Abstract
Inhibitive and adsorption properties of methanol extract of Leucas aspera leaves (MLA) on the corrosion of mild steel (MS) was studied using weight loss measurement, AC impedance spectroscopy, and potentiodynamic polarization spectroscopy, Fourier-transform infrared spectroscopy, field emission scanning electron microscope and energy-dispersive X-ray spectrometer, and GC–MS analysis. The results obtained indicated that the MLA is a good adsorption inhibitor for the corrosion of mild steel in HCl medium. The inhibition efficiency of MLA was found to increase with the increasing immersion of time and decreases with the increasing temperature. The polarization studies reveal that the extract acts as a mixed-type inhibitor. The adsorption of MLA on mild steel surface is a spontaneous and exothermic reaction and is best described by the Langmuir adsorption model. The values of activation and free energies obtained were within the range limits expected for the mechanism of physical adsorption. Examination of the FT-IR spectra of the corrosion products of mild steel and that of methanol extract of leaves of LA before and after adsorption indicates the adsorption of the inhibitor on MS surface. Field emission scanning electron microscopy confirms the adsorption of LA on the MS surface. The GC–MS analysis was used for the identification of active phytochemical compounds in MLA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Corrosion of metals is a major industrial problem that has attracted much investigations and researchers [1,2,3]. This is because some industrial processes such as acid cleaning, pickling, and etching facilitate contact between metal and aggressive medium (such as acid, base or salt), consequently metal is prone to corrosion. To reduce the menace due to corrosion of industrial installations, several steps have been adopted. However, one of the best options available for protecting metals against corrosion involves the use of corrosion inhibitors [4].
Most efficient inhibitors are organic compounds containing electronegative functional groups and π electrons in a triple or conjugated double bonds. The presence of heteroatoms such as sulfur, phosphorous, nitrogen, and oxygen as well as aromatic rings in their chemical structure act as the major centers for the adsorption of the inhibitor [5]. A large number of synthetic organic compounds are being used as inhibitors for a variety of purposes. Nevertheless, the use of synthetic organic compounds as an inhibitor is being restricted because of high cost and environmental issues like toxicity, and their ill effects on human and animal’s life.
Plant extract as natural organic inhibitors has received much more attention in recent years due to their high inhibitory efficiency which is explained by the presence of alkaloids, flavonoids, tannins and phenolic compounds [6]. A variety of plant extract has been reported as a corrosion inhibitor for metal and metal-based alloys in recent days [7,8,9,10,11,12,13,14,15,16,17]. MS is widely used as a variety of process in industries. Acid pickling is one of the important steps involved in the metal preparation. In acid pickling, HCl is commonly used. Hydrochloric acid apart from cleaning the metal surface eats away the metal during pickling resulting in a loss in the metal. Use of acid pickling inhibitors is one of the most commonly used methods for the prevention of metal loss. As mentioned above in the literature we find a variety of synthetic organic compounds and natural plant extracts as pickling inhibitors. In the present study to find out cost-effective and easily available inhibitor an attempt has been made to use a methanolic extract of Leucas aspera leaves as a corrosion inhibitor for MS in 1 M HCl medium. We report here for the first time the use of extract Leucas aspera (LA) plant leaves as a corrosion inhibitor. We have selected this plant because it is commonly available in plenty in our area (Coimbatore, India). Though it has medicinal value, since it is a weed it is economically viable to use the plant extract for corrosion inhibition studies. Phytochemical studies of this plant prove the presence of alkaloids, phytosterols, flavonoids, saponins, phenols and glycosides in chloroform and ethanol-soluble fractions and are responsible for its antioxidant property [18].
1.1 Plant Introduction
Leucas aspera (LA) is a botanical species belonging to the family Lamiaceae and is mainly distributed throughout India from the Himalaya down to Ceylon. The plant is harvested from the wild for local use, primarily as a medicine. The leaves extract also showed the antibacterial and cytotoxic effects of the phytochemicals of whole Leucas aspera [19].
2 Experimental Details
2.1 Specimen Preparation
The metal samples were procured from the local market and the specimen was analyzed using ARL- spark analyzer, in Pricol technology private limited, Coimbatore, India to establish the chemical composition. The chemical composition of MS is used as follows: (wt%) C 0.051%, Ni 0.004%, Mo 0.0076%, P 0.0099%, Si 0.423%, S 0.0033%, Cr 0.114%, Mn 1.159%, Cu 0.029% and the rest is Fe. The mild steel specimen of dimension 1 cm × 5 cm × 0.24 cm with an area of 12.99 cm2 was used for the mass loss measurement. The surface of the specimens was mechanically polished with different grades (600, 800, 1000, and 1200) of emery papers and then degreased with acetone and stored in a desiccator.
2.1.1 Preparation of the Extract
Fresh LA leaves were picked and then cleaned with tap water to remove ash and mud, dried in the shade and then ground into a powder. About 20 g of the dried and powdered LA leaves were taken in a Soxhlet and extracted with 140 ml of methanol in 500 ml RB flask for about 6 h. Thereafter, the solution was evaporated to about 50 ml on a water bath. The concentrated extract was dried to complete dryness. The dark brown solid residue (about 0.25 g) obtained was preserved in a desiccator. A stock solution of the methanol extract of Leucas aspera (MLA) was prepared by dissolving the required weight of the residue in methanol. Desired concentrations of the (0.05–0.40 g/L) were obtained by properly diluting the stock solution with distilled water. The test solution was freshly prepared before each experiment.
2.2 Mass Loss Measurements
This is one of the oldest and simplest methods for monitoring corrosion. Here, the mild steel (MS) specimens were polished with different grade emery papers and then degreased with acetone and stored in a desiccator. After weighing precisely using a digital balance with a sensitivity of ± 0.1 mg specimens in triplicate were immersed in the test solutions for specified periods of immersion in the absence and presence of MLA. After that, the specimens were removed, rinsed with water, dried in warm air using a hair drier then stored in a desiccator and then reweighed to determine the mass loss. The corrosion behavior of MS in the presence of MLA was also studied by varying the temperature from 303 to 333 K. The percentage of inhibition efficiency (% IE), corrosion rate (CR) and surface coverage (θ) were obtained using the following equations.
where W(b) and W(i) are the values of weight loss without and with inhibitor, respectively, ΔW is the weight loss in grams, ρ is the density of coupon in g cm−3, A is the area of the coupon in cm2 and t is the exposure period in hours.
2.3 Electrochemical Measurements
Electrochemical measurements including potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) were performed using CHI 608D model electrochemical analyzer of CH instruments. The experiments were carried out in a three-electrode cell assembly consisting of an MS coupon with an exposed area of 0.9785 cm2 as the working electrode. A saturated calomel electrode and a large area platinum mesh were used as a reference and auxiliary electrodes, respectively. All the experiments were performed in 100 ml of the test solution under non-stirred and naturally aerated conditions. Before each experiment, the MS surface was cleaned with a sequence of emery papers of different grades (600,800, 1000 and 1200) washed with distilled water and degreased with acetone. The polarization curves were obtained potentiodynamically between − 0.250 and − 0.750 V with the scan rate of 0.01 Vs−1. The inhibition efficiency (% IE) from PDP data was calculated using the following equation.
where Icorr(b) is the corrosion current density in the absence of inhibitor and Icorr(i) is the corrosion density in the presence of inhibitor.
The EIS experiments were conducted in the frequency range of 10 kHz–1 Hz at open –circuit potential by applying the alternating current signal of 0.005 V peak-to-peak. The inhibition efficiency (% IE) from EIS data was calculated using the following equation
where Rct(b) is the charge transfer resistance in the absence of inhibitor and Rct(i) is the charge transfer resistance in the presence of inhibitor.
2.4 Fourier-Transform Infrared Spectroscopy
Fourier-transform infrared spectroscopy (FT-IR) spectra were recorded in BRUKERALPHA II spectrometer, using the KBr disc technique. The pure solid MLA was mixed with KBr and made into a disc. The MS specimen after immersion in 1 M HCl with MLA for 5 h at 303 K (± 2 K) was cleaned with distilled water and then dried. Then, the thin adsorption layer formed on the steel surface was scrapped and the scrapped material was mixed with a small amount of KBr powder and made into a disc to study the nature of functional groups adsorbed on the metal surface.
2.5 Surface Analysis: FESEM and EDXS Analysis
The specimens used for the estimation of surface morphology were polished, immersed in 1 M HCl in the absence and presence of MLA at 303 K (± 2 K) for 5 h. and removed from the test solution, rinsed with distilled water, dried and then subjected to the surface examination. The analysis was performed using field emission scanning electron microscopy (FESEM) coupled with energy-dispersive X-ray spectroscopy (EDXS) model ZEISS sigma 5th version.
2.6 Gas Chromatography–Mass Spectrometry (GC–MS) analysis
Gas chromatography–mass spectrometry (GC–MS) analysis was carried out using a JEOL GCMATE II GC–MS data system which is a high resolution, double-focusing instrument The source options were electron impact (EI) and chemical ionization (CI).
2.7 Shelf life Studies
To assess the stability of the extract on storage, the performance of the MLA in 1 M HCl was studied every fifteen days for a duration of 4 months by storing them at room temperature and in the refrigerator. Every fifteen days the efficiency of the extract was evaluated by mass loss method.
3 Results and Discussion
3.1 Mass Loss Measurements
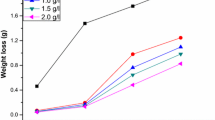
The values of corrosion rate (CR) and inhibition efficiency (%) obtained from mass loss measurement for various immersion times (1–7 h) at different concentrations of the MLA on mild steel in 1 M HCl at room temperature are illustrated in Table 1. It is observed that the corrosion rate decreases and % IE increases with concentrations at all immersion times from 1 to 5 h. The adsorbed layer of the inhibitor acts as a barrier between a steel surface and the aggressive solution, leading to a decrease in the corrosion rate. It is found that % IE is 89.78 in lM HCl medium for the highest inhibitor concentration of 0.40 g/L even at a longer immersion time of 5 h. The increase of % IE with increasing the inhibitor concentration indicates more inhibitor molecules are adsorbed on the surface of MS in 1 M HCl medium by blocking of more corrosion-active sites. Figure 1 shows the variation of % IE of MLA with its concentration in 1 M HCl. After 5 h of immersion period (i.e. 7 h), there was a decrease in IE from 89.78% to 77.56%. The decrease of % IE with increasing the immersion time indicates the inhibitor molecules are desorbed from the surface of MS on long standing.
3.2 Electrochemical Measurements
3.2.1 Electrochemical Impedance Spectroscopy
The corrosion behavior of MS in 1 M HCl solution in the absence and presence of different concentrations of MLA was investigated using EIS measurements. The Nyquist plot for MS in 1 M HCl without and with various concentrations of MLA is shown in Fig. 2a. The depressed semicircles of the Nyquist plots are probably due to heterogeneity of the metal sample which might be due to the increase in surface roughness, dislocations, distribution of the active sites, or the adsorption of the inhibitor molecules [20]. The diameter of the semicircle is observed to increase as the MLA concentration increased. This is an indication of the fact that the adsorption of the inhibitor molecules on the mild steel surface leads to the formation of a surface-protective film which reduces the corrosion-active sites on the metal surface thus enhancing its corrosion resistance [21].
The Randles equivalent circuits used for impedance studies are given in Fig. 2a inserted, where RS is a solution resistance, Cdl is the double-layer capacitance and Rct is the charge transfer resistance. The electrochemical parameters of Rct, Cdl, and % IE in the presence and absence of MLA in 1 M HCl are listed in Table 2. It is apparent from Table 2 that the Cdl values decrease and Rct values increase with increasing the inhibitor concentration. The increase in Rct in the presence of MLA is due to the fact that the added inhibitor molecules displace the water molecules at the interface of the double layer leading to the transfer of charge from solution to the metal surface.
The decrease in Cdl values from 8.648 10−5 to 3.352 10−5 Fcm−2 is due to the reduction in local dielectric constant and/or an increment in the thickness of the electrical double layer. The phenomenon proposed that the inhibitor molecule function by the adsorption at the metal surface. Thus, the change in Cdl is due to the gradual replacement of the water molecule by the adsorption of the inhibitor molecule on the metal surface, the decreasing magnitude of metal dissolution. The adsorption can occur directly on the basis of donor–acceptor interactions between the lone pair of electrons, π electrons in MLA and the vacant d orbital of iron atoms [22].
3.2.2 Potentiodynamic Polarization Method
The current potential relationships for MS corrosion in 1 M HCl with different concentrations of MLA was investigated through the polarization measurements and the electrochemical parameters such as corrosion potential (Ecorr), corrosion current density (Icorr), anodic slope (ba), and cathodic slope (bc), were obtained from Tafel plots, and % IE are tabulated in Table 2. Figure 2b explains the representative Tafel plots of the corrosion inhibition studies on mild steel with different concentrations of MLA. Tafel slopes were calculated by the linear extrapolation of the cathodic and anodic branch of the polarization curves. Corrosion current was calculated from the slope of the polarization curve (ba and bc) and linear polarization resistance Rp using the Stern–Geary equation which is given below:
The polarization data obtained in Table 2 shows the addition of the inhibitor alters both ba and bc values suggesting that the inhibitor reduces both anodic dissolutions of the metal and retard hydrogen evolution reaction. This indicates the mixed nature of the inhibitor. The Icorr values decrease while increasing the concentration of inhibitors which represents the higher surface coverage of the inhibitors [23,24,25,26].
3.3 Temperature Studies and Thermodynamic Parameters
To evaluate the nature of adsorption of MLA and activation parameters of the corrosion process of MS in 1 M HCl medium, mass loss measurements were accomplished in the temperature range of 303–333 K, in the absence and presence of MLA at different concentrations for 1 h immersion time and the obtained data are shown in Table 3. It clearly shows that the temperature is increased the % IE decreased from 84.78 to 62.39.
Figure 3 shows the variation of % IE with temperature for various concentrations of the selected inhibitor MLA in 1 M HCl. As the temperature is increased, the inhibition efficiency decreased at all concentrations. This shows that MLA prevents iron dissolution in aggressive acids through physical adsorption [27].
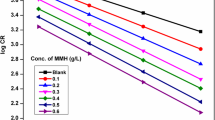
The correlation between the corrosion rate and the temperature is given by the Arrhenius equation
Where CR is the corrosion rate, Ea is the apparent activation energy, R is the molar gas constant (8.314 J mol−1 K−1), T is the temperature in K and A is the frequency factor. The Ea values can be determined from the slopes of Arrhenius plots (log CR vs. 1/T) (Fig. 4).
The activation energy (Ea) values are enumerated in Table 4. Ea values are higher for an inhibited solution than that for the uninhibited one, indicating a strong inhibitive action of MLA by increasing the energy barrier for the corrosion process. This is an indication of spontaneous adsorption of the inhibitor molecules on the MS surface and is attributed to physisorption [28]. The values of the free energy of adsorption (∆G)ads were obtained from Eq. 9 and are tabulated in Table 4.
where log B = − 1.74 − [− (∆G)ads/2.303 RT].C is the concentration of the inhibitor, θ is the surface coverage. It is described in the literature that a value of ∆Gads around − 20 kJ mol−1 or less negative implies that the adsorption is due to electrostatic interaction, i.e., physisorption, while a values of ∆Gads around − 40 kJ mol−1 or more negative suggests that the adsorption is chemisorption. Analysis of results presented in Table 4 shows that the values of ∆Gads are less negative than 20 kJ mol−1 proposing that the process of inhibition is through physisorption. The negative values of ∆Gads reflect the spontaneous adsorption of the inhibitor molecules on the metal surface [29].
From the basic thermodynamic reaction [30],
To have a better understanding of the adsorption process, the enthalpy of adsorption (∆Hads) and entropy of adsorption (∆Sads) were obtained from the ∆Gads vs. T plot. The intercept of the plot gives the values of ∆Hads and the slope gives the value of ∆Sads.
From Table 4, it is also clear that the ∆Hads values are negative. The negative value of ∆Hads indicates that the adsorption of inhibitor molecules on the metal surface is an exothermic process. It is observed that ∆Sads values in the presence of inhibitor are positive. This implies that the formation of the activated complex is the rate-determining step representing association rather than dissociation, indicating that a decrease in disorder on going on from reactants to the activated complex [31].
Basic information on the interaction between the inhibitor molecule and the mild steel surface can be obtained from the adsorption isotherm. For this purpose, the values of surface coverage (θ) at different concentrations (C) of MLA in HCl medium in the temperature range (303–333 K) have been used to construct a suitable isotherm. Attempts were made to fit the experimental data to assorted isotherms including Langmuir, Freundlich, Tempkin, Frumkin, El-awady, and Flory–Huggins. It has been found that the data fit well with the Langmuir adsorption isotherm (Fig. 5) with correlation coefficient nearing almost unity.
3.4 Analysis of the FT-IR Spectra
The FT-IR spectra of the crude LA and that of the scrapped products from the surface of MS after immersion in 1 M HCl containing 0.40 g/L of LA for 5 h are shown in Fig. 6a, b, respectively, provided information concerning the nature of the interaction between the metal surface and the inhibitor molecules. The broad peak at 3355.66 cm−1 in Fig. 6a is due to the O–H stretching of carboxylic acid. This disappears and a new peak is obtained at 3210.13 cm−1 Fig. 6b. Further, the peak at 1608 cm−1 in Fig. 6a is due to C=O stretching of carboxylic acid and this is shifted to 1621 cm−1 in Fig. 6b which is due to the C=O stretching of the aldehydic group. The results obtained show that the corrosion inhibition takes place through an adsorption process. Figure 6b shows the FT-IR spectrum of the scrapped material.
3.5 Surface Analysis
3.5.1 SEM Analysis
Figure 7 shows the SEM images recorded for (a) polished MS samples (b) MS samples exposed for 5 h in 1 M HCl and (c) MS samples exposed for 5 h in 1 M HCl with 0.40 g/L of MLA. The phytochemical constituents present in Leucas aspera plant extract having the polar groups OH−, COO− and the organic N, S groups in their structure. The MS surface contains Fe2+ atoms. Here, the lone pair of electrons on hetero atoms (O and N) of these functional groups get involved in forming a linkage with metal and block the active sites on it. This will protect the metal surface from further corrosion. The images clearly indicate that the damage to the MS surface is confidentially reduced by the presence of MLA advocating the adsorption of inhibited molecules on the metal surface.
3.5.2 EDXS Analysis
EDXS technique was used to get an idea about the nature of the protective film formed on the metal surface. The EDXS image of the uninhibited and inhibited MS samples, Fig. 8a shows the presence of the elements, namely Fe, Cl, C, and O. It shows in Table 5. The corresponding peak of iron 81.28% which is the main element constituting the MS sample and small peaks of Cl-1.38%, C-1.48%, O-15.86% were detected. The EDXS image of the inhibited MS sample Fig. 8b is also found to contain the elements Fe, Cl, C, O, Mn and Si. The percentages of these ions were Fe − 62.04%, Cl − 0.40%, C-5.27%, O-31.64%, Mn-0.15% and Si -0.50% respectively. From the above data, it is clear that the percentage of iron in the inhibited sample has been further decreased by Fe- 19%, indicating that the inhibitor molecules adsorbed on the MS surface.
3.6 GC–MS Analysis of LA
The GC–MS analysis of LA is shown in Fig. 9. Analysis of chromatogram shows that there are five individual components. The noticeable components are listed in Table 6. From the results obtained, it is evident that the separated compounds can be classified into alcohols (Estra-1, 3, 5(10)-trien-17a-ol, Phytol), acids (Oleic acid, (E)-13-Docosenoic acid) and ester (Docosenoic acid, methyl ester).
3.7 Shelf Life Studies
The results of the shelf life studies of MLA in 1 M HCl are shown in Fig. 10. From that Figure, it is clear that the performance of the inhibitor slowly declined, but the rate of decrease was very less. The refrigerated extract comparatively showed a better performance than the unrefrigerated extract. The decrease in the inhibition efficiency might be due to the degradation of the extract on standing. During storage, extract showed resistance towards the fungal and bacterial attack. There was no unpleasant odor or evolution of gas during storage. There was also no change in the color of the extract. Thus, MLA has found to have a reasonable shelf life of the order of four months.
4 Conclusion
-
The methanol extract of LA leaves is found to be a promising corrosion inhibitor for MS in HCl medium.
-
The adsorption of the inhibitor molecule on the MS surface followed Langmuir adsorption isotherm.
-
The results of the temperature studies indicate that the process of adsorption is by physisorption.
-
The polarization studies indicate that the MLA inhibitor acts as a mixed-type inhibitor.
-
The GC–MS analysis clearly indicates the involvement of the five major chemical constituents in the adsorption process.
-
The adsorption of the inhibitor molecules on the MS surface is confirmed by the results of FT-IR and EDXS studies.
References
Chauhan LR, Gunasekaran G (2007) Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros Sci 49(3):1143–1161
Satapathy AK, Gunasekaran G, Sahoo SC, Amit K, Rodrigues PV (2009) Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros Sci 51(12):2848–2856
Okafor PC, Ikpi ME, Uwah IE, Ebenso EE, Ekpe UJ, Umoren SA (2008) Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros Sci 50(8):2310–2317
El-Etre AY (2003) Inhibition of aluminum corrosion using Opuntia extract. Corros Sci 45(11):2485–2495
Gece G, Drugs A (2011) Review of promising novel corrosion inhibitors. Corros Sci 53(12):3873–3898
Nazeer AA, Shalabi K, Fouda AS (2015) Corrosion inhibition of carbon steel by Roselle extract in hydrochloric acid solution: electrochemical and surface study. Res Chem Intermed 41(7):4833–4850
Krishnaveni K, Ravichandran J (2015) A study on the inhibition of copper corrosion in sulphuric acid by aqueous extract leaves of Morinda tinctoria. J Fail Anal Prev 15(5):711–721
Perumal S, Muthumanickam S, Elangovan A, Karthik R, Mothilal K (2017) Bauhinia tomentosa leaves extract as green corrosion inhibitor for mild steel in 1 M HCl medium. J Bio Tribo-Corros 3(2):13
Ali AL, ShMahrous Y (2017) Corrosion inhibition in acidic media from fruiting bodies of Melia azedarach leaves extract and synergistic Ni2+ additive. RSC Adv 7(18):23687–23698
Aejitha S, Kasthuri PK, Jyothi S (2016) Corrosion inhibitory action of Commiphora caudata extract on the mild steel corrosion in 1 M H2SO4 acid medium. J Adhesion Sci Technol 30:784–802
Abboud Y, Tanane O, ElBouari A, Salghi R, Hammouti B, Chetouani A, Jodeh S (2015) Corrosion inhibition of carbon steel in hydrochloric acid solution using pomegranate leave extract. Corros Eng Sci Technol 51(8):557–565
El-Etre AY, Ali AI (2017) A novel green inhibitor for C-steel corrosion in 2.0 mol−1 hydrochloric acid solution. Chin J Chem Eng 25(3):373–380
Oguzie EE (2005) Inhibition of acid corrosion of mild steel by Telfairia occidentalis extract. Pigm Resin Technol 34(6):321–326
Mayakrishnan G, Pitchai S, Raman K, Vincent AR, Nagarajan S (2011) the Inhibitive action of Clematis gouriana extract on the corrosion of mild steel in acidic medium. Ionics 17(9):843–852
Li X, Deng S (2012) Inhibition effect of Dendrocalamus brandisii leaves extract on aluminum in HCl, H3PO4 solutions. Corros Sci 65:299–308
Rajendran A, Karthikeyan C (2012) The inhibitive effect of flowers of Cassia auriculata in 2 M HCl on the corrosion of aluminum and mild steel. Int J Plant Res 2(1):9–14
Njoku DI, Oguzie EE, Li Y (2017) Characterization, the electrochemical and theoretical study of the anticorrosion properties of Moringa oleifera extract. J Mol Liquids 237:247–256
Latha B, Rumaisa Y, Soumya CK, Shahul S, Sandhiya N (2013) Phytochemical studies on Leucas aspera. J Chem Pharm Res 5(4):222–228
Rahman MA, Islam MS (2013) Antioxidant, antibacterial and cytotoxic effects of the phytochemicals of whole Leucas aspera extract. Asian Pac J Trop Biomed 3(4):273
Shukla SK, Quraishi MA, Ebenso EE (2011) Adsorption and corrosion inhibition properties of cefadroxil on mild steel in hydrochloric acid. Int J Electrochem Sci 6:2912–2931
Jyothi S, Ravichandran J (2015) Inhibitive action of the acid extract of Luffa aegyptiaca leaves on the corrosion of mild steel in acidic medium. J Adhes Sci Technol 29(3):207–231
Saker S, Aliouane N, Hammache H, Chafaa S, Bouet G (2015) Tetraphosphoric acid on eco-friendly corrosion inhibitor on carbon steel in 3 N NaCl aqueous solutions. Ionics 21(7):2079–2090
Khaled KF (2003) The inhibition of benzimidazole derivatives on corrosion of iron in 1 M HCl solutions. Electrochim Acta 48(17):2493–2503
McCafferty E, Hackerman N (1972) Double layer capacitance of iron and corrosion inhibition with polymethylene diamines. J Electrochemical Soc 119(2):146–154
Raja PB, Sethuraman MG (2008) Natural products as a corrosion inhibitor for metals in corrosive media—a review. Mater Lett 62(1):112–116
Gunasekaran G, Chauhan LR (2004) Eco-friendly inhibitor for corrosion inhibition of mild steel in phosphoric acid medium. Electrochim Acta 49(25):4387–4395
Solomon M, Umoren MSA, Udosoro II, Udoh AP (2010) Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros Sci 52(4):1317–1325
Krishnaveni K, Ravichandran J, Selvaraj A (2013) Effect of Morinda tinctoria leaves extract on the corrosion inhibition of mild steel in acid medium. Acta Metall Sin (English Lett) 26(3):321–327
Kathiravan S, Ragul R, Raja G, Ravichandran J (2018) Theoretical and experimental studies about the inhibitive action of Ruellia tuberosa L on mild steel in HCl medium. J Bio-and Tribo-Corros 4(3):46
Roy P, Maji T, Dey S, Sukul D (2015) Adsorption behaviour of gluten hydrolysate on mild steel in 1 M HCl and its role as a green corrosion inhibitor. RSC Adv 5(75):61170–61178
Manimegalai S, Manjula P (2015) Thermodynamic and adsorption studies for corrosion inhibition of mild steel in aqueous media by Sargassum swartzii (Brown algae). J Mater Environ Sci 6(6):1629–1637
Acknowledgments
The authors acknowledge the Post Graduate and Research Department of Chemistry, Sri Ramakrishna Mission Vidyalaya College of Arts and Science, Coimbatore-20 for providing the facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kavitha, N., Kathiravan, S., Jyothi, S. et al. Adsorption and Inhibitive Properties of Methanol Extract of Leucas aspera Leaves for the Corrosion of Mild Steel in HCl Medium. J Bio Tribo Corros 5, 51 (2019). https://doi.org/10.1007/s40735-019-0244-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0244-6