Abstract
In the present study, CeO2 nanoparticles modified with stearic acid (mCeO2) were synthesized using the magnetic stirrer and subsequently vacuum dried for 12 h (MN12) and 24 h (MN24). Three different weight percent concentrations (0.1%, 0.3%, and 0.5%) of each MN12 and MN24 nanoparticle are added to the base lubricating oil (SAE15W40). The physicochemical properties of the synthesized nano-lubricants are investigated, including saponification, acid, calorific, flash and fire point, viscosity, and thermal stability. The tribological characteristics of the synthesized nano lubricants were evaluated at varying loads and under extreme pressure conditions. The nano lubricant added with MN24 has superior performance over base lubricating oil.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lubrication is a procedure or technique for reducing wear on one or both surfaces that move relative to each other. The lubrication is realized through interposing a substance termed lubricant between the surfaces to transport or assist in carrying the load (pressure generated) between the opposing surfaces. Various lubricants are placed between two moving objects in solid, liquid, and gas forms to reduce friction, wear rate, and surface deformation. Surface roughness, material type, component shape, load, sliding speed, temperature, and lubrication influence friction and wear rates. [1, 2]. Load and sliding speed control the attempt to change the friction and wear rate. The friction force is also a function of contact time and velocity, with the friction coefficient being lowest on smooth surfaces [3].
A typical automotive engine's mechanical losses account for around 40% of the total energy generated. The piston-cylinder system accounts for 50% of engine frictional losses, with piston rings accounting for 70 to 80% [4]. In internal combustion engines, lubrication is a fundamental technique for reducing frictional power losses, excess heat generation, exhaust emission, combustion, lower fuel consumption, enhanced heat transfer rate, and maintenance expenses. Inadequate lubrication in engine parts affects engine reliability by causing component wear, seizure, or catastrophic failure. Frictional losses also have a negative impact on the cost-effectiveness of fuel consumption. As a result, even a tiny reduction in frictional losses allows for cost-effective fuel usage, which is a considerable accomplishment. Also, automotive lubricants prevent oxidation and corrosion of the equipment, clean the engine and transport the impurities [5].
Synthetic oil has several advantages, including higher temperature performance, longer drain intervals, and improved fuel efficiency. Synthetic oils might initially be more expensive, but the benefits may outweigh the costs in the long run. Synthetic lubricants, as previously stated, can enhance both high and low-temperature performance. They feature lower pour points, allowing the oil to lubricate the engine quicker, helping it start easier on frosty mornings or in colder climates. The viscosity of a synthetic will be more constant at higher temperatures because of its high viscosity index. That, in turn, assists the formation of the lubricating film and provides a better lubrication effect.
Lubricant additives are often sold in performance packages mixed with base stocks to create formulated lubricants. The primary function of the base oil is to lubricate or create a thin film between moving components to minimize friction, wear, and heat. The base oil also serves as an additive carrier; therefore, it must be capable of retaining additives in solution during typical operating circumstances. Poor-quality base oils require more additives in larger quantities than good-quality base fluids. Similarly, applications that impose a greater demand on lubricants, such as automobile gear and lubricating oils, need higher-quality additives than those that do not. The majority of additives are used in lubricating oil[6]. Film formation, viscosity stabilizer, clotting, antiwear, anti-friction, anti-corrosion, and other additives are utilized to preserve the lubricant’s overall performance[7].
Nanoparticles help to reduce friction and wear on components [6,7,8]. Nano lubricants are made by mixing nanoparticles with a lubricant at a specific concentration. Furthermore, adding a low concentration of nanoparticles (between 0.2 and 3% vol.) into the lubricating oil improves tribological properties [8]. In addition, friction reduction and antiwear behaviours depend on nanoparticles' characteristics, such as size, shape, and concentration. The coefficient of friction and antiwear properties of nano-lubricant is significantly enhanced as compared to base lubricant due to various mechanisms such as ball-bearing [9, 10], mending [11], tribofilm formation [12], and surface polishing effects [10].
Nanoparticle dispersion enhances lubricant thermal conductivity, which is attributable to nanoparticle shape, size, and volume fraction. In addition, nano-lubricants' kinematic viscosity and density changed when nanoparticles were added. Nanoparticles with a smaller size and a higher volume % have a higher density. Metal and metal oxide nanoparticles are the most often utilized additions in lubricants to improve their characteristics [13]. Nanoparticles with a more significant specific heat increase the specific heat of nano lubricants. However, many metal and metal oxide-based nanoparticles decrease specific heat because of their low specific heat. The thermal conductivity of lubricating oils performs a predominant role in determining their cooling performance. In recent research, nanoparticles have increased heat conductivity [14].
Researchers developed nano-lubricants with various nanoparticles and nanomaterials, including BN, TiO2, SiO2, CuO, ZrO2, Al2O3, nano-diamond, and carbon nanotubes [15, 16]. Nanoparticles are mainly between 2 and 120 nm in size [19]. The addition of nanoparticles such as NiO, ZDDP, and MoS2, GNPS led to an increase in load carrying, a decrease in coefficient of friction, and an increase in wear resistance which is desirable [17, 18]. As an additive in mineral oil, Cu nanoparticles lowered the wear rate by 64% [19]. Adding a small amount of ZnO and Al2O3 to SAE15W40 engine oil increased the journal bearing's load-carrying capability [20]. Adding 0.1wt% nanoparticles to the tribology of Al2O3-TiO2 nanocomposites improved antiwear ability [21]. Ramakrishnan et al. [22] investigated the tribological performance of Cu–Zn mixed oxide nanoparticles and zinc oxide nanoparticles as additives in SAE20W40 engine oil. ZnO nanosuspensions showed the most significant reduction in friction and wear. Suspensions containing fewer than 0.02% of nanoparticles remained stable for more than 36 h. The coefficient of friction was decreased by 18.75% in nano zinc oxide-based solutions. Nanosuspensions with 0.02 wt% had better antiwear behavior. Suryawanshia and Pattiwarb [23] investigated the performance of the lubricant MOBIL grade (DTE 24, DTE 25, DTE 26) with 0.5% TiO2 measured at speeds ranging from 500 to 1000 rpm and at 1000 N load using a four-ball tester. Lubricants with TiO2 nanoparticle additions had better anti-friction and antiwear qualities. The viscosity of the lubricant was raised by 0.34 to 2.93%. In addition, the pressure distribution, frictional force, and attitude angle were improved by 0.23 to 1.10%, and load-carrying capacity by 0.34 to 2.93%. Anand Kumar Tripathi and Ravi Krishnan Vinu [24] characterized the thermal stability of synthetic (Shell Advance Ultra, 10W40) and semi-synthetic engine oils (Shell Advance AX7, 10W40) through thermo-oxidative degradation at 120 °C, 149 °C, and 200 °C for a different time interval. The synthetic oil lost its stability to an oxidation temperature of 120 °C for the first time but gained stability over time. Semi-synthetic oil formed a multitude of linear, branched, and cyclic hydrocarbons at an intermediate degradation temperature of 149 °C.
Muhammad Ilman Hakimi Chua Abdullahet al. [25] performed an experimental investigation on the potential of hexagonal boron nitride (hBN) nanoparticles as friction modifier and antiwear additive in diesel engine oil (SAE 15W40). An optimal composition (0.5 vol%) of 70 nm hBN nanoparticles was added to the base oil. The friction and wear rates between the contact surfaces were reduced by 53% (0.14 to 0.07) and 54% (11.21 × 10–9 to 5.19 × 10–9 mm3.s−1), respectively. Gautam Yadav et al. [26] experimented with engine oil lubricating properties. SAE15W40 lubricating oil samples were taken for viscosity testing, total acid number (TAN) test, total base number (TBN) test, and the presence of water in the oil test to estimate the degree of depletion of the engine oil's physical attributes. The oil viscosity with a service life of 3000 km was reduced to 108 mm2/s at 40 °C, compared to 111 mm2 for new oil. The water content and insoluble content of oil samples increased as the service life increased from 0.9 to 4.6% of 10 ml, respectively.
Khalid et al. [27] studied the effect of CuO and TiO2 nano additives on lubricating oil’s rheological and thermal properties (SAE 15W40). A bath ultrasonic was used to homogenize, disperse, and mix CuO and TiO2. Compared to parent oil, the flash points increased by 12.62% and 9.3% and thermal conductivity by 7.27%and 4.54%, respectively. CuO nanoparticles exhibited better functionality and influence on 15W40 oil. Ankit Kotia et al. [28] investigated the wear and performance of SAE15W40 engine oil with Nano lubricants Al2O3 and SiO2 in pin-on-disc test apparatus. Nanoparticles of 0.3 vol%, 0.6 vol%, and 0.9 vol% were added to the SAE15W40 oil. Adding 0.3 vol% Al2O3 nano lubricant resulted in the lowest frictional force (5 N), whereas 0.3% SiO2 nano lubricant had the least wear (10 μm). 0.3 vol % Al2O3 nano lubricant depicted lowest BSFC & highest BTE implying it's a better nano lubricant.
Nallusamy et al. [29] performed an experimental analysis on the influence of CuO nanoparticles on the lubricants SAE 15W40. Adding 0.1% CuO to the SAE 15W40 oil reduced the friction by 28% (0.21 to 0.15 at 60℃) & subsequent decrease in wear scar diameter by 65% (0.38 to 0.13 mm), respectively. Parjane et al. [17] investigated the mechanism to reduce friction/wear of diesel engines by adding NiO, ZDDP, and MoS2 nanoparticles to SAE15W40 oil. A 25% increase in load-carrying capacity (150 to 200 kg) and a 16% decrease in wear scar diameter (514 to 432 nm) were observed. Yeoh Jun Jie Jason et al. [18] investigated the tribological behaviour of graphene nanoplatelets (GNP) as an additive in SAE15W40 engine oil. Different concentrations of GNP, i.e. 0.01, 0.02, 0.05, 0.1, and 0.2 wt %, were taken. Base oil containing 0.05 wt. % GNPs exhibited the least friction and highest wear resistance. A 12.24% decrease in the coefficient of friction (0.055 to 0.049) was noted. Subsequently, a 5.14% reduction in wear scar diameter (746.2 to 709.7 m) was observed. Thulfiqar N Jaber et al. [30] investigated the properties of SAE15W40 oil that were added with MWCNTs and CuO as nano-additives at different concentrations of 0.1 wt%, 0.2 wt%, 0.5 wt%, and 1 wt%. The thermal conductivity was increased by 23% and 15.3%, and the flash point was increased by 19.54% and 15.9% at 1 wt% concentration of both additives as compared to the base oil. There was no change in pour point and viscosity at 0.1 wt% concentration.
Sathishkumar et al. [31] investigated the SAE15W40 oil tribological features by adding the Fe2O3 nano additives and pearlite in two different concentrations, i.e., Pearlite + 0.3 wt. % of Fe2O3 and Pearlite + 0.5wt % of Fe2O3. In all concentrations, nanoparticles showed a reduction in wear. The weight loss has been reduced to 75% (from 0.008 to 0.002 g) using a 0.5% concentration lubricant. Muballigh F et al. [32] experimented on the wear characteristics of SAE 15W40 and cerium oxide (CeO2) nanoparticles at concentrations varying between 0.2 and 0.4 wt%. The optimum RSM conditions are 0.3111% concentration., 5 kg load, and a sliding velocity of 6 m/s, which results in the least wear rate of 4.6201 [(mm3/Nm) × 106]. The wear rate lowers as the concentration of CeO2 nanoparticles in the fluid increases. A further increment in the high intensity of nanoparticles increased the wear rate.
Several studies have been conducted implementing different nanoparticles of various sizes and concentrations to maximize synthetic lubricants' wear and frictional performance. However, in past years, the chemical properties, friction, wear, or extreme pressure capabilities of surface-activated CeO2-based nano-lubricants were seldom documented. In this work, the base oil (SAE15W40) was mixed with different concentrations of surface-activated CeO2 nanoparticles to obtain the nano-lubricant mixture. The effect of surface-activated CeO2 nanoparticles on the physiochemical properties and tribological characteristics of nano lubricant was presented comprehensively.
2 Materials and Methods
2.1 Materials
Synthetic lubricating oil (Castrol CRB, SAE15W-40) was used as base oil. In this study, commercially available CeO2 was purchased from SRL Chemicals Pvt. Ltd, India, with an average size of 1 μm. In the chemical analysis, potassium hydroxide pellets (Rankem Chemicals), 0.5 N HCL (Rankem Chemicals), ethanol (Rankem Chemicals), and phenolphthalein solution from (S D Fine Chemicals Pvt. Ltd, India) were utilized.
2.2 Fabrication of Nanoparticles
The micron CeO2 particles had a typical disc-like shape with diameters of 0.6–1 μm and thickness in the order of 200–400 nm. CeO2 powder was mechanically milled in an alumina jar containing hardened alumina balls (Φ8 mm and Φ10 mm, 16 balls each). The mechanical milling [33] was performed in a horizontal oscillatory mill at 350 rpm for 4 h in an ambient atmosphere. The resulting CeO2 nanoparticles were used directly in the subsequent surface activation methods, as no milling media was added.
2.3 Surface Activation of CeO2 Nanoparticles
The surface charge immediately developed because of the high interfacial energy and specific surface area of the CeO2 nanoparticles, resulting in Van Der Waals interactions between the adjacent particles. As a result, the nanoparticles compressed, reducing the overall surface area and the excess Gibbs surface free energy. CeO2 nanoparticles were treated with stearic acid to improve their dispersion. The nanoparticles had a homogenous dispersion when the CeO2 nanoparticles to stearic acid mole ratio were 1:3. To begin, 8 g of ethanol and 32 mL of distilled water were mixed with 0.4 g of stearic acid; subsequently, 10 g of CeO2 nanoparticles were added. At 70 °C, the solution mixture was stirred using a magnetic stirrer for 60 min. The mixture was then filtered and vacuum-dried for 12 h / 24 h at a temperature of 50 °C. The respective mCeO2 nanoparticles were labelled as MN12 and MN24. After the particles had been modified with stearic acid, however, bound organic layers appeared surrounding them. The associated organic layer increased the particle spacing. Simultaneously, the major portion of the interfacial energy of the particles was reduced. The surface charges were removed, and the polarity of the surface was weakened. Hence, it was expected to get better dispersion characteristics. On the other hand, excessive levels of stearic acid did not assist with dispersion[34].
2.4 Preparation of Nano Lubricants
The nano-lubricants were prepared by adding mCeO2 at different weight concentrations of 0.1 wt. %, 0.3 wt. %, and 0.5 wt. %, to the lubricant base oil (SAE 15W40), as described in Table 1. A precise electronic balance with a least count of 0.0001 gm was used to determine the quantity of nanoparticles. Because of the high viscosity of the base fluid, mixing was conducted with an ultrasonic bath mixer, and the magnetic stirrer approach was performed to disperse mCeO2 into the parent fluid to make maximum desperation for nano lubricants. To sufficiently vibrate and scatter the particles, the mixer is placed in an Ultrasonicator for 1 h at 40 kHz. The sample was immediately transferred to a magnetic stirrer, swirling it for 60 min at a high speed of 1300–1400 rpm at a temperature of 85 °C.
2.5 Zeta Potential Analysis
The Zeta potential value of the synthesized nano lubricants was measured with dynamic light scattering equipment (Make: Malvern Panlytical; Model: Zetasizer) for evaluating the dispersion stability. Each experiment was repeated three times to guarantee the accuracy of the data. The mean value of the zeta potential was presented.
2.6 Acid Value Test
The acid number was determined by titrating the solution of the weighed oil in the solvent containing the indicator. The acid value test provides the number of milligrams of potassium hydroxide (KOH) necessary to neutralize all acidic components included in 1 g of the oil. Hydrolysis of oil increases the acid content with time, so acid value or acid number was used to determine the oil's age. One gram of oil was mixed with 50 ml of ethanol (solvent). Then, three drops of phenolphthalein (indicator) were added to the mixture. The solution was titrated with 0.1 N KOH until a sharp colour shift from colourless to pink occurred, indicating the titration point. The total acid value was calculated using Eq. (1)
2.7 Saponification Value
The saponification value is the quantity of alkali required to convert oil to soap. The test estimates the free fatty acid of oil after complete hydrolysis of 1 g of fat. In the test, 1 g of sample oil was mixed with 5 ml of ethanol (solvent) and stirred vigorously. The aforementioned solution was treated with 25 ml of 0.5 N KOH and placed in a reflux condenser. The setup was placed in a boiling water bath for 30 min and allowed to cool down to room temperature before adding two drops of phenolphthalein (indicator) to the existing mixture. Finally, the mixture was titrated against 0.5 N HCl. A sharp change from pink to colourless solution indicated the titration endpoint. The saponification value was calculated using Eq. (2)
2.8 Flash and Fire Point
A Cleveland open cup apparatus was used to determine the flash and fire point temperatures of the synthesized oils, according to ASTM D92. The Flashpoint is the lowest temperature at which vapours of fuel ignite. The fire point is the temperature at which vapours tend to burn for at least 5 s. These test results were carried out to address concerns regarding the safety of oils, as a lowered flash and fire point indicate a higher fire hazard.
2.9 Calorific Value
A bomb calorimeter is a commonly used instrument to find the calorific value of fuel or lubricant. One g mass of each oil was accurately weighed into the crucible, and a fuse wire (nickel) was stretched between the electrodes. The wire was set in close contact with the oil, and the setup was placed inside a closed shell (bomb). The bomb was then supplied pure oxygen at a pressure of 25 bar through the valve. The bomb was then placed in the calorimeter with the weighed amount of water. After completing the necessary electrical connections, the stirring began. The fuel was ignited as soon as the thermometer indicated a constant temperature. After firing the fuel, the temperature rise was noted at an interval of 20 s until a maximum temperature was reached. The bomb was removed, and the pressure was slowly released through the exhaust valve. The following Eq. (3) is used to find the calorific value of the prepared nano lubricants
where WW is the Weight of water in the calorimeter in kg, CW is the specific heat of water = 4.187 kJ/kg-K, Tf is the final temperature of the water in ºC, Ti is the initial temperature of water in ºC.
2.10 Thermogravimetric Analysis
The degradation behaviour of the oil was analyzed through thermogravimetric (TG) analysis. The thermogravimetric analysis was conducted using a TG-DSC system (Make: TA Instruments; Model: SDT 650). The TG analysis was performed using 2 mg of oil at a flow rate of 100 mL min-1 in a nitrogen atmosphere. A heating rate of 20 °C min-1 from room temperature to 400 °C was adopted to examine the thermal stability of the oils and the weight loss percentage. In a nitrogen atmosphere, the onset temperature was used to define the extent of thermal degradation.
2.11 Viscosity
The viscosity of the lubricating oil was determined using a standard Redwood viscometer (temperature range 30–95 °C). The oil temperature was regulated using a water bath surrounding the oil holder. The bottom of the oil holder was fitted with a polished agate discharge tube with an orifice of Φ1.70 mm. The time for dripping oil to fill the flask was noted, and viscosity was determined. Kinematic viscosity was calculated using Eq. (4)
where t = Redwood seconds. A = 0.26; B = 171.5
2.12 Fourier Transform Infrared Spectroscopy
Fourier transform infrared (FTIR) spectroscopy was performed to analyze the contaminants, oil degradation byproducts, and additives. FTIR spectroscope (Make: Thermo Nicolet; Model: IS10 FTIR-ATR) was used to characterize the nano lubricants using the attenuated total reflectance (ATR) method in the range of 400–4000 cm-1.
2.13 Wear Preventive Test
The four-ball tester (Make: Ducom; Model: TR-30L-PNU-IAS) was used to analyze the base oil and nano lubricants' tribological performance (wear preventive and friction coefficient). Three carbon-chrome steel balls with Φ 12.7 mm were associated in a ball-pot assembly along with the oil for tribological analysis in the four-ball tester. The fourth ball of the same dimension, coupled with a spindle through a unique clamp, was placed at the top of the ball pot. The top ball spun in contact with the three fixed balls under the action of load. Following the standard ASTM D-4172, the rotational speed, normal load, and temperature were modified. The wear preventive tests were performed at a constant spindle rotation speed of 1200 rpm, and ball-pot temperature of 75 °C at three different loadings, 600 N, 800 N, and 1000 N. Wear scar diameter of the balls was measured and characterized.
2.14 Extreme Pressure Test
The four-ball tester was used to determine the weld load for base oil and nano-lubricants through extreme pressure testing according to the standard ASTM D2783-03. At a temperature of 35 °C, the test was performed at 1600 rpm. The load was increased in steps of 100 N until the weld load was reached. Each step load was applied for 10 s. The associated coefficient of friction with each loading condition was determined.
2.15 Advanced Materials Characterization
The surface morphology of the worn specimens was observed using a high-resolution scanning electron microscope (SEM; Make: Zeiss Sigma; Model: Gemini). An energy-dispersive X-ray electron spectroscope (EDS; Make: Oxford Instruments; Model: Ultima Max) was used to determine the specimens' chemical composition and elemental map. An X-ray diffractometer (XRD; Make: Rigaku; Model: Ultima IV) was used to examine the fundamental phases of the base powder, fabricated, and surface-activated specimens (Make: Rigaku; Model: Ultima IV). Particles crystallinity and phase structures were investigated using XRD spectra that were recorded in continuous scanning mode utilizing Cu Kα radiation (λ = 1.54186 Å) at a scan rate of 0.5° per minute from 15 to 80º (2θ) [35].
3 Results and Discussions
3.1 Nanoparticles
Figures 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 summarize the morphology and elemental composition of CeO2 and surface-activated CeO2. Figures 1a–d and 2a–d demonstrate the morphology of CeO2 micro and nanoparticles, respectively, as recorded by SEM. The micron CeO2 particles had disc-like morphology, with diameters ranging between 0.6 and 1 µm and thicknesses ranging from 200 to 400 nm. The CeO2 nanoparticles had a spheroidal morphology and the desired profile for achieving particle-assisted rolling between two components flooded with lubricant. The average size of CeO2 nanoparticles was approximately 60 nm. A few particles of size ranging between 100 and 200 nm were also observed. The surface morphology of the CeO2 nanoparticles after the surface modification process (dried in the vacuum for 12 h and 24 h, respectively) are shown in Figs. 3a–d and 4a–d, respectively.
The specimens were subjected to elemental composition analysis using an EDS to assess the change in the composition of particles after the micro-milling and surface modification process. Figure 5a–d display the EDS spectra of micro CeO2, nano CeO2, MN12, and MN24 specimens, respectively. The spectra revealed the existence of Ce, O, and C elements. Micro CeO2 and nano CeO2 had dominant concentrations of Ce and O elements, as observed in Fig. 5a, b. Hence, the existence of CeO2 in the native form was confirmed after the mechanical milling process. However, the specimens MN12 and MN24 had additional peaks demonstrating the presence of C (Fig. 5c, d). The C peak was attributed to adhered stearic acid on the particles MN12 and MN24.
The elemental composition and presence of Ce, O, and C in the analytical area of as-received CeO2 (Fig. 6) synthesized CeO2 nanoparticles (Fig. 7), mCeO2 nanoparticles MN12 (Fig. 8), and mCeO2 nanoparticles MN24 (Fig. 9) was respectively analyzed using EDS. Carbon was observed in MN12 (Fig. 8) and MN24 (Fig. 9), indicating the presence of stearic acid. The diffractograms of four specimens in Fig. 10 show a collection of peaks identical to those of crystalline CeO2. Furthermore, the broadening of the peaks, apart from the micro, reveals the ultra-fine nature of the particles and the small crystallite size. There was no severe phase transformation after the surface alteration. An extra-wide band and peaks indicated the presence of stearic acid in MN12 and MN24 at a low diffraction angle of 2θ < 25º.
3.2 Dispersion Stability
The stability of the synthesized nano lubricants was assessed using zeta potential because the magnitude of zeta potential confirms the stability of colloidal dispersions. The dispersions with a higher zeta potential (negative or positive) are highly stable, whereas lower zeta potential tends to coagulate or flocculate. Conventionally, a feebly charged exterior could be distinguished from a strongly charged exterior by a value of 25 mV (positive or negative). The dispersion with a zeta potential between 30 and 40 mV is believed to possess consistent stability, and that between 40 and 60 mV is believed to possess good stability. But those with a zeta potential less than 25 mV are assumed to possess incipient stability. The zeta potential value of the synthesized nano lubricant with 0.1 wt% of MN12 and MN24 showed a lower zeta potential value, as given in Table 2. While 0.5 wt% of MN12 and MN24 showed higher zeta potential values, indicating consistent stability.
3.3 Acid Value
Figure 11 illustrates the variation in acid value for varied wt% nano lubricant and a comparison of SAE 15W40 base oil. For a good lubricant, the acid value should be low. The acid value of base oil was determined as 6.4. The addition of additives did alter the acid value of base oil, decreasing it by 34% for 0.1 wt% MN12, whereas 55% decrease for 0.1 wt% MN24. Among MN12 and MN24, MN24 exhibited low acid value as concentration increased. At a higher concentration of 0.5wt% MN24, the acid value decreased by 201% (2.124) as compared to a base oil (6.4).
3.4 Saponification Value
The saponification value describes soap formation ability, so the saponification value of a decent lubricant should be low. Compared to base oil, Fig. 12 demonstrates the saponification value of base oil with various additions. The saponification values decreased with an increase in concentrations of additives. The saponification value of base oil is 177.32. In comparison, the saponification value of 0.3 wt% shows the value of 171.71 for MN12, which is 3.1% lower.
3.5 Flashpoint and Fire Point
In the test, the flash point and fire point of the parent lubricating oil SAE15W40 were 210 °C and 222 °C. For 0.1 wt%, both MN12 and MN24 showed an increase of 3.8% and 6.1% for flash points and 2.2% and 5.9% for fire points, respectively. For 0.3 wt%, both MN12 and MN24 showed an increase of 5.2% and 7.1% for flash points and 5.4% and 6.7% for fire points, respectively, with base oil. The results revealed that nano lubricants of various concentrations had much greater flash and fire points than the base lubricant, as shown in Fig. 13a, b respectively. However, flash and fire points decreased at higher concentrations (0.5 wt%).
3.6 Calorific Value
The calorific value of the nano-lubricant decreased drastically for both MN12 and MN24 compared to the base oil, as shown in Fig. 14. Addition of 0.1 wt% CeO2 reduced the calorific value by up to 3%. The MN12 samples, compared to a base oil(33,663.48 kJ/kg), 0.1wt% had a 2.5% decrease(32,826.08 kJ/kg) in calorific value. At the same time, 0.3wt% had a 4.4% decrease(32,239.9 kJ/kg) in calorific value. Also, 0.5wt% had a 5.2% decrease (31,988.68 kJ/kg) in calorific value. For MN24 samples, As compared to a base oil(33,663.48 kJ/kg), 0.1wt% had a 3.2% decrease(32,481.12 kJ/kg) in calorific value. Whereas 0.3wt% had a 6.3% decrease (31,653.72 kJ/kg) in calorific value. Also, 0.5wt% had 7.4% decrease(31,318.76 kJ/kg) in calorific value. Overall, MN24 samples had the least calorific value among the tested specimens.
3.7 Thermogravimetric Analysis
Figure 15 shows the thermo-gravimetric (TGA) studies for various MN12 and MN24 nano-lubricants with 0.1 wt%, 0.3 wt%, and 0.5 wt% concentrations of nano-particles. Table 3 shows the six different oils, base oil, and their thermal breakdown start temperatures. The thermal stability of nano-lubricants was found to be high for 0.5 wt% of MN12 and MN24 compared to a base oil (SAE 15W40), with MN24 being higher. Among the investigated samples, the nano-lubricant containing 0.5 wt% MN12 CeO2 had the greatest onset temperature (194.34 °C). For MN12 Samples, as compared to a base oil (189.1 °C), 0.1wt% had a 13.6% decrease (166.36 °C) in temperature. Whereas, 0.3wt% had 0.9% decrease(187.39 °C) in temperature. Also, 0.5wt% had 0.5% increase(190.092 °C) in temperature. For MN24 Samples, as compared to a base oil (189.1 °C), 0.1wt% had a 1.86% increase (192.63 °C) in temperature. Whereas, 0.3wt% had 3.6% decrease(182.39 °C) in temperature. Also, 0.5wt% had 2.7% increase(194.347 °C) in temperature. There's an unusual trend in Onset temperature concerning concentrations. The onset temperature was typically at 2% weight loss to compensate for the presence of oil's relatively unstable and humid components.
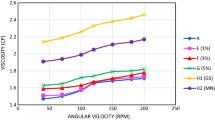
3.8 Viscosity
The kinematic viscosities of the nano-lubricant and base oil were determined using the Redwood Viscometer apparatus. The test was carried out at six different temperatures: 28 °C (room temperature), 35 °C, 45 °C, 55 °C, 65 °C, and 75 °C. The viscosity of SAE 15W40 oil (Base lubricant) with different CeO2 nanoparticle concentrations is shown in Fig. 16. For MN12 samples, at higher temperatures, i.e., 65 °C and 75 °C, as compared to base oil, 0.1wt% had a 55%(55.5cSt) and 85.3% (43.815cSt) increase in viscosity. Whereas, 0.3wt% had 53.7%(56cSt) and 72.9%(40.8cst) increase in viscosity. Also, 0.5wt% had 43.8%(52.4cSt) and 66.6%(39.4cSt) increase in viscosity.
In this test, we can see that MN24 performs better than MN12 in terms of objectives. As concentration increased, there was a decrease in viscosity. For MN24 samples, at higher temperatures, i.e., 65 °C and 75 °C, as compared to base oil, 0.1wt% had a 38.5%(50.5cSt) and 51%(35.7cSt) increase in viscosity. Whereas, 0.3wt% had 47%(53.6cSt) and 60%(37.9cst) increase in viscosity. Also, 0.5wt% had 58.6%(57.8cSt) and 88.1(44.4cSt) increase in viscosity. As concentration increased, there was an increase in viscosity. This is due to MN24 samples being more stable than MN12 samples.
3.9 Bond Analysis
Figure 17A shows FTIR spectra results for SAE15W40(Base oil). The highest absorption peak was observed at around 2923.78 cm-1, attributed to medium C–H stretching (butane) in stearic acid. The second-highest absorption peak was found at 2853.58 cm-1, which denoted a medium C–H stretching bond (butane). The third highest peak was observed at around 2726.28 cm-1, indicating the aldehyde compound's medium C–H stretching bond (2-butyne). The peak corresponding to 1701.34 cm-1 denoted a strong C=O stretching. The peak corresponding to 1155.24 cm-1 is attributed to a strong C–O stretching bond (Cyclohexane). The peak corresponding to 973.09 cm-1 denoted a strong C=C bending (1-Propene, 2-methyl-).
The final peak corresponding to 722.72 cm-1 is attributed to strong C–H bending (Butyne). Figure 17b, c show FTIR spectra results for modified CeO2—nano lubricant. For all three samples, the two highest absorption peaks were observed at around 2919 cm-1 & 2854 cm-1, attributed to medium C–H stretching bond (butane) in stearic acid. The third and fourth absorption peaks were observed at 1457 cm-1, which attributes to the C–H bending bond. The final peak was observed at around 725 cm-1, which also denoted a strong C–H bending bond (2-butyne).
Tribological analysis with varying load.
3.10 Coefficient of Friction
The values of the coefficient of friction with respect to different loads are shown in Fig. 18. As can be seen in the graph, the coefficient of friction decreases as the load increases. Furthermore, as compared to base oil, 0.1wt% MN24 had a reduced coefficient of friction. The coefficient of friction values for all three samples, except base oil, tend to rise once nanoparticles are introduced. However, there is not much variation in the trend. As seen in Fig. 18, the coefficient of friction increases significantly with a 1000 N load.
3.11 Wear Scar Diameter
Surface morphology analysis of carbon chrome balls subjected to wear preventive test at different loading conditions for base oil and 0.3 wt% MN24 sample were shown in Fig. 19.
Wear scar diameter data for MN24 samples at various loads (600 N, 800 N, 1000 N) are shown in Fig. 20. The 0.1 wt% MN24 sample had the most minor wear scar diameter at a load of 600 N. Furthermore, increasing the nanoparticle concentration for the same load resulted in a 12.7% increase in wear scar diameter. There is an uneven trend in wear scar diameters at 800 N, with the lowest being 0.3 wt% MN24 sample has a wear scar diameter of (0.727 ± 0.031) mm. Furthermore, with a load of 1000 N, 0.3 wt% MN24 had the lowest value, resulting in an 11.2 percent reduction in wear scar diameter compared to the base oil, SAE15W40. Ultimately, we can see from Figs. 19 and 20 that the MN24 sample has a smaller wear scar diameter than the base oil.
Figure 21a shows significant grooves on the surface of the balls. Similarly, we could observe grooves and delamination[36] in Fig. 21b. Moreover, as seen in Fig. 21c, many craters and cracks have emerged. Wedges and scratches are depicted in Fig. 21d.
Cracks and scratches in Fig. 22a, b indicate pits. Figure 22c clearly shows that grooves are significant in presence. Figure 22a–c also show the debris, but in Fig. 22d grooves and debris are significant comparatively.
Figure 23a shows the plough and debris. In Fig. 23b pits are more significant than other concentrations. Moreover, extensive delamination can be observed in Figs. 23c, d and a delaminated surface can be seen.
3.12 Extreme Pressure Test Using a Four-Ball Tester
The results of extreme pressure testing for several samples are shown in Fig. 24. The base oil could withstand a maximum load of 5300 N. The tests revealed significant effects after nanoparticles were added to the base oil. The maximum load withstanding capability of the 0.1wt % MN24 sample was 5000 N, while the 0.3 wt% MN24 sample was 5700 N. The sample containing 0.5wt% MN24 had the maximum load-withstanding capacity of 7300 N, which is approximately 27.4% greater than the base oil.
4 Conclusion
Nano-lubricants were prepared through ultrasonication and magnetic stirring techniques using surface-modified nanoparticles of CeO2 and SAE 15W40. Three different wt% concentrations of CeO2 nanoparticles are employed and compared the properties. The following conclusions are drawn from the analysis:
-
Viscosity increased as concentrations of mCeO2 increased, particularly at higher temperatures. MN24 is 88.175% higher viscosity than base oil at 0.5 percent wt.
-
Flash and fire point results revealed that nano lubricants of various concentrations had greater flash and fire points than the base lubricant. The 0.3% MN24 sample, in particular, performs around 7% higher than the base oil.
-
Chemical analysis revealed that the acid and saponification value of nano-lubricant decreased with increasing weight percent compared to base lubricant oil, which is a positive indicator. A definite trend declined 66% for acid value at a concentration of 0.5% wt.
-
Compared to base oil, the calorific value of CeO2 nano lubricants falls as the weight percent of nanoparticles increases, although not dramatically.
-
From the extreme pressure test, the 0.5 wt% MN24 sample showed the highest load-withstanding capacity compared to other samples.
-
The highest load-withstanding capability of 0.5wt percent MN24 was 7300 N, which is around 27.4% larger than base oil, according to the extreme pressure tribological analysis.
From the above consolidates, we can conclude that CeO2 modified for 24 h under the vacuum performs more significantly than 12 h of modification.
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code Availability
Not Applicable.
Abbreviations
- EDS:
-
Energy-dispersive spectroscopy
- SEM:
-
Scanning electron microscopy
- XRD:
-
X-ray diffraction analysis
- μm:
-
Micrometers
- nm:
-
Nanometers
- Wt%:
-
Weight percentage
- CeO2 :
-
Cerium oxide
- mCeO2 :
-
Modified nano-size cerium oxide
- MN12:
-
MCeO2 vacuum dried for 12 h
- MN24:
-
MCeO2 vacuum dried for 24 h
References
Ahmer S et al (2016) Experimental results of the tribology of aluminum measured with a pin-on-disk tribometer: testing configuration and additive effects. Friction 4(2):124–134
Du S, Sun J, Wu P (2018) Preparation, characterization and lubrication performances of graphene oxide-TiO2 nanofluid in rolling strips. Carbon 140:338–351
Gulzar M et al (2015) Improving the AW/EP ability of chemically modified palm oil by adding CuO and MoS2 nanoparticles. Tribol Int 88:271–279
Bathe R et al (2021) Impact of laser surface texturing (LST) on the tribological characteristics of piston rings and cylinder liners–a review. Part 1: development of LST technology. Trans Inst Met Finish (The International Journal of Surface Engineering and Coatings) 99(5):231–237
Choi Y et al (2009) Tribological behavior of copper nanoparticles as additives in oil. Curr Appl Phys 9(2):e124–e127
Rizvi S (2003) Fuels and Lubricants Handbook: Technology, Properties, Performance and Testing. In: Totten G (Ed). ASTM International: West Conshohocken, Chapter 20 pp:753–864
Mortier RM, Orszulik ST, Fox MF (2010) Chemistry and technology of lubricants, vol 107115. Springer, Dordrecht
Hwang Y et al (2011) Effect of the size and morphology of particles dispersed in nano-oil on friction performance between rotating discs. J Mech Sci Technol 25(11):2853–2857
Tao X, Jiazheng Z, Kang X (1996) The ball-bearing effect of diamond nanoparticles as an oil additive. J Phys D Appl Phys 29(11):2932
Rapoport L et al (2002) mechanism of friction of fullerenes. Ind Lubr Tribol. https://doi.org/10.1108/00368790210431727
Liu G et al (2004) Investigation of the mending effect and mechanism of copper nano-particles on a tribologically stressed surface. Tribol Lett 17(4):961–966
Zhou J et al (1999) Study on the structure and tribological properties of surface-modified Cu nanoparticles. Mater Res Bull 34(9):1361–1367
Kotia A et al (2018) Thermophysical and tribological properties of nanolubricants: a review. Heat Mass Transf 54(11):3493–3508
Karimi A et al (2015) Experimental investigation on thermal conductivity of water based nickel ferrite nanofluids. Adv Powder Technol 26(6):1529–1536
Wu Y, Tsui W, Liu T (2007) Experimental analysis of tribological properties of lubricating oils with nanoparticle additives. Wear 262(7–8):819–825
Liu L et al (2011) Lubrication effect of the paraffin oil filled with functionalized multiwalled carbon nanotubes for bismaleimide resin. Tribol Lett 42(1):59–65
Parjane MB, Dabade B (2020) Mechanism To reduce friction/wear of diesel engines by adding nanoparticles. Int J Eng Adv Technol 9(5):161–164
Jason YJJ et al (2021) Tribological behaviour of graphene nanoplatelets as additive in pongamia oil. Coatings 11(6):732
Borda FLG et al (2018) Experimental investigation of the tribological behavior of lubricants with additive containing copper nanoparticles. Tribol Int 117:52–58
Babu KS, Nair KP, Rajendrakumar P (2014) Computational analysis of journal bearing operating under lubricant containing Al2O3 and ZnO nanoparticles. Int J Eng Sci Technol 6(1):34–42
Luo T et al (2014) Tribology properties of Al2O2/TiO2 nanocomposites as lubricant additives. Ceram Int 40(7):10103–10109
Ramakrishnan B et al (2020) Investigation on tribological performance of NanoZnO and mixed oxide of Cu-Zn as additives in engine oil. SAE Technical Paper.
Suryawanshi S, Pattiwar J (2018) Effect of TiO2 nanoparticles blended with lubricating oil on the tribological performance of the journal bearing. Tribol Ind 40(3):370
Tripathi AK, Vinu R (2015) Characterization of thermal stability of synthetic and semi-synthetic engine oils. Lubricants 3(1):54–79
Abdullah MIHC et al (2016) The potential of hBN nanoparticles as friction modifier and antiwear additive in engine oil. Mech Ind 17(1):104
Yadav G et al (2017) Experimental investigation of lubricating properties of engine oil. Int J Eng Sci 7:10306
Sukkar KA, Karamalluh AA, Jaber TN (2019) Rheological and thermal properties of lubricating oil enhanced by the effect of CuO and TiO2 nano-additives. Al-Khwarizmi Eng J 15(2):24–33
Kotia A, Borkakoti S, Ghosh SK (2018) Wear and performance analysis of a 4-stroke diesel engine employing nanolubricants. Particuology 37:54–63
Nallusamy S, Logeshwaran J (2017) Experimental analysis on nanolubricants used in multi cylinder petrol engine with copper oxide as nanoparticle. Rasayan J Chem 10(3):1050–1055
Jaber TN, Sukkar KA, Karamalluh AA (2019) Specifications of heavy diesel lubricating oil improved by MWCNTs and CuO as nano-additives. IOP Conf Ser: Mater Sci Eng. https://doi.org/10.1088/1757-899X/579/1/012014
Sathishkumar B et al (2020) Triboengineered industrial lubricant using perlite loaded Fe2O3 nano-additives: a greener approach, vol 40, pp 2062–2070
Mohammad MF, Harne MS (2018) Experimental Investigation and Optimization of Wear Characteristics of Cerium Oxide (CeO2) Nanoparticle Based Nanolubricant under Boundary Lubrication Condition. Int Res J Eng Technol 10(8):02–508
Govindaraju M et al (2020) Investigations on the tribological behavior of functionally gradient iron-based brake pad material. Proc Inst Mech Eng C J Mech Eng Sci 234(12):2474–2486
Yao Y et al (2016) Mechanical, thermal, and ultraviolet resistance properties of polycarbonate/cerium oxide composites. Polym Sci Ser A 58(4):578–584
Bhardwaj V et al (2021) Investigations on the mechanical and tribological performance of nickel aluminum bronze-CaCO3 composite. IOP Conf Ser: Mater Sci Eng. https://doi.org/10.1088/1757-899X/1059/1/012059
Kannan KR et al (2019) Tribological performance of heavy-duty functionally gradient friction material (Cu-Sn-Fe-Cg-SiC-Al2O3) synthesized by PM route. In: AIP Conference Proceedings. 2019. AIP Publishing LLC.
Funding
The authors are thankful to Amrita Vishwa Vidyapeetham for providing financial assistance to carry out the research work through an internal seed grant for establishing “Four Ball Tester” in the campus.
Author information
Authors and Affiliations
Contributions
MKR: Investigation, Methodology, Writing—Original draft, Supervision. KM: Investigation, Formal Analysis, Writing—Review. RSC: Investigation, Formal analysis, Writing—Editing. PLKV: Investigation, Writing—Editing. KV: Data Curation. RVV: Conceptualization, Formal analysis, Validation, Writing—Review. MG: Resources, Formal analysis, Review. RSK: Formal analysis, Review. GSP: Resources, Formal analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest or competing interest in the research work.
Ethical Approval
Not Applicable.
Consent to Participate
Not Applicable.
Consent for Publication
The author transfers to the concerned publisher the non-exclusive publication rights and the warrants that his/her contribution is original and that he/she has full power to make this grant. The author signs for and accepts responsibility for releasing this material on behalf of any and all co-authors. This transfer of publication rights covers the non-exclusive right to reproduce and distribute the article, including reprints, translations, photographic reproductions, microform, electronic form (offline, online) or any other reproductions of similar nature.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reddy, M.K., Muralidharan, K., Chandra, R.S. et al. Influence of Nanophase Particles on the Physical, Chemical and Tribological Characteristics of SAE15W40. J Bio Tribo Corros 9, 20 (2023). https://doi.org/10.1007/s40735-022-00738-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-022-00738-1