Abstract
Prosopis juliflora, one of the most invasive species in arid and semi-arid environments, can sequester considerable amounts of carbon and provide fuel wood for domestic consumption. However, the lack of species-specific allometric models limits efforts to estimate forest biomass and its potential to store carbon. We conducted this study to determine the rate of expansion of this Prosopis in arid environments and develop robust allometric models to precisely predict tree-level biomass and its carbon storage potential. The satellite image analysis showed that Prosopis continues to expand rapidly along riverbanks and accounted for 69% of the available vegetation cover in the study area. The spatial coverage of Prosopis increased by 40% within 5 years from 2016 to 2021 and has been expanding at a rate of 8% per year. The rate of Prosopis expansion calls for the application of new utilization systems like massive charcoal production which may be a great blessing to the region. For such purpose, quantification of the biomass of Prosopis from easily measured variables is essential. Hence, after destructive sampling of 45 individual trees, the relationships between three variables (diameter at stump height, DSH; height, H; and wood density, ρ) and their total biomass of the aboveground parts were used to fit regression models in R software. These models were developed using DSH alone, DSH and height or in a combination of DSH, H and ρ. Although DSH alone explained most of the variations (91%), adding H and ρ as additional independent variables resulted in a much better prediction of biomass estimation with the lowest values of bias. Therefore, the best-selected model for the above-ground biomass (AGB) estimation of the species Prosopis is M9 (\({\text{AGB}}={\text{exp}}(-3.053+0.919\times {\text{ln}} ({\text{DSH}}^{2} \times H \times \rho ))\) which presented the highest adj. R2 value (0.985) and the lowest values of Akaike information criteria. Given narrow diameter ranges, the model can be applied beyond their valid data ranges and to other similar growth forms across the arid regions of Ethiopia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prosopis juliflora (hereafter Prosopis) is a drought-tolerant, fast-growing, evergreen and very thorny invasive species in arid and semi-arid areas (Ilukor et al. 2016; Gianvenuti et al. 2018; Rapinel et al. 2019). Prosopis was introduced to Ethiopia in the late 1970s as part of the green campaign afforestation program to curb land degradation (Mehari 2015; Birhane et al. 2017). However, this species has become naturalized, continuously invading pastoral areas by displacing native plants and forming a dominant vegetation type in the Afar region (Shiferaw et al. 2019a; Ravhuhali et al. 2021). Prosopis has been expanding at a rate of 50,000 ha year− 1 in the Afar region for a decade (Tilahun and Asfaw 2012; Wakie et al. 2014) and it invaded 1.17 million hectares (ha) of the landmass of the region within 35 years of its introduction (Shiferaw et al. 2019b).
Prosopis has been widely expanding in the tropical parts of the world (Oduor and Githiomi 2013; Abdulahi et al. 2017; Patnaik et al. 2017). This is because Prosopis can grow under different environmental conditions (Abdulahi et al. 2017) such as in any soil type (Patnaik et al. 2017), in areas that lie between 200 and 1500 m above sea level (a.s.l.) with a mean annual rainfall of 50–1500 mm and the air temperature below 50 °C (Pasiecznik et al. 2007). In addition, Prosopis has extensive root systems capable of tapping into the groundwater table up to 50 m depth (Ng et al. 2016, 2017; Askar et al. 2018), and it has large numbers of seeds which are dispersed through cattle wastes (Kyuma 2016). Moreover, rivers and water canals play a significant role in the dissemination of seeds to different areas (Abdulahi et al. 2017). The aforementioned belongings of Prosopis foster its adaptability and support the invasion of the species across various agro-ecosystems (Tilahun and Asfaw 2012; Mehari 2015). At present, Prosopis is found in 103 countries (Edrisi et al. 2020).
Prosopis forms a thick thorny structure and a canopy that ranges 20–95% (Berhanu and Tesfaye 2006) and expands at the expense of different land-use types. The major argument has been that Prosopis possesses allelopathic and allelochemical effects on other tropical plant species (Asrat and Seid 2017; Linders et al. 2019; Wakie et al. 2021) that affects biodiversity and ecosystem services, and removes a large amount of water resource through evapotranspiration (Shiferaw et al. 2021). The structural arrangements and biological characteristics of Prosopis limit the area coverage of multipurpose woody vegetation and grasslands (Wakie et al. 2014) which would, in turn, affect the livelihoods of agro-pastoralist (Meroni et al. 2017; Edrisi et al. 2020; Shackleton et al. 2014; Ilukor et al. 2016) reported that Prosopis negatively affects the native flora by invading grasslands, shrublands and woodlands. These researchers blamed Prosopis as an invasive species that has adverse impacts on the ecology, economy and society.
On the other hand, many researchers argued that Prosopis is a gift to the arid and semi-arid parts of the world because of its ability to grow in a harsh environment. This species offers direct tangible benefits, e.g., construction, fencing and craft materials (Abdulahi et al. 2017), and fodder for animals (Ravhuhali et al. 2021). It has high quality for firewood and charcoal (Oduor and Githiomi 2013) and becomes the main source of household energy (Patnaik et al. 2017; Gianvenuti et al. 2018). Prosopis also offers indirect benefits such as making the desert area more habitable by regulating the local temperature (Patnaik et al. 2017) and environmental services including carbon sequestration.
Assessing the benefits of Prosopis for carbon sequestration as well as biomass for domestic energy depends very much on accurate methods developed to estimate the biomass and carbon stock of a specific tree species. Technically, quantification of amounts of carbon stored in trees requires estimates of the dry weight of biomass, and then, the biomass estimates are converted to carbon and carbon dioxide equivalents (Mugasha et al. 2013; Chave et al. 2014). The biomass can be estimated either using stem volume information (Andreas et al. 2011) and biomass expansion factors (Guo et al. 2010; Bohdan et al. 2015) or based on allometric biomass models (Diédhiou et al. 2017; Kusmana et al. 2018). Biomass models comprise easily measurable tree variables, e.g., diameter at breast height (DBH), height, and wood density that are correlated to the biomass. Numerous allometric biomass models have been developed in recent years (Diédhiou et al. 2017; Dimobe et al. 2018; Sillett et al. 2019). However, due to differences in allometry and wood density, tree species-specific biomass models are often preferred (Mugasha et al. 2013).
Despite many studies carried out on Prosopis, there is no allometric biomass model for this species as to the researchers’ knowledge. In addition, quantitative information about the rate of spatial extent and trends of Prosopis is limited. These situations demand scientific evidence to provide a balanced view for decision-makers, environmentalists, farmers, and land-use planners. Therefore, the objectives of this study were to map the spatial extent and rate of invasiveness of Prosopis using remote sensing and GIS techniques and to develop allometric biomass models for the Prosopis species in the Afar region that can be used in the dry land of other tropical areas.
Materials and methods
Description of the study area
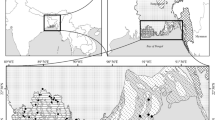
The study was carried out in the Afar region (eastern parts of Ethiopia) where Prosopis is vastly distributed. Geographically, the study area is located between 8.96° to 10.80° N and 39.79° to 40.83° E (Fig. 1) and covers an area of 13,420 km2. The topography of the area is relatively flat with an altitude varying between 551 and 1863 m a.s.l. According to the world reference base for soil resources (WRB 2014), the soil of the Afar region is classified as Lithic and Eutric Fluvisols. Acacia nilotica (L.) Delile. and Tamarix aphylla (L.) H. Karst. dominate the riverine forest along the Awash River. Other tree species such as Prosopis, Acacia senegal (L.) Willd., Dobera glabra (Forssk.) Poir., and Cadaba routundifolia Forssk. are common in the rangeland, particularly in areas with high saline soil. Sorghum, sugarcane, cotton and vegetables are the major crops in the study area. This area is mainly irrigated by the Awash River. The most important domestic animals are camels and goats which are considered the main sources of household income.
Mapping spatial extent of Prosopis
A satellite dataset of Sentinel-2 imageries taken during the dry season (March 2016 and 2021) was used to map the spatial extent and trends of Prosopis, because its spatial and spectral properties are suitable to detect and map this woody vegetation. The dry season is the optimum time to spectrally differentiate the evergreen Prosopis from other leaf-shedding vegetation. Rapinel et al. (2019) valued Sentinel-2 data for the identification of evergreen invasive species.
Spatial information on Prosopis coverage was produced using supervised classification technique, maximum-likelihood classifier and the normalized difference vegetation index (NDVI). The supervised classification method requires spatially explicit training sites—collected from each land-cover type—to train the machine. In the study area, five major land-cover types, namely woody vegetation, grazing area, cropland, water body and settlement were identified based on Ethiopian LULC classification standard (EMA 2018). A total of 100 training sites, specifically 55, 15, 12, 5, 6 and 7 training sites from Prosopis vegetation, non-Prosopis woody vegetation, cropland, water body, settlement and grazing area, respectively, were collected from the study area using a handheld Global Positioning System (GPS) with an accuracy of ± 3 m. The training sites were collected from the field where each land-cover type was not changed between 2016 and 2021. This was done through observation and by asking the elderly people about the coverage of each land-cover category in 2016. Moreover, the land-cover types of each training sites were validated using high spatial resolution Google Earth pictures. Based on these training data, the 2016 and 2021 imageries were classified into vegetative and non-vegetative areas using Sentinel Application Platform, ArcGIS 10.8 and ERDAS IMAGINE 2016 software and maximum-likelihood classifier. Furthermore, areas covered by Prosopis and non-Prosopis woody vegetation were identified using biomass indicators, expert knowledge and environmental variables including rivers, roads and settlement. The inclusion of expert knowledge is key, because the Prosopis expansion is influenced by different environmental variables. Biomass indicators help to detect and map Prosopis (Vidhya et al. 2017). Finally, areas covered by Prosopis and non-Prosopis woody vegetation were extracted using ArcGIS 10.8.
To discriminate the evergreen Prosopis from other leaf-shedding vegetation, a threshold value was determined using the NDVI, because Prosopis is evergreen and NDVI is a ration-based index. In the study area, the NDVI value between 0.3 and 0.8 represents Prosopis vegetation. Before analysing the produced Prosopis and non-Prosopis woody vegetation maps, its accuracy should be assessed. Therefore, 43 and 38 referenced data from Prosopis and non-Prosopis woody vegetation, respectively, were collected to assess the accuracy of the maps.
Allometric modelling
Selection of sample trees and measurements
Biomass data were collected from 45 plots of 5.64 m radius which were well spread over the Prosopis forest area. Within these plots, one tree is purposely selected for destructive sampling to match the target distribution across different diameters ranging from 2.5 to 8.3 centimeters (cm). In total, we selected 45 sample trees for measurements of biomass and subsequent development of allometric equations. Prior to the destructive procedure, all sample tree diameters at stump height (DSH) were measured with a diameter tape which is seemingly more consistent for repeated measures and total tree height (H) was measured with meter tape after felling. Both DSH (cm) and H (m) measurements were taken to the nearest 0.1. The reference level for diameter measurement was 30 cm, because the majority of the diameter of the trees are below 5 cm (Djomo and Chimi 2017). Trees selected for sampling were cut at the base using a saw.
The aboveground part was considered as all biomass above a stump height of approximately 30 cm and it was further divided into stems, branches and leaves. The branches were removed from the stem and trimmed into manageable billets for weighing using a mechanical hanging balance (0–100 kg) with an accuracy of 0.01 kg. All foliar material was separated from branches and collected into bags to facilitate weighing. Stems were cross cut into 1 m length and upper and lower diameters of each segment were measured using a diameter tape. The fresh weights of branches and leaves for each individual tree were separately determined in the field using an electronic balance (model YH-T3 multi-function weighing indicator) with a 600 kg capacity (precision 0.05 g). Depending on the stem length, two-to-four small disc samples (a disc of about 5 cm thick) were cut from each segment at 0.3, 0.6, 1.3 m, and thereafter, every 1 m along the stem and the fresh weight and volume of each disc was recorded directly in the field for the determination of basic wood density (Eq. 1). All collected aboveground samples (discs, sample branches and leaves) were transported to the laboratory where they were oven-dried at 105 °C (samples of branches and stems) or 75 °C (samples of leaves) to constant weight by monitoring changes in weight with intervals of 6 h until there was no change in weight. The dry weight of the samples was recorded immediately after removal from the oven. Wood basic density (g cm− 3) for each disc (and tree) was determined as the ratio of dry mass to green volume
where ρ = wood basic density (g cm− 3), Dry Mss = subsample oven-dried mass (g), and Green Vss = subsample fresh volume of wood (cm3).
For the stems and sample discs, diameters of the lower and upper levels of each segment were used to calculate the volume (V) using the Smalian formula (Dimobe et al. 2018; Sillett et al. 2019; Vinh et al. 2019)
where V represents the volume, L the length, and \({D}_{1}\) and \({D}_{2}\) the diameters at the lower and upper sides of each segment.
The dry weights (biomass) of tree components were obtained by multiplying the dry-to-green weight ratio by the green weight of the respective tree component. The total biomass for each tree component was obtained by the following formula:
The total aboveground dry weight of a tree was finally computed as the sum of the stem, branch and leaf dry weight (Ubuy et al. 2018).
Model development, selection and evaluation
The relationship between tree variables (DSH, H and ρ) and the total biomass of the aboveground woody parts were used to fit regression models using the dynamic fit module function in R-software (Version R-4.1.3). Prior to developing allometric equations, the relationship between aboveground biomass (dependent variable) and DSH and H (independent variables) was plotted using scatter plots. This helped to visualize the range and shape of the functional relationships of variables and to determine the type of model to be fitted (Picard et al. 2012; Kapinga et al. 2018). For the construction of the allometric equations, the data were tested for normality of residuals as determined by Shapiro–Wilk (p < 0.05) and homogeneity of variances (Djomo and Chimi 2017). We performed several regression models from the aboveground data on the basis of DSH only and in combination with H and/or ρ as predictor variables. These model forms have previously been applied in developing biomass models (see, e.g., Singh et al. 2011; Huy et al. 2016; Diédhiou et al. 2017; Djomo and Chimi 2017; Kapinga et al. 2018). For this study, 11 allometric models of the following form were evaluated for their fitness in estimating ABG:
M1 \({\rm AGB}=a+b\times {\rm DSH}\)
M2 \({\rm AGB}=a+b\times\left({\rm DSH}\right)+c\times\left({\rm DSH}^{2}\right)\)
M3 \({\rm AGB}={\rm exp}(a+b \times {\rm ln}\left({\rm DSH}\right))\)
M4 \({\rm AGB}={\rm exp}(a+b \times {\rm ln}\left({\rm DSH}\times H\right))\)
M5 \({\rm AGB}={\rm exp}(a+b\times {\rm ln}\left({\rm DSH}\times \rho \right))\)
M6 \({\rm ABG}={\rm exp}(a+b\times {\rm ln}({\rm DSH}^{2}\times H))\)
M7 \({{\rm AGB}=a\times {\rm DSH}}^{b}\)
M8 \({\rm AGB}={\rm exp}(a+b\times {\rm ln}({\rm DSH}^{2}\times \rho ))\)
M9 \({\rm AGB}={\rm exp}(a+b\times {\rm ln}({\rm DSH}^{2}\times H\times \rho ))\)
M10 \({\rm AGB}={\rm exp}(a+b\times {\rm ln}({\rm DSH}^{2}\left)\times H\times \rho \right)\)
M11 \({\rm AGB}={\rm exp}(a+b\times ln\left({\rm DSH})\times {\rm ln}(\rho \right)).\)
In which AGB is the response variable (total aboveground tree biomass) (kg tree− 1), DSH is the tree diameter at stump height (30 cm), H is the tree height (m), and ρ is the wood density (g cm− 3); a, b, c, d and e are model parameters.
The non-linear programming (NLP) procedure in R-software was used to estimate the model parameters (a, b, and c). The procedure produces the least-squares estimates of the parameters of a non-linear model through an iteration process. The selection of the best allometric models was obtained by comparing the values of the Akaike information criteria (AIC), root-mean-square error (RMSE), the adjusted coefficient of determination (adj. R2), and mean percentage error (MPE%) (Kapinga et al. 2018; Kusmana et al. 2018) based on the following formula:
In these formulae, MPE% is the mean percentage error; \({\widehat{y}}_{i}\) is the predicted aboveground biomass; \({y}_{i}\) is the observed aboveground biomass; \({\stackrel{-}{y}}_{i}\) is the mean value of the biomass; n is the number of samples; p is the number of parameters; logLik is the log-likelihood values of the non-linear regression model. Then, the best models are selected based on the smallest values of AIC, RMSE, and MPE and the biggest value of adj. R2 (Kusmana et al. 2018). Student's t tests were done to see whether the MPE% values were significantly different from zero. Models with non-significant parameter estimates were excluded during the selection process regardless of AIC and MPE% values.
Data analysis
All models were estimated and evaluated on the basis of linear and non-linear regression statistics, taking into account the classical criteria such as coefficient of determination (adj. R2) and standard error of estimate (SEE). The decisive criterion for judging model performance when comparing different models was AIC. All statistical and regression analysis, equation parameterization, and all graphics were done using R-software. Significant means were separated by Tukey’s test at the 5% probability level.
Results
Spatial distribution of Prosopis
The overall classification accuracy of Prosopis and non-Prosopis woody vegetation map was 95 and 97%, respectively. The analysis results showed that quite large parts of the study area are covered with Prosopis followed by indigenous species-dominated vegetation (Fig. 2). About 15% and 20% of the study area were covered with woody vegetation in 2016 and 2021, respectively (Table 1). Of the available woody vegetation resources, 59% in 2016 and 69% in 2021 were covered with Prosopis. This shows that the spatial coverage of Prosopis increased by 40% within 5 years and has been expanding at a rate of 8% per year. On the contrary, the area coverage of indigenous woody vegetation decreased by 23%. The distribution map shows that Prosopis is not uniformly distributed across the area; instead, it heavily invaded the banks of the Awash River and the western and central parts of the study area which are potentially suitable irrigation croplands (Fig. 2).
Allometric modelling
In total, 45 trees having a DSH ranging from 2.50 to 8.25 were destructed to determine the total aboveground biomass of Prosopis species in the Afar Region. The mean specific gravity of the sampled species was 0.63 ± 0.06 (g cm− 3), varying between 0.47 and 0.75 g cm− 3 (Table 2). The tree height averaged 5.03 ± 0.20, varying from 2.80 to 7.50 (Table 2). The scatter plot (Fig. 3) showed the relationship of AGB with the three predictor variables (DSH, H and ρ). As depicted in the figure, AGB did not show linear relation with all three predictors. As a result, square and log-transformed data were used in selecting the best-fitted models for AGB estimation of Prosopis forest.
Species-specific allometric models for estimating tree-level biomass (AGB) are shown in Table 3. These models were developed using DSH alone, DSH and H or in a combination of DSH, H and ρ. The analysis of the AGB models utilizing a combination of several independent variables revealed that the most effective variable was DSH. Diameter at stump height (30 cm) alone showed a good fit in AGB in this model development (adj. R2 = 0.875–0.911) (Table 3). The strongest model for AGB of a single tree using only one independent variable (DSH) explained 91% variability (model 2). Adding H as the second predictor gave a much better prediction with the lowest values of bias and AIC compared to DSH-alone (M6). When DSH and H are included in the prediction, it can explain 96% of the variability in AGB. However, the AGB model using DSH and ρ (M5, M8, and M11) did not improve the fit and was found slightly weaker, explaining 92–93% variability in the observed data. The best-fit equation for the AGB estimation of Prosopis was obtained by the combination of three predictor variables (DSH, H and ρ; M9) and by all metrics improved the model fitness. This model presented the highest adj. R2 value (0.985) and the lowest values of AIC statistics (Table 3). Therefore, M9 is the best model for the AGB estimation of the species Prosopis with the highest value of the coefficient of determination (adj. R2) and the lowest value of AIC. This good fit that is provided by the biomass prediction model to the data is confirmed by the regression fit of the total AGB against the predictors (DSH, H and ρ) (adj. R2 = 0.985, at 95% CI) (Fig. 4).
Discussion
Spatial distribution of Prosopis in arid and semiarid regions
NDVI values and high spatial resolution satellite images, the size, color, and shape of the canopy (Gunawardena et al. 2015) and ground truth information were considered to map the spatial distribution of Prosopis. The analysis showed that about 69% of the available woody vegetation resource is Prosopis which expands at a rate of 8% per year (Table 2). On the contrary, the non-Prosopis woody vegetation decreased at a rate of 0.6% per year which is below the national deforestation rate (0.93% per year; Mongabay 2019). We observed that Prosopis heavily invaded the grazing areas because of the dispersal agents of its seed such as goats and camels. In addition, Prosopis is densely distributed around the Awash River banks—highly suitable for irrigation and crop production—mainly due to the availability of water resources. This means that the species perform well in arid and semi-arid regions mainly in wetlands (Ng et al. 2016; Meroni et al. 2017). Water availability quickly led to the formation of dense thickets that decreased the cultivation fields (Walter and Armstrong 2014). Hence, the most vulnerable and threatened land use for the invasion of Prosopis is the potentially suitable irrigation fields followed by grazing lands.
Currently, the invasion rate of Prosopis is tremendously high. For example, Ilukor et al. (2016) found that in the Afar region, the expansion of Prosopis towards the wetlands increased by 44 km2 (122%) between 2000 and 2005 years. In Somaliland, the uncontrolled spread of Prosopis into the irrigation fields becomes a serious problem in the region (Meroni et al. 2017; Ng et al. 2016). In semi-arid areas of South Africa, Prosopis becomes highly invasive, creating extensive and invulnerable thickets mainly due to its high seed production ability and fast growth and high capability of seeds to stay in the soil for long period (Ravhuhali et al. 2021). Because of such behaviour, the species invaded about 12% of the study area in the last 5 years (Fig. 2). Similarly, in the Afar region, Prosopis invaded the area of grazing lands and shrub lands and negatively affects useful native grass and herb species (Wakie et al. 2016).
Realizing its invasion rate, there have been attempts to eradicate the trees from the banks of irrigation canals in several locations. However, it took only a few years for Prosopis to re-occupy the space again (Walter and Armstrong 2014). On the other hand, Prosopis provides fodder to livestock, and becomes a reliable source of construction materials, furniture, firewood, and medicinal purposes in the Afar region (Wakie et al. 2016), reducing soil erosion, and providing good quality charcoal. In the Afar Region, Prosopis has become a reliable source of fuelwood and charcoal compared to acacia trees (Ilukor et al. 2016). Researchers reported that in arid and semi-arid regions, Prosopis has a multipurpose ecosystem role including controlling soil erosion, providing fodder for livestock from its fruits and leaves and fuel energy resources, wood for furniture, and timber for construction (Gianvenuti et al. 2018; Linders et al. 2019; Edrisi et al. 2020). Studies showed that Prosopis species produce high-value charcoal that tends to burn very well. In particular, the calorific value of Prosopis (21 MJ kg− 1; Kumar and Chandrashekar 2016) was higher compared to the conventional biomass of wood (8.4–17 MJ kg− 1; Haile et al. 2018). This means that there is a possibility of utilizing Prosopis as a raw material for solid fuel production and its contribution to the energy supply for subsistence people in arid regions. Therefore, any control programme should not ignore its contribution to the smallholder livestock farmers in semi-arid areas.
Allometric equations for biomass estimation
The fundamental importance of species-specific model construction is to estimate the tree biomass using measured variables without destruction. Species-specific allometric equations were developed as a function of DSH, H and wood density (ρ) and performed well in predicting the AGB of the selected species (Abich et al. 2019). The selections of best models are based on the best fit to available independent variables. As proven in many allometric studies, DSH alone is the most powerful variable that always explains most of the variability in aboveground biomass estimation (Cienciala et al. 2013; Kapinga et al. 2018; Abich et al. 2019). This is also confirmed here for Prosopis, where the AGB model using DSH only as an independent variable explained 87–91% of the observed variability. This may be attributed to the high correlations between woody biomass and diameter which constitutes the largest portion of the total aboveground biomass (Kapinga et al. 2018). In the model, M1 shows a linear relationship between DSH and AGB, whereas other models show an exponential growth of AGB. The value b (i.e., the slope) of the equations describes the type of scaling relationship between AGB and other tree parameters mainly DSH. For example, AGB increases proportionally with DSH when the exponent is 1, but in the case of M7, AGB increases exponentially with an increase in DSH.
For a quick estimation of Prosopis biomass at a single tree level, simply using the measurement of one variable (DSH) is certainly an advantage. This is because the diameter is sufficient to yield reliable tree biomass (Kapinga et al. 2018). In addition, it reduces the difficulties in measurements of tree height (H) data in dense and closed-canopy Prosopis forests. According to Kusmana et al. (2018), researchers prefer to use only the diameter in developing allometric models, because the measurements of the diameter are an easier mensuration variable to do at the study site.
Adding tree height (H) as a second independent variable resulted in a much better prediction with the lowest values of bias and AIC than the model with DSH alone. Adding H to the DSH-based model decreased AIC and increased R2 values but not RMSE. Adding wood density (ρ) as the second predictor instead of H had no significant effect on the fit of the observed tree biomass (Table 3). Our findings conform to the findings of some authors (Djomo and Chimi 2017; Kapinga et al. 2018) who noted that including ρ or H does not substantially lead to an increase in the predictive ability of diameter based models. However, the best overall result for the AGB model was obtained using a combination of the three independent variables, namely DSH, H, and ρ. It explained 99% of the variability in the observed data (Table 3). At a 95% confidence interval (CI), the parameter estimates of all model forms were significant. We also found that the AIC of the models was substantially reduced when H and ρ covariates are included in the model (Table 3), indicating that H may be more important than ρ for reducing uncertainty in AGB estimates. This was consistent with Kusmana et al. (2018) that the model used the diameter and height variables which basically can increase the accuracy in biomass estimation, with the assumption that a tree would have the same wood density. This confirms the findings of the previous studies (Dimobe et al. 2018; Feyisa et al. 2018) that the inclusion of H in combination with basic wood density explains most of the variability in aboveground tree biomass. In general, the fitting statistics revealed that the M9 model containing three predictors accurately predicted AGB of Prosopis tree species with the RMSE of 2% and proposed for prediction.
Conclusions
The average wood-specific gravity found in this study (0.62 ± 0.01 g cm−3) is in the range reported elsewhere. This study has confirmed that Prosopis continues to expand rapidly along river banks and rangelands. The spread of this invasive species is greater than the existing management capacity and is impossible to eradicate by human power. The mismatch between management strategies and the rate of Prosopis expansion calls for the application of new utilization systems from this species like massive production of charcoal bricks and selling to other regions which may be a great blessing to the region. For such purpose, quantification of the biomass of Prosopis from easily measured variables is essential. Although DSH alone mostly explained the variation for biomass estimation of Prosopis species, adding tree height (H) and wood density (ρ) as additional independent variables resulted in a much better prediction with the lowest values of bias and AIC than the model with DSH alone. For practical purposes, using DSH alone as a predictor variable with the lowest bias is sufficiently robust to be applicable for Prosopis species across the arid regions of Ethiopia with similar growth forms.
Data availability
The dataset supporting the conclusions of this article is included within the article.
References
Abdulahi MM, Ute JA, Regasa T (2017) Prosopis juliflora L.: distribution, impacts and available control methods in Ethiopia. Trop Subtrop Agroecosyst 20:75–89
Abich A, Mucheye T, Tebikew M et al (2019) Species-specific allometric equations for improving aboveground biomass estimates of dry deciduous woodland ecosystems. J For Res 30:1619–1632. https://doi.org/10.1007/S11676-018-0707-5
Andreas J, Markus H, Martin R, Bernhard H (2011) Estimation of aboveground biomass in alpine forests: a semi-empirical approach considering canopy transparency derived from airborne LiDAR data. Sensors 11:278–295. https://doi.org/10.3390/s110100278
Askar NN, Phairuang W et al (2018) Estimating aboveground biomass on private forest using sentinel-2 imagery. J Sens. https://doi.org/10.1155/2018/6745629
Asrat G, Seid A (2017) Allelopathic effect of meskit (Prosopis juliflora (Sw.) DC) aqueous extracts on tropical crops tested under laboratory conditions. Momona Ethiop J Sci 9:32. https://doi.org/10.4314/mejs.v9i1.3
Berhanu A, Tesfaye G (2006) The prosopis dilemma, impacts on dryland biodiversity and some controlling methods. J Drylands 1(2):158–164
Birhane E, Treydte AC, Eshete A et al (2017) Can rangelands gain from bush encroachment? Carbon stocks of communal grazing lands invaded by Prosopis juliflora. J Arid Environ 141:60–67. https://doi.org/10.1016/j.jaridenv.2017.01.003
Bohdan K, Jozef P, Vladimír S (2015) Biomass functions and expansion factors for young trees of European ash and Sycamore maple in the Inner Western Carpathians. Aust J For Sci 131:1–26
Chave J, Réjou-Méchain M, Búrquez A et al (2014) Improved allometric models to estimate the aboveground biomass of tropical trees. Glob Change Biol 20:3177–3190. https://doi.org/10.1111/gcb.12629
Cienciala E, Centeio A, Blazek P et al (2013) Estimation of stem and tree level biomass models for Prosopis juliflora/pallida applicable to multi-stemmed tree species. Trees Struct Funct 27:1061–1070. https://doi.org/10.1007/s00468-013-0857-1
Diédhiou I, Diallo D, Mbengue A et al (2017) Allometric equations and carbon stocks in tree biomass of Jatropha curcas L. in Senegal’s Peanut Basin. Glob Ecol Conserv 9:61–69. https://doi.org/10.1016/j.gecco.2016.11.007
Dimobe K, Mensah S, Goetze D et al (2018) Aboveground biomass partitioning and additive models for Combretum glutinosum and Terminalia laxiflora in West Africa. Biomass Bioenergy 115:151–159. https://doi.org/10.1016/j.biombioe.2018.04.022
Djomo AN, Chimi CD (2017) Tree allometric equations for estimation of above, below and total biomass in a tropical moist forest: case study with application to remote sensing. For Ecol Manag 391:184–193. https://doi.org/10.1016/j.foreco.2017.02.022
Edrisi SA, El-Keblawy A, Abhilash PC (2020) Sustainability analysis of Prosopis juliflora (Sw.) DC based restoration of degraded land in North India. Land. https://doi.org/10.3390/land9020059
Ethiopian Mapping Agency (EMA) (2018) Ethiopian land use land cover classification and coding standard. Addis Ababa
Feyisa K, Beyene S, Megersa B et al (2018) Allometric equations for predicting above-ground biomass of selected woody species to estimate carbon in East African rangelands. Agrofor Syst 92:599–621. https://doi.org/10.1007/S10457-016-9997-9
Gianvenuti A, Farah I, Yasmin N et al (2018) Using Prosopis as an energy source for refugees and host communities in Djibouti, and controlling its rapid spread. Food and Agriculture Organization of the United Nations (FAO), Rome
Gunawardena AR, Fernando TT, Nissanka SP, Dayawansa NDK (2015) Assessment of spatial distribution and estimation of biomass of Prosopis juliflora (Sw.) DC. in Puttlam to mannar region of Sri Lanka using remote sensing and GIS. Trop Agric Res 25:228. https://doi.org/10.4038/tar.v25i2.8144
Guo Z, Fang J, Pan Y, Birdsey R (2010) Inventory-based estimates of forest biomass carbon stocks in China: a comparison of three methods. For Ecol Manag 259:1225–1231. https://doi.org/10.1016/j.foreco.2009.09.047
Haile M, Hishe H, Gebremedhin D (2018) Prosopis juliflora pods mash for biofuel energy production: implication for managing invasive species through utilization. Int J Renew Energy Dev 7:205–212. https://doi.org/10.14710/ijred.7.3.205-212
Huy B, Kralicek K, Poudel KP et al (2016) Allometric equations for estimating tree aboveground biomass in evergreen broadleaf forests of Viet Nam. For Ecol Manag 382:193–205. https://doi.org/10.1016/j.foreco.2016.10.021
Ilukor J, Rettberg S, Treydte A, Birner R (2016) To eradicate or not to eradicate? Recommendations on Prosopis juliflora management in Afar, Ethiopia, from an interdisciplinary perspective. Pastoralism 6:4–11. https://doi.org/10.1186/s13570-016-0061-1
Kapinga K, Syampungani S, Kasubika R et al (2018) Species-specific allometric models for estimation of the above-ground carbon stock in miombo woodlands of Copperbelt Province of Zambia. For Ecol Manag 417:184–196. https://doi.org/10.1016/j.foreco.2018.02.044
Kumar R, Chandrashekar N (2016) Study on fuelwood and carbonization characteristics of Prosopis juliflora. J Indian Acad Wood Sci. https://doi.org/10.1007/s13196-016-0171-9
Kusmana C, Hidayat T, Tiryana T et al (2018) Allometric models for above- and below-ground biomass of Sonneratia spp. Glob Ecol Conserv 15:e00417. https://doi.org/10.1016/j.gecco.2018.e00417
Kyuma RK (2016) Evaluation of Prosopis Juliflora productivity for carbon stocks and animal feeds in selected dry land sites, Magadi Sub-county, Kenya. University of Nairobi, Nairobi
Linders TEW, Schaffner U, Eschen R et al (2019) Direct and indirect effects of invasive species: biodiversity loss is a major mechanism by which an invasive tree affects ecosystem functioning. J Ecol 107:2660–2672. https://doi.org/10.1111/1365-2745.13268
Mehari ZH (2015) The invasion of Prosopis juliflora and Afar pastoral livelihoods in the Middle Awash area of Ethiopia. Ecol Process 4:1–9. https://doi.org/10.1186/s13717-015-0039-8
Meroni M, Ng WT, Rembold F et al (2017) Mapping Prosopis juliflora in West Somaliland with Landsat 8 Satellite Imagery and Ground Information. Land Degrad Dev 28:494–506. https://doi.org/10.1002/ldr.2611
Mongabay (2019) Deforestation statistics for Ethiopia. rainforests.mongabay.com. Accessed 10 Feb 2022
Mugasha WA, Eid T, Bollandsås OM et al (2013) Allometric models for prediction of above- and belowground biomass of trees in the miombo woodlands of Tanzania. For Ecol Manag 310:87–101. https://doi.org/10.1016/j.foreco.2013.08.003
Ng WT, Meroni M, Immitzer M et al (2016) Mapping Prosopis spp. with Landsat 8 data in arid environments: Evaluating effectiveness of different methods and temporal imagery selection for Hargeisa, Somaliland. Int J Appl Earth Obs Geoinf 53:76–89. https://doi.org/10.1016/j.jag.2016.07.019
Ng WT, Rima P, Einzmann K et al (2017) Assessing the potential of sentinel-2 and pléiades data for the detection of prosopis and vachellia spp. in Kenya. Remote Sens. https://doi.org/10.3390/rs9010074
Oduor NM, Githiomi JK (2013) Fuel-wood energy properties of Prosopis juliflora and Prosopis pallida grown in Baringo District, Kenya. Afr J Agric Res 8:2476–2481. https://doi.org/10.5897/AJAR08.221
Pasiecznik NM, Choge SK, Rosenfeld AB, Harris PJC(2007) Underutilised crops for famine and poverty alleviation: a case study on the potential of the multipurpose Prosopis tree. In: 5th international symposium on new crop uses their role a rapidly change world, pp 326–346
Patnaik P, Abbasi T, Abbasi SA (2017) Prosopis (Prosopis juliflora): blessing and bane. Trop Ecol 58:455–483
Picard N, Henry M, Mortier F et al (2012) Using Bayesian model averaging to predict tree aboveground biomass in tropical moist forests. For Sci 58:15–23
Rapinel S, Mony C, Lecoq L et al (2019) Evaluation of Sentinel-2 time-series for mapping floodplain grassland plant communities. Remote Sens Environ 223:115–129. https://doi.org/10.1016/J.RSE.2019.01.018
Ravhuhali KE, Mudau HS, Moyo B et al (2021) Prosopis species—an invasive species and a potential source of browse for livestock in semi-arid areas of South Africa. Sustainability 13:1–13. https://doi.org/10.3390/su13137369
Shackleton RT, Le Maitre DC, Pasiecznik NM, Richardson DM (2014) Prosopis: a global assessment of the biogeography, benefits, impacts and management of one of the world’s worst woody invasive plant taxa. AoB Plants 6:1–18. https://doi.org/10.1093/aobpla/plu027
Shiferaw W, Bekele T, Demissew S, Aynekulu E (2019) Prosopis juliflora invasion and environmental factors on density of soil seed bank in Afar Region, Northeast Ethiopia. J Ecol Environ 43:1–21. https://doi.org/10.1186/s41610-019-0133-4
Shiferaw H, Bewket W, Eckert S (2019b) Performances of machine learning algorithms for mapping fractional cover of an invasive plant species in a dryland ecosystem. Ecol Evol 9:2562–2574. https://doi.org/10.1002/ece3.4919
Shiferaw H, Alamirew T, Dzikiti S et al (2021) Water use of Prosopis juliflora and its impacts on catchment water budget and rural livelihoods in Afar Region, Ethiopia. Sci Rep 11:1–14. https://doi.org/10.1038/s41598-021-81776-6
Sillett SC, Van Pelt R, Carroll AL et al (2019) Allometric equations for Sequoia sempervirens in forests of different ages. For Ecol Manag 433:349–363. https://doi.org/10.1016/j.foreco.2018.11.016
Singh V, Tewari A, Kushwaha SPS, Dadhwal VK (2011) Formulating allometric equations for estimating biomass and carbon stock in small diameter trees. For Ecol Manag 261:1945–1949. https://doi.org/10.1016/j.foreco.2011.02.019
Tilahun SL, Asfaw A (2012) Modeling the expansion of Prosopis juliflora and determining its optimum utilization rate to control the invasion in Afar Regional State of Ethiopia. Int J Appl Math Res 1:726–743. https://doi.org/10.14419/ijamr.v1i4.200
Ubuy MH, Eid T, Bollandsås OM, Birhane E (2018) Aboveground biomass models for trees and shrubs of exclosures in the drylands of Tigray, northern Ethiopia. J Arid Environ 156:9–18. https://doi.org/10.1016/j.jaridenv.2018.05.007
Vidhya R, Vijayasekaran D, Ramakrishnan SS (2017) Mapping invasive plant Prosopis juliflora in arid land using high resolution remote sensing data and biophysical parameters. Indian J Geo-Mar Sci 46:1135–1144
Vinh T, Van, Marchand C, Linh TVK et al (2019) Allometric models to estimate above-ground biomass and carbon stocks in Rhizophora apiculata tropical managed mangrove forests (Southern Viet Nam). For Ecol Manag 434:131–141. https://doi.org/10.1016/j.foreco.2018.12.017
Wakie TT, Evangelista PH, Jarnevich CS, Laituri M (2014) Mapping current and potential distribution of non-native Prosopis juliflora in the Afar region of Ethiopia. PLoS One 9:3–11. https://doi.org/10.1371/journal.pone.0112854
Wakie TT, Laituri M, Evangelista PH et al (2021) Allelopathic effect of meskit (Prosopis juliflora (Sw.) DC) aqueous extracts on tropical crops tested under laboratory conditions. Appl Geogr 66:32. https://doi.org/10.4314/mejs.v9i1.3
Walter KJ, Armstrong KV (2014) Benefits, threats and potential of Prosopis in South India. For Trees Livelihoods 23:232–247. https://doi.org/10.1080/14728028.2014.919880
WRB (2014) World reference base for soil resources 2014. Food and Agriculture Organization of the United Nations, Rome
Acknowledgements
This study was supported by the Panafrica Geoinformation Service plc (Prosopis Juliflora energy potential estimation project in Afra region, Ethiopia). We acknowledge Panafrica Geoinformation Service plc for reasonable financial support. During the course of this research, many individuals were involved in biomass sampling; the authors would like to thank the Awash Office of Agriculture and the leaders of the forestry sector for their kind assistance in the field work. Comments from two anonymous referees and editor improved the quality of the original manuscript.
Funding
Funding was provided by Panafrica Geoinformation Service plc (Prosopis Juliflora energy potential estimation project_2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Assefa, D., Mekuriaw, A., Tesfaye, M. et al. Mapping of Prosopis juliflora rate of expansion and developing species-specific allometric equations to estimate its aboveground biomass in the dry land of Ethiopia. Model. Earth Syst. Environ. 9, 263–274 (2023). https://doi.org/10.1007/s40808-022-01495-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40808-022-01495-3