Abstract
Purpose of Review
In this review, we will explore the clinical and commercial focus on amniotic membranes (AMs) and their use in tissue engineering (TE). We will showcase the therapeutic potential of AM-isolated epithelial cells (hAECs) and the prospective use of their secreted factors, extracellular vesicles (EVs), in cell and non-cell therapies.
Recent Findings
The potential of the hAECs as a therapeutic source has been investigated in various preclinical models with some progressing into phase I clinical trials to evaluate their safety. Additionally, multiple animal studies showcase the therapeutic potential of EVs as non-cellular treatments.
Summary
The amniotic membrane (AM) has been used as a form of regenerative medicine in wound healing for burns and ulcerated surfaces and in ophthalmology for over a century. In the last few decades, research has looked to the use of the various stem cells that can be isolated from the AM. The use of AM-isolated hAECs has proven rather promising with phase I clinical trials currently underway across life-threatening diseases in both pediatric and adult populations. However, due to limitations of using cell-based therapies (e.g., cost of production, delivery restricted to major hospitals, etc.), attention has turned to investigating EVs secreted by the cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
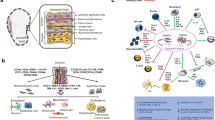
The amniotic membrane (AM) is a thin membrane on the inner side of the fetal placenta, consisting of an epithelial coat and a layer of mesenchymal connective tissue. It possesses unique biological features, including anti-inflammatory, anti-bacterial, anti-viral, and immunological characteristics as well as anti-angiogenic and pro-apoptotic qualities. The AM is commonly used clinically for wound treatment and ocular surface reconstruction. In more recent years, hAECs isolated from the AM have been investigated as a form of regenerative medicine given their immunomodulatory, anti-tumorigenic, and anti-inflammatory properties. In this review article, we focus on the hAECs and their role as a therapeutic source. First, we discuss the historical use of the AM and highlight more recent developments in amnion-derived products. Next, we provide insight into hAECs themselves. This is followed by a summary of preclinical models using hAECs and current clinical trials investigating the safety of hAECs for cell therapy. Lastly, we describe tissue engineering using the AM as a bio-scaffold and preclinical studies exploring extracellular vesicles for cell-free therapy. This is summarized in Fig. 1.
History of the Amniotic Membrane in Medical and Surgical Applications
The human placenta has been used traditionally for over centuries in Chinese medicine. The Compendium of Materia Medica, a Chinese record of substances with medicinal properties published in 1593 by Li Shi-Zhen, devotes an entire section to the uses of the human placenta as a medicine [1]. More than 3 centuries later came the first documented use of the fetal membrane/amniotic membrane as a surgical material in skin transplantation in 1910 [2] and 1913 [3, 4]. The AM was employed in skin grafting and gave superior results when compared to xenografts or cadaveric coverings [2]. In 1913, intact amniotic membranes were applied to varicose ulcers, burns, scalds, and denudations of traumatic origin. Dr. Maximillian Stern found that the amniotic cellular tissue was taken up by the raw surface of the wound without remarkable adverse effects [3]. It would be another 20 years before another paper was published using the AM for wound repair or surgery. In the late 1930s, the AM was used for genital reconstructive surgery [5]. Then in the 1940s, it was used for the first time in ophthalmology to repair conjunctival defects [6] and ocular burns [7, 8]. Since then, the AM has been further investigated with studies and a large number of clinical trials reconfirming its successful use for skin injuries and in various clinical indications, including reconstructive surgery (genital and abdominal) [9,10,11,12,13,14,15,16], prevention of adhesions [10, 17, 18], ulcers [19,20,21], and burns [22, 23].
More Recent Developments with Amnion-Derived Products for Wound Healing
Ophthalmologists embraced the use of AMs increasingly from the 1990s [24], and they are now routinely used as a graft, spread over the ocular surface to treat epithelial defects or ulcers, or as a bandage to promote healing [25]. More recently, the commercial market for AM-based products for skin wounds has boomed. There are multiple companies offering human amnion-derived products for wound healing, ranging from sheets to cover wound beds to particulates used to “fill” tunneled wounds (summarized in Table 1). Primarily these products are applied as wound coverings (acute and chronic wounds, burns, pressure ulcers, diabetic ulcers, venous stasis ulcers). Additional uses in surgical procedures include extremity, vascular, spine, orthopedic, urological, colorectal, and general surgery. Investigative clinical trials run with these commercially available products focus primarily on the treatment of diabetic foot ulcers and venous leg ulcers. The successful use of the AM in wound healing and surgical applications is in part due to its low immunogenicity (see the section “Clinical Trials”). The AM does not require preconditioning, attributed to the low expression of classical major histocompatibility complex (MHC) classes I and II molecules and high expression of HLA-G from hAECs (see the section “Stem-like Cells from Amniotic Membranes”—“Physical Characteristics”) and EVs [26].
Denuded Human Amniotic Membranes Can Be Used as a Substrate for Growth of Other Stem Cells
Potential applications of the denuded AM as scaffolds have been explored in both animal and human studies to target tissues such as the eye, cartilage, peripheral nerve, and skin tissue engineering (TE). The extracellular matrix of the human amnion is an effective conduit for peripheral nerve regeneration, and the AM is a biodegradable scaffold with unique biochemical and mechanical characteristics for nerve regeneration [39, 40]. Miyamoto et al. showed that primate embryonic stem cells had undifferentiated growth when cultured on human amniotic epithelial feeder cells, while Ueno et al. utilized the denuded AM as a feeder layer to direct the neuronal differentiation of stem cells [41, 42]. Another group investigated the denuded AM as a carrier matrix to promote chondrocyte growth and support cartilage regeneration [43]. When epithelial and mesenchymal cells were seeded on an AM scaffold, the cells became highly interconnected and capable of penetrating the porous structure of the amnion scaffold. This observation has led to the suggestion of a novel approach for repair of prematurely ruptured fetal membranes, a leading indication for preterm delivery [44]. The seeding of epithelial cells on an AM scaffold is frequently utilized for ocular surface and skin reconstruction [45, 46]. The AM is able to accelerate re-epithelization due to the presence of a basement membrane and therefore is compatible as a unique biological skin substitute for treating deep dermal and full-thickness wounds [47]. Similarly, endothelial cells have been seeded on an AM scaffold for vascular TE purposes [48].
Stem-like Cells from Amniotic Membranes
Amniotic epithelial cells (hAECs) and amniotic mesenchymal stromal cells (hAMSCs) are the major stem and stem-like cells of the human amnion. No invasive technique is required to harvest hAEC and hAMSC as they are isolated from the epithelial layer of the amniotic membrane, which is usually discarded after birth along with the rest of the placenta. Specialized protocols have been developed for their isolation. Both hAEC and hAMSC possess unique properties that are vital in regenerative medicine including their multipotent differentiation potential, low immunogenicity [49], and anti-fibrotic and anti-inflammatory properties [50]. In vitro, hAMSC and hAEC have shown to develop into cells with mesodermal, ectodermal, and endodermal lineages [50]. hAMSCs are a multipotent stem cell population that express the classical MSC markers including CD90, CD44, CD73, and CD105 [50, 51] while hAECs are pluripotent and express different markers including OCT-4, NANOG, SOX-2, and TRA-1-60 [52,53,54]. Typically, a healthy term placenta will yield >10 times more hAEC compared to hAMSC [55]. In this review, we will focus on the therapeutic effects of hAEC. hAECs have been explored as a potential therapy for fibrotic and inflammation-based disorders including respiratory [56, 57, 58•, 59], gastroenterological [60•, 61, 62], neurological [63, 64], and cardiac conditions [65, 66].
Physical Characteristics
hAECs are small circular cells that have either a central or eccentric nucleus with one or two nucleoli and an abundant cytoplasm [53]. hAEC express embryonic stem cell markers SSEA-3 and SSEA-4 (stage-specific embryonic antigen 3 and 4) and tumor rejection antigens 1-60 (TRA 1-60) and 1-81 (TRA 1-81). The pluripotency of hAECs has been attributed to the expression of these markers as well as the expression of pluripotent stem cell transcription factors, Oct-4, Sox-2, Nanog, and Rex-1 [52,53,54]. The low immunogenicity of hAEC can be attributed to the expression of the class I human leukocyte antigen, HLA-G. The soluble form of HLA-G is known to induce the apoptosis of activated CD8+ T cells and modulate natural killer cell [67] and allo-cytotoxic T cell responses [68]. The membrane-bound form of HLA-G has also been shown to inhibit natural killer cell and T cell-mediated cytolysis [69, 70] and allo-specific CD4+ T cell proliferation [71, 72] and induce a Th2 response [73, 74]. Furthermore, hAECs express low levels of HLA class I and II molecules (i.e., HLA-A, B, C, and DR) which usually stimulate allogenic rejection [49, 75]. The immunomodulatory effects of hAEC are evident through their ability to suppress the T cell response, inhibit neutrophil and macrophage infiltration, and induce macrophage polarization [54, 56].
In culture, hAECs exist in three subpopulations—adherent, intermediate (loosely adherent), and free floating [76]. The adherent population consists of cells that grow in a single layer in culture with cobblestone epithelial morphology [77], the intermediate population consists of cells that are weakly adherent, and finally, there are a population of cells that remain free floating in culture [76]. All three subpopulations have been shown to have varying expression levels of OCT4, NANOG, and stem cell surface markers SSEA-4, TRA1-60, and TRA 1-81 [76]. hAECs reach replicative senescence after 6–10 passages in cell culture [78] due to the activation of epidermal growth factor receptor (EGFR) and cell-cell interactions at high cell densities in culture [78].
Impact of Donors on hAEC Quality
A consequence of the poor proliferative capacity of hAECs necessitates ongoing tissue donation. As a result, it would be beneficial to assess donor-specific variations which potentially affect the therapeutic potency [50, 79]. The level of HLA-G in hAEC has been shown to vary between donors, with the greatest association with gestational age [54]. Larger studies to assess the impact of common maternal-fetal health complications and risk factors such as fetal growth restrictions, maternal smoking, or maternal obesity should be considered.
Preclinical Applications of hAEC
The therapeutic potential of hAEC has been evaluated in small and large animal models of cardiorespiratory diseases, brain injury and neurological disorders, liver disease, metabolic diseases, and autoimmune diseases (summarized in Table 2). hAECs have been shown to improve organ structure and function through their anti-inflammatory, anti-fibrotic, immunomodulatory, and regenerative effects as discussed earlier. Furthermore, the application of hAECs has progressed into clinical studies in diseases including bronchopulmonary dysplasia, chronic liver disease, and stroke.
Clinical Trials
In recent years, multiple phase I clinical trials using allogenic hAECs have commenced (Fig. 2).
Summary diagram of phase I clinical trials currently underway using allogenic hAECs. The hAEC and their anti-inflammatory, anti-fibrotic, and immunomodulatory properties as well as their ability to support angiogenesis are being employed as a form of regenerative medicine. hAEC therapies are treating bronchopulmonary dysplasia in premature infants and liver disease (cirrhosis and liver fibrosis), acute ischemic stroke, and fistulising perianal Crohn’s disease in adults (18–85 years old)
Bronchopulmonary dysplasia (BPD)
A first-in-human phase I safety study (ACTRN12614000174684; UTN: U1111-1151-8685) using allogenic hAECs in premature infants with BPD was successfully completed in 2018 [119]. Six premature infants with established severe BPD were enrolled to a single-center, open-label trial. Each infant received one million cells/kg by intravenous infusion. The study showed that allogeneic hAECs were well tolerated. A phase I dose escalation study was subsequently registered (ACTRN12618000920291) [120]. The study will include 24 infants, with the first 12 infants receiving a single infusion of 2 to 10 million/kg. Infants 13–18 will receive two infusions to achieve 20 million/kg and infants 19–24 will receive three infusions to total 30 million/kg. The study has recruited 14 infants to date.
Liver cirrhosis
In 2017, Lim et al. [121] published a study protocol for the first phase I trial evaluating the safety and tolerability of intravenously delivered allogenic hAEC in 12 patients with compensated liver cirrhosis (ACTRN12616000437460; UTN: U1111-1181-4339). This is a single-center, open-label, dose escalation of hAEC (0.5 to 3 million/kg) clinical trial with four cohorts of three patients each. The study has recruited 6 patients to date.
Ischemic stroke
hAECs are also currently evaluated in patients who have suffered an acute ischemic stroke (ACTRN12618000076279) [122]. Eligibility criteria for this trial include an ischemic stroke in the territory of the large main artery, within 24h of stroke onset, ineligible for clot retrieval and NIH stroke severity (NIHSS) scale between 6 and 15. This is a phase I, open-label, dose escalation 3+3 trial. Dosing starts at 2 million cells/kg with the final group receiving 32 million cells/kg. It is open for recruitment and has thus far treated 8 patients, with a target of 15 total.
Perianal fistulae
A phase I clinical trial (ACTRN12618001883202) was registered in 2018 to evaluate the safety of locally administered allogenic hAECs for the treatment of refractory perianal fistulising Crohn’s disease. This open-label study aims to recruit 10 adults with complex perianal Crohn’s fistulas who have previously failed one conventional treatment. The participants will receive a dose of 40 million cells/fistula, with up to 3 fistulas treated per participant (maximum 120 million cells). Four participants have been enrolled to date.
Interestingly, these clinical trials are not the first to investigate allogeneic hAEC clinically. The very first study in 1981 [123] was conducted in London, England, where a layer of amniotic epithelial cells were transplanted into seven volunteers (six men and one woman, ages 28–80 years old) without immunosuppressive treatment. The aim of the study was to assess the immunogenicity and survival of the amniotic epithelial membrane implants, with the intention of using the implants for treating patients with enzyme defects. Specifically, Adinolfi et al. [124] showed that hAECs produce lysosomal enzymes that are capable of correcting in vitro the enzymatic defects of patients with Hurler’s syndrome. None of the volunteers showed signs of acute rejection and hAECs were present in biopsies for up to 7 weeks post-implantation.
Niemann-Pick disease
In 1987, an Italian team published a successful treatment of Niemann-Pick disease type B by implanting a suspension of allogeneic amnion membrane tissue [125]. The tissue suspension was injected into a subcutaneous thoracic pouch under the armpit. A total of six implants were placed at intervals of 1–4 months. Following the fifth and sixth implantations, serous secretion from the wound was noted. This was thought to be due to host-versus-graft rejection due to the presence of donor fibroblasts and macrophages in the tissue suspension. The investigators postulated that a pure suspension of amniotic epithelial cells might bypass the immune reaction. Notably, they commented that “In vitro culture and cryopreservation of epithelial cells, …, should allow us to store and inject high numbers of non-immunogenic cells, thereby avoiding graft rejection.” In 1992, the same team published their findings using repeated implantations of hAECs to treat Niemann-Pick disease in five patients over 4 years [126]. The hAEC treatment resulted in the abolishment of recurring infections, mainly of the respiratory tract, and improvements to the general conditions of the patients.
Looking into the Future
Amniotic Membrane as Bioscaffolds
The interest in human AM for TE is on the rise due to its non-xenogeneic origin, inexpensive, highly abundant source and their regenerative properties. TE is defined as the development of biological substitutes for the purpose of restoring, maintaining, or improving tissue function. The three major pillars of TE are scaffolds, cells, and growth factors. An important component of TE is the supporting matrix upon which cells and tissues grow, also known as the scaffold [127•]. The special structure and biological nature of the AM allows it to be an ideal candidate for the fabrication of TE scaffolds.
One of the oldest biomaterials used for scaffolds is the AM as it is easily obtained, processed, and transported. The extracellular matrix (ECM) components of the basement membrane of the AM create an almost native scaffold for cell seeding and the AM itself has important biological properties including anti-inflammatory, anti-microbial, anti-fibrotic, low immunogenicity and provide mechanical stability. Scaffolds are developed to support cell seeding in TE, promoting their differentiation and proliferation in the formation of implantable tissue. Design and selection of the biomaterials used for scaffolding is a critical step because successful cell seeding of the scaffold depends on the type and source of the living cells as well as the ECM components of the scaffold.
A major prerequisite for choosing a TE scaffold is its biocompatibility. Furthermore, its mechanical properties should include permeability, stability, elasticity, flexibility, plasticity, and resorbability at a rate congruent with tissue replacement. Scaffolds should also allow cell adhesion and the potential for delivery of biological agents. Immunocompatibility is another important feature of AM as a TE scaffold as it can bypass the immunological complications of xenogenic biomaterials. The presence or absence of certain ECM molecules within any basement membrane also influences adhesion and growth of the overlying cells as they detect and respond to the ECM including the composition, adhesive ligands, matrix stiffness, and spatial and topological organization of integrins [127•]. The AM is a scaffold that can be used either with the epithelial layer intact or denuded or decellularized. The spongy layer on the stromal portion of the amnion has an abundance of hydrated proteoglycans and glycoproteins. The AM has a mechanical response that is inherently dependent on the stage of pregnancy, described as “viscoelasticity.” This is a critical scaffold property in a majority of tissues. One measure of elasticity is Young’s modulus (the ratio of applied stress to strain) which is 3.6 MPa in preterm human AM (26–36 weeks) compared to 2.29 MPa at full term (36–40 weeks) [128]. Therefore, preterm AM is stiffer than term AM and these properties are of interest for matching mechanical integrity in TE application where one such example is that stiff scaffolds will lack the viscoelasticity of arteries.
Despite all these advantages, there are some potential challenges that need to be addressed when applying any biologically derived material to TE uses. For example, transmission of infectious diseases is always a risk when using human tissues; therefore, precautions and safety criteria must be adhered to. Another issue with TE scaffolds is the possibility of invoking an inflammatory reaction upon implantation (i.e., foreign body reaction or immune rejection). AM has been reported to downregulate TGF-β and its receptor expression by fibroblasts and in doing so, reduces the risk of fibrosis and inflammation [127•]. The physical difficulty in handling and placement of thin AM sheets has limited their use in routine clinical care. In TE applications where AM function as a cell delivery matrix, strategies to improve AM bio-stability are often utilized as it could take longer for transplanted cells to home to the target site [47]. There is a plethora of investigations that focus on enhancing AM usage as a TE scaffold by employing surface modification targeted for various TE applications. When AM is expected to support the in vivo viability of the transplanted cells; therefore, treating AM with crosslinking agents may improve the bio-stability and mechanical strength of the scaffold. One such example is the study by Gobinathan et al. which showed that genipin-crosslinked AM has better bio-stability and the slowest degradation rate compared to decellularized and native AM [129].
Urethral Reconstruction
Xenografts of the urethra made with denuded human AM have been used to minimize potential rejection and maximize biocompatibility. Denuded human AM seeded with rabbit urethral epithelial cells were subcutaneously implanted in a rabbit model of urethral injury with resolution of urethral defects in 1 month. The cell-seeded denuded AM grafts were intact without obvious infiltration of inflammatory cells compared to intact AM patches [130].
Ocular Regeneration
The application of AM in ocular disorders is often limited by its relatively rapid degradation and resorption in vivo. To overcome this, crosslinking of AM has been used to reinforce the biomaterial structures. In particular, the fabrication of photo-crosslinked AM using UV irradiation has been developed as a scaffold for limbal stem cell culture. These physically crosslinked AM matrices exhibited negligible cytotoxicity to the corneal epithelial cells irrespective of the irradiation time and maintained the undifferentiated cell phenotype [131].
Wound Healing
Solubilized AM combined with a hyaluronic acid (HA-SAM) hydrogel was developed to provide a wound treatment that is easy to produce, store, and apply to wounds. Using murine and porcine models of full-thickness wound healing, HA-SAM significantly accelerated wound closure through re-epithelialization and prevented wound contraction [132, 133] and conformed to a non-uniform wound shape. A major benefit of using a hydrogel is its potential to match the rate of growth factor release to specific wound types, e.g., fast release hydrogel for acute burns and slow release hydrogel for diabetic wounds. Moreover, an aseptically processed human amnion and chorion allograft (AmnioBand) has been shown to be superior for wound healing in patients with diabetic foot ulcers compared to FDA-approved engineered skin substitutes (Apligraf). AmnioBand brings even greater value for the patients in terms of the healing efficacy endpoints, graft cost, and graft wastage [27].
Skin Regeneration
The poor mechanical and handling characteristics of AM have led to the development of a 3D skin substitute by reinforcing an AM scaffold with biodegradable polymer [134]. Silver nanoparticles incorporated with poly-[Lactide-co-Glycolide-co-Caprolactone] terpolymer (PLGC) and fibrin coating are used to reinforce the AM and to deliver bioactive molecules to the wound site. This combination scaffold has excellent biocompatibility and the addition of PLGC-silver nanoparticles is expected to provide excellent mechanical properties and potential benefit in treating infectious wounds due to their anti-microbial activity. A fibrin sealant can also act as a hemostat to minimize bleeding upon applying to the wound site; hence, a combined scaffold has potential use for dermal regeneration with better clinical handling.
Cartilage Regeneration
The collagen-rich ECM of AM has been investigated as a potential scaffold for cartilage regeneration. Fabrication of hybrid denuded AM-chitosan hydrogels has been proposed for articular cartilage TE as a rich source of collagen and the study has demonstrated that these hydrogels had a higher elastic modulus than chitosan or collagen hydrogels [135].
hAEC-Derived EVs
In the last decade, significant strides have been made in the regenerative medicine sector with a number of cellular therapies attaining market approval. While there are reports of stem cell-based therapies showing benefit in preclinical disease models, there is increasing evidence that the cells themselves are not critical to the functional outcome. Instead, stem cells serve as bio-factories releasing bioactive products including extracellular vesicles (EVs) (Fig. 3) and growth factors. EVs are naturally occurring nanoparticles (70–120nm) shed by virtually all cell types. Cells selectively package bioactive materials (e.g., proteins, miRNAs) into their exosomal cargo, which then serve as signaling packets to allow intercellular communication [136, 137]. Indeed, the so-called paracrine effects of stem cells are increasingly attributed to EV release and targeting [138, 139].
EVs have several advantages over cell therapies. Unlike their cells of origin, EVs are non-replicative, non-living bio-stable nanoparticles that do not require complex storage, transport, and handling [140]. The manufacturing, formulation, and clinical delivery of EV therapeutics are significantly simpler compared to cell therapies, thereby representing a more cost-effective form of regenerative medicine. EVs are enclosed by lipid bilayers and this protects and stabilizes their bioactive cargo compared to the direct delivery of growth factors, nucleic acids, or cells alone. EVs are easy to isolate and remain stable over long periods of time without the need for liquid nitrogen storage. There is therefore a potential to deliver regenerative medicine with a simplified cold chain and significantly lower cost of goods.
The topology of EVs is similar to that of cells, with lipid bilayer membranes decorated with extracellular receptors and ligands as well as cytoplasmic proteins and RNAs contained within. Membrane proteins such as tetraspanins and integrins are central to the identity and function of EVs, and these proteins could also be used to identify the cell type and influence EV uptake by recipient cells.
EVs from hAECs contain a myriad of growth and signaling factors that have immunomodulatory properties and can regulate cell differentiation. Recently, we have shown that hAECs release EVs that have similar regenerative properties to the cells themselves in the setting of experimental stroke and lung and liver fibrosis models [60•, 61, 141, 142•, 143]. We reported that hAEC-EVs exhibit anti-fibrotic properties by decreasing the number of activated hepatic stellate cells resulting in reduced collagen deposition. In addition, we have shown through potency assays that hAEC-EVs exert their anti-inflammatory effect by reducing neutrophil myeloperoxidase activity, suppressing CD3/CD28 activated T cell proliferation, increasing macrophage phagocytic activity, and shifting their polarization state [141, 142•]. These EVs are enriched with various microRNAs such as miR-27a, miR-23a, miR-203a, miR-34a, miR-150, and miR-194 which exert anti-fibrotic properties. The combination of the anti-fibrotic drug serelaxin and hAEC-EV in a model of experimental lung fibrosis demonstrated broader protection compared to pirfenidone, the standard of care. The therapeutic efficacy of hAEC-EV in treating basement membrane-induced fibrosis and related airway dysfunction can be enhanced by the co-administration of serelaxin [85].
Zhao et al. demonstrated that local injection of hAEC-EV could reduce collagen deposition in the rat full-thickness skin wound model [144•]. Another study also reported that hAEC-EV promoted proliferation and migration of fibroblasts and therefore could play an effective role in promoting scarless wound healing [145]. In a study by Zhang et al., the therapeutic potential of hAEC-EV in restoring ovarian functions following chemotherapy was investigated. They demonstrated an increased number of follicles and improved ovarian function in a murine premature ovarian failure model upon hAEC-EV transplantation [146].
Overall, EVs from hAECs contain cargo consistent with their biological properties to potentiate tissue regeneration, participate in immune modulation, and function as potential alternatives to stem cell therapy. As such, their untapped potential as cell-free therapeutics and the further possibility to bioengineer EVs to mediate specific biological functions, facilitate EV uptake, and EV targeting warrant future research exploration.
Conclusions
While hAECs have proven promising as a cell-based therapy, they remain a challenging treatment to integrate into the healthcare system due to their production cost and the challenges of delivering to smaller hospitals and remote communities. In order to overcome these geographical and socioeconomic barriers, the focus has shifted to investigating cell-free regenerative medicines that utilize the amniotic cells’ innate capacity to secrete EVs. Clinical application of EVs should be considered in the near future as numerous animal studies have shown the therapeutic potential of EVs as a cell-free form of regenerative medicine.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Young SM, Benyshek DC. In search of human placentophagy: a cross-cultural survey of human placenta consumption, disposal practices, and cultural beliefs. Ecol Food Nutr. 2010;49(6):467–84.
Davis J. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15.
Stern M. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituting skin grafts. J Am Med Assoc. 1913;60(13):973–4.
Sabella N. Use of fetal membranes in skin grafting. Med Rec. 1913;83:478–80.
Silini AR, et al. The long path of human placenta, and its derivatives, in regenerative medicine. Front Bioeng Biotechnol. 2015;3:162.
De Rotth A. Plastic repair of conjunctival defects with fetal membranes. Arch Ophthalmol. 1940;23:522–5.
Sorsby A, Symons HM. Amniotic membrane grafts in caustic burns of the eye (burns of the second degree). Br J Ophthalmol. 1946;30:337–45.
Sorsby A, Haythorne J, Reed H. Further experience with amniotic membrane grafts in caustic burns of the eye. Br J Ophthalmol. 1947;31(7):409–18.
Trelford-Sauder M, Trelford JD, Matolo NM. Replacement of the peritoneum with amnion following pelvic exenteration. Surg Gynecol Obstet. 1977;145(5):699–701.
Trelford-Sauder M, Dawe EJ, Trelford JD. Use of allograft amniotic membrane for control of intra-abdominal adhesions. J Med. 1978;9(4):273–84.
Silverton JS, Trelford JD, Roussere JT, Wolfe BM, Conti S. The use of amniotic membrane in acute massive full-thickness loss of the abdominal wall from clostridial myonecrosis. Ann Plast Surg. 1979;3(6):558–66.
Dhall K. Amnion graft for treatment of congenital absence of the vagina. Br J Obstet Gynaecol. 1984;91(3):279–82.
Nisolle M, Donnez J. Vaginoplasty using amniotic membranes in cases of vaginal agenesis or after vaginectomy. J Gynecol Surg. 1992;8(1):25–30.
Georgy M, Aziz N. Vaginoplasty using amnion graft: new surgical technique using the laparoscopic transillumination light. J Obstet Gynaecol. 1996;16:262–4.
Gharib M, Ure BM, Klose M. Use of amniotic grafts in the repair of gastroschisis. Pediatr Surg Int. 1996;11(2-3):96–9.
Kubanyi A. Prevention of peritoneal adhesions by transplantation of amnion. Br Med J. 1947;2(4514):55–6.
Muralidharan S, Gu J, Laub GW, Cichon R, Daloisio C, McGrath LB. A new biological membrane for pericardial closure. J Biomed Mater Res. 1991;25(10):1201–9.
Chao YC, Humphreys S, Penfield W. A new method of preventing adhesions. The use of amnioplastin after craniotomy. Br Med J. 1940;1(4134):517–538.1.
Troensegaard-Hansen E. Amniotic grafts in chronic skin ulceration. Lancet. 1950;1(6610):859–60.
Bennett JP, Matthews R, Faulk WP. Treatment of chronic ulceration of the legs with human amnion. Lancet. 1980;1(8179):1153–6.
Subrahmanyam M. Amniotic membrane as a cover for microskin grafts. Br J Plast Surg. 1995;48(7):477–8.
Gruss JS, Jirsch DW. Human amniotic membrane: a versatile wound dressing. Can Med Assoc J. 1978;118(10):1237–46.
Bose B. Burn wound dressing with human amniotic membrane. Ann R Coll Surg Engl. 1979;61(6):444–7.
Dua HS, Gomes JAP, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51–77.
Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24(10):299–307.
Morandi F, et al. Human amnion epithelial cells impair T cell proliferation: the role of HLA-G and HLA-E molecules. Cells. 2020;9(9):2123.
Glat P, Orgill DP, Galiano R, Armstrong D, Serena T, DiDomenico LA, et al. Placental membrane provides improved healing efficacy and lower cost versus a tissue-engineered human skin in the treatment of diabetic foot ulcerations. Plast Reconstr Surg Glob Open. 2019;7(8):e2371.
Snyder RJ, et al. A prospective, randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of care for the closure of chronic diabetic foot ulcer. Wounds. 2016;28(3):70–7.
Doucette M, et al. Early advanced therapy for diabetic foot ulcers in high amputation risk veterans: a cohort study. Int J Low Extrem Wounds. 2020;22:1534734620928151.
Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122–8.
Serena TE, Carter MJ, le LT, Sabo MJ, DiMarco DT, EpiFix VLU Study Group. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22(6):688–93.
Tettelbach W, Cazzell S, Reyzelman AM, Sigal F, Caporusso JM, Agnew PS. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: a prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2019;16(1):19–29.
Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502–7.
Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347–8 350-1.
Bianchi C, Cazzell S, Vayser D, Reyzelman AM, Dosluoglu H, Tovmassian G, et al. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix((R))) allograft for the treatment of venous leg ulcers. Int Wound J. 2018;15(1):114–22.
Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12(6):724–32.
Lavery L, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, et al. Open-label extension phase of a chronic diabetic foot ulcer multicenter, controlled, randomized clinical trial using cryopreserved placental membrane. Wounds. 2018;30(9):283–9.
Ananian CE, Dhillon YS, van Gils CC, Lindsey DC, Otto RJ, Dove CR, et al. A multicenter, randomized, single-blind trial comparing the efficacy of viable cryopreserved placental membrane to human fibroblast-derived dermal substitute for the treatment of chronic diabetic foot ulcers. Wound Repair Regen. 2018;26(3):274–83.
Mligiliche N, Endo K, Okamoto K, Fujimoto E, Ide C. Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration. J Biomed Mater Res. 2002;63(5):591–600.
Mohammad J, Shenaq J, Rabinovsky E, Shenaq S. Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Surg. 2000;105(2):660–6.
Miyamoto K, Hayashi K, Suzuki T, Ichihara S, Yamada T, Kano Y, et al. Human placenta feeder layers support undifferentiated growth of primate embryonic stem cells. Stem Cells. 2004;22(4):433–40.
Ueno M, Matsumura M, Watanabe K, Nakamura T, Osakada F, Takahashi M, et al. Neural conversion of ES cells by an inductive activity on human amniotic membrane matrix. Proc Natl Acad Sci U S A. 2006;103(25):9554–9.
Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng. 2007;13(4):693–702.
Portmann-Lanz CB, Ochsenbein-Kölble N, Marquardt K, Lüthi U, Zisch A, Zimmermann R. Manufacture of a cell-free amnion matrix scaffold that supports amnion cell outgrowth in vitro. Placenta. 2007;28(1):6–13.
Yang L, Shirakata Y, Shudou M, Dai X, Tokumaru S, Hirakawa S, et al. New skin-equivalent model from de-epithelialized amnion membrane. Cell Tissue Res. 2006;326(1):69–77.
Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci. 2004;45(3):800–6.
Farhadihosseinabadi B, et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif Cells Nanomed Biotechnol. 2018;46(sup2):431–40.
Tsai SH, Liu YW, Tang WC, Zhou ZW, Hwang CY, Hwang GY, et al. Characterization of porcine arterial endothelial cells cultured on amniotic membrane, a potential matrix for vascular tissue engineering. Biochem Biophys Res Commun. 2007;357(4):984–90.
Xu H, et al. Therapeutic potential of human amniotic epithelial cells on injuries and disorders in the central nervous system. Stem Cells Int. 2019;2019:5432301.
Magatti M, Vertua E, Cargnoni A, Silini A, Parolini O. The immunomodulatory properties of amniotic cells: the two sides of the coin. Cell Transplant. 2018;27(1):31–44.
Krause M, Lozano J, Lim R. The regenerative and reparative potential of amniotic membrane stem cells: biology, manufacturing and translational medicine. In: Perital Stem Cells; 2019. p. 9–26.
Fatimah SS, Tan GC, Chua KH, Tan AE, Hayati AR. Effects of epidermal growth factor on the proliferation and cell cycle regulation of cultured human amnion epithelial cells. J Biosci Bioeng. 2012;114(2):220–7.
Insausti CL, Blanquer M, García-Hernández AM, Castellanos G, Moraleda JM. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014;7:53–63.
McDonald CA, Payne NL, Sun G, Moussa L, Siatskas C, Lim R, et al. Immunosuppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J Neuroinflammation. 2015;12(1):112.
Lim R. Concise review: fetal membranes in regenerative medicine: new tricks from an old dog? Stem Cells Transl Med. 2017;6(9):1767–76.
Tan JL, Chan ST, Wallace EM, Lim R. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant. 2014;23(3):319–28.
Vosdoganes P, et al. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep.(Report). Am J Obstet Gynecol. 2011;205(2):156.e26–33.
• Lim R, et al. First-in-human administration of allogeneic amnion cells in premature infants with bronchopulmonary dysplasia: a safety study. Stem Cells Transl Med. 2018;7(9):628–35 Demonstrates the safety of hAECS with no adverse effects in this first-in-human clinical trial of hAECs in babies with BPD.
Zhu D, Tan J, Maleken AS, Muljadi R, Chan ST, Lau SN, et al. Human amnion cells reverse acute and chronic pulmonary damage in experimental neonatal lung injury. Stem Cell Res Ther. 2017;8(1):257.
• Hodge A, et al. Soluble factors derived from human amniotic epithelial cells suppress collagen production in human hepatic stellate cells. Cytotherapy. 2014;16(8):1132–44 Provides evidence that factors secreted by hAECs have anti-fibrotic effects in liver fibrosis in vivo, significantly decreasing the expression of pro-fibrotic cytokine TGF-β1 and reducing HSC collagen production.
Kuk N, Hodge A, Sun Y, Correia J, Alhomrani M, Samuel C, et al. Human amnion epithelial cells and their soluble factors reduce liver fibrosis in murine non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2019;34(8):1441–9.
Manuelpillai U, Lourensz D, Vaghjiani V, Tchongue J, Lacey D, Tee JY, et al. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS One. 2012;7(6):e38631.
Kakishita K, Nakao N, Sakuragawa N, Itakura T. Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res. 2003;980:48–56.
Roh D-H, Seo MS, Choi HS, Park SB, Han HJ, Beitz AJ, et al. Transplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in rats. Cell Transplant. 2013;22(9):1577–90.
Dong W, Chen H, Yang X, Guo L, Hui G. Treatment of intracerebral haemorrhage in rats with intraventricular transplantation of human amniotic epithelial cells. Cell Biol Int. 2010;34(6):573–7.
Roy R, et al. Epithelial-to-mesenchymal transition enhances the cardioprotective capacity of human amniotic epithelial cells. Cell Transplant. 2013;24(6):985–1002.
Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164(12):6100–4.
Marchal-Bras-Goncalves R, Rouas-Freiss N, Connan F, Choppin J, Dausset J, Carosella ED, et al. A soluble HLA-G protein that inhibits natural killer cell-mediated cytotoxicity. Transplant Proc. 2001;33(3):2355–9.
Kapasi K, Albert SE, Yie SM, Zavazava N, Librach CL. HLA-G has a concentration-dependent effect on the generation of an allo-CTL response. Immunology. 2000;101(2):191–200.
Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED. HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol. 2001;166(8):5018–26.
Riteau B, Menier C, Khalil-Daher I, Sedlik C, Dausset J, Rouas-Freiss N, et al. HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol. 1999;43(2):203–11.
Bainbridge DR, Ellis SA, Sargent IL. HLA-G suppresses proliferation of CD4(+) T-lymphocytes. J Reprod Immunol. 2000;48(1):17–26.
Kanai T, Fujii T, Kozuma S, Yamashita T, Miki A, Kikuchi A, et al. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod. 2001;7(2):195–200.
Kanai T, et al. Human leukocyte antigen-G-expressing cells differently modulate the release of cytokines from mononuclear cells present in the decidua versus peripheral blood. Am J Reprod Immunol. 2001;45(2):94–9.
Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900–7.
Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23(10):1549–59.
Parolini O, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–11.
Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133–42.
Atala A. Perinatal stem cells: research and therapy. Cambridge: Academic Press; 2018.
Moodley Y, Ilancheran S, Samuel C, Vaghjiani V, Atienza D, Williams ED, et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Respir Crit Care Med. 2010;182(5):643–51.
Murphy S, Lim R, Dickinson H, Acharya R, Rosli S, Jenkin G, et al. Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplant. 2011;20(6):909–24.
Murphy SV, Shiyun SC, Tan JL, Chan S, Jenkin G, Wallace EM, et al. Human amnion epithelial cells do not abrogate pulmonary fibrosis in mice with impaired macrophage function. Cell Transplant. 2012;21(7):1477–92.
Vosdoganes P, Wallace EM, Chan ST, Acharya R, Moss TJM, Lim R. Human amnion epithelial cells repair established lung injury. Cell Transplant. 2013;22(8):1337–49.
Tan JL, et al. Amnion cell-mediated immune modulation following bleomycin challenge: controlling the regulatory T cell response. Stem Cell Res Ther. 2015;6(1):1–12.
Royce SG, Patel KP, Mao W, Zhu D, Lim R, Samuel CS. Serelaxin enhances the therapeutic effects of human amnion epithelial cell-derived exosomes in experimental models of lung disease. Br J Pharmacol. 2019;176(13):2195–208.
Geng L, Chen Z, Ren H, Niu X, Yu X, Yan H. Effects of an early intervention using human amniotic epithelial cells in a COPD rat model. Pathol-Res Prac. 2016;212(11):1027–33.
Vosdoganes P, et al. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. Am J Obstet Gynecol. 2011;205(2):156.e26–33.
Hodges RJ, et al. Human amnion epithelial cells reduce ventilation-induced preterm lung injury in fetal sheep. Am J Obstet Gynecol. 2012;206(5):448.e8–448.e15.
Vosdoganes P, Lim R, Koulaeva E, Chan ST, Acharya R, Moss TJM, et al. Human amnion epithelial cells modulate hyperoxia-induced neonatal lung injury in mice. Cytotherapy. 2013;15(8):1021–9.
Zhu D, Tan J, Maleken AS, Muljadi R, Chan ST, Lau SN, et al. Human amnion cells reverse acute and chronic pulmonary damage in experimental neonatal lung injury. Stem Cell Res Ther. 2017;8(1):257.
Fang C-H, Jin J, Joe JH, Song YS, So BI, Lim SM, et al. In vivo differentiation of human amniotic epithelial cells into cardiomyocyte-like cells and cell transplantation effect on myocardial infarction in rats: comparison with cord blood and adipose tissue-derived mesenchymal stem cells. Cell Transplant. 2012;21(8):1687–96.
Song Y-S, Joo HW, Park IH, Shen GY, Lee Y, Shin JH, et al. Transplanted human amniotic epithelial cells secrete paracrine proangiogenic cytokines in rat model of myocardial infarctio. Cell Transplant. 2015;24(10):2055–64.
Yawno T, Schuilwerve J, Moss TJM, Vosdoganes P, Westover AJ, Afandi E, et al. Human amnion epithelial cells reduce fetal brain injury in response to intrauterine inflammation. Dev Neurosci. 2013;35(2-3):272–82.
Yawno T, Sabaretnam T, Li J, Mcdonald C, Lim R, Jenkin G, et al. Human amnion epithelial cells protect against white matter brain injury after repeated endotoxin exposure in the preterm ovine fetus. Cell Transplant. 2017;26(4):541–53.
Barton SK, Melville JM, Tolcos M, Polglase GR, McDougall ARA, Azhan A, et al. Human amnion epithelial cells modulate ventilation-induced white matter pathology in preterm lambs. Dev Neurosci. 2015;37(4-5):338–48.
Leaw B, et al. Human amnion epithelial cells rescue cell death via immunomodulation of microglia in a mouse model of perinatal brain injury. Stem Cell Res Ther. 2017;8(1):1–17.
van den Heuij LG, Fraser M, Miller SL, Jenkin G, Wallace EM, Davidson JO, et al. Delayed intranasal infusion of human amnion epithelial cells improves white matter maturation after asphyxia in preterm fetal sheep. J Cereb Blood Flow Metab. 2019;39(2):223–39.
Evans MA, Lim R, Kim HA, Chu HX, Gardiner-Mann CV, Taylor KWE, et al. Acute or delayed systemic administration of human amnion epithelial cells improves outcomes in experimental stroke. Stroke. 2018;49(3):700–9.
Liu T, Wu J, Huang Q, Hou Y, Jiang Z, Zang S, et al. Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock. 2008;29(5):603–11.
Zhou H, et al. HAEC in the treatment of brain hemorrhage: a preliminary observation in rabbits. Int J Clin Exp Pathol. 2015;8(6):6772.
Liang H, Guan D, Gao A, Yin Y, Jing M, Yang L, et al. Human amniotic epithelial stem cells inhibit microglia activation through downregulation of tumor necrosis factor-α, interleukin-1β and matrix metalloproteinase-12 in vitro and in a rat model of intracerebral hemorrhage. Cytotherapy. 2014;16(4):523–34.
Kim HA, et al. Systemic treatment with human amnion epithelial cells after experimental traumatic brain injury. Brain Behav Immun Health. 2020;5:100072.
Wu Z-Y, et al. Transplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injury. Chin Med J. 2006;119(24):2101–7.
Meng XT, et al. Co-transplantation of bFGF-expressing amniotic epithelial cells and neural stem cells promotes functional recovery in spinal cord-injured rats. Cell Biol Int. 2008;32(12):1546–58.
Xue H, et al. Development of a chemically extracted acellular muscle scaffold seeded with amniotic epithelial cells to promote spinal cord repair. J Biomed Mater Res A. 2013;101(1):145–56.
Wang T-G, et al. Human amniotic epithelial cells combined with silk fibroin scaffold in the repair of spinal cord injury. Neural Regen Res. 2016;11(10):1670.
Sankar V, Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience. 2003;118(1):11–7.
Kim KY, Suh Y-H, Chang K-A. Therapeutic effects of human amniotic epithelial stem cells in a transgenic mouse model of Alzheimer’s disease. Int J Mol Sci. 2020;21(7):2658.
Yang X, Song L, Wu N, Liu Z, Xue S, Hui G. An experimental study on intracerebroventricular transplantation of human amniotic epithelial cells in a rat model of Parkinson’s disease. Neurol Res. 2010;32(10):1054–9.
Kakishita K, Nakao N, Sakuragawa N, Itakura T. Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res. 2003;980(1):48–56.
Kuk N, Hodge A, Sun Y, Correia J, Alhomrani M, Samuel C, et al. Human amnion epithelial cells and their soluble factors reduce liver fibrosis in murine non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2019;34(8):1441–9.
Manuelpillai U, Tchongue J, Lourensz D, Vaghjiani V, Samuel CS, Liu A, et al. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4-treated mice. Cell Transplant. 2010;19(9):1157–68.
Hong S-B, Seo MS, Park SB, Seo YJ, Kim JS, Kang KS. Therapeutic effects of human amniotic epithelial stem cells in Niemann–Pick type C1 mice. Cytotherapy. 2012;14(5):630–8.
Skvorak KJ, Dorko K, Marongiu F, Tahan V, Hansel MC, Gramignoli R, et al. Placental stem cell correction of murine intermediate maple syrup urine disease. Hepatology. 2013;57(3):1017–23.
Skvorak KJ, Dorko K, Marongiu F, Tahan V, Hansel MC, Gramignoli R, et al. Improved amino acid, bioenergetic metabolite and neurotransmitter profiles following human amnion epithelial cell transplant in intermediate maple syrup urine disease mice. Mol Genet Metab. 2013;109(2):132–8.
Liu YH, Vaghjiani V, Tee JY, To K, Cui P, Oh DY, et al. Amniotic epithelial cells from the human placenta potently suppress a mouse model of multiple sclerosis. PLoS One. 2012;7(4):e35758.
Tan B, Yuan W, Li J, Yang P, Ge Z, Liu J, et al. Therapeutic effect of human amniotic epithelial cells in murine models of Hashimoto’s thyroiditis and systemic lupus erythematosus. Cytotherapy. 2018;20(10):1247–58.
Zhang Q, Huang Y, Sun J, Gu T, Shao X, Lai D. Immunomodulatory effect of human amniotic epithelial cells on restoration of ovarian function in mice with autoimmune ovarian disease. Acta Biochim Biophys Sin. 2019;51(8):845–55.
Lim R, Malhotra A, Tan J, Chan ST, Lau S, Zhu D, et al. First-in-human administration of allogeneic amnion cells in premature infants with bronchopulmonary dysplasia: a safety study. Stem Cells Transl Med. 2018;7(9):628–35.
Baker EK, Malhotra A, Lim R, Jacobs SE, Hooper SB, Davis PG, et al. Human amnion cells for the prevention of bronchopulmonary dysplasia: a protocol for a phase I dose escalation study. BMJ Open. 2019;9(2):e026265.
Lim R, Hodge A, Moore G, Wallace EM, Sievert W. A pilot study evaluating the safety of intravenously administered human amnion epithelial cells for the treatment of hepatic fibrosis. Front Pharmacol. 2017;8:549.
Phan TG, Ma H, Lim R, Sobey CG, Wallace EM. Phase 1 trial of amnion cell therapy for ischemic stroke. Front Neurol. 2018;9:198.
Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003–5.
Adinolfi M, Akle CA, McColl I, Fensom AH, Tansley L, Connolly P, et al. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982;295(5847):325–7.
Scaggiante B, et al. Successful therapy of Niemann-Pick disease by implantation of human amniotic membrane. Transplantation. 1987;44(1):59–61.
Bembi B, Comelli M, Scaggiante B, Pineschi A, Rapelli S, Gornati R, et al. Treatment of sphingomyelinase deficiency by repeated implantations of amniotic epithelial cells. Am J Med Genet. 1992;44(4):527–33.
• Niknejad H, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99 Discusses the biological properties of the amniotic membrane that makes it an ideal candidate for creating scaffolds that can be used in tissue engineering.
Benson-Martin J, Zammaretti P, Bilic G, Schweizer T, Portmann-Lanz B, Burkhardt T, et al. The Young’s modulus of fetal preterm and term amniotic membranes. Eur J Obstet Gynecol Reprod Biol. 2006;128(1-2):103–7.
Gobinathan S, Zainol SS, Azizi SF, Iman NM, Muniandy R, Hasmad HN, et al. Decellularization and genipin crosslinking of amniotic membrane suitable for tissue engineering applications. J Biomater Sci Polym Ed. 2018;29(17):2051–67.
Wang F, Liu T, Yang L, Zhang G, Liu H, Yi X, et al. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med Sci Monit. 2014;20:2430–8.
Lai JY. Photo-cross-linking of amniotic membranes for limbal epithelial cell cultivation. Mater Sci Eng C Mater Biol Appl. 2014;45:313–9.
Murphy SV, Skardal A, Nelson RA Jr, Sunnon K, Reid T, Clouse C, et al. Amnion membrane hydrogel and amnion membrane powder accelerate wound healing in a full thickness porcine skin wound model. Stem Cells Transl Med. 2020;9(1):80–92.
Murphy SV, Skardal A, Song L, Sutton K, Haug R, Mack DL, et al. Solubilized amnion membrane hyaluronic acid hydrogel accelerates full-thickness wound healing. Stem Cells Transl Med. 2017;6(11):2020–32.
Ramakrishnan R, Krishnan LK, Nair RP, Krishnan KV. Reinforcement of amniotic membrane with fibrin coated poly-[Lactide-co-Glycolide-co-Caprolactone] terpolymer containing silver nanoparticles for potential wound healing applications. Int J Polym Mater Polym Biomater. 2020;69(12):810–9.
Toniato TV, Stocco TD, dos Martins DS, Santanna LB, Tim CR, Marciano FR, et al. Hybrid chitosan/amniotic membrane-based hydrogels for articular cartilage tissue engineering application. Int J Polym Mater Polym Biomater. 2019;69(15):961–70.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–88.
van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cells paracrine signalling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26(9):617–31.
Riazifar M, Pone EJ, Lötvall J, Zhao W. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125–54.
Kusuma GD, Barabadi M, Tan JL, Morton DAV, Frith JE, Lim R. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front Pharmacol. 2018;9:1199.
Tan JL, Lau SN, Leaw B, Nguyen HPT, Salamonsen LA, Saad MI, et al. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7(2):180–96.
• Alhomrani M, et al. The human amnion epithelial cell secretome decreases hepatic fibrosis in mice with chronic liver fibrosis. Front Pharmacol. 2017;8:748 Provides novel evidence that extracellular vesicles secreted by hAECs significantly reduces liver fibrosis and macrophage infiltration, further highlighting the therapeutic potential of hAEC-derived EVs.
Broughton B, Tran TN, Lim R, Wallace E, Kemp-Harper B. A8321 Delayed post-stroke administration of human amnion epithelial cell-derived exosomes improve outcomes. J Hypertens. 2018;36:e51.
• Zhao B, et al. Exosomal microRNAs derived from human amniotic epithelial cells accelerate wound healing by promoting the proliferation and migration of fibroblasts. Stem Cells Int. 2018;2018:5420463 Demonstrates the role exosomal microRNA from hAECs play in promoting the wound healing response by reducing collagen deposition in vivo.
Zhao B, Zhang Y, Han S, Zhang W, Zhou Q, Guan H, et al. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J Mol Histol. 2017;48(2):121–32.
Zhang Q, Sun J, Huang Y, Bu S, Guo Y, Gu T, et al. Human amniotic epithelial cell-derived exosomes restore ovarian function by transferring microRNAs against apoptosis. Mol Ther Nucleic Acids. 2019;16:407–18.
Acknowledgements
Figures 1 and 2 were created with BioRender.com.
RHMS is supported by NHMRC project grant GNT1144265. MG is supported by an MRFF Stem Cell Mission grant. DZ is supported by NHMRC project grant GNT1141946. GDM is supported by the Rebecca L. Cooper Medical Research Foundation. RL is supported by an NHMRC career development fellowship. This work is supported by the Victorian Government’s Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Prenatal Therapies
Renate H. M. Schwab and Mihiri Goonetilleke are joint first authors.
Rights and permissions
About this article
Cite this article
Schwab, R.H.M., Goonetilleke, M., Zhu, D. et al. Amnion Epithelial Cells — a Therapeutic Source. Curr Stem Cell Rep 7, 13–29 (2021). https://doi.org/10.1007/s40778-021-00187-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-021-00187-5