Abstract
Green organic inhibitors have become a more efficient alternative to inorganic inhibitors in mild steel (MS) corrosion in industries due to their cheap and eco-friendly characteristics. Gasometric techniques were used to investigate the adsorption and inhibitive qualities of a methyl alcohol extract of Solanum macrocarpon (SM) leaves as a green inhibitor for the corrosion of MS in 2-M sulfuric acid solutions, respectively, at 303–323 K. Fourier Transform Infrared (FTIR) and qualitative phytochemical analysis were done to elucidate the functional group and organic compounds present in the leaf extract. The inhibition efficiency (IE) increased with concentration and decreased with temperature. The results showed that at a temperature of 303 K, 91.4% IE was reached at 0.5% w/v extract concentration, and thus indicating that methanol extract of Solanum macrocarpon leaves is suitable for corrosion inhibition. Furthermore, the inhibitory potential of the plant extract was ascertained due to the existence of organic compounds containing heteroatoms in their functional groups. The corrosion mechanism was shown to be physical adsorption using thermodynamics parameters (\({E}_{\text{a}}\) and \(\Delta {G}_{\text{ads}}\)). According to the findings, SM methanol extract has the potential to be an affordable corrosion inhibitor for industrial use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Corrosion involves the degradation of materials in their surroundings [1, 2]. The degrading process is usually gradual, resulting in economic waste [3, 4]. Regarding industrial tasks, the two materials most commonly used in industries for duties related to industry are mild steel and hydrochloric acids [5]. Because of this, researchers are currently examining how corrosion inhibitors impact MS in an acidic environment [6,7,8]. Corrosion inhibitors are necessary when using acid solutions for cleaning and descaling metals in industrial settings to prevent metal corrosion [9, 10]. Chemical substances known as inhibitors help to reduce a material’s corrosion rate (CR) [11, 12]. For decades, inhibitors of inorganic origin have been employed in industries to reduce the spread of material corrosion [9, 13]. Their use has dropped owing to their hazardous nature, expensive cost, and non-environmentally friendly nature [12, 14,15,16]. In corrosion control, plant extracts have recently been an important research topic due to their eco-friendliness, non-toxicity, and cost. Numerous studies have utilized plant extracts as environmentally friendly inhibitors [6, 7, 17, 18]. The extract from plants consists of phytochemicals containing atoms that are not carbon or hydrogen in their functional groups [19,20,21]. The atoms that are not carbon or hydrogen function as the adsorption process’s active centers [19, 22, 23]. The heteroatom lone pair electrons facilitate the electron transfer between the inhibitor and the metal surface, forming a shield that reduces corrosion [20, 21].
Acalypha chamaedrifolia was utilized as an organic inhibitor for MS inhibition in 1 M HCl [24]. The study gave an optimum IE of 85.11%. Commiphora caudate was employed for inhibition studies for MS in 1-M H2SO4, with a maximum IE of 90.1% obtained [25]. Tithonia diversifolia displayed excellent IE in MS in 1-M HCl with 94.55% [23].
These studies have demonstrated the capabilities of using plant extracts in corrosion inhibition applications due to their cheap, eco-friendly, and nontoxic properties [26,27,28]. Little or no work has employed the gasometric technique to investigate the corrosion inhibition of SM methanol leaf extract on MS in 2-M H2SO4. This study contributes to our attempt to promote awareness of the growing interest in corrosion inhibitors that are environmentally beneficial. In this study, the inhibitive characteristics of methanol extract of SM leaves as a green inhibitor for MS corrosion were examined and evaluated using the gasometric method.
2 Material and Methods
2.1 Materials
The MS was procured from the market and contains the following composition by weight (%): 0.2, 0.1, 0.01, 0.01, and 0.1 for C, Si, S, P, and Mn, respectively, and the balance Fe. A sheet of MS sized 4.0 by 3.0 by 0.12 cm was used to press and cut each coupon. To suspend each coupon in the corrosive liquid, a tiny 3-mm hole was drilled near the upper edge. Before being placed in a desiccator, each coupon was cleaned with ethanol, rinsed with acetone, and air-dried [29]. The reagents and chemicals used for the investigations were of analytical grade and acquired from Sigma-Aldrich in the USA. The study was conducted using 2-M H2SO4.
2.2 Extraction and Preparation of Plant Leaves Extracts
SM leaves were bought at a nearby market. The leaves were identified at the herbarium of Nnamdi Azikiwe University in Awka. Following a clean water wash, the leaves were shade-dried, ground into a powder, and then the soxhlet extraction method was used to extract the methanol from them. A 2-M H2SO4 solution was used to prepare 0.1–0.5% w/v of the extract for the experiment.
2.3 Phytochemical Analysis
The crude extract was subjected to phytochemical analysis using the methods [30, 31].
2.4 FTIR Experiment
About 2 g of plant extract and 0.5 g of KBr were crushed in a mortar. To make a paste, 1 mL of nujol was poured into the sample using a syringe. To obtain the paste’s spectral lines, it was put in the instrument sample mold and scanned using a Buck 530 infrared spectrophotometer between 600 and 4000 nm.
2.5 Gasometric Experiment
This experiment was carried out using a gasometric assembly, in accordance with Iloamaeke [32]. The apparatus is made up of Erlenmeyer flask connected to a burette by a flexible air pipe that is 10 mm long. Paraffin oil was filled in a burette to a predetermined capacity. During the corrosion monitoring tests using this method, 2 M of 100-mL sulfuric acid was introduced into the Erlenmeyer flask and a burette was used to measure the initial volume of air. A glass stopper was used to quickly seal the Erlenmeyer flask after inserting an existing weight and a 4.0 × 3.0 × 0.12 cm MS coupon into the acidic solution (blank). The quantity of H2 gas produced by the corrosion reaction was measured at 5-min interval for 25 min using the paraffin oil volume variations in the graduated burette. In a separate experiment, fresh coupons were immersed in corrodent, while several concentrations of SM leaf extract were added (0.1, 0.2, 0.3, 0.4, and 0.5% w/v, proportionately). At the same intervals as the blank, the amount of hydrogen gas produced by every sample was measured. The experiment was conducted at 303, 313, and 323 K using a thermostat water bath. The following characteristics, such as IE and corrosion rate, were computed using the volume of hydrogen evolution [18, 32].
The volumes of H2 gas given off in uncontrolled and controlled solutions are denoted as \({V}_{0}\) and \({V}_{i}\), respectively.
The H2 gas volume given off for the uncontrolled and controlled solutions are denoted as \({V}_{0}\) and \({V}_{i}\), respectively.
Equation 3 is used to calculate the rate of evolution of hydrogen.
\({V}_{\text{t}}\) and \({V}_{i}\) denote the H2 gas volumes at time intervals tt and ti, correspondingly.
2.6 Thermodynamic and Kinetic Considerations
The Arrhenius equation [33] was used to evaluate the reaction’s activation energy.
where MS corrodes at the fastest rates (CR1 and CR2) at temperatures T1 (323 K) and T2 (303 K), respectively. R denotes the molar gas constant (8.314 kJ/mol), and \({E}_{\text{a}}\) is the activation energy of the reaction.
The Arrhenius equation’s transition state was used to calculate the enthalpy (ΔH) and entropy (ΔS) variations of activation for the inhibition reaction using equation [34]:
where h = Planck’s constant (6.626 × 10−34 JS), R = gas constant (8.314 J mol−1 K−1), and N = Avogadro’s Number (6.022 × 10–23 mol).
Equations 6 and 7 were used to calculate the adsorption's free energies [35].
where R = molar gas constant, T = temperature (303 K), molar concentration of water = 55.50, and equilibrium constant = Kads.
3 Results and Discussion
3.1 Phytochemical Screening
The phytochemical results of the methanol extract of the leaves of SM are shown in Table 1. The results revealed that the phytochemical constituents are contained in the leaf extract and were responsible for the inhibition properties [8]. The phytochemical components contain heteroatoms that create chemical linkages between the extract and the MS [28]. Because these aromatic compounds contain polar functional groups and N, O, P, and S, heteroatoms are typically found in organic compounds employed as corrosion inhibitors [22, 23]. The lone pair of electrons helps form a protective shield on the metal surface, thereby inhibiting the CR [3, 25]. Similar findings were also observed [16, 27, 28]. The findings indicated that the presence of phytochemical compounds with heteroatoms demonstrated the inhibition properties of the extract.
3.2 FTIR Results
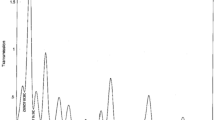
The FTIR spectrum (Fig. 1) of the methanol leaf extract of SM reveals the presence of the following functional groups (Table 2).
The extract contains functional groups with heteroatoms, which are known to have a good inhibiting property [3, 4]. They also act as active centers of adsorption in corrosion inhibition processes [27]. The FTIR results displayed are in agreement with other works [2, 16, 24].
Therefore, the FTIR study showcased the outstanding inhibiting characteristics of the leaf extract of SM for the corrosion of MS due to the presence of these functional groups with heteroatoms, which are embedded in the organic compounds.
3.3 Hydrogen Evolution Data
Figures 2, 3, and 4 illustrate the volume of hydrogen given off at 303–323 K in uncontrolled and controlled solutions. The amount of hydrogen gas emitted increased with longer immersion times while decreasing with greater inhibitor doses. Furthermore, the results demonstrated that the MS corroded in a 2-M H2SO4 solution; however, because the extract had a shielding effect on the metal surface, the acid’s inhibitor reduced the metal deterioration level [21]. Remarkable outcomes were noted by [27, 28, 36]. Therefore, in a 2-M H2SO4 solution, SM leaf extract can function as a highly effective green inhibitor for MS corrosion.
Figure 5 shows the relationship between temperature and evolved hydrogen gas volume. The findings also demonstrated that, as a result of the metal’s susceptibility to corrosion due to temperature increases, the evolved hydrogen gas volume increased [2]. An increase in temperature had a significant impact on the inhibitor’s inhibitory potential. Comparable findings have been demonstrated [16, 37].
3.4 Inhibition Efficiency (IE) Data
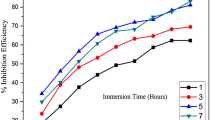
Figures 6, 7, and 8 show the fluctuation of IE with extract concentrations in 2-M H2SO4 acid solutions at 303–323 K. The extract’s IE increased as its inhibitor concentration was increased in the acid solution, which was observed in previous results [5, 26]. This implied that the extract molecule’s concentration adsorbed in to the surface of the metal increased with an increase in the concentration’s extract [2]. As a result, these inhibitor molecules covered a larger percentage of the metal surface, stopping the aggressive anions in the acid from further corroding the metal [26]. Similar findings have been reached by other researchers [18, 21]. At 303 K (Fig. 6), the maximum IE of 91.4% was recorded at an inhibitor concentration of 0.5% w/v. The extract’s capacity to stop corrosion decreases as temperature rises (Fig. 9). This is attributed to an increase in the kinetic energy of the inhibitor due to the faster transfer of protons and sulfate anions, which weakens the binding site’s strength of adsorption where the inhibitor and MS interact [32]. Thus, physical adsorption was identified as the method of inhibition. Similar findings were reported by [32, 38, 39].
The comparison study of several plant extracts used in corrosion inhibition studies of MS is shown in Table 3. A higher IE was observed in previous studies compared to the present study [16, 23]. Results lower than the IE of the present study were obtained by [3, 22, 24, 26,27,28]. Maximum IE similar to the present study was reported by [5, 25]. Therefore, the extract displayed excellent IE for MS corrosion.
3.5 Corrosion Rate (CR) Data
Figures 10, 11, and 12 show the CR of the MS at varied extract concentrations at 303–323 K. Based on the data shown in the graphs, it was discovered that the presence of SM leaf extract as an inhibitor in the acidic medium slowed the CR, since it gradually decreased as the extract concentration increased. Similar findings were reached by other researchers [18, 29, 38]. The CR decreases as the time of immersion increases, because of desorption on the MS by the inhibitor molecules [26]. The temperature effect on the inhibition process is illustrated in Fig. 13. Both inhibited and uninhibited solutions show a progressive rise in MS corrosion rates, which could be the result of the anodic and cathodic sites quicker reaction kinetics [2, 18].
3.6 Degree of Surface Coverage (Ɵ) Result
The extent of the corrosion process's surface coverage (Ɵ) at different temperatures is shown in Fig. 14. From the results, an increase in inhibitor concentration results in an increase in surface coverage at different temperatures, indicating a higher number of inhibitor molecules adsorbed on the MS when the concentration of the extract increases [22, 28]. The desorption of the extract molecules on the MS surface was responsible for the decrease in the surface coverage (Ɵ) proportion with increasing temperature [3, 25]. Similar observations were reported [2, 18].
3.7 Kinetic and Thermodynamic Data
The results of activation energy are reported in Table 4. There was variation in the activation energies (\({E}_{\text{a}}\)) between 57.64 and 62.99 kJ mol−1. The readings are greater than the blank’s values of 46.36 kJ mol−1, indicating that the extract slowed corrosion. Chemisorption requires activation energies of less than 80 kJ mol−1 [40]. The physical adsorption procedure is thus followed by the SM extract’s adsorption on an MS surface [40].
The results of the free energy of adsorption \({\Delta \text{G}}_{\text{ads}}\) indicated that it was less than or equal to the –40 kJ \({\text{mol}}^{-1}\) needed for the chemical adsorption, indicating spontaneous adsorption and that the method used was physical adsorption [16, 39]. Plotting the log of CR/T against 1/T (Transition state) in Fig. 15 yielded a straight line graph with an intercept of Iog (R/Nh) + ΔS/2.303R and a slope equal to − ΔH/2.303R. The enthalpy values (Table 4) were higher with inhibitor presence than without the inhibitor (blank) due to the energy barrier presence in the inhibition process [41]. In addition, the enthalpy values were all positive, indicating the endothermic nature of the reaction [20, 21]. The entropy values (Table 4) in the inhibited system were higher than the uninhibited system, which is in agreement with other studies [22, 23]. The negative entropy values depicted a slow adsorption process, indicating that a reduction in disorderliness happened on going from reactants to the activated complex [42].
4 Conclusion
This investigation has shown that adding SM leaf extract to 2-M H2SO4 solutions significantly slowed the CR of the MS in the acid solutions. The extract’s IE rises exponentially with concentration but falls with temperature. The phytochemical substances present in the extract have heteroatoms in their functional groups, which contributes to their inhibitory activity. The values of \(\Delta {G}_{\text{ads}}\) and \({E}_{\text{a}}\) obtained indicate that the adsorption of the extract is spontaneous, validating the mechanism of physical adsorption. This study concluded that SM is an inexpensive, environmentally friendly inhibitor that can be employed in industries to reduce metal corrosion.
Data Availability
No datasets were generated or analyzed during the current study.
References
Eduok UM, Etim UJ, Akpakpan AE, Umoren SA (2012) Corrosion inhibitors and Coco nucifera L. coir dustfor mild steel in 1M HCl synergistic effect of iodide ions. Int J Adv Sci Res Technol 2(1):338–360
Aralu CC, Chukwuemeka-Okorie HO, Akpomie KG (2021) Inhibition and adsorption potentials of mild steel corrosion using methanol extract of Gongronema latifolium. Appl Water Sci 11(22):1–7. https://doi.org/10.1007/s13201-020-01351-8
Adah CA, Adejo SO, Gbertyo JA, Ogwuche AA (2021) Comparative studies of inhibitive properties of Ficuspolita and Ficusplatyphylla on corrosion inhibition of mild steel in acidic medium. Ovidius Univ Ann Chem 32(1):40–45
Tezeghdenti M, Dhouibi L, Etteyeb N (2015) Corrosion inhibition of carbon steel in 1 M sulphuric acid solution by extract of eucalyptus globulus leaves cultivated in Tunisia arid zones. J BioTribo-Corros 1(3):16
Faiz M, Zahari A, Awang K, Hussin H (2020) Corrosion inhibition on mild steel in 1 M HCl solution by Cryptocarya nigra extracts and three of its constituents (alkaloids). RSC Adv 10:6547–6562
Onen AI, Nwufo BT, Ebenso EE (2011) The effect of Cassia Siamealam root extract on the corrosion and kinetics of corrosion process of copper alkaline solutions. E J Chem 8(4):1708–1713
Saratha R, Devi S, Meenakshi HN, Shyamala R (2011) Enhanced corrosion resistance of Tecomastans extract on the mild steel in 0.5M H2SO4 solution. Int J Curr Res 2(1):092–096
Onwumelu HA, Aralu CC, Egwuatu CI (2018) Inhibition of mild steel corrosion in HCl solution by Gongronema latifolium methanol extract. IOSR J Appl Chem 2(2):35–44. https://doi.org/10.9790/5736-1111013544
Singh A, Quraishi MA (2012) Azwain (Trachyspermum copticum) seed extract as an efficient corrosion inhibitor for Aluminum in NaOH solution. J Recent Sci 1:57–61
Amitha BE, Bharathi BJ (2011) Green inhibitors for corrosion protection of metals and alloys: an overview. Int J Corros 2011:1–15
Douadi T, Issaadi S, Chafaa S (2014) Adsorption and corrosion inhibition of new synthesized thiophene Schiff base on mild steel X52 in HCl and H2SO4 solutions. Corros Sci 79:50–58
Fouda AS, Bad AH (2013) Aqueous extract of propolis as corrosion inhibitor for carbon steel in aqueous solutions. Afr J Pure Appl Chem 7(10):350–359
Abdel-GaberAM RHT, Beqai FT (2020) Eucalyptus leaf extract as a eco-friendly corrosion inhibitor for mild steel in sulfuric and phosphoric acid solutions. Int J Ind Chem. https://doi.org/10.1007/s40090-020-00207-z
Solmaz R (2014) Investigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-dimethylaminobenzylidene) rhodanine. Corros Sci 79:169–176
Krishnegowda PM, Venkatesha VT, Krishnegowda PKM, Shivayogiraju SB (2013) Acalypha torta leaf extract as green corrosion inhibitor for mild steel in hydrochloric acid solution. Ind Eng Chem Res 52:722–728
Abdel-Gaber AM, Rahal HT, Beqai FT (2020) Eucalyptus leaf extract as a eco-friendly corrosion inhibitor for mild steel in sulfuric and phosphoric acid solutions. Int J Ind Chem. https://doi.org/10.1007/s40090-020-00207-z
Eduok UM, Umoren SA, Udoh AP (2012) Synergistic inhibition effects between leaves and stem extracts of Sida acuta and iodide ion for mild steel corrosion in 1 M H2SO4 solutions. Arab J Chem 5:325–337
Obot IB, Umoren SA, Obi-Egbedi NO (2011) Corrosion inhibition and adsorption behaviour for Aluminum by extract of Aningeria robusta in HCl solution: synergistic effect of iodide ions. J Mater Environ Sci 2(1):60–70
Mobin M, Khan MA, Parveen M (2011) Inhibition of mild steel corrosion in acidic medium using starch and surfactants additives. J Appl Polym Sci 121:1558–1565
Khadom AA, Yaro AS, Musa AY, Mohamad AB, Abdul AH (2012) Corrosion inhibition of copper-nickel alloy: experimental and theoretical studies. J Korean Chem Soc 54(4):406–416
Nnanna LA, Owate IO, Nwadiuko OC, Dike II, Isu JO (2014) Inhibition of mild steel corrosion by Aspilia africana in acidic solution. Am J Mat Sci 4(3):144–149
Ogunleye OO, Arinkoola AO, Eletta OA, Agbede OO, Osho YA, Morakinyo AF, Hamed JO (2020) Green corrosion inhibition and adsorption characteristics of Luffa cylindrica leaf extract on mild steel in hydrochloric acid environment. Heliyon 6:e03205
Divya P, Subhashini S, Prithiba A, Rajalakshmi R (2019) Tithonia diversifolia flower extract as green corrosion inhibitor for mild steel in acid medium. Mater Today Proc 18:1581–1591
Arthur DE, Abechi SE (2019) Corrosion inhibition studies of mild steel using Acalypha chamaedrifolia leaves extract in hydrochloric acid medium. SN Appl Sci 1(9):1089
Aejitha S, Kasthuri PK, Geethamani P (2015) Comparison of the corrosion inhibition efficiencies of mild steel in different acidic mediums using commiphora caudata plant extract. Int J Adv Technol Eng Sci 3(5):75–85
Dehghani A, Bahlakeh G, Ramezanzadeh B (2019) Green Eucalyptus leaf extract: a potent source of bio-active corrosion inhibitors for mild steel. Bioelectrochemistry 130:107339
Alibakhshi E, Ramezanzadeh M, Haddadi SA, Bahlakeh G, Ramezanzadeh B, Mahdavian M (2019) Persian Liquorice extract as a highly efficient sustainable corrosion inhibitor for mild steel in sodium chloride solution. J Clean Prod 210:660–672
Akinbulumo OA, Odejobi OJ, Odekanle EL (2020) Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results Mater 5:100074
Ihebrodike MM, Uroh AA, Okeoma BK, Alozie GA (2010) The inhibitive effect of Solanum melongena L. leaves extract on the corrosion of Aluminum in H2SO4 acid. Afr J Pure Appl Chem 4(8):158–165
Harborne JB (1998) Phytochemical methods; a guide to modern techniques of plant analysis, 3rd edn. Chapman and Hall, London, pp 33–243
Sofowora AO (1993) Medicinal plants and traditional medicine in Africa; screening plants for bioactive agents, 2nd edn. Spectrum Books Ltd, Ibadan, pp 134–320
Iloamaeke IM, Onuegbu TU, Umeobika UC, Onyema CT (2013) A comparative study of Vitexdoniana and Pterocarpus soyauxias corrosion inhibitors of mild steel in HCl medium. J Atoms Mol 3(2):509–519
Gopal JI, Shukla SK, Dwivedi P, Sundaram S, Ebenso EE, Prakesh R (2012) Parthenium hysterophorus plant extract as efficient green corrosion inhibitor for mild steel in acidic environment. Int J Electrochem Sci 7:9933–9945
Al-Mhyawi SR (2014) Inhibition of mild steel corrosion using Juneperus plants as green inhibitor. Afr J Pure Appl Chem 8(1):9–22
Odiongenyi AO, Enengedi IS, Ukpong EJ et al (2015) Inhibition of the corrosion of zinc in 0.1M HCl by ethanol extract of honey. Int J Chem Mat Environ Res 2(1):16–25
Eddy NO, Odiongenyi AO, Ameh PO, Ebenso EE (2012) Corrosion inhibition potential of Daniella Oliverri gum exudate for mild steel in acidic medium. Int J Electrochem Sci 7:7425–7439
Eddy NO, Momoh-Yahaya H, Oguzie EE (2014) Theoretical and experimental studies on the corrosion inhibition potentials of some purines for aluminum in 0.1 M HCl. J Adv Res 6(2):203–217
Eddy NO, Awe F, Ebenso EE (2010) Adsorption and inhibitive properties of ethanol extract of leaves of Solanum melongena for the corrosion of mild steel in 0.1M HCl. Int J Electrochem Sci 5:1996–2011
Iloamaeke IM, Onuegbu TU, Ajiwe VIE, Umeobika UC (2012) Corrosion inhibition of mild steel by Pterocarpus soyauxi leaves extract in HCl medium. Int J Plant Anim Environ Sci 2:22–28
Umoren SA, Eduok UM, Israel AU, Obot IB, Solomon MM (2012) Coconut coir dust extract: a novel eco-friendly corrosion inhibition for Al in HCl solutions. Green Chem Lett Rev 5:303–309
Akpan IA, Ofong NO (2015) Electrochemical linear polarization studies of amodiaquine drug as a corrosion inhibitor for mild steel in 0.1M HCL. Solut Chem Mater Res 7(1):17–20
Nair R (2018) Kinetic Thermodynamic, adsorption and electrochemical studies for corrosion behaviour of aluminium AA6063 alloy in ethanolic extract of Lawsoniaalba Lam. leaves. Int J Tech Innov Mod Eng Sci 4:2455–2585
Funding
The work received no funding.
Author information
Authors and Affiliations
Contributions
S.I.E wrote the main manuscript text and C.C.A and V.C.E edited the work. All authors reviewed the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ezugha, S.I., Aralu, C.C. & Eze, V.C. Inhibition Potentials of Solanum macrocarpon Leave Extract as a Green Corrosion Inhibitor of Mild Steel in an Acidic Solution. J Bio Tribo Corros 10, 81 (2024). https://doi.org/10.1007/s40735-024-00885-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-024-00885-7