A.: Analysis of the fractional order dengue transmission model: a case study in Malaysia. Adv. Differ. Equ. 2019, 1–13 (2019)
Hamdan, N., Kilicman, A.: Sensitivity analysis in a dengue fever transmission model: a fractional order system approach. J. Phys. Conf. Ser. 1366, 012048 (2019)
Hoan, L.V.C., Akinlar, M.A., Inc, M., Gomez-Aguilar, J.F., Chu, Y.M., Almohsen, B.: A new fractional-order compartmental disease model. Alex. Eng. J. 59, 3187–3196 (2020)
Houas, M., Samei, M.E.: Existence and Mittag–Leffler–Ulam-stability results for duffing type problem involving sequential fractional derivatives. Int. J. Appl. Comput. Math. 8, 185 (2022)
Kawada, H., Futami, K., Higa, Y., Rai, G., Suzuki, T., Rai, S.K.: Distribution and pyrethroid resistance status of Aedes aegypti and Aedes albopictus populations and possible phylogenetic reasons for the recent invasion of Aedes aegypti in Nepal. Parasit. Vectors 13, 2–13 (2020)
Kermack, W., McKendrick, A.: A contribution to mathematical theory of epidemics. Proc. R. Soc. Lond. A 115, 700–721 (1927)
Kermack, W., McKendrick, A.: Contributions to the mathematical theory of epidemics-II—The problem of endemicity. Bull. Math. Biol. 53(1/2), 57–87 (1991)
Li, C., Zeng, F.: The finite difference methods for fractional ordinary differential equations. Numer. Funct. Anal. Optim. 34(2), 149–179 (2013)
Li, Y., Chen, Y., Podlubny, I.: Stability of fractional-order nonlinear dynamic systems: Lyapunov direct method and generalized Mittag–Leffler stability. Comput. Math. Appl. 59(5), 1810–1821 (2010)
Li, H.L., Zhang, L., Hu, C., Jiang, Y.L., Teng, Z.: Dynamical analysis of a fractional-order predator-prey model incorporating a prey refuge. J. Appl. Math. Comput. 54, 435–449 (2017)
Mandal, M., Jana, S., Nandi, S.K., Kar, T.K.: Modelling and control of a fractional-order epidemic model with fear effect. Energy Ecol. Environ. 5(6), 421–432 (2020)
Martcheva, M.: An Introduction to Mathematical Epidemiology, 61. Springer, Berlin (2015)
Matignon, D.: Stability results for fractional differential equations with applications to control processing. Comput. Eng. Syst. Appl. 2, 963–968 (1996)
Miller, K.S.: An Introduction to Fractional Calculus and Fractional Differential Equations. Wiley, New York (1993)
MoHP: Government of Nepal. Ministry of Health and Population. www.edcd.gov.np/ewars January–December (2019)
Odibat, Z.M., Moamni, S.: An algorithm for the numerical solution of differential equations of fractional order. J. Appl. Math. Inform. 26, 15–27 (2008)
Odibat, Z.M., Shawagfeh, N.T.: Generalized Taylor’s formula. Appl. Math. Comput. 186, 286–293 (2007)
Pandey, B.D., Costello, A.: The dengue epidemic and climate change in Nepal. The Lancet 394(10215), 2150–2151 (2019)
Pandey, B.D., Rai, S.K., Morita, K., Kurane, I.: First case of dengue virus infection in Nepal. Nepal Med. Coll. 6(2), 157–159 (2004)
Pandey, B.D., Morita, K., Khanal, S.R., Takasaki, T., Miyazaki, I., Ogawa, T., Inoue, S., Kurane, I.: Dengue virus, Nepal. Emerg. Infect. Dis. 14(3), 514–515 (2008)
Peters, W., Dewar, S.C.: A preliminary record of the megarhine and culicine mosquitoes of Nepal with notes on their taxonomy (Diptera: Culicidae). Indian J. Malariol. 10, 37–51 (1956)
Peters, W., Dewar, S.C., Bhalla, B.D., Manadhar, T.L.: A preliminary note on the Anophelini of the Rapti valley area of the Nepal Terai. Indian J. Malariol. 9, 207–212 (1955)
Petras, I.: Fractional-Order Nonlinear Systems: Modeling, Analysis and Simulation. Higher Education Press, Beijing (2011)
Phaijoo, G.R., Gurung, D.B.: Modeling impact of temperature and human movement on the persistence of dengue disease. Comput. Math. Methods Med. 9 (2017)
Phaijoo, G.R., Gurung, D.B.: Mathematical model of dengue disease transmission dynamics with control measures. J. Adv. Math. Comput. Sci. 23, 1–12 (2017)
Podlubny, I.: Fractional Differential Equations, vol. 198. Academic Press, Cambridge (1999)
Pongsumpun, P.: Mathematical model of dengue disease with the incubation period of virus. World Acad. Sci. 44, 328–332 (2008)
Pooseh, S., Rodrigues, H., Torres, D.: Fractional derivatives in dengue epidemics. In: Simos, T., Psihoyios, G., Tsitouras, C., Anastassi, Z. (eds.) Numerical Analysis and Applied Mathematics, ICNAAM, pp. 739–42. American Institute of Physics, Melville (2011)
Pun, S.B.: Dengue: an emerging disease in Nepal. J. Nepal Med. Assoc. 51(184), 203–208 (2011)
Rahman, M., Maxwell, K.B., Cates, L.L., Banks, H.T., Vaidya, N.K.: Modeling zika virus transmission dynamics: parameter estimates, disease characteristics and prevention. Sci. Rep. 9, 10575 (2019)
Rezapour, S., Mohammadi, H., Samei, M.E.: SEIR epidemic model for COVID-19 transmission by Caputo derivative of fractional order. Adv. Differ. Equ. 2020, 1–19 (2020)
Rijal, K.R., Adhikari, B., Ghimire, B., Dhungel, B., Pyakurel, U.R., Shah, P., Bastola, A., Lekhak, B., Banjara, M.R., Pandey, B.D., Parker, D.M., Ghimire, P.: Epidemiology of dengue virus infections in Nepal, 2006–2019. Infect. Dis. Poverty 10, 1–10 (2021)
Samei, M.E., Rezapour, S.: On a system of fractional q-differential inclusions via sum of two multi-term functions on a time scale. Bound. Value Probl. 2020, 1–26 (2020)
Sardar, T., Rana, S., Chattopadhyay, J.: A mathematical model of dengue transmission with memory. Commun. Nonlinear Sci. Numer. Simul. 22(1–3), 511–525 (2014)
Shah, N.H., Thakkar, F.A., Yeolekar, B.M.: Dynamics of parking habits with punishment: an application of SEIR model. J. Basic Appl. Res. Int. 19(3), 168–174 (2016)
Side, S., Noorani, S.M.: A SIR model for spread of dengue fever disease (simulation for South Sulawesi, Indonesia and Selangor, Malaysia). World J. Model. Simul. 9, 96–105 (2013)
Srivastav, A.K., Ghosh, M.: Assessing the impact of treatment on the dynamics of dengue fever: a case study of India. Appl. Math. Comput. 362(124533), 1–17 (2019)
Srivastav, A.K., Ghosh, M., Chandra, P.: Modeling dynamics of the spread of crime in a society. Stoch. Anal. Appl. 37, 991–1011 (2019)
Subedi, D., Taylor-Robinson, A.W.: Epidemiology of dengue in Nepal: history of incidence, current prevalence and strategies for future control. J. Vector Borne Dis. 53, 1–7 (2016)
Sun, H., Zhang, Y., Baleanu, D., Chen, W., Chen, Y.: A new collection of real world applications of fractional calculus in science and engineering. Commun. Nonlinear Sci. Numer. Simul. 64, 213–231 (2018)
Vaidya, N.K., Wang, F.B.: Persistence of mosquito vector and dengue: impact of seasonal and diurnal temperature variations. Discrete Contin. Dyn. Syst. (2021)
van den Driessche, P.: Reproduction numbers of infectious disease models. Infect. Disease Model. 2, 288–303 (2017)
van den Driessche, P., Watmough, J.: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180, 29–48 (2002)
Velasco, M.P., Usero, D., Jiménez, S., Vázquez, L., Vázquez-Poletti, J.L., Mortazavi, M.: About some possible implementations of the fractional calculus. Mathematics 8, 893 (2020)
World Health Organization: Online news on dengue-and-severe-dengue. www.who.int, 2 March (2020)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
The author’s has equal contributions.
Corresponding author
Ethics declarations
Competing interests
There is not any conflict of interest with co-authors.
Ethics approval
Approval was taken from Kathmandu University Research Committee of the school of science and the secondary data was taken from Epidemiology and diseases control division, Ministry of Health and Population, Government of Nepal after taking permission from the concern person.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, H.R., Phaijoo, G.R. & Gurung, D.B. Fractional-Order Dengue Disease Epidemic Model in Nepal. Int. J. Appl. Comput. Math 8, 259 (2022). https://doi.org/10.1007/s40819-022-01459-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s40819-022-01459-2