Abstract
Purpose of review
In this manuscript, we review the diagnosis, causes, and management of cardiac thrombosis in children with congenital and acquired heart disease. Indications for invasive management and surgery are discussed. The differential diagnostic considerations and management of cardiac thromboses will be reviewed, including those that occur as a consequence of repair and palliation of congenital heart disease.
Recent findings
The definition of venous thromboembolism has recently been expanded to include thromboses within both the right and left heart chambers. Direct thrombin inhibitors (DTIs) and direct oral anticoagulants (DOACs) are emerging as treatment alternatives to the heparins and vitamin K antagonists for the management of thrombosis in pediatric heart disease.
Summary
Thrombosis within the heart occurs as a consequence of abnormalities and alterations in blood flow resulting in stasis and turbulence. Children with acquired and congenital heart disease carry a number of risk factors in Virchow’s triad. Thrombosis can occur throughout any part of the cardiovascular system in congenital heart disease and after surgery for repair or palliation of defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
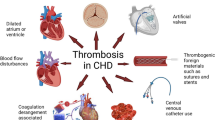
Thrombosis is a common morbidity in children with congenital and acquired heart disease that is often life threatening. The risk factors for thrombosis described by Rudolph Virchow nearly 150 years ago are ever present in children with heart disease: hypercoagulability, venous stasis, and endothelial injury. High-risk populations in pediatric cardiology often manifest all three risk factors, especially after congenital heart surgery (Fig. 1). Additionally, infants and children maintain hemostatic balance in the context of developmental hemostasis with different levels of coagulation proteins, which only reach normal adult levels in adolescence [1]. Thrombosis can occur throughout the circulation; however, most attention is directed at venous thromboembolism (VTE) which is estimated at 58 cases per 10,000 pediatric hospital admissions [2]. VTE has traditionally been defined as thrombosis affecting the deep veins of the extremities or pelvis and pulmonary embolism [3]. However, more recent definitions for pediatric VTE have included intracardiac thromboses of both right and left heart chambers [4]. In this review, we will discuss the management of intracardiac thrombosis in children with congenital and acquired heart disease. Pharmaceutical treatment options, including contemporary therapies, will be discussed (Table 1).
Right heart and cavopulmonary connections
Thrombosis within the right heart is often due to an extension of or in association with VTE caused by deep vein thrombosis, pulmonary embolism, or the presence of a central venous or intracardiac catheter [5]. Other causes are typically acquired in the context of atrial dilatation or derangements to right heart blood flow. This can be seen in association with atrial arrhythmias, cardiomyopathies (e.g., dilated, constrictive, restrictive), myocarditis, and tricuspid valve disease [6]. Post-operative right heart thrombosis can be seen after the closure of septal defects or tricuspid valve repair and replacement.
The diagnosis of right heart thrombosis is made commonly by echocardiography and also by computed tomography (CT) or cardiac magnetic resonance imaging (CMR). Non-mobile masses fixed to the endocardium can often be difficult to differentiate from myocardial tissue by echocardiography. In these scenarios, contrast-enhanced CMR can aide in differentiating intracardiac thrombus from other masses or the myocardium itself [7]. The diagnosis of right heart thrombosis is likely under-appreciated as they only appear when symptomatic or during screening in high-risk scenarios such as the following: concurrent VTE (pulmonary embolism, in particular), atrial arrhythmia, central line dysfunction, and myocarditis or cardiomyopathy. Additionally, right heart thrombi may be found incidentally during routine surveillance echocardiography for myocarditis and cardiomyopathy, or after congenital heart surgery (in particular after surgical repair within the atrium or when redundant atrial septal tissue remains).
Right ventricular (RV) thrombi can also be seen related to stasis and altered blood flow dynamics in the context of single ventricle palliation. During any stage of single ventricle palliation, thrombosis is seen in relation to single RV myocardial dysfunction or tricuspid valve dysfunction. Transthoracic echocardiography is, again, the primary imaging modality in the diagnosis of RV thrombus (both in 2-ventricle and single ventricle disease); CT or CMR may be helpful.
Right heart thrombosis can also be seen in the cavopulmonary connections of palliated single ventricle disease. Thrombi are present both at the superior (Glenn) and inferior (Fontan) cavopulmonary connections. In contrast to pulmonary embolism in the 2-ventricle heart, cavopulmonary thrombosis is rarely an extension of a more peripheral VTE. Rather, palliated patients with Glenn shunts or Fontan conduits present with primary thrombosis due to venous stasis and altered flow dynamics. For this reason, it is recommended that patients with cavopulmonary connections receive some form of antithrombotic therapy, with aspirin antiplatelet prophylaxis being the most frequently used [1]. Anticoagulation can also be considered if there are additional risk factors (family or personal history of prior thrombosis, prolonged indwelling central venous catheter dependence, anastomotic narrowing, atrial hypertension and ventricular dysfunction, AV valve dysfunction, and/or protein-losing enteropathy) [1]. It is important to note that children with palliated single ventricle disease will have, at least in part, an obligatory right-to-left shunt placing them at risk for direct thromboembolic stroke in the setting of right heart thrombosis. Patients who undergo non-fenestrated Fontan completion procedures may be somewhat protected, but those with fenestrations maintain a risk for embolic stroke due to right-to-left shunt. Though diagnosis of cavopulmonary thrombosis is often made during surveillance echocardiography, CT or CMR may be indicated for confirmation. Notably, post-operative pleural and pericardial effusions should prompt consideration for associated upper extremity VTE, especially in the presence of central venous access. Furthermore, post-operative chylothorax is associated with VTE and should prompt evaluation.
Medical management
Right heart and pulmonary arterial thromboses without right ventricular dysfunction are managed primarily with systemic anticoagulation. Chemical thrombolysis with alteplase may be considered but is, in general, rarely the first and urgent choice for therapy. The exception to this is the scenario of right heart thrombosis associated with pulmonary embolism. In general, the risks of systemic thrombolysis with alteplase are reserved for children with pulmonary embolism and right heart thrombosis who demonstrate associated right ventricular failure, hemodynamic instability, or collapse [8••]. Otherwise, catheter-directed alteplase therapy is considered for patients with right ventricular dysfunction prior to the onset of hemodynamic collapse.
Right heart thrombosis without pulmonary embolism is managed primarily with systemic anticoagulation. Traditionally, the initial anticoagulant of choice in children is heparin. Infusions of unfractionated heparin offer the usual convenience of titration and short half-life. The benefit of rapid discontinuation of the infusion, short half-life, and availability of a reversal agent is often important in patients at risk for post-operative bleeding or if an upcoming invasive procedure is anticipated. However, unfractionated heparin has the disadvantages of high protein binding and dependence on endogenous antithrombin [9, 10]. Heparin exerts its anticoagulant effect by potentiation of antithrombin. As a consequence, neonates and infants demonstrate relative heparin resistance due to low circulating antithrombin concentrations due to developmental hemostasis [11••]. Particularly problematic in inflamed states (such as infection or wound healing), heparins are susceptible to enhanced clearance by heparin-binding proteins produced as a consequence of inflammation. The release of pro-inflammatory cytokines also induces enhanced expression of endothelial tissue factor, thus increasing thrombin generation [12]. Finally, unfractionated heparin, via antithrombin, binds both factor Xa and thrombin. Thus, the excess of thrombin generation in inflamed states can result in relative heparin resistance. This relative resistance can be overcome by the use of low-molecular weight heparin (LMWH), which binds primarily factor Xa (also via antithrombin) with little action on thrombin [10]. Therefore, the right heart thrombi in hemodynamically stable patients (not requiring surgery) are often treated with LMWH.
More recently, the use of parenteral direct thrombin inhibitors (DTIs) is becoming common in the management of pediatric VTE and right heart thrombosis. Both bivalirudin and argatroban are FDA approved for anticoagulation in adults with heparin-induced thrombocytopenia (HIT). By in large, DTIs are considered as alternatives (off-label) to heparin anticoagulation in infants and children for relative heparin resistance (in addition to HIT). Though DTI use in children is off-label, their use even as first-line therapy (particularly bivalirudin) is becoming more common. Many who do so cite studies demonstrating early clot resolution with bivalirudin (relative to heparin) due to its unique ability to inhibit clot-bound thrombin [13]. Experience with DTIs continues to mount by virtue of their use in children supported by mechanical circulatory support (extracorporeal membrane oxygenation (ECMO) and ventricular assist device (VAD)) and during the transcatheter intervention [14,15,16,17,18]. Both bivalirudin and argatroban are generally well tolerated without significant hemorrhagic complication [19, 20]. An important consideration when choosing DTI therapy over heparin for intracardiac thrombosis, however, is the metabolism of bivalirudin predominately via proteolytic cleavage (80%). Circulating protease metabolism offers some advantage in children with renal dysfunction; however, there exists a potential for robust clearance in areas of blood stasis. In general, most avoid the use of bivalirudin as the sole anticoagulant for right heart thrombosis if there is stasis and the potential for rapid proteolytic cleavage (e.g., in patients supported by ECMO with severe right heart failure). Alternatively, fondaparinux is a parenteral factor Xa inhibitor that is used as a treatment for adult VTE and PE that may be considered for right heart thrombosis.
The standard of care for long-term outpatient anticoagulation for VTE and right heart thrombi is with either LMWH or vitamin K antagonist (VKA) therapy with warfarin. The recommended duration of treatment, irrespective of the resolution of thrombus, is 3 months (primarily for the prevention of post-thrombotic syndrome) [21]. In line with an adult practice, however, the use of direct oral anticoagulant (DOAC) agents is becoming more common and accepted in the treatment of thrombosis in children. Apixaban, rivaroxaban, and edoxaban are oral factor Xa inhibitors and dabigatran is an oral direct thrombin inhibitor. Though no controlled trials of DOACs in children have been completed, clinical trials are actively ongoing and case series have demonstrated safety and resolution of refractory intracardiac thromboses [22•].
Invasive management
Invasive management in right heart thrombosis in the 2-ventricle heart is reserved for patients with associated right heart failure. Specifically, pulmonary embolism with right ventricle dysfunction (not requiring systemic alteplase for hemodynamic collapse) can be managed with catheter-directed thrombolysis with alteplase. No specific criteria or guidance exist for catheter-directed thrombolytic therapy for right heart thrombi, but its consideration would be reasonable based in the context of the risk profile of invasive catheter placement and alteplase therapy. Surgical embolectomy may be considered in patients with massive pulmonary embolism, but surgical intervention solely for right heart thrombi in the absence of pulmonary embolism and right ventricular dysfunction is generally avoided. If surgical pulmonary embolectomy is considered, it should be noted that distal emboli may be more difficult to access surgically, in comparison with proximal pulmonary emboli. Surgical right ventricular thrombectomy may be considered in selected circumstances surrounding congenital heart surgery [23]. The feasibility of transcatheter embolectomy, whether in the right atrium or ventricle, is often limited (by the size of the patient, catheter or equipment required, and risk of further embolization). However, percutaneous devices such as the AngioVac [24, 25] and AngioJet [26,27,28,29] may provide approaches for percutaneous embolectomy at experienced centers. Notably, these devices may be more effective at the site of proximal thrombosis, rather than an area of multiple distal emboli.
Cavopulmonary thrombosis after palliated single ventricle disease is a known complication and risk. Early thrombosis after surgical palliation often warrants aggressive intervention to ensure adequate and safe progression for the patient after surgery. Late thrombosis (remote from surgery) may portend embolic stroke risk or risk for failure of single ventricle palliation. For these reasons, intervention for cavopulmonary thrombosis is not rare and catheter-directed thrombolysis with alteplase is most common [30]. In addition, attention must be directed at correcting any anatomic causes of increased venous stasis (e.g., percutaneous balloon and stent angioplasty for cavopulmonary stenosis). Systemic thrombolysis may be considered in extreme circumstances of acute thrombosis, but these scenarios are rare. Alternatives to systemic alteplase therapy should be considered for non-acute thrombus where the risk of systemic alteplase may exceed the potential benefit over long-term systemic anticoagulation. Operative thrombectomy is also rare, unless another concurrent indication for cardiac surgery exists. Notably, a history of Fontan thrombosis warrants particular attention and consideration of the risk of embolism of calcified thrombus at the time of any surgical revision or transplant.
Left atrium
Left atrial (LA) thrombosis is relatively rare in children. LA thrombi in adults are most commonly seen at the atrial appendage in the context of atrial fibrillation or flutter. Importantly, electrical cardioversion for atrial tachycardias results in an acute increased risk of thromboembolism from the LA appendage thought to be related to LA appendage “stunning” and reduced flow (evidenced by Doppler interrogation), rather than the prior hypothesis that LA thrombi are dislodged by the act of cardioversion [31••].
Diagnosis is most commonly made by echocardiography. Transesophageal echocardiography is the imaging modality of choice for evaluation of the left atrium and its appendage [31••]. Diagnosis may also be made via computed tomography [32, 33].
LA thrombi are also seen in association with pathologies resulting in LA dilatation (and altered atrial flow mechanics with stasis). In adults, mitral valve disease (regurgitation, stenosis, and prolapse) can be a cause of LA enlargement and associated thrombosis, particularly in the context of rheumatic heart disease or bacterial endocarditis. LA dilatation in children is seen secondary to myocarditis or cardiomyopathies.
In children, LA thrombosis can also be seen, albeit rarely, after the closure of an atrial septal defect (ASD), whether by transcatheter device or surgical patch closure. After transcatheter device closure of an ASD, thromboprophylaxis with aspirin is recommended for at least 6 months [1] to allow for endothelialization of the device along the atrial septum. It is not typical for aspirin thromboprophylaxis after surgical repair of a secundum or primum defect (though aspirin may be prescribed after repair of a sinus venosus ASD). Upon diagnosis of mass on the LA septum (particularly in the absence of repair of an ASD), atrial myxoma must be considered in the differential diagnosis.
Medical management
The primary goal of treatment for LA thrombosis is the prevention of thromboembolism and stroke. Published statements provide guidance for risk-based anticoagulant selection in adults with atrial fibrillation [34]. The traditional and yet the most widely accepted anticoagulant of choice for atrial fibrillation with and without LA thrombus is VKA therapy with warfarin. However, numerous studies have demonstrated the efficacy and safety of many DOACs in this context [34] and their use is largely becoming the standard of care for atrial fibrillation anticoagulation. DOACs provide advantages over warfarin for their lack of monitoring. Importantly, DOACs are contraindicated for mechanical heart valve prophylaxis based on currently available data evaluating its use in adults [35]. Their applicability for use in children has yet to be determined, but clinical trials are ongoing.
Anticoagulation remains the mainstay of treatment of LA thrombi. The focus should be directed at treating the underlying cause, but anticoagulation should continue until resolution of the thrombus and, perhaps, until resolution or treatment of the underlying pathology. Again, VKA therapy is the treatment of choice with LMWH and DOACs in consideration, based on the clinical context and risk profile.
It may be reasonable to continue aspirin in conjunction with anticoagulation for added platelet inhibition for the indications of ventricular systolic dysfunction, coronary artery disease, or device closure of ASD. However, as with any adjunctive antiplatelet therapy, there may be an increased risk of bleeding adverse events.
Invasive management
In adults with atrial fibrillation, percutaneous occlusion of the LA appendage can be considered. There exists, however, no data to suggest its safety and efficacy in children with LA appendage thrombosis. Transcatheter left atrial or ventricular mechanical thrombolysis is relatively contraindicated for its risk of embolism and stroke.
In selected cases, where the risk of acute thromboembolism and stroke is deemed high and outweighing the risk of hemorrhage, a low-dose systemic alteplase infusion for thrombolysis may be considered [36]. Again, systemic thrombolysis with alteplase should be reserved for acute thrombosis. Notably, a case describing catheter-directed thrombolysis with alteplase to the pulmonary artery for LA thrombus is reported [37]. Safety and efficacy data supporting thrombolysis for LA thrombosis are lacking, particularly in children.
Surgical management of LA and mitral valve infective endocarditis and associated thrombosis may be indicated in cases of refractory cases of progressive disease despite antibiotic and antithrombotic therapy. The decision on timing of surgical intervention, however, is extremely complex with reasons for early intervention and reasons for conservative management in respect to surgery. Some guidance on indications for surgery and considerations for timing are provided [38,39,40,41]; however, little evidence and guidance exist for the timing of surgery for infectious thrombotic disease of the mitral valve.
Operative thrombectomy is rare and often not indicated in the absence of another indication for cardiac surgery or thromboembolic complications despite anticoagulation (such as stroke). In both children and adults undergoing cardiac surgery for another indication, concurrent surgical occlusion of the LA appendage may be considered [34].
Left ventricle
Thrombus formation in the left ventricle (LV) can be attributed to altered LV flow dynamics and stasis caused by myocardial dysfunction. Thrombi are frequently seen in the LV apex. Diagnosis is made by transthoracic echocardiography and transesophageal imaging is not beneficial due to the remote location of the LV apex related to the esophagus [31••]. In children, LV thrombosis is commonly seen in myocarditis and cardiomyopathy (dilated, in particular) that induce altered blood flow and ejection. In adults with acute coronary syndromes, regional wall motion abnormalities associated with ischemia can precipitate LV thrombosis. For these reasons, children and adults with LV dysfunction commonly receive primary thromboprophylaxis with aspirin, at a minimum.
Mechanical support (left ventricular assist device (LVAD) or ECMO) poses high risk for thrombosis and embolism, for which early and consistent systemic anticoagulation is delivered in majority of cases. LVAD thrombosis can present both as acute device dysfunction and also with progressive thrombosis or hemolysis [42]. LVAD dysfunction and inadequate LV drainage can also result in LV apical thrombosis. Inadequate right heart drainage by inadequate ECMO flow can result in stasis and distension of the left heart (especially in the cases of severe ventricular dysfunction). In contrast, LV thrombosis during veno-venous ECMO is less common owing to the requisite normal right heart, pulmonary, and left heart blood flow needed for this type of ECMO support.
Medical management
Left ventricular and LVAD thrombosis requires urgent and aggressive anticoagulation to mitigate the risk of acute cerebral thromboembolism. Again, the traditional inpatient therapy for LV thrombi is heparin [43], but the DTIs are emerging for the treatment of left heart thrombosis as well. The benefits of inhibiting clot-bound thrombin may effectively induce thrombolysis [44]. Importantly, due to the proteolytic degradation of bivalirudin, it should be avoided in scenarios of impaired LV ejection and resultant stasis.
Pharmacologic thrombolysis can be considered for LV thrombosis. Though not reported in the literature, a low-dose continuous infusion of alteplase may be effective at hastening lysis. In an emergent organ- or life-threatening circumstance of acute thrombosis, a bolus of systemic alteplase may be given, similar to the strategy with unstable massive pulmonary embolism. Both strategies of thrombolysis may be considered for LV thrombosis in mechanical circulatory support, but the significant risk of hemorrhage must be weighed.
Subacute and chronic management of LV apical thrombosis can be maintained either with LMWH or VKA therapy [45]. The off-label use of DOACs, however, is avoided in the treatment of LV apical thrombosis, at this time, due to an association with stroke when compared with VKA therapy [46•].
Invasive management
A number of cases of successful operative LV thrombectomy are described in both children and adults, but surgery is commonly reserved for mobile pedunculated LV thrombi [47]. Surgical thrombectomy for mural thrombi (whether flat to the endocardium or with some protrusion) is often avoided. Alternatively, robot- or video-assisted surgical thrombectomy and LV mass resection are described with an acceptable risk of stroke in adults [48]. One case of successful trans-aortic video-assisted LV thrombectomy in a teenage child is identified in the literature [49]. Surgical thrombectomy, however, is often considered if an LV thrombus is present at the time of VAD implantation (to avoid the complication of inflow obstruction).
LVAD thrombosis or LVAD-associated apical thrombosis may require surgical device exchange if medical management fails. Catheter-directed intraventricular thrombolysis with alteplase may be considered. In such cases, the infusion catheter is positioned retro-aortic at the site of LV thrombosis [50, 51].
In VA ECMO, LV thrombosis due to inadequate cardiac decompression may be an indication for percutaneous atrial septostomy or surgical LA vent placement [52]. Thrombolysis with alteplase (whether catheter directed or systemic) for LV thrombosis while on ECMO is not reported.
Coronary arteries
Coronary artery thrombosis and myocardial infarction (MI) in children are relatively uncommon. However, a commonly appreciated etiology for coronary thrombosis is Kawasaki disease with coronary artery ectasia or aneurysm. Childhood MI can also occur as a consequence of congenital heart disease and its repair or palliation, congenital coronary artery anatomic abnormalities, myocarditis and cardiomyopathies, transplant coronary arteriopathy, inherited metabolic disease caused by congenital hyperlipidemia, vasculitides, and drug intoxication (e.g., cocaine). Any cause of MI in a child can be complicated by thrombosis (in addition to the intravascular change caused by primary disease).
Thrombosis in Kawasaki disease and other vasculitides (e.g., Takayasu arteritis, Behcet’s disease, and systemic lupus erythematosus) is caused by irregularities in coronary artery caliber resulting in flow turbulence and stasis. Invariably, there is also endothelial dysfunction and hypercoagulability, completing Virchow’s triad. Diagnosis of thrombosis is made either by echocardiography or coronary CT angiography.
Coronary artery thrombosis secondary to congenital heart disease can be seen in some pre-operative states, for example, pulmonary atresia with intact ventricular septum can be associated with right ventricle–dependent coronary artery circulation and consequent ostial atresia. Myocardial ischemia can be seen after surgical reimplantation or repair of congenital coronary abnormalities, but thrombosis is rare in comparison with ostial stenosis. Thrombosis is reported after arterial switch operation for transposition of the great arteries [53] and repair of the anomalous left coronary artery from the pulmonary artery (ALCAPA) [54]. Though the incidence is not reported, the risk of coronary thrombosis after surgeries requiring reimplantation is appreciated (e.g., aortic valve replacement and the Ross procedure). Coronary artery thrombosis is also reported after palliation for single ventricle disease and can also be seen in the Damus-Kaye-Stansel anastomosis or reconstructed neo-aorta [55, 56]. Again, diagnosis of coronary thrombosis after congenital heart surgery is often made by echocardiography; however, coronary CT angiography is often needed to further profile the extension and severity of thrombosis.
Medical management
Coronary artery thrombosis nearly always requires urgent or emergent intervention. The largest experience in the management of pediatric coronary thrombosis is in the context of giant aneurysms in Kawasaki disease [57••]. Percutaneous coronary intervention (PCI) should be considered but is often not feasible due to a number of factors (e.g., patient size and availability of invasive cardiologist experienced in PCI). If PCI is not pursued, thrombolytic therapy with alteplase should be initiated. For acute coronary thrombosis posing a risk of coronary ischemia, most recommend a low-dose infusion with consideration of uptitration if thrombolysis is not achieved. An emergent high-dose bolus of alteplase is generally not recommended. As is usual, thrombolytic therapy should be followed with systemic anticoagulation and antiplatelet therapy should resume. Such a regimen of low-dose systemic thrombolytic therapy can be applied to the medical management of coronary artery thrombosis from other etiologies. However, thrombolytic therapy for coronary thrombosis may be contraindicated in the setting of recent surgery; this risk must be weighed with the severity of myocardial ischemia.
Invasive management
PCI in Kawasaki disease is indicated when signs and symptoms of ischemia are evident or if progression to myocardial failure occurs. Many of the techniques utilized in adult PCI can be considered in coronary thrombosis secondary to Kawasaki disease. Examples include percutaneous transluminal coronary revascularization with thrombolysis, rotational atherectomy, and balloon and stent angioplasty [58]. Published experience in transcatheter intervention for coronary artery thrombosis in congenital heart disease is limited, but the balloon and stent angioplasty is the most common PCI [59]. Surgical coronary artery bypass surgery is well described for coronary artery thrombosis and MI in children with Kawasaki disease and after congenital heart surgery [60,61,62].
Aortopulmonary shunt thrombosis
Thrombosis of aortopulmonary shunts in the staged palliation of single ventricle disease and ductal-dependent congenital heart disease is a known risk and complication. Thrombosis of all types of shunts (modified Blalock Taussig Thomas, Sano, and central) is reported. The reported incidence of aortopulmonary shunt thrombosis is between 3.3 and 33% [63•, 64, 65]. Thrombosis of these aortopulmonary shunts occurs at two distinct time points: early post-operative occlusion (hours) and late occlusion (weeks). Though these shunts originate in the arterial circulation, all three of Virchow’s risk factors exist at the site of shunt anastomosis. These aortopulmonary shunts are often the sole source of pulmonary blood flow in these patients and thrombosis results in rapidly progressive, and often acute, life-threatening impairment to pulmonary blood flow. The diagnosis of shunt thrombosis is often made clinically with signs of suddenly decreased pulmonary blood flow resulting in acute refractory hypoxemia and hypercarbia (with low exhaled carbon dioxide). Shunt obstruction caused by thrombosis, narrowing, or anatomic disruption is confirmed by echocardiography but often cannot be differentiated without angiography.
Medical management
Rapid emergent recanalization of shunt flow is the primary goal in shunt thrombosis. Though emergent transcatheter or surgical intervention is warranted, these are often not feasible in the setting of a sudden loss of pulmonary blood flow. The initial medical management in the resuscitation of shunt thrombosis includes a bolus of unfractionated heparin while the patient’s hemodynamics are stabilized. An infusion of heparin may be considered but often progression to either mechanical circulatory support with ECMO or referral for invasive intervention takes precedent. Reports of systemic thrombolysis for acute shunt thrombosis are found in the literature [66].
Invasive management
Aortopulmonary shunt thrombosis requires urgent invasive intervention, whether via transcatheter embolectomy with angioplasty or with surgical shunt revision (more desirable in some circumstances, such as early post-operative shunt thrombosis, often thought to be the result of a technical complication at the anastomotic site) [63•]. Transcatheter intervention can include mechanical thrombolysis, catheter-directed pharmacologic thrombolysis, and/or stent angioplasty [67, 68].
Conclusion
Thrombosis within the heart is as a consequence of abnormalities in blood flow and the risk factors of Virchow’s triad. Extension of VTE is a common cause of right heart thrombosis and its management parallels that of pulmonary embolism. Ventricular dysfunction and atrial dilatation are primary risk factors for cardiac chamber thrombosis. Kawasaki disease is a common cause of coronary artery thrombosis and experience in management is extrapolated from adult acute coronary syndromes. Thrombosis can occur throughout any part of the cardiovascular system in congenital heart disease and after surgery for repair or palliation of defects. Heparin and VKA therapy are the mainstays of anticoagulation for cardiac thrombosis and VTE, but direct thrombin inhibitors for inpatient management and DOACs for outpatient management are emerging in the treatment of specific locations of thrombosis.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Giglia TM, Massicotte MP, Tweddell JS, Barst RJ, Bauman M, Erickson CC, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;128:2622–703.
Raffini L, Huang Y-S, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–8.
Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464–74.
Mahajerin A, Branchford BR, Jaffray J, Vasquez B. Children’s Hospital-Acquired Thrombosis Database (CHAT): a multi-institutional database for prospective identification of independent risk factors. Blood. 2016;128(22):2597.
Ögren M, Bergqvist D, Eriksson H, Lindblad B, Sternby NH. Prevalence and risk of pulmonary embolism in patients with intracardiac thrombosis: a population-based study of 23,796 consecutive autopsies. Eur Heart J. 2005;26(11):1108–14.
Panidis IP, Kotler MN, Mintz GS, Ross J. Clinical and echocardiographic features of right atrial masses. Am Heart J. 1984;107(4):745–58.
Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152(1):75–84.
•• Monagle P, Cuello CA, Augustine C, Bonduel M, Brandão LR, Capman T, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2:3292–316 This clinical guideline document authored by experts in the field of pediatric thrombosis from the American Society of Hematology provides the most current recommendations and evidence for the management of pediatric venous thromboembolism (VTE).
Young E, Prins M, Levine MN, Hirsh J. Heparin binding to plasma proteins, an important mechanism for heparin resistance. Thromb Haemost. 1992;67(6):639–43.
Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337(10):688–98.
•• Manlhiot C, Gruenwald CE, Holtby HM, Brandão LR, Chan AK, Van Arsdell GS, et al. Challenges with heparin-based anticoagulation during cardiopulmonary bypass in children: impact of low antithrombin activity. J Thoracic Cardiovasc Surg. 2016;151(2):444–50 This study highlights the importance of relative antithrombin deficiency in neonates and infants in the management of heparin-based anticoagulation in children during cardiopulmonary bypass. The impact of relative antithrombin deficiency in infants, related to developmental hemostasis, is of critical importance in the hemostatic and anticoagulant management in children with congenital heart disease.
Levi M, van der Poll T, ten Cate H. Tissue factor in infection and severe inflammation. Mammen EF, Levi M, editors. Semin Thromb Hemost. 2006;32(1):33–9.
Young G. Anticoagulants in children and adolescents. Hematology Am Soc Hematol Educ Program. 2015;2015:111–6.
Buck ML. Bivalirudin as an alternative to heparin for anticoagulation in infants and children. J Pediatr Pharmacol Ther. 2015;20(6):408–17.
• Young G. How I treat pediatric venous thromboembolism. Blood. 2017;130(12):1402–8 In this invited commentary by Dr. Young, he describes his specific diagnostic and management approaches to cases of pediatric VTE, including selection of anticoagulant pharmacotherapies. Of particular importance to children with congenital and acquired heart disease, he presents an algorithm for the evaluation and management of catheter-related VTE.
Nagle EL, Dager WE, Duby JJ, Roberts AJ, Kenny LE, Murthy MS, et al. Bivalirudin in pediatric patients maintained on extracorporeal life support. Pediatr Crit Care Med. 2013;14(4):e182–8.
Rutledge JM, Chakravarti S, Massicotte MP, Buchholz H, Ross DB, Joashi U. Antithrombotic strategies in children receiving long-term Berlin Heart EXCOR ventricular assist device therapy. J Heart Lung Transplant. 2013;32(5):569–73.
Forbes TJ, Hijazi ZM, Young G, Ringewald JM, Aquino PM, Vincent RN, et al. Pediatric catheterization laboratory anticoagulation with bivalirudin. Catheter Cardiovasc Interv. 2011;77(5):671–9.
Young G, Boshkov LK, Sullivan JE, Raffini LJ, Cox DS, Boyle DA, et al. Argatroban therapy in pediatric patients requiring nonheparin anticoagulation: an open-label, safety, efficacy, and pharmacokinetic study. Pediatr Blood Cancer. 2011;56(7):1103–9.
Young G, Tarantino MD, Wohrley J, Weber LC, Belvedere M, Nugent DJ. Pilot dose-finding and safety study of bivalirudin in infants <6 months of age with thrombosis. J Thromb Haemost. 2007;5(8):1654–9.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–52.
• Esch JJ, Hellinger A, Friedman KG, Van der Pluym CJ. Apixaban for treatment of intracardiac thrombosis in children with congenital heart disease. Interact Cardiovasc Thorac Surg. 2020;30(6):950–1 In this case series, Drs. Esch, VanderPluym, and colleagues describe their use of apixaban for the treatment of refractory intracardiac thrombosis in children with congenital heart disease. These cases highlight the emergence of the direct oral anticoagulants (DOACs) as effective and safe therapies for cardiac and venous thrombosis.
Cetin Iİ, Ekici F, Ünal S, Kocabaş A, Sahin S, Yazıcı MU, et al. Intracardiac thrombus in children: the fine equilibrium between the risk and the benefit. Pediatr Hematol Oncol. 2014;31(5):481–7.
Griffith KE, Jenkins E, Copenhaver W, Williams DM. Novel use of the AngioVac® system to remove thrombus during simultaneous extracorporeal membrane oxygenation life support. Perfusion. 2016;31(2):164–8.
Alsamendi J, Doshi M, Bhatia S, Bordegaray M, Arya R, Morton C, et al. Single center experience with the 637 AngioVac Aspiration System. Cardiovasc Intervent Radiol. 2015;(38)4:998–1004.
Qureshi AM, Petit CJ, Crystal MA, Liou A, Khan A, Justino H. Efficacy and safety of catheter-based rheolytic and aspiration thrombectomy in children. Catheter Cardiovasc Interv. 2016;87(7):1273–80.
Feldman JP, Feinstein JA, Lamberti JJ, Perry SB. Angiojet catheter-based thrombectomy in a neonate with postoperative pulmonary embolism. Cathet Cardiovasc Intervent. 2005;66(3):442–5.
Nicholson GT, Kogon B, Vincent R. AngioJet rheolytic thrombectomy in a neonate with pulmonary artery thrombus. Catheter Cardiovasc Interv. 2013;82(5):E704–7.
Fleming GA, Khan M, Janssen D, Doyle T. Angiojet rheolytic thrombectomy in infants following cardiac surgery. Catheter Cardiovasc Interv. 2010;76(2):233–40.
Malagon I, Evens FCM, Freund MW. Life threatening cavopulmonary thrombus: resolution within 24 h with tissue plasminogen activator. Pediatr Anesth. 2008;18(4):343–4.
•• Saric M, Armor AC, Arnaout MS, Chaudhry FA, Grimm RA, Kronzon I, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr. 2016;29(1):1–42 The American Society of Echocardiography presents guidelines for the use of echocardiography for the evaluation of cardiac thrombosis. Though the authors present guidelines for evaluation in adults, no similar publication exists with such broad guidance in children.
Mosleh W, Sheikh A, Said Z, Ahmed MAA, Gadde S, Shah T, et al. The use of cardiac-CT alone to exclude left atrial thrombus before atrial fibrillation ablation: efficiency, safety, and cost analysis. Pacing Clin Electrophysiol. 2018;41(7):727–33.
Guglielmo M, Baggiano A, Muscogiuri G, Fusini L, Andreini D, Mushtaq S, et al. Multimodality imaging of left atrium in patients with atrial fibrillation. J Cardiovasc Comput Tomogr. 2019;13(6):340–6.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–51.
Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–14.
Asante-Korang A, Sreeram N, McKay R, Arnold R. Thrombolysis with tissue-type plasminogen activator following cardiac surgery in children. Int J Cardiol. 1992;35(3):317–22.
Pollert L, Prikrylova Z, Berousek J, Mosna F, Lischke R. Direct thrombolysis of multiple thrombi in both right and left heart atrium in a patient on extracorporeal membrane oxygenation support following urgent double-lung transplantation: a case report. Ther Clin Risk Manag. 2016;12:1003–8.
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–128.
Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–86.
Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010;121(9):1141–52.
Venn RA, Ning M, Vlahakes GJ, Wasfy JH. Surgical timing in infective endocarditis complicated by intracranial hemorrhage. Am Heart J. 2019;216:102–12.
Raffa GM, D’Ancona G, Sciacca S, Pietrosi A, Hernandez Baravoglia CM, Turrisi M, et al. Systemic or endoventricular thrombolysis to treat HeartWare left ventricle assist device thrombosis: a clinical dilemma. Artif Organs. 2015;39(6):526–9.
Paç FA, Cağdaş DN. Treatment of massive cardiac thrombi in a patient with protein C and protein S deficiency. Blood Coagul Fibrinolysis. 2007;18(7):699–702.
Weeks P, Sieg A, Rajapreyar I, Nathan S, Jumean M, Patel M, et al. Bivalirudin for left ventricular assist device thrombosis. J Thromb Thrombolysis. 2018;46(4):496–501.
Choi S-H, Jeong SI, Yang J-H, Kang I-S, Jun T-G, Lee H-J, et al. A single-center experience with intracardiac thrombosis in children with dilated cardiomyopathy. Pediatr Cardiol. 2010;31(2):264–9.
• Robinson AA, Trankle CR, Eubanks G, Schumann C, Thompson P, Wallace RL, et al. Off-label Use of direct oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA Cardiol. 2020;5(6):685–95 Though the option of the DOACs continues to demonstrate promise in the treatment of both adult and pediatric antithrombosis, this study provides a reminder of the importance of continued study of DOACs for the management of cardiac thrombosis.
Dechant M-J, Siepe M, Stiller B, Grohmann J. Surgical thrombectomy of two left ventricular thrombi in a child with acute myocarditis. Pediatrics. 2013;131(4):e1303–7.
Soylu E, Kidher E, Ashrafian H, Stavridis G, Harling L, Athanasiou T. A systematic review of left ventricular cardio-endoscopic surgery. J Cardiothorac Surg. 2017;12(1):41.
Mazza IL, Jacobs JP, Aldousany A, Chang AC, Burke RP. Video-assisted cardioscopy for left ventricular thrombectomy in a child. ATS. 1998;66(1):248–50.
Dang G, Epperla N, Muppidi V, Sahr N, Pan A, Simpson P, et al. Medical management of pump-related thrombosis in patients with continuous-flow left ventricular assist devices: a systematic review and meta-analysis. ASAIO J. 2017;63(4):373–85.
Thenappan T, Anderson AS, Jeevanadham V, Rich JD, Shah AP. Treatment of left ventricular assist device thrombosis with extended catheter-directed intraventricular thrombolytic therapy. Circ Heart Fail. 2013;6(3):e27–9.
Weber C, Deppe A-C, Sabashnikov A, Slottosch I, Kuhn E, Eghbalzadeh K, et al. Left ventricular thrombus formation in patients undergoing femoral veno-arterial extracorporeal membrane oxygenation. Perfusion. 2018;33(4):283–8.
Bacha EA, Quinones J, Kahana MD, Baron JM, Hijazi ZM. Embolic coronary occlusion after the arterial switch procedure. J Thorac Cardiovasc Surg. 2001;122(5):1028–30.
Freud LR, Koenig PR, Russell HM, Patel A. Left ventricular thrombus formation after repair of anomalous left coronary artery from the pulmonary artery. World Journal for Pediatric and Congenital Heart Surgery. 2014;5(2):342–4.
Mesher AL, Hermsen JL, Rubio AE, Chen JM, McMullan DM. Neoaortic thrombus after Norwood procedure: complication of extracorporeal life support? Ann Thorac Surg. 2015;99(2):709–10.
Keraliya AR, Murphy DJ, Steigner ML, Blankstein R. Thrombus in hypoplastic aorta: an uncommon cause of acute myocardial infarction. J Cardiovasc Comput Tomogr. 2016;10(3):263–4.
•• Burns JC, El-Said H, Tremoulet AH, Friedman K, Gordon JB, Newburger JW. Management of myocardial infarction in children with giant coronary artery aneurysms after Kawasaki disease. J Pediatr. 2020;221:230–4 Drs. Burns, Newburger, and colleagues describe, in this contemporary commentary, their experience and highlight their world-renowned expertise in the diagnosis and management of acute myocardial infarction (MI) after Kawasaki disease. Specific management of alteplase and anticoagulation dosing is described in a manner that can be applied to local institutional practice.
Ishii M, Ueno T, Akagi T, Baba K, Harada K, Hamaoka K, et al. Guidelines for catheter intervention in coronary artery lesion in Kawasaki disease. Pediatrics international: official journal of the Japan Pediatric Society. 2001;43:558–62.
Callahan R, Lock JE, Shah PB, Marshall AC. Transcatheter intervention of coronary obstructions in infants, children, and young adults. Pediatr Cardiol. 2018;39(7):1299–307.
Kitamura S. Pediatric coronary artery bypass surgery for congenital heart disease. Ann Thorac Surg. 2018;106(5):1570–7.
Kitamura S, Tsuda E, Kobayashi J, Nakajima H, Yoshikawa Y, Yagihara T, et al. Twenty-five-year outcome of pediatric coronary artery bypass surgery for Kawasaki disease. Circulation. 2009;120(1):60–8.
Manlhiot C, Menjak IB, Brandão LR, Gruenwald CE, Schwartz SM, Sivarajan VB, et al. Risk, clinical features, and outcomes of thrombosis associated with pediatric cardiac surgery. Circulation. 2011;124(14):1511–9.
• Patregnani JT, Sochet AA, Zurakowski D, Klugman D, Diab Y, Berger JT, et al. Cardiopulmonary bypass reduces early thrombosis of systemic-to-pulmonary artery shunts. World Journal for Pediatric and Congenital Heart Surgery. 2018;9(3):276–82 This is a retrospective cohort study evaluating patients for early shunt thrombosis, specifically after modified Blalock Taussig Thomas (BTT) shunt. They identify a number of risk factors for early thrombosis, most of which are not modifiable. In addition to an excellent review of the literature studying aortopulmonary shunt thrombosis, the authors highlight the importance of consideration for early antiplatelet therapy.
Li JS, Yow E, Berezny KY, Rhodes JF, Bokesch PM, Charpie JR, et al. Clinical outcomes of palliative surgery including a systemic-to-pulmonary artery shunt in infants with cyanotic congenital heart disease: does aspirin make a difference? Circulation. 2007;116(3):293–7.
O’Connor MJ, Ravishankar C, Ballweg JA, Gillespie MJ, Gaynor JW, Tabbutt S, et al. Early systemic-to-pulmonary artery shunt intervention in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2011;142(1):106–12.
Diaz F, Sasser WC, Law MA, Alten JA. Systemic thrombolysis with recombinant tissue plasminogen activator for acute life-threatening Blalock-Taussig shunt obstruction. Indian J Crit Care Med. 2016;20(7):425–7.
Giglia TM, DiNardo J, Ghanayem NS, Ichord R, Niebler RA, Odegard KC, et al. Bleeding and thrombotic emergencies in pediatric cardiac intensive care: unchecked balances. World. J Pediatr Congenital Heart Surg. SAGE Publications. 2012;3(4):470–91.
Diab YA, Ramakrishnan K, Alfares FA, Hynes CF, Chounoune R, Shankar V, et al. Transcatheter treatment of thrombosis in the single ventricle pathway: an institutional experience. Congenit Heart Dis. 2016;11(1):39–44.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology/CT Surgery
Rights and permissions
About this article
Cite this article
Kim, J.S., Loi, M.M., Stone, M.L. et al. Medical and Invasive Management of Cardiac Thrombosis in Children with Congenital and Acquired Heart Disease. Curr Treat Options Peds 6, 283–298 (2020). https://doi.org/10.1007/s40746-020-00214-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-020-00214-3