Abstract
Purpose of review
In this manuscript, we review the modifications to the Fontan operation along with the incidence, etiology, and outcomes of atrial tachyarrhythmias in adults with single ventricle heart disease. Medical, interventional, device, and surgical management of these arrhythmias is discussed in detail.
Recent findings
Atrial tachyarrhythmias are the most common late complication following the Fontan operation present in > 50% of patients 20 years after palliation. Management of these arrhythmias is difficult, but advances in device and electrophysiology technology have made treatment more successful. For patients with atrio-pulmonary Fontan circulation, Fontan conversion surgery may be warranted.
Summary
Atrial tachyarrhythmias in adults with single ventricle heart disease are very common and associated with poor outcomes. The etiology of these dysrhythmias is multifactorial and change over time. Treatment of atrial tachyarrhythmias in this population is challenging, involving both medical and invasive therapies, and recurrence is common. These complex patients are best served by a multi-disciplinary team involving adult congenital cardiologists and congenital heart disease interventionalist and surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of the Fontan palliation in 1968 [1], survival of patients with single ventricle congenital heart disease has improved, with the current 20-year survival after Fontan completion around 75–85% [2,3,4,5,6]. With improved survival, there is an ever-growing number of these patients entering adulthood. In 2018, the estimated worldwide population of patients with Fontan circulation was between 50,000 and 70,000, with 40% of these patients > 18 years of age [7]. Despite advances in surgical and medical therapy, adults with single ventricle heart disease have significant multisystem morbidities secondary to their Fontan circulation, including exercise intolerance, heart failure, hepatic, gastrointestinal, renal, and pulmonary disease, thromboembolic events, and tachyarrhythmias. Of these, tachyarrhythmias are the most common long-term complication and the focus of this review [8•].
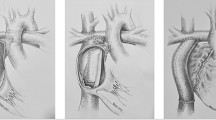
An understanding of the surgical modifications of the Fontan palliation is necessary when evaluating the risk of tachyarrhythmias in this group of patients. The original Fontan procedure involved an atrio-pulmonary (AP) connection from the right atrial appendage to the proximal right pulmonary artery (RPA) with the superior vena cava (SVC) anastomosed to the distal right pulmonary artery [1]. While there have been multiple modifications, the two major technical modifications since the original procedure are the lateral tunnel (LT) technique introduced by de Laval et al. in 1988 and the extracardiac (EC) technique introduced by Marcelletti et. al in 1989 [9, 10]. The LT technique involves creation of an intra-cardiac pathway utilizing most commonly a Gore-Tex baffle such that IVC blood flow is channeled to the pulmonary artery either through creation of an atrial-pulmonary artery anastomosis following bi-directional Glenn stage two palliation or through the creation of an atrial-pulmonary artery communication following hemi-Fontan stage two palliation. The EC Fontan operation involves the creation of a connection between the IVC and the pulmonary arteries utilizing a size-selected Gore-Tex graft and most commonly involves creation of a fenestration between the conduit and atrium. Proponents of bi-directional Glenn and EC Fontan palliation favor this approach as it can be performed with a beating heart while others favor hemi-Fontan and intra-cardiac lateral tunnel palliation given perceived improvements in augmentation of pulmonary artery diameter and orientation.
Atrial tachyarrhythmias
Atrial tachyarrhythmias are the most common late complication following the Fontan operation, with freedom from tachyarrhythmia between 23 and 50% at 20 years post-Fontan operation [2, 5, 6, 11,12,13]. The type of Fontan surgery and the duration of follow-up dictate the incidence of tachyarrhythmias in these patients. The incidence of atrial tachyarrhythmias is clearly highest in the AP Fontan group, with an incidence approaching 100% in some studies by 25 years following surgery [14]. While the occurrence of atrial tachyarrhythmias in the LT and EC Fontan populations is less, the incidence is still reported between 10 and 20% [2, 5, 13,14,15,16,17,18,19]. In addition, most of these studies evaluated a still largely pediatric or adolescent population with follow-up periods of 10 years or less. The results comparing risk of late atrial tachyarrhythmias between the LT and EC Fontan are conflicting and few in number. While there seems to be a higher risk of early post-operative tachyarrhythmias following LT Fontan, the prevalence of late atrial tachyarrhythmias between the 2 groups is similar [14,15,16, 20,21,22]. While it is likely that surgical modifications have contributed to decreased incidence of atrial tachyarrhythmias in these patients with particular attention to the preservation of the crista terminalis and strategic placement of atrial incisions and suture lines, there is the potential that the long-term incidence of this complication remains unknown within an increased aging population and that atrial tachyarrhythmias may significantly increase over time.
The most common atrial tachyarrhythmia in adult Fontan patients is intra-atrial reentrant tachycardia (IART), a type of macroreentrant tachycardia that accounts for up to 80% of atrial tachyarrhythmias in these patients [11, 13, 14, 23,24,25,26]. IART is also referred to as “incisional tachycardia” and is classified in this way to distinguish it from typical atrial flutter that occurs in structurally normal hearts. Adult Fontan patients also experience atrioventricular nodal reentry tachycardia, focal atrial tachycardia, and atrial fibrillation (AF), but to a lesser degree than IART. However, as this population ages, the incidence of AF increases [13, 27,28,29] and is present in almost half of patients who present for Fontan conversion surgery [27].
The etiology of atrial tachycardias in these patients is likely multi-factorial and includes injury to the sinus node or its arterial supply, effects of long-term cyanosis, atrial scar and suture lines, chronic atrial dilation, hypertrophy and elevated atrial pressure, and inherently abnormal atrial tissue related to the underlying congenital heart defect [8•, 13, 18, 25, 30]. Multiple risk factors for the development of atrial tachycardias have been identified. These include older age at Fontan repair, heterotaxy syndrome, ventricular dysfunction, more than mild atrioventricular valve regurgitation, previous history of tachyarrhythmia, and presence of atrial tachyarrhythmias in the early post-operative period [5, 14, 18, 19, 31, 32]. Above all, the most important risk factors for the development of atrial tachycardia are duration of Fontan circulation and Fontan type, with the AP Fontan being associated with significantly higher risk [2, 6, 13, 14, 18, 19, 22, 25, 31]. While there is data showing decreased incidence of atrial tachyarrhythmias in patients with LT and EC Fontan compared to AP Fontan circulation, the follow-up period for these patients is less [20, 22, 30, 31, 33, 34]. It is unclear whether the higher incidence of tachyarrhythmias seen in the AP Fontan cohort is most strongly influenced by surgical technique or rather the longer duration of Fontan circulation. At this time, it is unknown whether LT and EC Fontan patients will develop the same steep increase in atrial tachyarrhythmia burden seen in the AP Fontan population 20–25 years following palliation. Ultimately, longer follow-up time will help further elucidate tachyarrhythmia risk factors in these patients.
There is clear evidence that tachyarrhythmias in adult Fontan patients are associated with worse outcomes. Development of dysrhythmias is associated with increased rates of hospitalization, worse patient-reported quality of life, atrial thrombus formation and subsequent thromboembolic events, and protein losing enteropathy [4, 31, 35, 36]. Atrial tachycardias can lead to progressive compromise of ventricular function and accelerate the time to Fontan circuit failure [37,38,39]. Carins et al. showed the 10-year freedom from Fontan failure after onset of tachycardia to be on 50% [17]. Finally, multiple studies have shown atrial tachyarrhythmias in adult Fontan patients to be an independent predictor of death or cardiac transplantation [17, 40, 41]. Given the high incidence of atrial tachyarrhythmias in these patients and the strong association with increased morbidity and mortality, timely recognition and optimal management of these arrhythmias are crucial.
Management
The management options for atrial tachyarrhythmias in the adult Fontan population includes periodic cardioversions, anti-arrhythmic medications, catheter ablation, atrial pacing (both anti-bradycardic and anti-tachycardic), Fontan conversion with arrhythmia surgery, and heart transplantation [42•]. Determining the appropriate therapy is a complex and multi-factorial decision that depends on the patient’s hemodynamics and severity of symptoms in the tachyarrhythmia, along with their surgical history, baseline hemodynamics, comorbidities, and arrhythmia frequency and progression. Any Fontan patient who presents with new onset tachyarrhythmia mandates a thorough evaluation, including a detailed history, comprehensive exam, 12-lead electrocardiogram, transthoracic or transesophageal echocardiogram, and a low threshold for cross-sectional imaging and cardiac catheterization to obtain invasive hemodynamic data [43•, 44•]. The involvement of a multi-disciplinary team involving adult congenital heart disease (ACHD) specialists, cardiac intensivists, electrophysiologists, congenital interventionalists, and congenital heart disease surgeons is critical in managing these complex patients.
Medical management
Patients with hemodynamic instability secondary to IART or AF warrant urgent electrical cardioversion to terminate the tachycardia regardless of duration of tachycardia and anticoagulation status. Anterior–posterior pad positioning may be needed in the setting of significant atrial dilation [44•]. The team planning electrical cardioversion must be prepared for the presence of long sinus pauses or severe sinus bradycardia following rhythm restoration as many Fontan patients have sinus node dysfunction [25]. Patients with atrial or dual chamber pacemakers or defibrillators may undergo overdrive pacing to terminate the tachycardia, although one must ensure that ventricular pacing is maintained in pacemaker-dependent patients. For patients who present with preserved hemodynamics in an atrial tachyarrhythmia, acute management includes initiation of anticoagulation, pharmacologic rate control, and cardioversion. While a minimum of 3 weeks of anticoagulation is recommended prior to cardioversion for adults with 2 ventricles who have been in a tachyarrhythmia for ≥ 48 h or unknown duration [45, 46], patients with Fontan circulation are unlikely to tolerate persistent tachyarrhythmia and may develop congestive heart failure within a few days. Therefore, prompt cardioversion within 24–48 h after presentation of tachycardia is recommended for these patients [25, 27, 42•]. Transesophageal echocardiogram (TEE) is recommended to evaluate for atrial thrombus formation prior to cardioversion if tachycardia duration is ≥ 48 h or unknown [25, 27, 42•, 43•, 44•]. Given Fontan patients are predisposed to thrombus formation even in the absence of atrial tachyarrhythmias, some argue for TEE prior to cardioversion even if the IART/AF is < 48-h duration [47, 48].
There is limited data regarding the use of antiarrhythmic medications for both pharmacologic cardioversion and long-term use in adults with Fontan circulation, and no randomized controlled trials exist. Many of these studies have grouped patients with either various congenital heart disease lesions or different tachycardia mechanisms, making interpretation of the results challenging [25, 26, 49,50,51,52,53]. Regardless, antiarrhythmic medications are prescribed for these patients, and specific recommendations for their individual use do exist in the various ACHD guidelines [43•, 44•, 54].
There is no consensus regarding pharmacologic cardioversion for adults with single ventricle heart disease. One should proceed with caution given the pro-arrhythmia side effects of many antiarrhythmics, including ventricular tachycardia with class IA and IC drugs and torsades de pointes with class III drugs. Type 1C drugs are generally avoided given the potential to slow atrial arrythmia cycle length and allow for 1:1 atrioventricular (AV) conduction [55]. Pharmacologic cardioversion should be done in an ICU setting with continuous monitoring and preparation for potential resuscitation. Intravenous calcium channel blocking medication, like diltiazem, can help to slow the ventricular response or even break the tachycardia [42•]. Intravenous ibutilide, a class III drug, has been used successfully to cardiovert IART and AF in adults with CHD, including those with Fontan circulation [51, 56]. Side effects include QTc prolongation and ventricular arrhythmias, including torsades de pointes. Magnesium and potassium levels should be repleted prior to administration, and its use is contraindicated when the QTc is > 440 ms. Sotalol, a class III anti-arrhythmic with class II effects, has also been used for pharmacologic cardioversion for atrial tachycardias in patients with congenital heart disease, including Fontan patients [53]. Side effects include QTc prolongation, torsades de pointes, and bradycardia. It should be avoided if the QTc is > 450 ms. Amiodarone, another class III drug, can be given as an IV bolus followed by a continuous infusion to achieve sinus rhythm or slow ventricular response if cardioversion fails [25, 56]. Dosing recommendations for acute pharmacologic therapy for atrial tachyarrhythmias can be found in Table 1.
Overall, long-term success rates of chronic pharmacologic therapy for atrial tachycardias in the Fontan population have not been stellar, leading to most centers preferring non-pharmacologic therapies for arrhythmia control [26, 44•]. However, there are several instances when chronic pharmacologic therapy is pursued secondary to multiple factors, including previous unsuccessful ablation attempt, feasibility of a procedure, arrhythmia frequency, comorbidities, and patient preference. No guidelines for long-term pharmacologic management of arrhythmias in adults with Fontan circulation exist, but rather, recommendations are based on general ACHD guidelines, which are derived from small retrospective studies or expert opinion. Long-term use of class 1 antiarrhythmics is not recommended given their pro-arrhythmia side effects and association with mortality in both structurally normal hearts and CHD [44•, 57, 58]. Amiodarone is successful in achieving rhythm control and is recommended as a first-line agent for adults with CHD with IART or AF and ventricular hypertrophy, ventricular dysfunction, or coronary artery disease [25, 44•]. However, long-term therapy is limited by side effects such as hepatic and pulmonary toxicity, adverse cardiac effects, and thyroid dysfunction (hypothyroidism and hyperthyroidism). These adverse effects, especially amiodarone-induced thyrotoxicosis, are especially common in adults with single ventricle circulation [59, 60]. Given the high incidence of organ toxicity in Fontan patients, many experts do not recommend its use other than as a bridge to more definitive therapy. Deal et al. [13, 42•] recommend a combination of sotalol with lose-dose beta blockade to be most effective chronic pharmacologic therapy for atrial tachyarrhythmias in patients with Fontan circulation. Sotalol has been shown in several studies to elicit complete or partial rhythm control in ACHD patients with atrial tachyarrhythmias, including those with Fontan circulation. However, the risk of significant bradyarrhythmias is higher in single ventricle patients with sotalol [61,62,63]. The use of sotalol carries class IIb indication as a first-line anti-arrhythmic agent for maintenance of sinus rhythm in adults with CHD [44•]. Dofetilide, a class 3 anti-arrhythmic drug, has been used successfully for long-term rhythm control in adults with various congenital heart disease lesions, including single ventricle heart disease [49, 64, 65]. However, given the 5–10% incidence of torsades de pointes with dofetilide initiation, inpatient monitoring with daily electrocardiograms to follow QTc and surveillance of renal function for a minimum of 3 days is mandated by the Food and Drug Administration. Dofetilide has a class IIa recommendation as a reasonable alternative to amiodarone in adults with CHD and atrial tachyarrhythmias [44•]. Dosing recommendations for chronic pharmacologic therapy are summarized in Table 2. Despite pharmacologic therapy, recurrence of atrial tachyarrhythmias exceeds 50% in adult Fontan patients. [2, 24, 31, 66]. Thus, given poor long-term freedom from arrhythmia, exploration of non-pharmacologic therapies is strongly recommended.
Patients with Fontan palliation are at high risk of thromboembolic complications given a multitude of factors, including stasis of blood flow, presence of prosthetic material, altered levels of clotting and fibrinolytic factors, hepatic congestion, protein-losing enteropathy, ventricular dysfunction, and tachyarrhythmias [67,68,69]. Despite this, no consensus regarding thromboprophylaxis in this group exists and there are significant institutional variations in practice. However, long-term anticoagulation in adult Fontan patients with IART or AF for the prevention of thromboembolic complications is a class I recommendation from both the AHA/ACA ACHD and PACES/HRS arrhythmias in ACHD guidelines [44•, 54]. Warfarin is the recommended drug of choice and most centers target an INR of 2–3. Despite a class III recommendation from the PACES/HRS guidelines, many institutions have started using non-vitamin K oral anticoagulants (NOACs) for thromboembolism prophylaxis in adults with congenital heart disease, including the Fontan population [70,71,72,73]. These studies show NOACs have equal efficacy and safety when compared to vitamin K antagonists for thromboembolism prophylaxis. Further studies are necessary before more uniform recommendation of NOACs for thromboembolism prophylaxis in Fontan palliated patients can be made.
Catheter-based intervention
For patients with recurrent atrial tachyarrhythmias, catheter ablation should be considered. Acute success rates for catheter ablation are lower in Fontan patients when compared to other forms of congenital heart disease and recurrence rates are higher [74,75,76]. This is secondary to several factors, including anatomic challenges, multiple re-entrant circuits or foci, and evolving arrhythmogenic substrate over time. However, with improvements in technology, including 3D electroanatomic mapping along with the use of irrigated-tip and large-tip ablation catheters, the acute success rate has improved. Recent studies have reported acute ablation success rates greater than 75% for adult Fontan patients with various atrial tachyarrhythmias [12, 23, 77, 78]. As mentioned previously, the vast majority of atrial tachyarrhythmias in adult Fontan patients are re-entry type, including IART, typical atrial flutter, and AF, with focal atrial tachycardias less frequently observed. Most of these tachycardias are due to macro-reentrant circuits bordered by scar tissue, suture lines, prosthetic materials, or anatomic structures such as the inferior vena cava or the systemic atrioventricular valve annulus [23, 79, 80]. Multiple reentrant circuits are often possible given considerable substrate. Electropathologic alterations in the atrial myocardium, atrial pressure and/or volume overload, and progressive scarring along previous atriotomy sites or suture lines are responsible for intra-atrial conduction delay, changes in atrial refractoriness, and other conduction abnormalities. These anomalies, paired with a higher incidence of premature atrial contractions, make these patients vulnerable to tachyarrhythmia development [81].
The location of the IART circuit may depend on the type of Fontan palliation, with the anastomosis from the right atrium to the pulmonary artery, atriotomy incision, or caval veins serving as electrical barriers in those with AP Fontan patients [52, 77, 79]. However, circuits involving the cavotricuspid isthmus (CTI) or other areas within the pulmonary venous atrium do exist in AP Fontans as well [12, 76, 80]. IART circuits in LT and EC Fontan patients more commonly involve reentry around the systemic atrioventricular valve annulus, as well as atriotomy scar in the right atrial free wall. There are various anatomic challenges in addressing arrhythmia substrate in the Fontan population, especially when reentrant circuits involve atrial tissue excluded from the Fontan circuit. For example, with CTI-dependent flutter, there are essentially 2 separate isthmuses —one between the tricuspid valve and the conduit/baffle and the second from the conduit/baffle to the inferior vena cava, with the former not accessible via standard catheter placement in the systemic veins. When electroanatomic mapping suggests a portion of a circuit or arrhythmogenic substrate is not accessible with systemic venous mapping alone, access to the area of interest can be obtained in a few different ways. These include crossing a pre-existing fenestration, retrograde via the aorta to the subaortic ventricle and across the AV valve, or transconduit, transbaffle, or transcaval puncture into the atrium [77, 78, 82, 83]. When direct puncture is needed, transesophageal or intracardiac echocardiography is used in addition to biplane angiography to accurately identify the ideal puncture site. A transseptal needle is used, although radiofrequency energy is also sometimes needed to successfully access the atrium. For patient with an EC Fontan, the standard transconduit approach is best performed at the lower portion of the conduit. Otherwise, when enough overlap between the inferior vena cava and the pulmonary venous atrium is present, a transcaval approach is feasible [84, 85]. Because of the potential for catastrophic intrathoracic hemorrhage with forceful needle puncture, these procedures should only be done in centers with considerable experience with adult congenital heart disease and ready availability of congenital heart surgeons.
Device intervention
Implantation of an antitachycardia pacing (ATP) device is an option for atrial tachyarrhythmia management and prevention in adult Fontan patients, especially in those with sinus bradycardia or junctional bradycardia [27, 39, 55]. Permanent pacing, ideally with a device with antitachycardia pacing properties, is a PACES/HRS class IIa recommendation for adults with CHD and sinus or junctional bradycardia for the prevention of recurrent IART [44•]. These dual-chamber devices are not only able to pace terminate episodes of IART but are also able to prevent atrial tachyarrhythmia occurrence via 2 mechanisms. The first is atrial preference pacing, which essentially paces the atrium slightly faster than the intrinsic sinus rate to suppress tachycardia. The second is atrial rate stabilization, which increases the pacing rate following a premature atrial beat to prevent long pauses, which can be proarrhythmic [86]. Several studies have shown successful termination of atrial tachyarrhythmias with ATP and reduction in need for cardioversion in adults with congenital heart disease, including those with Fontan palliation [86,87,88]. Implantation of a dual-chamber device is preferred given the potential need for ventricular pacing over time, but devices should be programmed for atrial pacing only unless there is atrioventricular block. Fontan palliation type influences device implantation approach (surgically versus transvenous) with an epicardial surgical approach often needed, especially in EC Fontan patients. Transvenous pacing is possible, although technically challenging, and chronic anticoagulation is necessary given thrombosis risk [89, 90]. Finally, anti-tachycardia pacing devices are routinely implanted in patients undergoing Fontan conversion to further help prevent and treat atrial tachyarrhythmias over time [13, 42•, 87].
Surgical intervention
Surgical risk factors for the development of post-Fontan tachydysrhythmia have been established as: an older age at initial repair, early post-Fontan arrhythmia, double-inlet left ventricle, AV valve regurgitation, and abnormal AV valve morphology [26].
The surgical treatment of arrhythmia following Fontan palliation has aimed both to address predisposing anatomic and hemodynamic factors while also incorporating established ablative techniques to minimize arrhythmia burden following Fontan conversion. In addition to post-Fontan ablative techniques and structural conversion, longitudinal knowledge of post-Fontan arrhythmia risk has supported the avoidance of intra-atrial suture lines with an extra-cardiac conduit palliation and potential incorporation of prophylactic ablation from the atriotomy across the anterior atrial wall to the right-sided valve annulus at the time of lateral tunnel palliation [91]. While the more historical technique of Fontan palliation with the performance of an atrial-pulmonary artery anastomosis has been largely abandoned, sinus node dysfunction has been reported in up to 40% of atriopulmonary connection patients and remains a primary indication for corrective Fontan conversion surgery with many advocating for the concomitant performance of right atrial reduction, bi-atrial MAZE procedures, and the addition of dual chamber antitachycardia pacing systems for nearly all patients at the time of Fontan conversion [36, 92, 93]. While variable results are reported, meta-analysis has demonstrated a recurrence of tachyarrhythmia in 76% of patients during short-term follow-up post-Fontan revision alone prompting many centers to incorporate concomitant ablation at the time of Fontan revision with reported recurrence as low as 8.8–12.8% at 56 months of follow-up [42•]. While current outcome measures are limited by heterogenous adoption of preoperative electrophysiologic mapping, variable lesion set application, and use of both radiofrequency and cryoablative techniques, reported recurrence rates are superior to that reported for catheter-based techniques [42•, 74, 94].
Advancements in surgical technique with the adoption of lateral tunnel and extracardiac cavopulmonary connections operations have lowered the incidence of post-Fontan atrial dysrhythmias to 25%. Comparative analysis has demonstrated equivocal rates of supraventricular atrial arrhythmias when comparing lateral tunnel versus total cavopulmonary Fontan palliation techniques in short-term follow-up [21, 95]. Systematic reviews, however, have favored the extra-cardiac conduit technique for decreased late arrhythmia risk with higher rates demonstrated within the lateral tunnel technique population (OR 1.96; 95% CI 1.64–2.35; p < 0.01) [96]. Proposed factors for preservation of sinus rhythm following extra-cardiac conduit palliation include the avoidance of exposure of the right atrium to elevated systemic pressures, lack of need for atrial incisions and suture lines, absence of sinus node manipulation, and elimination of cross-clamp and associated ischemic times [97]. Modifications to the lateral tunnel technique have been adopted; however, in an attempt to minimize the post-Fontan arrhythmia burden and include a right atriotomy extending to the coronary sinus to block the slow conduction isthmus, cryoablation performed between the right atriotomy and right AV valve annulus, avoidance of the crista terminalis, and use of a sandwich technique in closure of the atriotomy incorporating the GoreTex baffle [98]. Furthermore, absence of sinus rhythm at the conclusion of the Fontan palliation has been established as a primary predictor for post-Fontan atrial tachydysrhythmia development supporting the placement of an atrial pacemaker at the Fontan operation in these select patients [99]. While promising early results have been published, long-term data remain limited, supporting prospective comparative analyses for lateral tunnel versus extra-cardiac conduit Fontan techniques and concomitant approaches to atrial ablation.
As reviewed, an ever-increasing and aging post-Fontan population has afforded a growing body of knowledge regarding long-term risk and predisposing risk factors to the development of post-Fontan atrial dysrhythmia. Surgical technique modification, Fontan conversion indications, and defined prophylactic and therapeutic ablative techniques will be dependent upon standardized approaches to longitudinal follow-up and technical comparisons to ultimately advance and improve our surgical treatment strategies for this complex patient population.
Conclusion
Atrial tachyarrhythmias are the most common late complication following Fontan operation and a significant source of morbidity and mortality in these patients. Treatment includes medical, interventional, and surgical options, and therapy should consider each individual patient’s symptoms, comorbidities, and risk factors. As this patient population ages into adulthood, the incidence of atrial tachyarrhythmias is likely to increase. Given the complexities of adults with Fontan circulation, including the challenges of managing tachyarrhythmias, these patients should be cared for in centers with a multi-disciplinary team including ACHD cardiologists, congenital heart disease surgeons, and interventionalists and electrophysiologists with congenital heart disease expertise. This unique, challenging, and growing patient population will continue to need focused attention and research to advance care and improve outcomes.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–8.
d’Udekem Y, et al. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation. 2014;130(11 Suppl 1):S32–8.
Downing, T.E., et al., Long-term survival after the Fontan operation: twenty years of experience at a single center. J Thorac Cardiovasc Surg, 2017. 154(1): p. 243–253 e2.
Khairy P, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117(1):85–92.
Ono M, et al. Clinical outcome of patients 20 years after Fontan operation–effect of fenestration on late morbidity. Eur J Cardiothorac Surg. 2006;30(6):923–9.
Pundi KN, et al. 40-year follow-up after the Fontan operation: long-term outcomes of 1,052 patients. J Am Coll Cardiol. 2015;66(15):1700–10.
Schilling C, et al. The Fontan epidemic: population projections from the Australia and New Zealand Fontan Registry. Int J Cardiol. 2016;219:14–9.
• Rychik J, et al. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation, 2019: p. CIR0000000000000696. Scientific statement from the American Heart Association summarizing the current state of knowledge on the Fontan circulation and its consequences. This document reviews in detail the various complications adults with Fontan circulation suffer and discusses various treatment options. It also proposes a surveillance testing tool kit that provides recommendations for follow-up care. Finally, it highlights gaps in knowledge for this patient population and suggests areas of focus moving forward to improve quality of life for these individuals.
de Leval MR et al. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations Experimental studies and early clinical experience. J Thorac Cardiovasc Surg, 1988. 96(5): 682–95.
Marcelletti C, et al. Inferior vena cava-pulmonary artery extracardiac conduit A new form of right heart bypass. J Thorac Cardiovasc Surg. 1990;100(2):228–32.
Song MK, et al. Intra-atrial reentrant tachycardia in adult patients after Fontan operation. Int J Cardiol. 2015;187:157–63.
Weipert J, et al. Occurrence and management of atrial arrhythmia after long-term Fontan circulation. J Thorac Cardiovasc Surg. 2004;127(2):457–64.
Deal BJ. Late arrhythmias following fontan surgery. World J Pediatr Congenit Heart Surg. 2012;3(2):194–200.
Quinton E, et al. Prevalence of atrial tachyarrhythmia in adults after Fontan operation. Heart. 2015;101(20):1672–7.
Balaji S, et al. An international multicenter study comparing arrhythmia prevalence between the intracardiac lateral tunnel and the extracardiac conduit type of Fontan operations. J Thorac Cardiovasc Surg. 2014;148(2):576–81.
Bossers SS et al. Comprehensive rhythm evaluation in a large contemporary Fontan population. Eur J Cardiothorac Surg, 2015. 48(6): p. 833–40; discussion 840–1.
Carins TA et al. Long-term outcomes after first-onset arrhythmia in Fontan physiology. J Thorac Cardiovasc Surg, 2016. 152(5): p. 1355–1363 e1.
Stephenson EA, et al. Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol. 2010;56(11):890–6.
d’Udekem Y, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation. 2007;116(11 Suppl):I157–64.
Azakie A, et al. Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: impact on outcomes. J Thorac Cardiovasc Surg. 2001;122(6):1219–28.
Hakacova N, Lakomy M, Kovacikova L. Arrhythmias after Fontan operation: comparison of lateral tunnel and extracardiac conduit. J Electrocardiol. 2008;41(2):173–7.
Nurnberg JH et al. New onset arrhythmias after the extracardiac conduit Fontan operation compared with the intraatrial lateral tunnel procedure: early and midterm results. Ann Thorac Surg, 2004. 78(6): 1979–88; discussion 1988.
de Groot NM, et al. Long-term outcome of ablative therapy of postoperative supraventricular tachycardias in patients with univentricular heart: a European multicenter study. Circ Arrhythm Electrophysiol. 2009;2(3):242–8.
Egbe AC, et al. Outcomes in adult Fontan patients with atrial tachyarrhythmias. Am Heart J. 2017;186:12–20.
Karbassi A, et al. Atrial tachyarrhythmia in adult congenital heart disease. World J Cardiol. 2017;9(6):496–507.
Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115(4):534–45.
Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart. 2012;98(14):1098–104.
Kollengode M, et al. Successful atrial fibrillation ablation without pulmonary vein isolation utilizing focal impulse and rotor mapping in an atriopulmonary Fontan. HeartRhythm Case Rep. 2018;4(6):241–6.
Labombarda F, et al. Increasing prevalence of atrial fibrillation and permanent atrial arrhythmias in congenital heart disease. J Am Coll Cardiol. 2017;70(7):857–65.
Lasa JJ, et al. Prevalence of arrhythmias late after the Fontan operation. Am J Cardiol. 2014;113(7):1184–8.
Ghai A, et al. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol. 2001;37(2):585–92.
Stamm C, et al. Long-term results of the lateral tunnel Fontan operation. J Thorac Cardiovasc Surg. 2001;121(1):28–41.
Kverneland LS, Kramer P, Ovroutski S. Five decades of the Fontan operation: a systematic review of international reports on outcomes after univentricular palliation. Congenit Heart Dis. 2018;13(2):181–93.
Cecchin F, et al. Effect of age and surgical technique on symptomatic arrhythmias after the Fontan procedure. Am J Cardiol. 1995;76(5):386–91.
Mertens L et al. Protein-losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg, 1998. 115(5): 1063–73.
van den Bosch AE, et al. Long-term outcome and quality of life in adult patients after the Fontan operation. Am J Cardiol. 2004;93(9):1141–5.
Khairy P, et al. Arrhythmias in adult congenital heart disease. Expert Rev Cardiovasc Ther. 2006;4(1):83–95.
Khairy P, Poirier N, Mercier LA. Univentricular heart. Circulation. 2007;115(6):800–12.
Pundi KN, et al. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit Heart Dis. 2017;12(1):17–23.
Diller GP, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31(24):3073–83.
Giannakoulas G, et al. Atrial tachyarrhythmias late after Fontan operation are related to increase in mortality and hospitalization. Int J Cardiol. 2012;157(2):221–6.
Deal BJ, Mavroudis C, Backer CL. Arrhythmia management in the Fontan patient. Pediatr Cardiol. 2007;28(6):448–56.
Hernandez-Madrid A, et al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace. 2018;20(11):1719–53.
• Khairy P et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm, 2014. 11(10): p. e102–65. (Consensus statement from the PACES and HRS on the recognition and management of arrhythmias in adult congenital heart disease. This detailed report critically appraises and synthesizes the available data to present evidence-based recommendations (when available) and expert opinon on the strategies for arrhythmia management in this complex patient population including medical treatment, catheteter-based interventions, device therapy, and surgical options. In addition, there is a specific section dedicated to adults with single ventricle physiology.)
January CT, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071–104.
Kirchhof P, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962.
Idorn L, et al. Thromboembolic complications in Fontan patients: population-based prevalence and exploration of the etiology. Pediatr Cardiol. 2013;34(2):262–72.
Wan D et al. Atrial arrhythmias and thromboembolic complications in adults post Fontan surgery. Open Heart, 2020. 7(2).
El-Assaad I, et al. Use of dofetilide in adult patients with atrial arrhythmias and congenital heart disease: a PACES collaborative study. Heart Rhythm. 2016;13(10):2034–9.
Fujita S, et al. Management of late atrial tachyarrhythmia long after Fontan operation. J Cardiol. 2009;53(3):410–6.
Hoyer AW, Balaji S. The safety and efficacy of ibutilide in children and in patients with congenital heart disease. Pacing Clin Electrophysiol. 2007;30(8):1003–8.
Moore JP, Khairy P. Adults with congenital heart disease and arrhythmia management. Cardiol Clin. 2020;38(3):417–34.
Rao SO, et al. Atrial tachycardias in young adults and adolescents with congenital heart disease: conversion using single dose oral sotalol. Int J Cardiol. 2009;136(3):253–7.
Stout KK, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(14):e637–97.
Zentner D, et al. Management of people with a Fontan circulation: a Cardiac Society of Australia and New Zealand Position statement. Heart Lung Circ. 2020;29(1):5–39.
Page RL, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):e27–115.
Fish FA, P.C. Gillette, and D.W. Benson, Jr., Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide. The Pediatric Electrophysiology Group. J Am Coll Cardiol, 1991. 18(2): 356–65.
January CT, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-76.
Moore BM, et al. Adverse effects of amiodarone therapy in adults with congenital heart disease. Congenit Heart Dis. 2018;13(6):944–51.
Thorne SA, et al. Amiodarone-associated thyroid dysfunction: risk factors in adults with congenital heart disease. Circulation. 1999;100(2):149–54.
Miyazaki A, et al. Efficacy and safety of sotalol for refractory tachyarrhythmias in congenital heart disease. Circ J. 2008;72(12):1998–2003.
Moore BM, et al. Efficacy and adverse effects of sotalol in adults with congenital heart disease. Int J Cardiol. 2019;274:74–9.
Koyak Z, et al. Efficacy of antiarrhythmic drugs in adults with congenital heart disease and supraventricular tachycardias. Am J Cardiol. 2013;112(9):1461–7.
Banchs JE, et al. Clinical efficacy of dofetilide for the treatment of atrial tachyarrhythmias in adults with congenital heart disease. Congenit Heart Dis. 2014;9(3):221–7.
Wells R, et al. Dofetilide for atrial arrhythmias in congenital heart disease: a multicenter study. Pacing Clin Electrophysiol. 2009;32(10):1313–8.
Egbe AC, et al. Recurrent sustained atrial arrhythmias and thromboembolism in Fontan patients with total cavopulmonary connection. Int J Cardiol Heart Vasc. 2021;33:100754.
Egbe AC, et al. Thrombotic and embolic complications associated with atrial arrhythmia after fontan operation: role of prophylactic therapy. J Am Coll Cardiol. 2016;68(12):1312–9.
McCrindle BW, et al. Factors associated with thrombotic complications after the Fontan procedure: a secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J Am Coll Cardiol. 2013;61(3):346–53.
Rosenthal DN et al. Thromboembolic complications after Fontan operations. Circulation, 1995. 92(9 Suppl): p. II287–93.
Georgekutty J, et al. Novel oral anticoagulant use in adult Fontan patients: a single center experience. Congenit Heart Dis. 2018;13(4):541–7.
Kawamatsu N, et al. Direct oral anticoagulant use and outcomes in adult patients with Fontan circulation: a multicenter retrospective cohort study. Int J Cardiol. 2021;327:74–9.
Pujol C, et al. Usefulness of direct oral anticoagulants in adult congenital heart disease. Am J Cardiol. 2016;117(3):450–5.
Yang, H., et al., Non-vitamin K antagonist oral anticoagulants in adults with a Fontan circulation: are they safe. Open Heart, 2019. 6(1): p. e000985.
Kannankeril PJ, et al. Frequency of late recurrence of intra-atrial reentry tachycardia after radiofrequency catheter ablation in patients with congenital heart disease. Am J Cardiol. 2003;92(7):879–81.
Triedman JK, et al. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39(11):1827–35.
Yap SC, et al. Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol. 2010;56(19):1589–96.
Moore BM, et al. Ablation of atrial arrhythmias after the atriopulmonary Fontan procedure: mechanisms of arrhythmia and outcomes. JACC Clin Electrophysiol. 2018;4(10):1338–46.
Moore JP, et al. Catheter ablation of supraventricular tachyarrhythmia after extracardiac Fontan surgery. Heart Rhythm. 2016;13(9):1891–7.
Collins KK, et al. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol. 2000;86(9):969–74.
Yap SC, et al. Evolving electroanatomic substrate and intra-atrial reentrant tachycardia late after Fontan surgery. J Cardiovasc Electrophysiol. 2012;23(4):339–45.
de Groot NMS, Bogers A. Development of tachyarrhythmias late after the Fontan procedure: the role of ablative therapy. Card Electrophysiol Clin. 2017;9(2):273–84.
Correa R, et al. Transbaffle mapping and ablation for atrial tachycardias after mustard, senning, or Fontan operations. J Am Heart Assoc. 2013;2(5):e000325.
El Yaman MM, et al. Methods to access the surgically excluded cavotricuspid isthmus for complete ablation of typical atrial flutter in patients with congenital heart defects. Heart Rhythm. 2009;6(7):949–56.
Moore JP, et al. Ten-year outcomes of transcaval cardiac puncture for catheter ablation after extracardiac Fontan surgery. Heart Rhythm. 2020;17(10):1752–8.
Moore JP, et al. Transcaval puncture for access to the pulmonary venous atrium after the extracardiac total cavopulmonary connection operation. Circ Arrhythm Electrophysiol. 2015;8(4):824–8.
Kramer CC, et al. Safety and efficacy of atrial antitachycardia pacing in congenital heart disease. Heart Rhythm. 2018;15(4):543–7.
Tsao S, et al. Device management of arrhythmias after Fontan conversion. J Thorac Cardiovasc Surg. 2009;138(4):937–40.
Stephenson EA, et al. Efficacy of atrial antitachycardia pacing using the Medtronic AT500 pacemaker in patients with congenital heart disease. Am J Cardiol. 2003;92(7):871–6.
O’Leary E, et al. Transvenous approach to pacemaker lead implantation for sinus node dysfunction after extracardiac lateral tunnel Fontan conduit placement. HeartRhythm Case Rep. 2016;2(6):495–8.
Moore JP, Shannon KM. Transpulmonary atrial pacing: an approach to transvenous pacemaker implantation after extracardiac conduit Fontan surgery. J Cardiovasc Electrophysiol. 2014;25(9):1028–31.
Ohye RG, et al. Primary surgical prevention of post-operative atrial re-entry tachycardia. Prog Pediatr Cardiol. 2002;14(3):223–8.
Kim SJ, et al. Outcome of 200 patients after an extracardiac Fontan procedure. J Thorac Cardiovasc Surg. 2008;136(1):108–16.
Mavroudis C et al. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. 111 Fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg, 2007. 84(5): p. 1457–65; discussion 1465–6.
Jais P, HL, Sanders P et al. Determinants of the type of recurrent arrhythmia after ablation of chronic atrial fibrillation. . Heart Rhythm Suppl 2004. 1.
Durongpisitkul K, et al. Predictors of early- and late-onset supraventricular tachyarrhythmias after Fontan operation. Circulation. 1998;98(11):1099–107.
Li D, et al. Arrhythmias after Fontan operation with intra-atrial lateral tunnel versus extra-cardiac conduit: a systematic review and meta-analysis. Pediatr Cardiol. 2017;38(4):873–80.
Kogon B. Is the extracardiac conduit the preferred Fontan approach for patients with univentricular hearts? The extracardiac conduit is the preferred Fontan approach for patients with univentricular hearts. Circulation, 2012. 126(21): p. 2511–5; discussion 2515.
Lim HG, Lee JR, Kim YJ. The effects of modification to lateral tunnel Fontan procedure for prophylactic arrhythmia surgery. Ann Thorac Surg. 2017;104(1):197–204.
Fishberger SB, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113(1):80–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology/CT Surgery
Rights and permissions
About this article
Cite this article
SooHoo, M.M., Stone, M.L., von Alvensleben, J. et al. Management of Atrial Tachyarrhythmias in Adults With Single Ventricle Heart Disease. Curr Treat Options Peds 7, 187–202 (2021). https://doi.org/10.1007/s40746-021-00231-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-021-00231-w