Abstract
Purpose of Review
Clinical implementation of personalized cancer therapy necessitates translational cancer models that faithfully represent the molecular and cellular features of human cancer. Current patient-derived preclinical models, including cell line and xenograft models, are limited by incomplete recapitulation of parental tumor heterogeneity and long induction times, impeding their ability to directly inform clinical decision-making. Newly emerging patient-derived organoids (PDOs) of solid tumors retain the intra-tumoral heterogeneity lost in many preclinical models and mirror the therapeutic responsiveness of their parent tumors. Herein, we explore the origins and rationale for organoid cancer modeling, the creation of PDO models through an illustrative example of glioma organoids, and their downstream use in comprehensive drug screens to guide oncologic therapy selection.
Recent Findings
Cancer organoid models have been generated through numerous techniques, producing PDOs of brain, pancreatic, breast, and gastrointestinal cancer, among others. Recent evidence supports the creation of PDOs using a minimally processed approach, whereby manually parcellated tissue can produce viable organoids in the absence of tissue dissociation, an artificial extracellular matrix, and exogenous growth factors. Refinement of these models thus allows PDOs to serve as patient avatars, and early evidence demonstrates similar responses to chemotherapy and radiotherapy as the parent tumor.
Summary
The retention of key molecular, histopathologic, and phenotypic features of numerous human cancers offers compelling support for the use of PDOs as translational cancer models. Given the ability to rapidly create these models following tumor resection, PDOs can be used as platforms for personalized drug screens to guide the selection of oncologic therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solid cancers are often treated with maximal surgical resection, systemic therapy, and/or adjuvant radiotherapy aimed at disrupting tumor maintenance and proliferation [1]. While this paradigm remains standard, modern oncology has placed a growing emphasis on the development of targeted therapies and patient-specific treatment regimens. The advent of advanced genomic technology, such as next-generation sequencing (NGS) and transcriptomic analysis, allows for the rapid identification of the molecular characteristics of individual tumors [2]. Furthermore, increased appreciation for the heterogeneity of solid cancers has resulted in a push towards personalized oncology — treatment based on a tumor’s molecular, metabolic, physiologic, and environmental factors [3]. In this regard, several genomic tools have emerged that can aid in personalizing cancer treatment decisions, including use of chemotherapy (ex. Oncotype DX in breast cancer) and radiation therapy (ex. GARD) [4,5,6,7]. However, these predictive tools were developed from aggregated data and thus may not fully capture the unique characteristics of any single tumor. As further technological advances have increased the feasibility of developing faithful ex vivo models, the ability to test treatment strategies on individual patients’ tumor models is an exciting advance in facilitating personalized cancer treatment. A critical component of this approach is the need for translational models that reliably demonstrate the array of molecular phenotypes appreciated in complex human cancers [8].
Traditional laboratory cancer models have provided tremendous value in cancer research. The high throughput capacity of two-dimensional cell cultures facilitates rapid testing of novel therapeutics and provides the rationale for in vivo experimentation [9,10,11]. Also, patient-derived xenografts in mice have been utilized to study the biology of human cancer tissue and have guided many aspects of preclinical therapeutic development [12, 13]. Further, the breadth of tools to create genetically engineered mouse models (GEMMs) allows for the temporal and spatial control of tumor formation in vivo [13,14,15]. However, these models carry several limitations that may, in part, contribute to their shortcomings in establishing cancer therapy efficacy [16]. The selective pressures of serial cell culture often manifest in oligoclonal cell populations that lose the genotypic and phenotypic heterogeneity of the parent tissue and ultimately diverge from the primary sample and parental tumor [17,18,19]. For cancers with diverse intratumoral complexity, such as malignant glioma, breast cancer, and pancreatic cancer, the absence of tumor-tumor, tumor-parenchyma, and tumor-stroma interaction limits modeling of diverse cellular states and hierarchies [20,21,22,23]. Additionally, the requisite use of immunocompromised mice in patient-derived xenograft models limits study of the tumor-immune interface [15, 24]. While GEMMs enable cancer formation to occur in immunocompetent mice, species-specific differences may pose challenges in modeling some cancers, as seen with differential phenotypes in mice following aberrations in classical driver mutations of human cancer, such as APC, BRCA1/2, and RB [14, 25]. Similarly, tumors in GEMMs trend towards a homogeneous state compared to the heterogeneity seen with the progressive accumulation of genetic alterations in human tumors [26, 27]. Finally, murine models are time- and resource-intense and are relatively low-throughput models for preclinical drug screens. Thus, new modeling approaches, particularly in the realm of translational research and personalized oncology, may identify promising therapeutics more efficiently and accurately.
Cancer organoid models have emerged as preclinical and translational models. Organoids are heterogeneous, three-dimensional, self-organizing structures that can model the architecture and function of native organs and neoplasms [28]. In 2009, Sato et al. created the first organoids by developing crypt-villous structures from Lgr5 + intestinal stem cells [29•]. By embedding cells in Matrigel, an artificial extracellular matrix, and culturing in media enriched with epidermal growth factor (EGF), r-spondin 1, and Noggin, organoids resembling crypt and villous domains of freshly isolated small intestine crypts were produced and persisted for greater than 8 months [29•]. To date, numerous organoid models of human organs have been created, including brain, blood vessels, endometrium, fallopian tubes, intestine, kidney, lung, ovaries, pancreas, retina, stomach, taste buds, and testicles [29•, 30•, 31,32,33,34,35,36,37,38,39,40]. Following generation of many of these primary tissue models, cancer organoid models were produced, with an emphasis on their creation from primary patient tissue samples [22]. The resulting patient-derived organoids (PDOs) faithfully demonstrated many genotypic and phenotypic features of their native tissue. PDOs have been developed for bladder, breast, colorectal, gastric, metastatic gastrointestinal, liver, pancreatic cancer, and glioblastoma, among others [41, 42•, 43,44,45,46,47,48,49,50,51]. The translational implications for PDOs are most apparent in tumors characterized by inter-tumoral heterogeneity, diverse tumor cell states, and complex tumor microenvironments integral to tumor behavior and response to treatment. As such, PDOs may complement classical cancer models to support personalized predictions of human tumor responses to specific treatments.
Glioma, the most common primary malignant tumor of the central nervous system (CNS), exists along a histopathologic spectrum from WHO grade 1 to grade 4 [52, 53••]. The most aggressive of these is glioblastoma (GBM, IDH-WT, WHO grade 4), characterized by its infiltration of the brain parenchyma, cellular heterogeneity, and dismal clinical prognosis [54]. While GBM cell lines have provided insight into the cellular hierarchy of glioma cell populations, these models are time intensive and challenging to establish [10, 50, 55,56,57]. Furthermore, the overrepresentation of GBM cell lines has limited the study of lower-grade lesions that frequently progress to higher-grade tumors [58, 59]. Given these challenges in utilizing two-dimensional in vitro models to recapitulate the characteristics of gliomas of various grades, the development of PDOs for glioma modeling has already improved the ability to study tumor biology and develop new therapies for these tumors.
PDOs of cancer recapitulate the architectural, cellular, and molecular features of a diverse array of tumors with the potential to take personalized and translational oncology from bench-to-bedside to a bedside-to-bench-and-back model [60]. Herein, we review the creation and applications of PDOs of cancer through the lens of glioma, followed by a discussion of the impact of PDOs on clinical oncology.
Creation and Utilization of Patient-Derived Organoids of Glioma
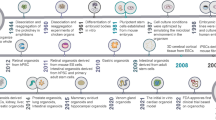
A critical advance in the development of glioma organoids came from Lancaster et al. with the creation of cerebral organoids [30•]. To generate cerebral organoids, human embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs) were exposed to a series of stepwise media conditions and nutrient supplements [61]. The hESCs or iPSCs were cultured with basic fibroblast growth factor (bFGF) and rho-associated protein kinase (ROCK) inhibitor to induce the formation of embryoid bodies (EB). These EB were kept in suspension and subsequently cultured in neural induction media — composed of DMEM/F12, N2 supplement, GlutaMax, non-essential amino acids (NEAAs), and heparin — to induce the formation of neuroepithelial tissue. The primitive neuroepithelial tissue is then embedded in Matrigel droplets and cultured in a differentiation medium — composed of DMEM/F12, Neurobasal, N2 supplement, insulin, GlutaMax, NEAAs, antibiotics, 2-mercaptoethanol, and B27 supplement — for 4 days, and finally, they were transferred to a spinning bioreactor and differentiation medium containing vitamin A. The newly generated cerebral organoids developed primitive regions analogous to ventricles, choroid plexus, and retina, as well as functional and structural cortical organization [30•]. In addition, the cerebral organoids displayed regionalization of discrete primitive brain regions [30•]. Utilization of the in vitro cerebral organoid model, effectively mini-brains, stimulated interest using similar approaches to model brain cancer, leading to an expansion of glioma organoid models (Table 1). These models can be developed via co-culture of glioma stem-like cells (GSCs) and cerebral organoids, genetic engineering of cerebral organoids, or direct culture of minimally processed glioma tissue samples (Fig. 1) [62••, 63•, 64••, 65, 66].
Several studies have genetically manipulated cerebral organoids to model glioma [63•, 65, 66]. Using sleeping beauty transposon-mediated gene insertion for oncogene amplification and CRISPR/Cas9-mediated mutagenesis, Bian et al. introduced combinations of 15 clinically relevant genomic alterations seen in GBM, among other CNS tumors, to generate neoplastic cerebral organoids (NeoCOR) [65]. Three combinations of genetic alterations, including those affecting CDKN2A/B, NF1, PTEN, TP53, and epidermal growth factor receptor variant III (EGFRvIII), were then identified and classified as GBM-1, GBM-2, and GBM-3. These NeoCOR of GBM demonstrated similar cellular identities and transcriptomic signatures to analogous human GBMs [65]. Similarly, Ogawa et al. introduced the oncogene HRasG12V at the TP53 locus to simultaneously knock out the tumor suppressor gene and introduce an oncogene [66]. Both models demonstrated invasive phenotypes on xenograft transplantation in mice as well as a propensity to undergo epithelial-mesenchymal transition, a transition seen in GBM tissue that is associated with a more aggressive and infiltrative phenotype [65, 66, 74, 75].
Adapting the approach to generate cerebral organoids, Hubert et al. developed the first PDOs of GBM utilizing GSCs from surgically excised human GBM tissue [62••]. Tissue samples were either finely minced and plated in a Matrigel suspension or dissociated into single-cell suspensions [62••]. Isolated cells were then maintained as tumorspheres and sorted for CD133, a marker of the GSC population [56, 76, 77]. CD133 + cells were plated in a complete medium — containing EGF, bFGF, B27 supplement, glutamine, sodium pyruvate, and antibiotics — and resultant tumorspheres were cultured in a Matrigel matrix and allowed to form three-dimensional organoids [62••]. Their GSC-based PDO model recapitulated hallmark features of GBM, including radio-resistance of GSCs, radio-sensitivity of non-GSC populations, single-cell infiltration that is lost in many xenograft models, and gradients of GSC density and hypoxia, all hallmarks of in vivo human GBMs [62••].
Similarly, Linkous et al. developed a PDO model of GBM by co-culturing GSCs with cerebral organoids termed glioma cerebral organoids (GLICO) [63•]. The GSCs are isolated from the primary tissue and maintained in serum-free media supplemented with EGF and bFGF as previously described [78, 79]. Mature GSCs are then placed in culture with cerebral organoids and are rapidly and efficiently engrafted into the organoid. Considerable bulk tumor growth was observed after 1 week, and at 2 weeks the GLICO demonstrated histopathologic features of human disease and generated a network of microtubes associated with invasion and proliferation [63•, 80]. Similarly, Krieger et al. utilized a GBM cerebral organoid model and transcriptomic analysis of receptor-ligand pairing to highlight the importance of the tumor microenvironment in microtube formation and GBM invasion [71].
In defining the inter- and intra-tumoral heterogeneity associated with GBM, single-cell RNA sequencing of GBM identified the presence of four cellular states, neural progenitor-like cells (NPC), oligodendrocyte progenitor-like cells (OPC), astrocyte-like cells (AC), and mesenchymal-like cells (MES) [81••]. Analysis of the patient-derived GLICO model developed by Linkous et al. revealed an enrichment in the NPC/OPC signature [68]. Furthermore, the enriched NPC cellular state was lost following organoid dissociation and analysis of the two-dimensional culture, providing more evidence as to the value of the tumor microenvironment in maintaining the diverse cellular states of glioma [68]. Despite the value of GSC-based organoid models, the dependence of the models on exogenous growth factors (EGF and bFGF), and the use of an artificial extracellular matrix rather than native extracellular matrix introduces laboratory conditions that could lead to drift in the cellular and molecular features of these cultures.
To maintain the native cellular hierarchy and tumor architecture, Jacob et al. successfully generated GBM organoids from minimally processed primary tissue samples [64••, 72]. The tissue is acquired from the operating room and taken directly to the laboratory, where it is parcellated into approximately 1-mm-diameter pieces [64••]. A critical advantage over prior techniques is the absence of single-cell dissociation, added growth factors, such as EGF and bFGF, or artificial Matrigel extracellular matrix. After 1 week in culture, the tissue formed well-rounded spheres, and within 2–4 weeks a mature and cell-rich organoid is achieved [64••, 72]. Importantly, the GBM organoids retained histopathologic features of their parent tumor including nuclear atypia, hypoxia gradients, and high Ki67 indices [64••]. Similarly, organoids retained their intracellular heterogeneity, maintaining CD31+ vascular cell populations, markers for glial cells such as GFAP and S100B, and markers of neural progenitor cells and GSCs such as DCX, NESTIN, SOX2, and OLIG2 [64••]. Furthermore, the GBM organoids retained features at the 4-week time point, as well as after freezing and reanimation, indicating the ability to create clinically relevant biobanks [64••, 72]. Given the profound inter-cellular heterogeneity seen in GBM, RNA, and exome sequencing analyses were performed on the PDOs and revealed similarities in gene expression, copy number variants, and somatic variants [64••]. The GBM PDOs also retained tumor subregion features, including mutations in PTEN and EGFR from different regions of the same primary tumor, indicating their ability to maintain intra-tumoral heterogeneity [64••, 72].
Recently, our group successfully generated the first PDO models of lower-grade glioma (LGG), including CNS WHO grade 1–3 disease [73••]. With modifications to the minimally processed glioma sample approach, namely organoid culture at intracranial physiologic oxygen tension, we demonstrated a 91% success rate in all grades of glioma organoid formation and an 87% success rate for grade 1–3 tumors [73••]. As such, the high fidelity of the workflow is unlikely to systematically exclude subsets of tumors from being made into organoids. The basis for these modifications was the extracellular environment of the intracranial vault, wherein oxygen tension is shown to be less than ambient room air [82,83,84]. These findings are consistent with the role of hypoxia in promoting GSC expansion [85]. While grade 4 tumors have likely reached a mutational burden that limits their sensitivity to perturbations of environmental conditions, we hypothesized that lower-grade tumors require culture conditions that better mirror physiologic conditions in the human brain [73••]. Additionally, in vitro culture conditions that better recapitulate physiologic conditions may produce more biologically relevant results. Similar to prior PDO models of GBM, LGG organoids maintained markers of stemness, proliferation, and vascularity. Genomic and metabolomic analyses of the organoids demonstrated maintenance of common mutations in LGG, such as those in IDH1/2, TP53, NOTCH1/2, CIC, and ATRX, retention of copy number variations, and similar levels of the IDH1-R132H oncoprotein and the oncometabolite 2-hydroxyglutarate [73••]. There is emerging data, particularly in glioma, that tumors harbor significant differences in biological features at the time of recurrence following treatment than at diagnosis [86, 87]. However, most existing models are derived from end-stage disease, given that these highly aggressive tumor cells are most likely to grow in vitro or in xenografts. Thus, treatment-naïve models of LGG can be used to study glioma biology independent of the effects of prior therapy.
While recent advances in GBM organoid models are promising, questions regarding the applicability of these models to studying some key aspects of glioma biology remain. For example, Jacob et al. reported an organoid success rate for IDH-wildtype tumors > 90% while IDH1-mutant tumors were generated at a much lower success rate of 66.7% [64••]. Furthermore, detailed characterization of the vascular and immune cell compartments in minimally processed samples is not yet available. While markers of vascular endothelial and immune cells are maintained in minimally processed glioma PDOs, it is possible that these non-glioma cell populations are not maintained as efficiently in long-term cultures relative to glioma cells. Contrary to cell-line models or GSC-derived organoids, minimally processed patient-derived LGG organoids cannot be expanded over time, thereby limiting the scale of experiments that can be performed with each model. Thus, while they present an excellent model for personalized oncology screening, they may not support high-throughput experimental approaches. Despite these limitations, the remarkable fidelity of PDOs underscores their utility for studying glioma biology in the laboratory setting.
Applications to Personalized Oncology and Research
Ideal patient-derived cancer models are reliable, moderate- to high-throughput, and able to replicate the cellular and molecular phenotypes of their parent tumors. Current in vitro models are limited in their clinical utility due to the homogeneity of the tumor cell populations and long induction times. Conversely, the relatively rapid rate at which PDO models can be generated for many cancers enables experimentation within a clinically relevant timeline. Three-dimensional organoid architecture permits tumor-tumor and tumor-stroma interactions that are critical for the development of cellular hierarchies and tumor cell proliferation. The ability of PDOs to retain the genetic and transcriptomic signatures of their parent tumors allows them to act as patient avatars and may provide value for preclinical drugs screens [88, 89]. Growing evidence for the reliability and efficiency of PDOs as preclinical translational models supports potential uses of these models as avatars in oncology trials. If successfully implemented, one could use tissue from a patient’s biopsy or resection specimen to create a faithful PDO, screen this PDO across multiple treatments including systemic therapies and/or radiation, and use the information from this screen to guide personalized treatment recommendations for the patient. Together with established tumor characterization methods and treatment pipelines, a comprehensive report of histopathologic features, genetic alterations, and responsiveness of PDOs to various therapies has the potential to guide evidence-based and patient-specific oncology care (Fig. 2).
Critical to PDO use in personalized clinical drug screens are reliable, efficient, and timely protocols for organoid generation. Given that the primary tissue sample is a limiting resource, any PDO model must prioritize high success rates for organoid creation. While there is variability between models and cancer types, success rates for producing organoid models are generally higher than for producing cell lines, ranging from 60 to 95% [42•, 50, 64••, 73••, 90,91,92, 93•, 94]. Additionally, to generate the appropriate number of replicates for organoid-based drug screens, successful splitting and expansion of organoids from a single sample expand their use and longevity [95]. Compared with the time to generate and assay patient-derived xenografts and genetically engineered mice, most organoid models can be established in less than four weeks [96, 97]. Prior studies have completed rapid comprehensive drug screens, with Yan et al. screening responses of PDOs of gastric cancer to 37 drugs in under 2 weeks, ideal for timely prediction of patient responses to treatment [45, 98]. Tiriac et al. outline a similar timeline for drug screening utilizing PDOs of pancreatic cancer, with organoid creation to screening being completed in around 6 weeks [99].
Several studies have examined the feasibility of PDOs in conducting patient-specific drug screens [42•, 47, 48, 64••, 93•, 94, 100,101,102,103,104]. Wetering et al. utilized colorectal cancer organoids to screen 83 therapeutics, including chemotherapies and targeted therapies for treatment response [102]. Jacob et al. targeted tumor-specific genetic mutations affecting EGFR, NF1, PI3K, and EGFRvIII using gefitinib, trametinib, and everolimus, respectively, and used EGFRvIII-specific CAR-T cells to target organoids carrying this mutation [64••]. Sachs et al. screened 6 drugs targeting EGFR and HER2 signaling pathways, including downstream inhibitors of PI3K, AKT, and mTORC1/2, in their breast cancer PDOs [42•]. Furthermore, cancer organoids are shown to respond to treatment based on their genetic alterations, as seen with BRCA2-mutant organoids responding to olaparib, EGFR-mutant organoids to erlotinib, and EGFR-mutant/MET-amplified organoids to crizotinib [94]. Additional PDO-based drug screens have been conducted in bladder, ovarian, endometrial, pancreatic, lung, and other gastrointestinal cancers, among others [44, 45, 47, 93•, 94, 99, 105, 106]. Similar to parent tissue, PDOs retained features of chemoresistance and radioresistance, providing faithful representation of the parent tumor phenotype [42•, 67]. Furthermore, many studies cite the ability of their PDO models to be biobanked and later reanimated, thus generating stored clinical databases for future screening and analysis [42•, 45, 64••, 102, 107]. From a clinical standpoint, the ability to create and screen organoids in less than 6 weeks is optimal as it allows PDO formation and screening to occur in the period between surgical resection and initiation of subsequent therapy [108]. In the case of GBM, the drug screening protocol can overlap with the receipt of the standard of care temozolomide chemotherapy and radiotherapy and guide additional therapeutic considerations upon recurrence or progression [109].
Conclusions
Recapitulation of the three-dimensional native tissue structure, maintenance of molecular and cellular heterogeneity, and rapid generation of patient-derived models present significant advantages for organoids relative to classical cancer models. The advent of organoid development from human cancer samples, either by isolation of cancer stem cells or minimally processed primary tissue specimens, has paved the way for PDO models. Relative to other cancer models, the rapid speed of generation and more faithful representation of human cancer offer new opportunities for personalized oncology. Over the course of the last decade, cancer organoid models have been shown to retain histopathologic and molecular features of their primary tumors, as well as demonstrating treatment responses akin to those of the parent tumor. As such, PDOs are a potential model for high-throughput drug screens to guide the selection of oncologic therapies. The translational implications for PDOs are most apparent in cancers with limited therapeutic options and those characterized by patient-to-patient heterogeneity, diverse intra-tumoral cellular states, and complex tumor microenvironments integral to tumor behavior, as illustrated here in our discussion of malignant glioma. Despite the promising prospects of current studies, the use of PDOs in clinical oncology care is not without drawbacks. Variability in organoid generating methodologies, wide ranges of organoid creation success rates, and incomplete characterization of non-tumor cell populations in organoid models are key limitations. While there is a need for standardization of PDO models, current data suggest their continued expansion and utility as translational cancer models. PDOs are poised to support the development and implementation of personalized oncology treatment programs.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
DeVita VT, Chu E. A history of cancer chemotherapy. Can Res. 2008;68(21):8643–53. https://doi.org/10.1158/0008-5472.Can-07-6611.
Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat Rev. 2020;86:102019-. https://doi.org/10.1016/j.ctrv.2020.102019.
Goetz LH, Schork NJ. Personalized medicine: motivation, challenges, and progress. Fertil Steril. 2018;109(6):952–63. https://doi.org/10.1016/j.fertnstert.2018.05.006.
Bear HD, Wan W, Robidoux A, Rubin P, Limentani S, White RL Jr, et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J Surg Oncol. 2017;115(8):917–23. https://doi.org/10.1002/jso.24610.
Pease AM, Riba LA, Gruner RA, Tung NM, James TA. Oncotype DX(®) Recurrence score as a predictor of response to neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26(2):366–71. https://doi.org/10.1245/s10434-018-07107-8.
Scott JG, Berglund A, Schell MJ, Mihaylov I, Fulp WJ, Yue B, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202–11. https://doi.org/10.1016/S1470-2045(16)30648-9.
Scott JG, Sedor G, Ellsworth P, Scarborough JA, Ahmed KA, Oliver DE, et al. Pan-cancer prediction of radiotherapy benefit using genomic-adjusted radiation dose (GARD): a cohort-based pooled analysis. Lancet Oncol. 2021;22(9):1221–9. https://doi.org/10.1016/S1470-2045(21)00347-8.
Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017;7(5):462–77. https://doi.org/10.1158/2159-8290.Cd-16-1154.
Mirabelli P, Coppola L, Salvatore M. Cancer cell lines are useful model systems for medical research. Cancers. 2019;11(8):1098. https://doi.org/10.3390/cancers11081098.
Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–17. https://doi.org/10.1101/gad.261982.115.
Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44(12):2144–51. https://doi.org/10.1016/j.biocel.2012.08.022.
Lai Y, Wei X, Lin S, Qin L, Cheng L, Li P. Current status and perspectives of patient-derived xenograft models in cancer research. J Hematol Oncol. 2017;10(1):106. https://doi.org/10.1186/s13045-017-0470-7.
Hicks WH, Bird CE, Traylor JI, Shi DD, El Ahmadieh TY, Richardson TE, et al. Contemporary mouse models in glioma research. Cells. 2021;10(3). https://doi.org/10.3390/cells10030712.
Cheon DJ, Orsulic S. Mouse models of cancer. Annu Rev Pathol. 2011;6:95–119. https://doi.org/10.1146/annurev.pathol.3.121806.154244.
Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7(9):645–58. https://doi.org/10.1038/nrc2192.
Ledur PF, Onzi GR, Zong H, Lenz G. Culture conditions defining glioblastoma cells behavior: what is the impact for novel discoveries? Oncotarget. 2017;8(40):69185–97. https://doi.org/10.18632/oncotarget.20193.
Allen M, Bjerke M, Edlund H, Nelander S, Westermark B. Origin of the U87MG glioma cell line: good news and bad news. Sci Transl Med. 2016;8(354):354re3. https://doi.org/10.1126/scitranslmed.aaf6853.
Auman JT, McLeod HL. Colorectal cancer cell lines lack the molecular heterogeneity of clinical colorectal tumors. Clin Colorectal Cancer. 2010;9(1):40–7. https://doi.org/10.3816/CCC.2010.n.005.
Gillet J-P, Varma S, Gottesman MM. The clinical relevance of cancer cell lines. J Natl Cancer Inst. 2013;105(7):452–8. https://doi.org/10.1093/jnci/djt007.
Prager BC, Bhargava S, Mahadev V, Hubert CG, Rich JN. Glioblastoma stem cells: driving resilience through chaos. Trends Cancer. 2020;6(3):223–35. https://doi.org/10.1016/j.trecan.2020.01.009.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94. https://doi.org/10.1038/nrclinonc.2017.166.
Aboulkheyr Es H, Montazeri L, Aref AR, Vosough M, Baharvand H. Personalized cancer medicine: an organoid approach. Trends Biotechnol. 2018;36(4):358–71. https://doi.org/10.1016/j.tibtech.2017.12.005.
Langer EM, Allen-Petersen BL, King SM, Kendsersky ND, Turnidge MA, Kuziel GM, et al. Modeling tumor phenotypes in vitro with three-dimensional bioprinting. Cell Rep. 2019;26(3):608-23.e6. https://doi.org/10.1016/j.celrep.2018.12.090.
Kamb A. Wha’’s wrong with our cancer models? Nat Rev Drug Discovery. 2005;4(2):161–5. https://doi.org/10.1038/nrd1635.
Karim BO, Huso DL. Mouse models for colorectal cancer. Am J Cancer Res. 2013;3(3):240–50.
Becher OJ, Holland EC. Genetically engineered models have advantages over xenografts for preclinical studies. Can Res. 2006;66(7):3355–9. https://doi.org/10.1158/0008-5472.Can-05-3827.
Kersten K, de Visser KE, van Miltenburg MH, Jonkers J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol Med. 2017;9(2):137–53. https://doi.org/10.15252/emmm.201606857.
Li M, Izpisua Belmonte JC. Organoids — preclinical models of human disease. N Engl J Med. 2019;380(6):569–79. https://doi.org/10.1056/NEJMra1806175.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5. https://doi.org/10.1038/nature07935. This article was the first to create the organoid model system by developing three-dimensional crypt-villous structures from Lgr5+ intestinal stem cells.
Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–9. https://doi.org/10.1038/nature12517. This article outlined methodology for creation of the first cerebral organoids from human embryonic stem cells and induced pluripotent stem cells.
Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19(5):568–77. https://doi.org/10.1038/ncb3516.
Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14(1):53–67. https://doi.org/10.1016/j.stem.2013.11.010.
Takasato M, Er PX, Chiu HS, Little MH. Generation of kidney organoids from human pluripotent stem cells. Nat Protoc. 2016;11(9):1681–92. https://doi.org/10.1038/nprot.2016.098.
Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019;14(2):518–40. https://doi.org/10.1038/s41596-018-0104-8.
Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, et al. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc Natl Acad Sci. 2014;111(46):16401–6. https://doi.org/10.1073/pnas.1409064111.
Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4. https://doi.org/10.7554/eLife.05098.
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–6. https://doi.org/10.1038/nature09941.
Wimmer RA, Leopoldi A, Aichinger M, Kerjaschki D, Penninger JM. Generation of blood vessel organoids from human pluripotent stem cells. Nat Protoc. 2019;14(11):3082–100. https://doi.org/10.1038/s41596-019-0213-z.
Hohwieler M, Illing A, Hermann PC, Mayer T, Stockmann M, Perkhofer L, et al. Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut. 2017;66(3):473–86. https://doi.org/10.1136/gutjnl-2016-312423.
Alzamil L, Nikolakopoulou K, Turco MY. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2021;28(1):35–51. https://doi.org/10.1038/s41418-020-0565-5.
Bolck HA, Corrò C, Kahraman A, von Teichman A, Toussaint NC, Kuipers J, et al. Tracing clonal dynamics reveals that two- and three-dimensional patient-derived cell models capture tumor heterogeneity of clear cell renal cell carcinoma. Eur Urol Focus. 2021;7(1):152–62. https://doi.org/10.1016/j.euf.2019.06.009.
Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(1):373-86.e10. https://doi.org/10.1016/j.cell.2017.11.010. This article outlines successful creation of patient-derived breast cancer organoids with similar in vitro and in vivo drug responses.
Rosenbluth JM, Schackmann RCJ, Gray GK, Selfors LM, Li CM-C, Boedicker M, et al. Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nature Communications. 2020;11(1):1711. https://doi.org/10.1038/s41467-020-15548-7.
Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science (New York, NY). 2018;359(6378):920–6. https://doi.org/10.1126/science.aao2774.
Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. cell stem cell. 2018;23(6):882–97.e11. https://doi.org/10.1016/j.stem.2018.09.016.
Yamazaki S, Ohka F, Hirano M, Shiraki Y, Motomura K, Tanahashi K, et al. Newly established patient-derived organoid model of intracranial meningioma. Neuro Oncol. 2021. https://doi.org/10.1093/neuonc/noab155.
Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173(2):515-28.e17. https://doi.org/10.1016/j.cell.2018.03.017.
Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarró LM, Bradshaw CR, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424–35. https://doi.org/10.1038/nm.4438.
Weeber F, van de Wetering M, Hoogstraat M, Dijkstra KK, Krijgsman O, Kuilman T, et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci USA. 2015;112(43):13308–11. https://doi.org/10.1073/pnas.1516689112.
Pernik MN, Bird CE, Traylor JI, Shi DD, Richardson TE, McBrayer SK, et al. Patient-derived cancer organoids for precision oncology treatment. J Pers Med. 2021;11(5). https://doi.org/10.3390/jpm11050423.
Baker LA, Tiriac H, Clevers H, Tuveson DA. Modeling pancreatic cancer with organoids. Trends Cancer. 2016;2(4):176–90. https://doi.org/10.1016/j.trecan.2016.03.004.
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-oncology. 2015;17 Suppl 4:iv1-iv62. https://doi.org/10.1093/neuonc/nov189.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021. https://doi.org/10.1093/neuonc/noab106. This article contains the World Health Organization 2021 classification guidelines for central nervous system tumors, containing updates pertinent to the role of molecular features in diagnostic algorithms.
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–50. https://doi.org/10.1001/jama.2013.280319.
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Can Res. 2006;66(16):7843–8. https://doi.org/10.1158/0008-5472.Can-06-1010.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60. https://doi.org/10.1038/nature05236.
Seidel S, Garvalov BK, Acker T. Isolation and culture of primary glioblastoma cells from human tumor specimens. In: Rich IN, editor. Stem Cell Protocols. New York, NY: Springer New York; 2015. p. 263–75.
Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, Schildkraut JM, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurgical Focus FOC. 2015;38(1):E6. https://doi.org/10.3171/2014.10.Focus12367.
Li Y, Wang H, Sun T, Chen J, Guo L, Shen H, et al. Biological characteristics of a new human glioma cell line transformed into A2B5(+) stem cells. Molecular cancer. 2015;14:75-. https://doi.org/10.1186/s12943-015-0343-z.
Kastner C, Hendricks A, Deinlein H, Hankir M, Germer C-T, Schmidt S, et al. Organoid models for cancer research-from bed to bench side and back. Cancers. 2021;13(19):4812. https://doi.org/10.3390/cancers13194812.
Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014;9(10):2329–40. https://doi.org/10.1038/nprot.2014.158.
Hubert CG, Rivera M, Spangler LC, Wu Q, Mack SC, Prager BC, et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Can Res. 2016;76(8):2465–77. https://doi.org/10.1158/0008-5472.Can-15-2402. This study successfully generated organoids from CD133+ glioma stem cells isolated from primary patient tumor samples, creating the first PDO model of glioma.
Linkous A, Balamatsias D, Snuderl M, Edwards L, Miyaguchi K, Milner T, et al. Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep. 2019;26(12):3203-11.e5. https://doi.org/10.1016/j.celrep.2019.02.063. This article provided methodology for creation of organoids via co-culture of GSCs and cerebral organoids.
Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH, et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180(1):188–204 e22. https://doi.org/10.1016/j.cell.2019.11.036. This study demonstrated creation of minimally processed PDOs of glioblastoma which retained molecular and histopathologic features of the parent tumor.
Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, et al. Genetically engineered cerebral organoids model brain tumor formation. Nat Methods. 2018;15(8):631–9. https://doi.org/10.1038/s41592-018-0070-7.
Ogawa J, Pao GM, Shokhirev MN, Verma IM. Glioblastoma model using human cerebral organoids. Cell Rep. 2018;23(4):1220–9. https://doi.org/10.1016/j.celrep.2018.03.105.
Sundar SJ, Shakya S, Barnett A, Wallace LC, Jeon H, Sloan A, et al. Three-dimensional organoid culture unveils resistance to clinical therapies in adult and pediatric glioblastoma. Translational oncology. 2021;15(1): 101251. https://doi.org/10.1016/j.tranon.2021.101251.
Pine AR, Cirigliano SM, Nicholson JG, Hu Y, Linkous A, Miyaguchi K, et al. Tumor microenvironment is critical for the maintenance of cellular states found in primary glioblastomas. Cancer Discov. 2020;10(7):964–79. https://doi.org/10.1158/2159-8290.Cd-20-0057.
Goranci-Buzhala G, Mariappan A, Gabriel E, Ramani A, Ricci-Vitiani L, Buccarelli M, et al. Rapid and efficient invasion assay of glioblastoma in human brain organoids. Cell Rep. 2020;31(10): 107738. https://doi.org/10.1016/j.celrep.2020.107738.
da Silva B, Mathew RK, Polson ES, Williams J, Wurdak H. Spontaneous glioblastoma spheroid infiltration of early-stage cerebral organoids models brain tumor invasion. SLAS discovery : advancing life sciences R & D. 2018;23(8):862–8. https://doi.org/10.1177/2472555218764623.
Krieger TG, Tirier SM, Park J, Jechow K, Eisemann T, Peterziel H, et al. Modeling glioblastoma invasion using human brain organoids and single-cell transcriptomics. Neuro Oncol. 2020;22(8):1138–49. https://doi.org/10.1093/neuonc/noaa091.
Jacob F, Ming GL, Song H. Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nat Protoc. 2020;15(12):4000–33. https://doi.org/10.1038/s41596-020-0402-9.
Abdullah KG, Bird CE, Buehler JD, Gattie LC, Savani MR, Sternisha AC, et al. Establishment of patient-derived organoid models of lower grade glioma. Neuro Oncol. 2021. https://doi.org/10.1093/neuonc/noab273. This article was the first to generate minimally processed PDOs of WHO grades 1-3 glioma which retained histopathologic and metabolic features of the parent tumor.
Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science (New York, NY). 2012;338(6110):1080–4. https://doi.org/10.1126/science.1226929.
Hara T, Chanoch-Myers R, Mathewson ND, Myskiw C, Atta L, Bussema L, et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell. 2021;39(6):779-92.e11. https://doi.org/10.1016/j.ccell.2021.05.002.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Can Res. 2003;63(18):5821–8.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. https://doi.org/10.1038/nature03128.
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. https://doi.org/10.1016/j.ccr.2006.03.030.
Kim S-S, Pirollo KF, Chang EH. Isolation and culturing of glioma cancer stem cells. Curr Protoc Cell Biol. 2015;67:23.10.1–23.10. https://doi.org/10.1002/0471143030.cb2310s67.
Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–8. https://doi.org/10.1038/nature16071.
Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178(4):835-49.e21. https://doi.org/10.1016/j.cell.2019.06.024. This study utilized bulk and single-cell RNA sequencing to reveal four unique cell subpopulations in primary patient GBM samples.
Evans SM, Jenkins KW, Jenkins WT, Dilling T, Judy KD, Schrlau A, et al. Imaging and analytical methods as applied to the evaluation of vasculature and hypoxia in human brain tumors. Radiat Res. 2008;170(6):677–90. https://doi.org/10.1667/rr1207.1.
Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell cycle (Georgetown, Tex). 2009;8(20):3274–84. https://doi.org/10.4161/cc.8.20.9701.
Jagannathan L, Cuddapah S, Costa M. Oxidative stress under ambient and physiological oxygen tension in tissue culture. Curr Pharmacol Rep. 2016;2(2):64–72. https://doi.org/10.1007/s40495-016-0050-5.
Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene. 2009;28(45):3949–59. https://doi.org/10.1038/onc.2009.252.
Barthel FP, Johnson KC, Varn FS, Moskalik AD, Tanner G, Kocakavuk E, et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576(7785):112–20. https://doi.org/10.1038/s41586-019-1775-1.
Varn FS, Johnson KC, Wade TE, Malta TM, Sabedot TS, Barthel FP, et al. Longitudinal analysis of diffuse glioma reveals cell state dynamics at recurrence associated with changes in genetics and the microenvironment. bioRxiv. 2021:2021.05.03.442486. https://doi.org/10.1101/2021.05.03.442486.
Pasch CA, Favreau PF, Yueh AE, Babiarz CP, Gillette AA, Sharick JT, et al. Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(17):5376–87. https://doi.org/10.1158/1078-0432.Ccr-18-3590.
Bleijs M, van de Wetering M, Clevers H, Drost J. Xenograft and organoid model systems in cancer research. EMBO J. 2019;38(15):e101654. https://doi.org/10.15252/embj.2019101654.
Centenera MM, Hickey TE, Jindal S, Ryan NK, Ravindranathan P, Mohammed H, et al. A patient-derived explant (PDE) model of hormone-dependent cancer. Mol Oncol. 2018;12(9):1608–22. https://doi.org/10.1002/1878-0261.12354.
Ganesh K, Wu C, O’Rourke KP, Szeglin BC, Zheng Y, Sauvé C-EG, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nature Medicine. 2019;25(10):1607–14. https://doi.org/10.1038/s41591-019-0584-2.
Shi R, Radulovich N, Ng C, Liu N, Notsuda H, Cabanero M, et al. Organoid cultures as preclinical models of non–small cell lung cancer. Clin Cancer Res. 2020;26(5):1162–74. https://doi.org/10.1158/1078-0432.Ccr-19-1376.
Driehuis E, van Hoeck A, Moore K, Kolders S, Francies HE, Gulersonmez MC, et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc Natl Acad Sci USA. 2019;116(52):26580–90. https://doi.org/10.1073/pnas.1911273116. This study conducted extensive drug screens to identify unique drug sensitivities in patient PDO models of pancreatic cancer.
Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10(1):3991. https://doi.org/10.1038/s41467-019-11867-6.
Driehuis E, Kretzschmar K, Clevers H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat Protoc. 2020;15(10):3380–409. https://doi.org/10.1038/s41596-020-0379-4.
Kondo J, Inoue M. Application of cancer organoid model for drug screening and personalized therapy. Cells. 2019;8(5):470. https://doi.org/10.3390/cells8050470.
Ruiz-Garcia H, Alvarado-Estrada K, Schiapparelli P, Quinones-Hinojosa A, Trifiletti DM. Engineering three-dimensional tumor models to study glioma cancer stem cells and tumor microenvironment. Front Cell Neurosci. 2020;14:558381-. https://doi.org/10.3389/fncel.2020.558381.
Jin W-L, Jin M-Z, Tu Y-Y. Organoids: a platform ready for glioblastoma precision medicine? Trends Cancer. 2020;6(4):265–7. https://doi.org/10.1016/j.trecan.2020.01.016.
Tiriac H, Belleau P, Engle DD, Plenker D, Deschênes A, Somerville TDD, et al. organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8(9):1112–29. https://doi.org/10.1158/2159-8290.Cd-18-0349.
Liu HD, Xia BR, Jin MZ, Lou G. Organoid of ovarian cancer: genomic analysis and drug screening. Clin Transl Oncol. 2020;22(8):1240–51. https://doi.org/10.1007/s12094-019-02276-8.
Kopper O, de Witte CJ, Lõhmussaar K, Valle-Inclan JE, Hami N, Kester L, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25(5):838–49. https://doi.org/10.1038/s41591-019-0422-6.
van de Wetering M, Francies Hayley E, Francis Joshua M, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–45. https://doi.org/10.1016/j.cell.2015.03.053.
Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21(8):1041–51. https://doi.org/10.1038/s41556-019-0360-z.
Saito Y, Muramatsu T, Kanai Y, Ojima H, Sukeda A, Hiraoka N, et al. Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma. Cell Rep. 2019;27(4):1265-76.e4. https://doi.org/10.1016/j.celrep.2019.03.088.
Li L, Knutsdottir H, Hui K, Weiss MJ, He J, Philosophe B, et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI insight. 2019;4(2): e121490. https://doi.org/10.1172/jci.insight.121490.
Kiyohara Y, Yoshino K, Kubota S, Okuyama H, Endo H, Ueda Y, et al. Drug screening and grouping by sensitivity with a panel of primary cultured cancer spheroids derived from endometrial cancer. Cancer Sci. 2016;107(4):452–60. https://doi.org/10.1111/cas.12898.
Calandrini C, Schutgens F, Oka R, Margaritis T, Candelli T, Mathijsen L, et al. An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat Commun. 2020;11(1):1310. https://doi.org/10.1038/s41467-020-15155-6.
Chen H, Zhuo Q, Ye Z, Xu X, Ji S. Organoid model: a new hope for pancreatic cancer treatment? Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2021;1875(1):188466. https://doi.org/10.1016/j.bbcan.2020.188466.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. https://doi.org/10.1056/NEJMoa043330.
Acknowledgements
The authors would like to thank Melissa Logies for the illustration of the figures.
Funding
Funding was provided through Eugene P. Frenkel, M.D. Endowed Scholar Program (K. G. A), National Cancer Institute (NCI) R01CA238586 (K. G. A. and S. K. M.), Cancer Prevention and Research Institute of Texas (CPRIT) RP210140 (K. G. A.), CPRIT RR190034 (S. K. M.), NCI K22CA237752 (S. K. M), V Foundation for Cancer Research V2020-006 (S. K. M.), Sontag Foundation Distinguished Scientist Award (S. K. M.), Oligo Nation (K. G. A.), and a gift from the Jonesville Foundation (S. K. M.).
Author information
Authors and Affiliations
Contributions
Conceptualization: SKM and KGA; literature review: WHH, CEB, LCG, MES; writing—original draft preparation: WHH; writing—review and editing: WHH, CEB, LCG, MES, JIT, DDS, SKM, KGA; supervision: SKM and KGA; funding acquisition: SKM and KGA. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors. Informed consent was not required.
Disclaimer
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Radiation Biology
Rights and permissions
About this article
Cite this article
Hicks, W.H., Bird, C.E., Gattie, L.C. et al. Creation and Development of Patient-Derived Organoids for Therapeutic Screening in Solid Cancer. Curr Stem Cell Rep 8, 107–117 (2022). https://doi.org/10.1007/s40778-022-00211-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-022-00211-2