Abstract
At elevated temperatures, coating develops protective oxide scales on the surfaces, ensuring prolonged stability. In this investigation, ASTM-SA213-T-22 (T22) boiler tube steels underwent coating with NiCrAlY/Cr3C2/h-BN/Mo matrix using plasma spray to resist oxidation and hot corrosion. The study involved 50 cycles at 700 °C, evaluating hot corrosion in a molten salt atmosphere (Na2SO4–60%V2O5) and oxidation in static air. Oxidation kinetics was determined via thermogravimetric analysis, while XRD and SEM/EDAX methods characterized the produced oxide scales. The NiCrAlY/Cr3C2/h-BN/Mo coated T22 substrate demonstrated reduced oxidation rates compared to uncoated steels, with gradual scale growth kinetics and surface oxides of NiO and Cr2O3 in air. In molten salt, accelerated oxidation resulted in metastable Cr2O3 formation. Cr, Al, B, and Mo preferentially oxidized along the nickel-rich splat boundary, hindering oxygen penetration through pores and voids, thereby ensuring stable oxidation. The uncoated steel experienced rapid corrosion, characterized by the detachment and flaking off of the unprotected Fe2O3 oxide layer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The harsh operational conditions within coal-fired power plant boilers, characterized by high temperatures, lead to accelerated material degradation, particularly in superheater tubes.To overcome these challenges, components like heat transfer pipes require exceptional resistance to high-temperature corrosion and oxidation [1,2,3,4]. Surface modification of alloy components with coatings is a common strategy to enhance their resistance to high-temperature oxidation and hot corrosion. Thermal spraying has emerged as a prominent method for depositing protective corrosion coatings on alloys [5,6,7,8]. These coatings find wide-ranging applications in various industries, including aerospace, automotive, and mining, for safeguarding critical metal components in aero and industrial gas turbines, high-temperature power plants, and more [9,10,11]. Among thermal spraying techniques, plasma thermal spray stands out as a popular choice due to its versatility, cost-effectiveness, and ability to produce thick and uniform coatings of superior quality [12,13,14,15].
For machinery like boilers, gas turbines, and waste incinerators that operate at high temperatures, hot corrosion presents a serious problem. It causes abrupt and erratic material deterioration, which reduce the component load-bearing capacity and eventually results in catastrophic failures [16,17,18,19,20]. Because of cost concerns as well as the depletion of premium fuels, residual fuel oil is now widely used in power generation systems. However, sulfur, vanadium, and salt are among the contaminants found in residual fuel oil. The consequences of hot corrosion are exacerbated when these contaminants combine to produce compounds with low melting points, which speed up the rate of oxidation when deposited on surfaces [21,22,23,24,25].
Applying thin, wear, corrosion, chemical, and oxidation-resistant coatings with superior thermal conductivity is one way to overcome these obstacles [26,27,28]. A way to make materials usable at high temperatures is through coatings. When applied in high-temperature environments, coatings need to be thick enough to act as a shield against the spread of corrosive agents onto the substrate. Additionally, coatings should be sufficiently dense to fill gaps and increase the development of protective oxides [29,30,31]. Metallic coatings have been deposited on surfaces using a variety of techniques. For high-temperature applications, thermal spray techniques like flame, arc, plasma, detonation gun, and high-velocity oxy-fuel (HVOF) spraying are frequently utilised [32,33,34,35,36].
The protection of underlying substrates against oxidation, hot corrosion, and erosion is achieved via thermal sprayed coatings, which rely on NiCrAlY powder-based coatings [13, 37,38,39,40]. When the coating temperature reaches 700 °C, these NiCrAlY coatings start to develop protective oxide scales of Cr2O3 or Al2O3. However, outside of this temperature range, alumina does not regenerate, therefore the coatings may deteriorate more quickly. Cr and Al are being depleted as a result of elemental inter-diffusion and which is the cause of this deterioration [14,15,16, 26]. Furthermore, serious corrosion and coating spalling can only be avoided with high mechanical strength and hardness [41, 42]. Hard minerals like molybdenum, chromium carbide (Cr3C2), and solid lubricant boron nitride (BN), in powder form, can be added to further enhance the properties of resistance to corrosion, heat, chemicals, and wear [18, 19, 29, 30].
It is possible to apply protective coatings to boiler tube steels that can withstand the corrosive effects of high temperatures. A mixture of metals and ceramics can be applied to a surface using plasma spraying technology, producing coatings with remarkable corrosion resistance, chemical resistance, and thermal stability. These coatings are composed of elements such as nickel-based alloys, chromium carbide, molybdenum, and boron nitride. Boiler tube systems are protected from oxidation, hot corrosion, and other types of degradation by the synergistic combination of these materials [20,21,22,23,24]. By acting as a shield against oxidation and hot corrosion wear, these coatings protect the tube surfaces from the working environment [31,32,33]. Protective coatings can be specifically designed to handle issues that arise in boiler applications, like high temperatures, corrosive gases, and abrasive particles [34].
In addition to the coating materials, the impact of various salts on substrate materials at elevated temperatures is crucial. Prominent salts such as sodium sulfate (Na2SO4) and vanadium pentoxide (V2O5) are known to cause severe hot corrosion. Sodium sulfate can lead to the formation of a molten salt layer that promotes rapid oxidation and scale formation. Vanadium pentoxide, commonly found in combustion environments, can form eutectic mixtures with other salts, exacerbating the corrosion process. These salts penetrate the protective oxide layers, accelerating degradation and leading to significant material loss and structural integrity issues.
The enhancement of alloy coatings with elements such as molybdenum, chromium carbide (Cr3C2), and solid lubricant boron nitride (BN) has been the subject of extensive research. These additions are known to improve the high-temperature performance and corrosion resistance of coatings [5,6,7]. Molybdenum, for instance, has been shown to enhance the hardness and wear resistance of coatings. Chromium carbide (Cr3C2) contributes to the formation of a stable and protective oxide layer, significantly improving oxidation resistance. Solid lubricant boron nitride (BN) is recognized for its lubricating properties, reducing friction and wear at high temperatures [3,4,5,6].
Upon reviewing the publicly accessible literature, it was observed that there is insufficient information regarding the performance of NiCrAlY/Cr3C2/h-BN/Mo plasma-sprayed coatings at elevated temperatures. The objective of this research was to evaluate the oxidation resistance and corrosion resistance of NiCrAlY/Cr3C2/h-BN/Mo plasma-sprayed coatings applied on T22 boiler steel alloys at 700 °C for 50 cycles, in a high-temperature molten salt environment (Na2SO4–60%V2O5). Utilizing cutting-edge coating materials such as solid lubricants (h-BN), molybdenum, and Cr3C2, the study aimed to improve the resistance against typical high-temperature degradation mechanisms present in industrial boiler setups. The research outcomes showed that the coating resistance to hot corrosion and oxidation was greatly increased by the addition of Cr3C2, Mo, and h-BN, extending their lifespan under harsh operating circumstances. To simulate the operating environment of industrial components, which frequently experience cyclic operations, the experiments were carried out under cyclic parameters. The findings of this study will contribute to assessing the feasibility of utilizing plasma spray coatings on boiler tubes.
2 Materials and Method
2.1 Preparation of Coatings
A Fe-based alloy steel, namely 2.44Cr –0.97Mo steel ASME-SA213-T-22 (Grade T22), was chosen as the substrate material for the current study. In Indian power plants, this alloy is frequently utilised as a tube material for the boilers. Samples that were rectangular and measuring 25 × 25 × 5 mm3 were taken from a wire EDM machine. Before coating, the specimens underwent careful surface preparation to guarantee the best possible adherence and homogeneity. SiC emery abrasive sheets were used to precisely grind the substrate surface, and then alumina powders were used for grit blasting. By using this technique, contaminants were guaranteed to be eliminated and the substrate surface was given the perfect roughness profile to improve coating adherence.
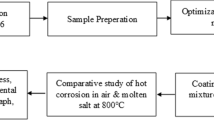
Commercially available feedstock powders, including 50%NiCrAlY/30%Cr3C2/10%h-BN/10%Mo, were selected for their composition and desired properties in oxidation, chemical and heat resistance. The coatings were applied onto the T22 boiler steel substrate using the plasma spray technique. This method ensured precise control over coating deposition parameters such as temperature, velocity, and feed rate, resulting in the desired coating thickness and microstructure. Detailed information on the experimental setup and conditions employed during the coating deposition process can be found in Table 1, facilitating reproducibility and comparison of results across experiments. Additionally, Fig. 1 outlines the workflow of the study.
2.2 Coating Thickness Measurement
The cross-sectional analysis of the coating involves evaluating its thickness and examining its profiles transversely. This allows for direct measurement of the film thickness using both optical and scanning electron microscopes (SEM). The analysis comprises three main phases: sample preparation (including sampling, embedding, and lapping), SEM microscopic inspection, and data processing (using specialized software to quantify image dimensions). Sample preparation is particularly crucial and time-consuming, as it is prone to introducing artifacts that could compromise further investigations. Therefore, meticulous attention to detail during sample preparation is essential to ensure the accuracy and reliability of coating thickness measurements.
2.3 Hot Corrosion and Oxidation Test
Understanding material behavior, particularly in harsh industrial environments such as coal-fired thermal power plants and incinerators, relies heavily on studying hot corrosion. To replicate real-world conditions faced by boiler tubes and other components, both coated and uncoated substrates are subjected to a controlled molten salt environment (40%Na2SO4-60% V2O5) at 700 °C for approximately 50 cycles and each cycle consists of one hour of isothermal heating at 700 °C, followed by a 20-min cooling period at ambient temperature. The oxidation test chamber's precise visual is shown in Fig. 2, an IFC model that offers a thorough view of the chamber's structure to aid in experimental setup and analysis This experimental setup is accurate as it mimics the corrosive conditions typically encountered in such environments. A high-temperature silicon carbide tube furnace with an error range of 5 °C is used to ensure precise temperature control, which guarantees the accuracy of the experimental data. Specimens are carefully prepared, including surface cleaning and exact weight and dimension measurements, before being exposed to the hot environment. These steps are essential to guarantee constant substrate adhesion and salt coating, which produce repeatable and trustworthy test results. It is also necessary to apply a salt coating within a particular thickness range and thoroughly clean the salt mixture in order to preserve consistency between specimens and avoid differences in the corrosion process.
To ensure that the test conditions accurately simulate the anticipated high-temperature environment, the heating stages are designed to remove any remaining moisture from the salt-coated specimens. The process of cyclic exposure entails heating and cooling repeatedly, which makes it possible to examine long-term corrosion behaviour and determine oxidation kinetics. Frequent weight change measurements for every cycle yield a quantitative indicator of corrosion rates and provide important information about how materials deteriorate over time. By enabling the identification of any obvious indications of scale damage or spallation, visual inspections support these measures and contribute to our understanding of corrosion mechanisms. For each test, the test specimen’s appearance, color changes, cracking, and spalling were meticulously recorded. All of the oxidized and corroded samples were then characterized using XRD, SEM/EDS, and EDS mapping analysis.
Similarly, cyclic oxidation tests are performed on both the substrate and coated samples at 700 °C for 50 cycles utilizing a tubular furnace from IFC. Before testing, the surface area of each sample is measured. Each cycle involves heating at 700 °C for 1 h followed by cooling at ambient temperature for 20 min. The weight change for each cycle is meticulously recorded using an electronic balance with a sensitivity of 0.001 mg. These cyclic oxidation tests are aim to mimic real-world conditions experienced in thermal power plants, including frequent power failures.
2.4 Coating Characterisation
All the coated samples were subjected to X-ray analysis utilizing an XRD machine fitted with a CuKα radiation-powered XPERT Pro system diffractometer. The 2θ range of 10° to 100° was scanned at a pace of 2°/min to record the peaks. The coating surface morphology, cross-sectional morphology, and EDS mapping were assessed using Genesis software and an FESEM with an EDS attachment (located at TUV Bangalore, India). Using Image J image processing software, porosity was measured from surface SEM micrographs; an average of ten measurements for the porosity were reported.
3 Results
3.1 Powder Morphology
Figure 3 micrograph displays uneven dispersion of components in the powder matrix, showing various particle sizes and shapes. Phases corresponding to Cr3C2 carbides, h-BN, and Mo particles, and NiCrAlY alloy are evident, highlighting the blend's complexity. The micrograph also reveals particle surfaces and boundaries, indicating potential bonding sites within the matrix. This SEM analysis is crucial for understanding particle distribution in coating materials, impacting final coating properties. Additionally, it provides insights into reinforcement effects, enhancing coating performance. A cross-sectional study Fig. 3a reveals a coating thickness of 310 µm, assessed at 200× magnification with an optical microscope.
The Fig. 4 SEM image, combined with energy-dispersive X-ray spectroscopy (EDX) analysis of the T22 boiler steel alloy substrate, provides a comprehensive insight into its elemental composition and microstructural features. Through the SEM images, we can visualize the surface morphology and structural characteristics of the substrate material. Meanwhile, the EDX data offers quantitative information about the elemental distribution across the surface and within the bulk of the substrate. By correlating the SEM images with the EDX data, we can precisely identify the presence and distribution of specific elements within the substrate. This analysis enables us to characterize the composition of the substrate material accurately, identifying key elements and their spatial distribution. Additionally, the microstructural features revealed by the SEM images allow for a detailed examination of the substrate's surface morphology and texture, providing valuable insights into its structural properties and potential areas of interest for further investigation..
3.2 Morphology of As-Sprayed Coatings
The combination of scanning electron microscopy (Fig. 5a) and energy-dispersive X-ray spectroscopy (Fig. 5b) provides valuable insights into the microstructure and elemental composition of plasma-sprayed NiCrAlY/Cr3C2/h-BN/Mo coatings on T22 boiler steel. SEM imaging (Fig. 4a), reveals the surface morphology and uniform microstructure of the coating, which exhibits dense packing, a well-adhered coating-substrate interface, and minimal porosity. The coatings primarily consist of nickel, chromium, aluminum, carbon, boron, molybdenum, and nitrogen, with their spatial distribution mapped throughout the coating. EDAX analysis Fig. 4b offers quantitative data on the elemental composition, enabling the identification and distribution of specific elements within the coatings. Understanding the chemical composition aids in evaluating characteristics such as mechanical strength, corrosion resistance, and thermal stability. Components like carbon and boron from h-BN and Cr3C2 impart lubricating, chemical, and wear-resistant properties, and nitrogen from h-BN may contribute to nitride phase formation, further enhancing coating performance and molybdenum (Mo) improves the chemical and hot resistance due to its high melting point.
3.3 XRD Analysis of the Uncoated and Coated Substrate
As seen in Fig. 6, the substrate material (T22 boiler steel) underwent X-ray diffraction (XRD) investigation, which produced unique diffraction patterns. Prior to hot cyclic oxidation and corrosion testing, the steel alloy crystalline planes are represented by the peaks seen in the XRD spectrum.
3.4 Hot Corrosion
The hot corrosion test results show the kinetics of a 40% Na2SO4—60% V2O5 eutectic combination. Figure 7 depicts photos of samples from the hot cyclic corrosion test. Uncoated T22 steel resulted in a higher corrosion rate, significant weight gain, and severe scale peeling. This enhances oxygen absorption by allowing oxygen to diffuse through the liquid salt layer.
An X-ray diffraction (XRD) study is necessary to comprehend the hot corrosion and oxidation resistance of the NiCrAlY/Cr3C2/h-BN/Mo coating that is plasma-sprayed on the T22 boiler steel alloy. The phase composition, crystallographic structure, and phase transitions that occur in the coatings under corrosive and high-temperature circumstances are all clarified by this analytical approach. Through examination of the coated specimens, XRD provides information on the formation of protective oxides, the evolution of crystalline phases, and the stability of the coating microstructure. Different crystallographic phases in the coatings and substrate, such as metallic and ceramic phases, are represented by the distinctive diffraction peaks in the XRD patterns. Environmentally-induced phase transitions or lattice distortions are indicated by changes in peak intensity, location, or width. Phase abundance and crystallite size, which are critical for evaluating coating performance and stability, can also be quantitatively determined via XRD analysis as depicted in Fig. 8. The uncoated T22 substrate exhibited the most significant increase in weight gain during hot corrosion tests. Deviation from the parabolic rate observed for uncoated steels indicates resistance against weight gain at 700 °C due to oxide layers. However, the uncoated substrate showed the highest weight gain as presented in Fig. 9a, with the formed scale being brittle and experiencing severe spalling. Conversely, NiCrAlY/Cr3C2/h-BN/Mo-coated steels demonstrated significantly lower weight gain in a Na2SO4–60%V2O5 molten salt environment. Solid crystalline phases, such as Cr2O3 and NiO, Cr2Mo3O12, and Y2(Si2O7) create protective scales that protect coated components from oxidation and corrosion, extending their lifespan. On the other hand, the growth of Fe2O3 oxide on the uncoated substrate suggests degradation mechanisms, such as phase dissolution or oxide spallation, that compromise coating integrity and functionality. In the presence of molten salt, the parabolic rate constant (Kp) values were determined as 0.00791 mg^2 cm^− 4 s^− 1 for the uncoated T22 substrate and 0.000113 mg^2 cm^− 4 s^− 1 for the NiCrAlY/ Cr3C2/h-BN/Mo substrate as shown in Fig. 9b. The parabolic rate constant (Kp) consistently decreased across the coated steels compared to the uncoated ones, suggesting effective protection provided by the plasma-sprayed NiCrAlY/Cr3C2/h-BN/Mo coatings. Similar conclusions from many studies support the superior corrosion resistance of plasma-sprayed coatings over substrate boiler steels and Fe-based superalloys exposed to Na2SO4-60%V2O5 environments at 700℃ [22,23,24,25].
a Graph depicting the change in weight per unit area versus the number of cycles for both uncoated and plasma-sprayed T22 steel under cyclic corrosion conditions for 50 cycles at 700℃. b: Plot illustrating the (Weight change/area)2 Vs. Time for both the uncoated and plasma-sprayed NiCrAlY/Cr3C2/h-BN/Mo coated substrate under cyclic corrosion conditions for 50 cycles at 700 °C
Figure 10a and b displays the SEM pictures that depict the surface scale morphology and EDA analysis at a specific location of interest on both the coated and uncoated steel samples. These images were after exposure to a molten salt environment at a temperature of 700 °C. The coated steel surface (Fig. 10b) exhibits a surface scale that is free from cracks, while the untreated substrate surface (Fig. 10a) shows significant surface spallation. The EDA analysis reveals an elevated percentage of Cr2O3, NiO, Ni3(VO4)2and MoO3 on the coated surface. Elemental X-ray mapping of uncoated and NiCrAlY/Cr3C2/h-BN/Mo-coated specimens exposed to corrosion in a molten salt environment of 40% Na2SO4-60% V2O5 is shown in Fig. 11a and b. For an uncoated substrate, oxides of ferrous (Fe) cover the majority of the top layer; for coated substrates, oxides of molybdenum, chromium (Cr), and aluminum (Al) are produced on the top layer.
The presence of Cr2O3 and NiO is demonstrated by the combined mapping for chromium (Cr) and oxygen (O), which successfully prevents the migration of oxygen and other oxidizing agents into the coating material [17]. The distribution of Na and V within the dominating oxide scale is also disclosed by the result. This distribution indicates that the combination of Na2SO4-60% V2O5 may cause an acidic fluxing phenomenon to occur. The existing thermodynamic data indicates that Na2SO4 can interact with V2O5, increasing the melt's acidity by forming vanadate’s, especially NaVO3, which contributes to the environment's increased acidity.
3.5 High-Temperature Cyclic Oxidation
Uncoated T22 steel (Fig. 12a) showed notable evidence of deterioration throughout the 700 °C hot cyclic oxidation investigation, including uneven scaling, cracks, and spalling, which became more severe after the third cycle. On the other hand, a color shift and the formation of dark lines in the NiCrAlY/Cr3C2/h-BN/Mo coated sample during the first cycle suggested different surface chemistry and oxidation processes. No information on scale spallation was found for the coated sample in spite of increased interactions with the oxidizing environment, suggesting improved resistance to deterioration (Fig. 12b). The significance of protective coatings in preventing oxidation-induced degradation and preserving material integrity at higher temperatures is highlighted by these results [43, 44]. Iron, nickel, and cobalt boiler steels are subjected to protective oxidation of aluminum or chromium in order to create oxide layers that prevent the entry of corrosive substances and increase service life.
Figure 13a and b display two important graphs that plot the weight change per unit area versus the number of cycles for T22 steel samples that are plasma-sprayed, uncoated, and undergo 50 cycles of cyclic oxidation at 700 °C. Plot 13a provides insights into the oxidation kinetics and the subsequent specimen deterioration by showing the weight change per unit area progression during the oxidation cycles. Early cycle weight changes in both the coated and uncoated samples are negligible, indicating a relatively steady oxidation behaviour. However, the weight change patterns of the coated and uncoated samples diverge noticeably as the number of cycles increases.
a Graph depicting the relationship between weight change per area and the number of cycles for both uncoated and plasma-sprayed T22 steel under cyclic oxidation conditions for 50 cycles at 700 °C. b Illustrates the plot of (Weight change/area)2 Vs. Time plot for both uncoated and plasma sprayed NiCrAlY/Cr3C2/h-BN/Mo coated substrates undergoing cyclic oxidation for 50 cycles at 700 °C
The weight change per unit area of the uncoated T22 steel increases dramatically under severe cyclic oxidation conditions, indicating a quicker rate of oxidation and degradation. However, as evidenced by the much lesser weight change per unit area over the course of the cyclic oxidation period, the plasma-sprayed T22 steel coatings show superior resistance to oxidation. Plot 13b further clarifies these trends by showing the square of the weight change per unit area against the number of cycles. This figure facilitates the comparison of the oxidation kinetics of the coated and uncoated specimens, highlighting the superior performance of the plasma-sprayed T22 steel in avoiding oxidation-induced weight gain during extended exposure cycles. The results highlight the extent to which the plasma-sprayed coatings work to improve T22 steel's oxidation resistance and increase its service life in a hot environment.
Figure 13b illustrates how the coatings' oxidation behavior in air can be approximately described as parabolic. A linear least-squares fit approach was used to calculate the values of the parabolic rate constant (Kp). Kp was determined to be 0.00197 mg^2 cm^− 4 s^− 1 for the untreated substrate and 0.0000180 mg^2 cm^− 4 s^− 1 for the NiCrAlY/Cr3C2/h-BN/Mo coated substrate. These findings show that the plasma-sprayed coatings significantly outperform the uncoated T22 steel with regard to the oxidation resistance, suggesting a longer service life in high-temperature conditions.
The SEM/EDS analysis combined with EDS analysis provides insights into the microstructural and elemental composition changes in the T22 boiler steel specimens, both uncoated and plasma-sprayed coated, following exposure to 50 cycles of cyclic oxidation at 700ºC. SEM images in Fig. 14a reveal notable surface characteristics such as fractures, uneven scaling, and spalling in the uncoated T22 boiler steel, indicating severe deterioration due to oxidation.
The existence of oxygen-rich phases in the EDS study supports the hypothesised corrosion mechanisms that these surface defects correspond with. On the other hand, the NiCrAlY/Cr3C2/h-BN/Mo coated T22 boiler steel is shown in Fig. 14b SEM images with a smoother surface morphology, suggesting a stronger resistance to oxidation-induced degradation. By acting as a barrier, the protective coating reduces the creation of hazardous oxides and prevents the spread of corrosive species. The coated specimen's more uniform elemental distribution and the presence of protecting elements like boron, nickel aluminium, and chromium are further evidence of the surface coating's ability to reduce oxidation, according to EDS analysis.
The spatial distribution of elements within a material can be understood by SEM/EDS elemental mapping, which helps with understanding microstructural improvements and compositional homogeneity assessment. The SEM/EDS elemental mapping of the NiCrAlY/Cr3C2/h-BN/Mo samples that were plasma-sprayed onto the substrate (T22 boiler steel alloy) is shown in Fig. 15a & b. These maps show how different components are arranged and concentrated in space within the coated samples, making it possible to analyze how these elements are distributed throughout the surface. Any compositional changes within the coating structure can be found by comparing the elemental maps with SEM images. This information is useful in assessing the integrity and homogeneity of the coatings as well as how well the plasma spray method achieves the required elemental composition.
After being subjected to 50 cycles of cyclic high-temperature oxidation at 700 °C, the uncoated and NiCrAlY/Cr3C2/h-BN/Mo coated T22 specimens, respectively, show the X-ray elemental mapping results in Fig. 15a and b. The coated T22 sample in Fig. 15b displays a scale primarily composed of aluminium, chromium, molybdenum, oxygen, nickel, yttria, and boron. Oxygen is prominently present in the thicker band at the top of the scale, while aluminum forms the subscale area just below. Additionally, aluminum and chromium are observed at the boundaries of nickel-rich plasma spray splats, with scattered boron nitride (BN) particles within the scale. In contrast, Fig. 15a shows the uncoated substrate with the presence of iron and oxygen.
After 50 cycles of cyclic high-temperature oxidation at 700 °C, Fig. 16 shows X-ray powder diffraction patterns showing the top scale produced on the substrate both with and without coating. Fe2O3-indicating peaks for the damaged T22 substrate are visible on the scale. On the other hand, the major phases on the NiCrAlY/Cr3C2/h-BN/Mo coated substrate are Cr2O3, NiO, and AlNi3, with BN and yttria present as minor phases.
4 Discussion
The study of plasma-sprayed NiCrAlY/ Cr3C2/h-BN/Mo coating's resistance to hot corrosion and oxidation on T22 boiler steel alloy provides valuable insights into its potential for mitigating degradation in high-temperature environments. Cyclic oxidation experiments were conducted in air at 700 °C for both uncoated and coated steel. The weight gain of coated substrate was 9.3 times lower than the uncoated T22 substrate. The oxide layer on the uncoated steel exhibited spallation and material peeling, attributed to Fe2O3 presence. Surface SEM/EDS analysis of NiCrAlY/ Cr3C2/h-BN/Mo-coated T22 boiler steel revealed globular particles in the scale, with elevated levels of O, Cr, Al, Y, Mo, and Ni. Predominant phases include NiO, Cr2O3, Y2O3, Cr2Mo3O12, and Y2(Si2O7), as confirmed by XRD patterns. Cr2O3 presence in the oxide scale of NiCrAlY/Cr3C2/h-BN/Mo-coated substrate is protective against oxidation, reducing hot cyclic oxidation rates. These oxide formations along splat boundaries and within coating pores likely created diffusion barriers against corrosive species. Also, the presence of Cr2O3, MO, and oxides of Ni contributes to oxidation protection.
When exposed to oxygen at high temperatures, the oxidation reaction of NiCrAlY usually results in the formation of oxides of nickel (Ni), chromium (Cr), aluminium (Al), and yttrium (Y).
This reaction produces nickel oxide (Ni2O3), chromium oxide (Cr2O3), aluminum oxide (Al2O3), and yttrium oxide (Y2O3), respectively, from the interaction of nickel (Ni), chromium (Cr), aluminum (Al), and yttrium (Y) in the NiCrAlY alloy with oxygen (O2) in the air. On the surface of the coated substrate, these oxides usually form a protective oxide scale that aids in preventing additional oxidation and material degradation at elevated temperatures.
When oxygen is exposed to boron nitride at elevated temperatures, boron oxide and nitrogen oxides are formed. Similarly, chromium carbide Cr3C2 oxidation reactions usually result in the development of chromium oxides (Cr2O3). Eqs 2 & 3) can be used to represent the oxidation process. Equ. [4], represents the molybdenum's oxidation reaction when it reacts with oxygen.
NiCrAlY/Cr3C2/h-BN/Mo coated T22 substrate demonstrated significantly lower weight gain as compared to the uncoated substrate when exposed to a Na2SO4-60%V2O5 molten salt environment at 700℃. After undergoing 50 cycles of exposure, the weight gain of NiCrAlY/Cr3C2/h-BN/Mo coated T22 substrate is 8.6 times less than that of the uncoated substrate of the same type. Furthermore, the parabolic rate constant (Kp) consistently decreases across all the coated substrates compared to the uncoated steels. These results indicate that the plasma sprayed NiCrAlY/ Cr3C2/h-BN/Mo coatings effectively offer the required protection to the substrate. SEM analysis reveals a homogeneous, low-porosity microstructure crucial for effective barrier properties against corrosive chemicals. The EDAX results of corrosion-exposed samples reveals the distribution of Na and V within the oxide scale, indicating a potential acidic fluxing effect due to the combination of Na2SO4-60%V2O5. Thermodynamic data suggests that Na2SO4 can react with V2O5, increasing the melt's acidity by forming vanadate, particularly NaVO3, as described by the following reaction at 700 °C.
NaVO3 is a strong acid, which causes the quick oxide formation of the alloy elements it comes into contact with. Holt and Kofstad [24] have documented interactions between sulfur dioxide (SO2) and iron, which may also accelerate steel corrosion. The potential reactions reported are as follows.
XRD analysis identifies crystalline phases, highlighting the presence of protective oxides like Cr2O3, NiO, Y2(Si2O7), and, Cr2Mo3O12 which improve corrosion resistance. The inclusion of carbides and nitrides enhances mechanical strength and wear resistance, prolonging service life in harsh conditions. The significance of these findings for industrial applications, particularly in power generation, is discussed, emphasizing the role of plasma-sprayed coatings in increasing boiler component longevity, reducing maintenance needs, and improving operational performance and cost-efficiency. Experimental findings demonstrate enhanced resistance compared to uncoated T22 alloy, attributed to synergistic effects of various components. Incorporation of h-BN, Mo, and Cr3C2 forms protective oxides and borides, preventing oxygen migration and corrosive species infiltration, while improving mechanical and thermal stability, thus enhancing overall robustness [24,25,26,27,28,29,30,31,32].
5 Conclusions
The study of plasma-sprayed NiCrAlY/Cr3C2/h-BN/Mo coatings on T22 boiler steel alloys has thrown insight into how well these coatings perform under harsh operating circumstances, including hot corrosion and oxidation resistance.
-
1.
Using plasma thermal spraying, NiCrAlY/Cr3C2/h-BN/Mo alloy was successfully applied to T22 boiler tube steels. With the specified spraying conditions, an apparent compact laminar-structured coating with an average thickness of 280 µm and a porosity of less than 5% was produced.
-
2.
The addition of ceramic such as Mo, Cr3C2, and solid lubricant h-BN results in a notable improvement in resistance to heat corrosion and oxidation when compared to uncoated surfaces. These aid in the development of protective oxide scales and boron-rich layers that function as defences against corrosive attack and oxygen diffusion.
-
3.
Cr3C2 hard reinforcement improves mechanical properties and thermal stability, extending the lifetime and durability of coatings in high-temperature conditions.
-
4.
Surface morphology investigations using SEM and EDS techniques confirm the homogeneity and integrity of the coatings.
-
5.
XRD analysis validates the production of protective coatings, providing insight into the crystalline phases present.
-
6.
Elemental mapping reveals the uniform distribution of component elements within the coatings, contributing to overall performance and stability.
-
7.
The lower parabolic rate constant (Kp) of NiCrAlY/ Cr3C2/h-BN/Mo coatings compared to uncoated T22 substrate indicates reduced oxide rates in the coated steels. Uncoated T22 substrate, on the other hand, exhibits severe spalling and peeling of oxide scale from the topmost surface.
-
8.
In molten salt environments, NiCrAlY/ Cr3C2/h-BN/Mo coated steels develop a robust protective oxide layer mainly composed of nickel (Ni) and chromium (Cr) oxides, effectively resisting hot corrosion.
-
9.
During initial oxidation stages, aluminum (Al) and chromium (Cr) preferentially oxidize along the nickel-rich splat border, forming a barrier against oxygen and corrosion species penetration, thereby stabilizing the oxidation rate over time.
Overall, plasma-sprayed NiCrAlY/ Cr3C2/h-BN/Mo coatings show encouraging potential for usage in high-temperature, severe boiler systems. These coatings increase protection against oxidation and heat corrosion, extending the life of boiler components and lowering maintenance expenses. Subsequent studies can concentrate on enhancing coating composition and processing parameters to enhance efficacy and appropriateness for particular industrial uses.
Data Availability
Since no new data were generated or examined in this study, data accessibility is not relevant to this publication.
References
Singh G (2016) High-temperature oxidation behaviour of HVOF thermally sprayed NiCrAlY coating on boiler tube steel. Mater Today: Proc. https://doi.org/10.1016/j.matpr.2017.05.035
DeMasi-Marcin JT, Gupta DK (1994) Protective coatings in the gas turbine engine. Surf Coat Technol 68:1–9
Stringer J (1998) Coatings in the electricity supply industry: past, present, and opportunities for the future. Surf Coat Technol 108:1–9
Nicholls JR, Deakin MJ, Rickerby DS (1999) A comparison between the erosion behaviour of thermal spray and electron beam physical vapour deposition thermal barrier coatings. Wear 233:352–361
Murthy JKN, Rao DS, Venkataraman B (2001) Effect of grinding on the erosion behaviour of a WC–Co–Cr coating deposited by HVOF and detonation gun spray processes. Wear 249:592–598
Sidhu TS, Agrawal RD, Prakash S (2005) Hot corrosion of some superalloys and role of high-velocity oxy-fuel spray coatings—a review. J Surf Coat Technol 198:441–446
Sidhu BS, Prakash S (2006) Evaluation of the corrosion behaviour of plasma-sprayed Ni3Al coatings on steel in oxidation and molten salt environments at 900 C. Surf Coat Technol 166:89–100
Eliaz N, Shemesh G, Latanision RM (2002) Hot corrosion in gas turbine components. Eng Fail Anal 9:31–43
Sidhu TS, Malik A, Prakash S, Agrawal RD (2007) Oxidation and hot corrosion resistance of HVOF WC-NiCrFeSiB coating on Ni- and Fe-based superalloys at 800 °C. J Therm Spray Technol 16:844–849
Zhao WM, Wang Y, Dong LX, Wu KY, Xue J (2005) Corrosion mechanism of NiCrBSi coatings deposited by HVOF. Surf Coat Technol 190:293–298
Sidhu TS, Prakash S, Agrawal RD (2005) Studies on the properties of high-velocity oxy-fuel thermal spray coatings for higher temperature applications. Mater Sci 41:805–823
Heath GR, Hiemgartner P, Irons G, Miller R, Gustafsson S (1997) An assessment of thermal spray coating technologies for high temperature corrosion protection. In materials science forum. Mater Sci Forum 251–254:809–816
Choi H, Yoon B, Kim H, Lee C (2002) Isothermal oxidation of air plasma spray NiCrAlY bond coatings. Surf Coat Technol 150(2–3):297–308
Li Z, Qian S, Wang W (2011) Characterization and oxidation behavior of NiCoCrAlY coating fabricated by electrophoretic deposition and vacuum heat treatment. Appl Surf Sci 257:4616–4620
Wang B, Gong J, Wang AY, Sun C, Huang RF, Wen LS (2002) Oxidation behaviour of NiCrAlY coatings on Ni-based superalloy. Surf Coat Technol 149:70–75
Ren X, Wang F (2006) Knowledge management for teams and projects. Surf Coat Tech 201:30–37
Zhao L, ParcoMandLugscheider E (2004) Wear behaviour of Al2O3 dispersion strengthened MCrAlY coating. Surf Coat Tech 184:298–306
Zhu H, Niu Y, Lin C, Huang L, Ji H, Zheng X (2013) Microstructures and tribological properties of vacuum plasma sprayed B4C–Ni composite coatings. Ceram Int 39(1):101–110
Durga Prasad C, Jerri A, Ramesh MR (2020) Characterization and Sliding wear behavior of iron based metallic coating deposited by HVOF process on low carbon steel substrate. J Bio Tribo-Corros. https://doi.org/10.1007/s40735-020-00366-7
Li WZ, Yao Y, Wang QM, Bao ZB, Gong J, Sun C, Jiang X (2008) Improvement of oxidation-resistance of NiCrAlY coatings by application of CrN or CrON interlayer. J Mater Res 23:341–352
Madhusudana Reddy G, Prasad CD, Patil P, Kakur N, Ramesh MR (2023) High temperature erosion performance of NiCrAlY/Cr2O3/YSZ plasma spray coatings. Trans IMF. https://doi.org/10.1080/00202967.2023.2208899
MadhuSudana Reddy G, Durga Prasad C, Kollur S, Lakshmikanthan A, Suresh R, Aprameya C (2023) Investigation of high temperature erosion behaviour of NiCrAlY/TiO2 plasma coatings on titanium substrate. JOM Journal Min Metal Mater Soc 75:3317–3323. https://doi.org/10.1007/s11837-023-05894-4
Praveen AS, Sarangan J, Suresh S, Subramanian JS (2015) Erosion wear behaviour of plasma sprayed NiCrSiB/Al2O3 composite coating. Int J Refract Metal Hard Mater 52:209–218
Prasad CD, Kollur S, Nusrathulla M, SatheeshBabu G, Hanamantraygouda MB, Prashanth BN, Nagabhushana N (2024) Characterisation and wear behaviour of SiC reinforced FeNiCrMo composite coating by HVOF process. Trans IMF 102(1):22–28. https://doi.org/10.1080/00202967.2023.2246259
Schernau U, Hueser B, Weber K (1992) Modern analytical techniques for coating and coating materials. In: Golton W (ed) Analysis of paints and related materials: current techniques for solving coatings problems. ASTM International, West Conshohocken
Kumar SS, Prasad CD, Hanumanthappa H (2024) Role of thermal spray coatings on erosion, corrosion, and oxidation in various applications: a review. J Bio Tribo-Corros 10(2):22. https://doi.org/10.1007/s40735-024-00822-8
Lakkannavar V, Yogesha KB, Prasad CD, Mruthunjaya M, Suresh R (2024) A review on tribological and corrosion behaviour of thermal spray coatings. J Inst Eng (India): Ser D. https://doi.org/10.1007/s40033-024-00636-5
Ramesh MR (2024) Cyclic oxidation and hot-corrosion behavior of HVOF-sprayed NiCrAl coating on industrial boiler tube steels. JOM. https://doi.org/10.1007/s11837-024-06526-1
Sidhu TS, Prakash S, Agrawal RD (2006) Characterisations of HVOF sprayed NiCrBSi coatings on Ni- and Fe-based superalloys and evaluation of cyclic oxidation behaviour of some Ni-based superalloys in molten salt environment. Thin Solid Films 515:95. https://doi.org/10.1016/j.tsf.2.005.12.041
Kannan AR, Rajkumar V, Prasad CD, Shanmugam NS, Yoon J (2023) Microstructure and hot corrosion performance of stainless steel 347 produced by wire arc additive manufacturing. Vacuum 210:111901. https://doi.org/10.1016/j.vacuum.2023.111901
Nithin HS, Nishchitha KM, Pradeep DG, Durga CP, Mathapati M (2023) Comparative analysis of CoCrAlY coatings at high-temperature oxidation behavior using different reinforcement composition profiles. Weld World 67(3):585–592. https://doi.org/10.1007/s40194-022-01405-2
Holt A, Kofstad P (1989) High temperature corrosion of iron in O2+ 4% SO2/SO3 at 500–800 °C. Mater Sci Eng A 120:101–104
Kannan AR, Prasad CD, Rajkumar V, Shanmugam NS, Lee W, Yoon J (2023) Hot oxidation and corrosion behaviour of boiler steel fabricated by wire arc additive manufacturing. Mater Charact 203:113113. https://doi.org/10.1016/j.matchar.2023.113113
Lakkannavar VK, Yogesha KB (2023) Microstructure and corrosion characteristics of composite coating developed on T22 steel using plasma thermal spray method. SAE International, Warrendale
Reddy GMS, Prasad CD, Patil P, Shetty G, Ramesh MR, Rao TN (2022) Investigation of thermally sprayed NiCrAlY/TiO2 and NiCrAlY/Cr2O3/YSZ cermet composite coatings on titanium alloys. Eng Res Express 4:025049
Bala N, Singh H, Prakash S (2009) High-temperature oxidation studies of cold-sprayed Ni–20Cr and Ni–50Cr coatings on SAE 213–T22 boiler steel. Appl Surf Sci 255:6862–6869
Reddy GMS, Prasad CD, Shetty G, Ramesh MR, Rao TN, Patil P (2022) High-temperature oxidation behavior of plasma-sprayed NiCrAlY/TiO2 and NiCrAlY/Cr2O3/YSZ coatings on titanium alloy. Weld World 66:1069–1079
Mathapati M, Ramesh MR, Doddamani M (2018) Cyclic oxidation behavior of plasma sprayed NiCrAlY/WC/Co/cenosphere coating. AIP Publishing, Woodbury
Naik T, Mathapati M, Prasad CD, Nithin HS, Ramesh MR (2022) Effect of laser post-treatment on microstructural and sliding wear behavior of Hvof-sprayed NiCrC and NiCrSi coatings. Surf Rev Lett 29:2250007
Reddy MS, Durga Prasad G, Shetty C, Ramesh G, Rao MR, Patil TN (2021) High-temperature oxidation studies of plasma-sprayed NiCrAlY/TiO 2 and NiCrAlY/Cr 2 O 3/YSZ cermet composite coatings on MDN-420 special steel alloy. Metall Microstruct Anal 10:642–651
Kumar M, Singh H, Singh N (2014) Production of nanocrystalline Ni-20Cr coatings for high-temperature applications. J Thermal Spray Technol. https://doi.org/10.1007/s11666-014-0055-8
Reddy M, Prasad CD, Patil P, Ramesh MR, Rao N (2021) Hot corrosion behavior of plasma-sprayed NiCrAlY/TiO2 and NiCrAlY/Cr2O3/YSZ cermets coatings on alloy steel. Surf Interfaces 22:100810. https://doi.org/10.1016/j.surfin.2020.100810
Hanumanthlal S (2021) Microstructural characterization and hot corrosion behavior of plasma-sprayed Fe17Cr2Ni0. 18C/Fly ash cenosphere-based composite coating. SAE Int J Mater Manuf 14(3):259–274
Lakkannavar V, Yogesha K (2023) Microstructure and corrosion characteristics of composite coating developed on T22 steel using plasma thermal spray method. SAE Techn Paper. https://doi.org/10.4271/2023-01-5071
Funding
This work is not sponsored by any funding agencies.
Author information
Authors and Affiliations
Contributions
V.L: investigation and prepared figures with manuscript writing, K.B.Y: methodology, C.D.P: investigation & manuscript writing, C.P: prepared tables & figures, all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interests that the author has disclosed.
Ethical Approval
The paper accurately and thoroughly reflects the authors' research and analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lakkannavar, V., Yogesha, K.B., Prasad, C.D. et al. Investigation of Cyclic Oxidation and Hot Corrosion Behaviour of Plasma-Sprayed NiCrAlY/Cr3C2/h-BN/Mo Coatings on T22 Boiler Steel Alloy. J Bio Tribo Corros 10, 85 (2024). https://doi.org/10.1007/s40735-024-00890-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-024-00890-w