Abstract
Advancement in the field of biosensor/bioelectronics technology has been exploited in the biomedical field for diagnostics and therapy. The potential of this technology in identifying molecular targets has become projected as new generation diagnostics particularly in cancer diagnostics. Cancer being the second cause of death among all diseases in the US and worldwide, the early diagnosis becomes the growing research area in this field. The conventional techniques to detect cancer are expensive, painful, time-consuming, and low sensitivity and specificity. Recently, investigators have developed several types of biosensors to detect specific molecular markers of cancer. Biosensors are classified as point of care/diagnostic tools that can be conducted at home by the patient. This review describes a detailed investigation of different cancer biomarkers and various biosensors developed to detect the biomarkers, the principles, and detection limits, which could assist to detect cancer in early stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is the main health issue in the United States and the world. It is the second cause of morbidity and mortality worldwide, about 8.8 million people died in 2015. According to the World Health Organization Lung (1.76 million deaths), Colorectal (862,000 deaths), Stomach (783,000 deaths), Liver (782,000 deaths), and Breast (627,000 deaths) are the most widespread cancers that lead to death [1].
Cancer is caused by the uncontrolled division of cells continuously. Cancer can spread around tissues through blood and it can start in any part of the human body. Unlike normal cells, cancer cells follow an unregulated cell division leads to a tumor mass [2]. There are two types of tumors, one is malignant tumors that can spread around tissues and the human body, and the other is benign tumors that do not spread around tissues and the human body. Cancer is genetic and inherited disease [3]. It is caused by altering the genes that monitor the function of the cells. Moreover, cancer is related to environmental exposure such as radiation, UV rays, and chemicals in smoke. These factors lead to damage to the DNA.
Early diagnosis of cancer is very important that can increase the chance of cure and decrease the cancer death rate [4, 5]. For this aim, current techniques to detect cancer include CT scan, ultrasound, MRI, PET scan, X-ray, and biopsy [2]. However, some of these methods are expensive, painful, time-consuming, and low sensitivity [6, 7]. In addition, genomic detection methods such as polymerase chain reaction (PCR) [8] and DNA sequencing [9] are used to detect cancer biomarkers. Recently, investigators use immunoassays techniques such as ELISA to detect cancer biomarkers that indicate the presence of the cancers [10]. These techniques are very selective and sensitive, but they are high cost, prolonged and sometimes they can not detect a low concentration of the biomarkers that are present in the early stages of the cancers [11]. Table 1 demonstrates the limitation of the current methods for the early detection of cancers.
Hence, developing a new low-cost, highly sensitive, and rapid method for the early diagnosis of cancers is required. In the current era, investigators have developed biosensors to detect cancers by using different biomarkers found in blood, sputum, urine, and other biofluids. Biosensors have great advantages that raise medical care and develop the quality of health and life. In this review, we will discuss the advancements in the field of biosensors that are used to detect various types of cancer biomarkers in the early stages, including breast cancer, lung cancer, and prostate cancer.
1.1 Cancer Biomarkers
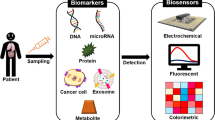
Biomarkers are significant tools that assist in detecting cancer in the early stages. Biomarkers are biological molecules that indicate the condition and the state of the diseases, and they are found in cancer cells, blood, urine, and other biofluids. These molecules could be DNA, RNA, proteins, and enzymes [19, 20]. Biomarkers also play a crucial role in monitoring the effect of the treatment in the cancers such as chemotherapy or radiation [21], because the alternation in the expression of the specific protein can be indicated by biomarkers that are measured in the biofluids. There are many cancer biomarkers that can detect different kinds of cancers. Figure 1 shows some common cancer biomarkers [22,23,24,25,26,27].
Biomarkers can be classified into two main categories: Protein biomarkers and DNA biomarkers. Protein biomarkers are widely used to detect cancers because they are prognostic markers. DNA markers are also used to detect cancers; they give information about the development of tumor growth, but they can’t be detected in the early stage of the cancers [28, 29]. For instance, the most common proteomic biomarker for breast cancer is the human epidermal growth factor receptor 2 (Her-2) which is a member of the EGFR family, because it is a prognostic marker that can be detected in breast cancer at the early stages [30, 31]. Her-2 promotes breast cancer growth. The normal levels of Her-2 in the blood are 2–15 ng/mL, but in breast cancer, the levels might be increased to 15–75 ng/mL [32]. The Her-2 levels are considered to diagnose cancer stage and tumor size [33]. There is the additional proteomic biomarker for advanced breast cancer which is cancer antigen 15–3 (CA 15–3). It is a member of the mucins family [34]. It is mostly used to monitor the therapy of breast cancer [35,36,37,38,39]. The normal concentration levels of CA 15–3 in the serum are less than 30 U/mL, in breast cancer, the levels will increase in the serum from stage 1 to stage 4 of cancer [39]. Furthermore, BRCA1 is a DNA biomarker. BRCA1 can be destroyed by mutation so that leads to raising the risk of having breast cancer [40]. There are other markers that are related to breast cancer such as BRCA2, CAE, and CA 27.29 [11].
In addition, prostate-specific antigen (PSA) is the current superior serum marker for prostate cancer detection and monitoring the patient after the therapy of prostate cancer. PSA is created inside the duct and acinar cells of the prostate. The normal levels of PSA in the body between the range 0.5–2 ng/mL, so 4–10 ng/mL levels of prostate cancer occurs [19]. GSTP-1 is a new biomarker for prostate cancer which was recently found in prostate cancer patients [41].
Moreover, lung cancer is detected by multiple biomarkers because it is difficult to indicate lung cancer by one specific marker [42]. The most popular proteomic biomarker for detecting lung cancer is a carcinoembryonic antigen (CEA). It is used to distinguish between malignant and benign tumors. In healthy people, the range levels of CEA in serum are 2–2.5 ng/mL, but above these levels, lung cancer occurs [43]. The high expression of CEA biomarkers can also cause breast or ovarian cancer [21]. The higher amount of the CEA in the patient’s body, the lower chance of survival [44]. Neuron-specific enolase (NSE) is another biomarker for lung cancer. NSE is a glycolytic enzyme which is located in the cytoplasm and cell membrane. The levels of the NSE normally are 9 ng/ml and the levels can be higher in cancer patients [45].
The biomarker levels are very low in the early stages of the cancers. Thus, the techniques are used for cancer detection must be sensitive, selective, and specific. Biosensors are promising methods for cancer detection in the early stages with high sensitivity and specificity.
2 Biosensor
The biosensor is a bioanalytical device that is utilized to detect analytes by converting them into an electrical signal to be analyzed in an electronic form [46]. In 1962, the first biosensor is introduced by Clark and Lyons, it was the first glucose biosensor to detect the level of glucose in the human body [47]. Since then, the biosensor has been improved to be an efficient detectable device for multiple applications in the medical, industrial, and environmental fields.
In the medical field, the biosensor is a novel technique because it is classified as a point of care/diagnostic tool that can be conducted at home by the patient, so they can monitor their health. For instance, blood-glucose biosensors are very common to use by diabetic patients at home to diagnose and monitor the level of blood glucose [48]. Recently, biosensors are being used widely for cancer detection in the early stages because biosensors provide better sensitivity and stability than other methods [4]. Furthermore, biosensors play a significant role in autoimmune diseases’ detection. An impedance biosensor is used to detect systemic lupus erythematosus (SLE), which is an autoimmune disease; vascular cell adhesion molecule-1 (VCAM 1-) is utilized as a marker to indicated SLE [49]. The biosensor is being used pervasively in the medical field to detect and diagnose pathogens and infectious diseases [50]
In the food industry, biosensors are applied to provide healthy food for the customers and detect the pathogens or chemical agents in the food that cause diseases [51, 52]. Enzyme-based biosensors and immunosensors are very popular to use in the food industry [53]. Foodborne and waterborne pathogens are a crucial aspect of public healthcare. Approximately 420,000 people died annually due to foodborne and waterborne diseases [54]. For this reason, many studies have been done to detect Foodborne and waterborne pathogens by applying biosensors. The electrochemical immune sensor was developed to detect Salmonella, the biosensor was able to detect salmonella with high sensitivity, stability, and selectivity [55]. E-coli bacteria are a major issue in food contamination and health hazard. Enzyme-based biosensors can detect E-coli successfully [56].
In the environmental field, the detection of the contamination caused by air, water, and the soil is important to protect human health. Different kinds of biosensors can detect a toxic levels of heavy metals [57]. In addition, the application of biosensor in pesticide detection is currently established [58]. Organophosphates are common to utilize as pesticides; it damages the biodiversity by killing the beneficial microbes and insects which exist in the soil. Enzyme-based biosensors help to indicate and monitor the level of organophosphates [59]. The main reason for all these applications of biosensors is to protect humans by detecting any hazards that can affect human health and life. The biosensors are used in broad applications because it is a new, economical, highly sensitive, and rapid technique that will improve the quality of humans’ life. This review will focus on the different types of biosensors and their application in cancer biomarker detection.
2.1 The Structure of the Biosensor
The biosensor consists of two main parts which are receptor recognition elements and transducer. Receptor recognition elements such as nucleic acid, protein, enzyme, antibody, or antigen which attract the analytes and convert them into electrical energy [60]. The transducer is the device used to transform the electrical energy to a signal such as electrochemical, optical piezoelectric, and thermal (calorimetric) transduction. Figure 2 illustrates the principle of the biosensor. Each type of transducers has features. For instance, the electrochemical transducer makes use of potentiometric, amperometric, or conductimetry/impedimetric principles. The electrochemical biosensor measures the change in impedance. This transducer is the most common to use because it is easy, rapid, and cost-effective [61]. However, currently, optical and acoustic (QCM) transducers have become popular to use in multiple applications because most of the electrochemical transductions still need labels like enzymes, but optical and QCM can provide label-free test easily [11]. The optical transducer is based on light, and it has many types of biosensors such as fluorescence, surface plasmon resonance (SPR), and spectroscopy of optical waveguides [62]. Piezoelectric is a different type of biosensor, it uses a quartz crystal microbalance (QCM) which is a device that measures the change in the mass by measuring the frequency change of quartz crystal resonator [63]. QCM devices are very common, especially in the medical field [52]. Thermal transduction is a rare mechanism, and it is based on calorimetry that measures the change of temperature [64].
Schematic representation of the transduction system of the biosensor (1) The analyte attracts to the receptor (2) The receptor could be an enzyme, protein, nucleic acid, or antibody. Electrical energy is generated by the chemical interaction between the analyte and the receptor. (3) The signal is being transduced by the transducer (electrochemical, optical, or piezoelectric) to the processor
2.2 Types of Biosensors
2.2.1 Electrochemical Biosensor
The electrochemical biosensor is widely used in the medical field especially for cancer detection and bioengineering filed because it is inexpensive, rapid, easy to use, and simple. It is also classified as a point-of-care device [11]. The electrochemical biosensor transforms biorecognition molecules and the biomarker interaction to electrochemical signals that can be measured (conductance, potential, impedance, current) [65]. The electrochemical transducer consists of three electrodes systems such as the working electrode (WE), reference electrode (RE), and counter electrode (CE) (Fig. 3a). The interaction between a target molecule and a bioreceptor occurs at the working electrode surface (gold area) [18] (Fig. 3). Electrochemical biosensor uses different techniques to obtain the readout such as electrochemical impedance spectroscopy (EIS), stripping voltammetry (SWSV), cyclic voltammetry (CV), the field-effect transistor (FET), or square wave voltammograms (SWV) [66]. Currently, EIS is the best technique because it gives awareness to biomolecular interaction out of their impact on electron transfer resistance (Ret) [18]. In addition, there are different nanomaterials recently used in electrochemical biosensor including graphene oxide (GO) [67], gold nanoparticle (AuNP), multi-walled carbon nanotubes (MWCNTs) [68], GO with MWCNTs [69] and GO with gold nanoparticle [70] to enhance the efficiency of the method. GO is the most common nanomaterial to use because it is high sensitivity and affinity for biochemical material [71, 72]. Even though there is advancement in electrochemical biosensors due to different nanomaterial strategies, the fabrication of biosensors is still challenging in terms of size, shape, and number of nanoparticles. Also, signal amplification methods are still under investigation to improve the detection sensitivity.
Showing schematic representation of a electrochemical biosensor, b surface plasmon resonance-based biosensor. The gold film is coated with Biorecognition molecules, which are Antibodies. The Biorecognition molecules interact with the biomarkers. The interaction between the antibodies and antigens results in changes in the refractive index that leads to a shift in the angle of SPR c showing the principle of colorimetric biosensor based on gold nanoparticles, d chemiluminescence-based biosensor. The interaction between Recognition elements such as antibody and the biomarkers results in Light emission and e the structure and the principle of the piezoelectric biosensor
2.2.2 Optical Biosensor
The optical biosensor is a light emission/ absorption-based sensor which measures the alteration in the wavelength of light [73,74,75,76]. The change in wavelength is measured by the optical biosensor. Many different optical biosensors have been developed for cancer detection in the early stages. There are many different types of optical biosensors.
2.2.3 Surface Plasmon Resonance (SPR)/Utilize Localized Surface Plasmon Resonance (LSPR) Biosensors
Surface plasmon resonance-based biosensor is an optical method based on the detection of the biomarkers at the surface of the metal (typically gold). Biorecognition molecules, such as the antibody, are immobilized on the surface of the metal, then they interact with the biomarker. This interaction results in the change in the refractive index and the mass of the sensing medium on the metal and it causes a shift in the angle of SPR [77, 78] (Fig. 3b). In addition, some optical biosensors utilize localized surface plasmon resonance (LSPR). LSPR happens when the surface plasmon gets excited by the light on the surface of the gold that causes the creation of scattering peak and spectral absorption [79].
2.2.4 Fluorescence-Based Biosensor
Fluorescence is a kind of luminescence light that is emitted from molecules following the absorption of light. The fluorophore is a molecule that can absorb light at a shorter wavelength to emit it at a longer wavelength [41]. One of the most significant fluorescence-based biosensors is the fluorescence resonance energy transfer (FRET). It is a non-radiative energy transfer between an excitation fluorophore (donor) and an absorption fluorophore (acceptor). The distance between them is very short, 10–100 Å only [80, 81]. FRET-based biosensor usually utilizes nanoparticle like quantum dots (QD) because it is most common for cancer detection. Moreover, it has high fluorescent for semiconducting nanoparticle, and the surface of QD forms bio conjugate with antibodies easily. Also, QD has unique properties that can resist photo blinking and photo bleaching [82,83,84,85,86].
2.2.5 Colorimetric Based Biosensor
The colorimetric biosensor is based on color change on gold (AuNPs) when the analyte presence or absent [87]. It is a simple and rapid operation for cancer detection [66] (Fig. 3c). AuNPs are most common to use in the colorimetric biosensor as a color development because of their property of rapid color changing that can give visual signal quickly [88,89,90,91].
2.2.6 Chemiluminescence Based Biosensor
Luminescence is light emission when an excited electron of molecules coming back to the ground state. While an electron in the excited state, chemiluminescence happens during the chemical reactions [92]. Chemical luminescence-based biosensors measure the light emitted by a bio-chemiluminescence, and it is based on the absorption of light. Light emission occurs when biomarker binds to the recognition element such as antibodies (Fig. 3d). The advantages of the chemiluminescence-based biosensor are high detectability and simple measurement tool. The photons are generated by a chemical reaction, then they are measured effectively and easily without nonspecific signals [93].
2.2.7 Piezoelectric Biosensor
The piezoelectric biosensor is used as a quartz crystal microbalance (QCM) device that measures the mass change and the frequency change of quartz crystal resonators [94] (Fig. 3e). Quartz is the most common and most suitable crystal for analytical applications such as electrical, mechanical, and chemical applications. QCM can detect small objects such as protein, nucleic acid, viruses, bacteria, and cells [95]. The fabrication of a piezoelectric biosensor is done by the vapor deposition of silver or gold that serves as an electrode [66]. The piezoelectric biosensor is popular in the medical field because it is easy to make, rapid analysis, economic, and highly sensitive [96].
For the development of biosensors, involving functional biomolecules includes complex processing methods which can cause the destruction of the biomolecules and which dramatically reduce the sensitivity of the biosensor. More detailed investigations are still underway to optimize the desirable biomolecule immobilization approaches to achieve the efficicacy of the optical biosensor.
3 Application Biosensor for Cancers Detections
3.1 Breast Cancer
Breast cancer is a major health issue for women. It is the most widespread cancer among females in the US and worldwide, accounting for 627,000 deaths annually [1]. There are 15–20 glands in each breast called lobes, which are the milk-producing glands. These lobes are linked to the nipple by ducts. The lobes are the place where breast cancer usually starts. The breast also contains lymph nodes and vessels, so sometimes cancer can spread to other organs of the body through the lymph nodes and vessels [97]. As mentioned before, there are various biomarkers for breast cancer detection. There are many studies have done to detect different kinds of breast cancer biomarkers by multiple types of biosensors.
The electrochemical biosensor is widely used for breast cancer detection. Selwyn et al. [98] have developed the electrochemical immunosensor for breast cancer detection (cancer antigen CA 15–3) using gold nanoparticle-based on CA 15:3 antibody and antigen interaction. CA 15:3 antibodies were immobilized on the gold nanoparticle, then antigen was added and tagged with the antibody, electrochemical impedance spectroscopy (EIS), and potentiostat was performed to read out the results. Experimental results showed that immunosensor can detect 5–75 U/mL of CA 15–3. There is also a different study for CA 15–3 detection that was done by using an electrochemical immunosensor based on graphene oxide and magnetic silica. The study used a sandwich method in which the anti-CA 15–3 antibody was immobilized on the electrode that contains the graphene oxide. Magnetic silica and graphene oxide were utilized as the signal label. A cyclic voltammogram was performed for the measurement. Electrochemical immunosensor was able to detect CA 15–3 concentration 10−3–200 U/mL with the detection limit of 2.8 × 10−4 U/mL [99]. Another study was done by Zhu et al. [100] to detect human epidermal growth factor receptor 2 (HER2) and SK-BR-3 breast cancer cells by electrochemical immunosensor combined with the gold electrode (AuNPs) and hydrazine advanced silver enhancement. SWSV was applied to analyze the results. As a result, the immunosensor was able to distinguish between HER-2 negative breast cancer and HER-2 positive breast cancer. Moreover, this sensor can indicate 26 cells/mL of SK-BR-3 breast cancer cells in the human blood serum, and able to detect breast cancer successfully in this study. In addition, the sandwich electrochemical biosensor was developed for the detection of breast cancer cells (MCF-7). This sensor was based on a gold electrode and polyadenine-aptamer. The gold electrode was coated with a mixture of AptMUC1 and DTT. Then, the MCF-7 solution was added. MCF-7 cells were sunken in an AptMUC1 functionalized graphene and gold solution. The result was obtained by cyclic voltammetry measurements and electrochemical impedance spectroscopy (EIS). The results suggested that sandwich electrochemical biosensors can be used as a point-of-care device to diagnose breast cancer. Electrochemical biosensor had a high selectivity in discriminating MCF-7 cells towards normal cells and other cancer cells, the detection limit was 8 cells/mL, and the linear range was 10–105 cells/mL [101] (Fig. 4a). Azimadeh et al. [102] have developed electrochemical nano biosensors based on oligo-hybridization to detect the miRNA-155 biomarker. The miRNA-155 is overexpressed in breast cancer. The sensor consists of gold nanorod and graphene oxide. This sensor can detect a low amount of miRNA-155. The linear range of detection was 2.0 fM–8.0 pM with the detection limit of 0.6 fM. In fact miRNA plays an important role in monitoring and controlling different development of cancer and cellular processes. miRNA is a small non-coding RNA molecule that consists of 19–24 molecules [103].
Breast cancer biosensors: a schematic procedure of a sandwich electrochemical biosensor to detect MCF-7 cell and b fabrication of piezoelectric biosensor by using DNA sequence and BRACA1 for breast cancer detection. The hybridization happens between unhybridized DNA-r on the AuNPc cluster and DNA-t. Lung cancer biosensor: c the schematic shows the procedure of the electrochemical aptasensor used for the detection of VEGF biomarker detection
Furthermore, the optical biosensor is used to detect breast cancer biomarkers. Liang et al. [104] have reported surface plasmon resonance-based biosensor with Au/ZnO thin film to detect carbohydrate antigen CA15-3 in the saliva of the human. They used two different surface plasmon resonance (SPR) systems which are SPR biosensor based on thin-film Au/ZnO and the Biacore SPR system to measure the presence of the tumor biomarker CA15-3 in human saliva. The detection range of CA15-3 was 2.5–20 U/mL with the SPR system based on thin-film Au/ZnO, and the detection range of CA15-3 was 40–300 U/mL with the biacore SPR system. As a result, the SPR system based on thin-film Au/ZnO can be used to detect CA15-3 in saliva because it has more sensitivity. FRET biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheet was developed by Shi et al. [105] to detect epithelial cell adhesion molecule (EpCAM), which is a glycosylated protein that can be expressed on the circulating tumor cells surface (CTCs). Breast cancer overexpresses EpCAM, and also is overexpressed by some other cancers. In this work, the donor molecules were PEGylated GQDs, they can raise the emission intensity and block non-specific adsorption of PEGylated GQDs on the surface of the MoS2. Then, MoS2 quenched GQD fluorescence signal. The linear detection range was 3-54 nM, and the detection limit was about 450 pM. This FRET biosensor can detect EpCAM successfully. It was used to detect the expression of EpCAM in MCF-7 cells.
Another type of biosensor was used for breast cancer biomarkers detection which is the Piezoelectric biosensor. Rasheed and Sandhyarani [106] developed a Piezoelectric biosensor which is a quartz crystal microbalance-based genosensor to detect breast cancer 1 gene (BRCA1). They applied a sandwich assay by combining probe DNA with AuNPs, and that results in increasing the mass at the surface and enhancing the sensitivity of magnitude. The result was obtained by using cyclic voltammetric (CV) and chronoamperometry measurement. The biosensor can detect BRCA1 successfully, the detection limit was 10 aM of BRCA1; therefore, the piezoelectric biosensor has good sensitivity, and it will be a potential tool for BRCA 1 detection (Fig. 4b).
A different type of piezoelectric biosensor was developed that is piezoelectric microcantilever sensors (PEMS) to detect HER2 of the breast cancer in the serum of the patients. piezoelectric microcantilever sensors (PEMS) were functionalized with HER2 antibodies against HER2 biomarker, which made the biosensor high sensitivity. This biosensor was able to detect > 2 ng/mL of the HER2 biomarker in the serum [107].
3.2 Lung Cancer
Lung cancer is a serious health concern and the most common cancer in the US and around the world. It is the first cause of death among all cancers, accounting for 1.69 million deaths annually. The treatment of lung cancer is difficult, and it takes a long time with a little improvement in the patient’s health. Early diagnosis of lung cancer increases the survival rate and leads to successful treatment [108]. For this aim, different kinds of biosensors have developed for lung cancer detection in the early stages. Lung cancer has various biomarkers.
The electrochemical biosensor has been developed for the detection of lung cancer biomarkers. Recent researches showed successful results of lung cancer detection by multiple types of electrochemical biosensors. Recent work has developed an electrochemical biosensor based on graphene for Carcinoembryonic antigen (CEA) detection. The graphene was grown on copper (Cu) and employing the chemical vapor deposition (CVD) method. Anti-CEA was immobilized in the biosensor (graphene/copper electrode) to make it specific for the CEA biomarker. To measure and analyze the result, electrochemical impedance spectroscopy (EIS) was performed. The biosensor shows the linear range 1.0–25.0 ng/mL with a limit of detection of 0.23 ng/mL. The result indicated that the electrochemical biosensor based on graphene is selective and sensitive to detect CEA biomarkers [109]. Altintas et al. [110] reported magnetic particle-modified capacitive sensor to detect the lung cancer biomarkers which are carcinoembryonic antigen (CEA), cancer antigen CA15-3 and epidermal growth factor receptor (hEGFR). The levels of concentrations of the biomarkers to indicate lung cancer are higher than 5 ng/mL for CEA, 64 ng/mL for hEGFR, and 50 U/mL for CA15-3. The biosensor can detect CEA and hEGFR successfully in the concentration range of 5 pg/mL to 1 ng/mL, whereas it can detect CA15-3 in the concentration range of 1–200 U/mL. From this study, biosensors are a potential method to detect lung cancer in the early stages. Moreover, a high sensitive electrochemical aptasensor based on the carbon-gold nanocomposite modified screen-printed electrode was developed by Tabrizi et al. [111] to detect vascular endothelial growth factor (VEGF165) in serum. AntiVEGF165 was immobilized on the electrode surface to capture VEGF165 marker in the serum, the interaction between the antibody and the marker leads to a change in the charge of transfer resistance. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were used to analyze the result. VEGF165 can be detected by the biosensor in the linear range of 10–300 pg/mL with the detection limit of 1.0 pg/mL. The aptasensor can detect VEGF165 in serum successfully with high sensitivity and selectivity (Fig. 4c).
In addition, various studies were done to detect lung cancer biomarkers by optical biosensors. Surface plasmon resonance-based immunosensor was developed for CEA detection. The immunoassay was used in this study. A self-assembled monolayer was immobilized on the gold surface by using 11-mercaptoundecanoic acid. Then, the antibody was immobilized to capture the antigen. The SPR based biosensor can detect CEA biomarker with a linear range 3–400 ng/mL. The detection limit was 3 ng/mL [112]. Li et al. [113] reported the FRET-based biosensor between upconverting nanoparticles (UCPs) and palladium nanoparticles (PdNPs) for CEA detection. CEA aptamers were used as biorecognition molecules, and it was bind to hexanedioic acid (HDA) and modified UCPs (HDA-UCPs). The interaction between CEA aptamer and PdNPs made UCPs and PdNPs very close to each other, thus the fluorescence quenching of UCPs reached up to 85%. Biosensor showed that the linear detection range of CEA was 2–100 pg/mL with the detection limit of 0.8 pg/mL in the aqueous buffer. The linear detection range was 4–100 pg/mL in diluted human serum. Furthermore, the colorimetric biosensor was developed to detect the CEA biomarker. This biosensor-based gold Nanoparticle-Decorated Bi2Se3 Nanosheets. The biosensor can detect CEA biomarkers with low concentration as 160 pg/mL [87].
Another type of biosensor was developed for lung cancer detection is the chemiluminescent biosensor. Qu et al. [114] reported chemiluminescent immunosensor along with immunomagnetic separation for lung cancer biomarker (CEA) detection in human serum. HRP labeled-CEA antibody and immunomagnetic beads (IMBs) with target protein CEA formed a sandwich assay structure which is a IMBs-CEA-HRP labeled antibody. IMBs were used to immobilize CEA on the magnetic field. HRP produced optical signals. These signals can be detected by a luminometer which measures the concentration of CEA in the human serum. The results showed that the linear range of detection was 0–50 ng/mL, and the limit of detection was lower than 5.0 pg/mL. As a result, this biosensor is highly sensitive for lung cancer detection.
3.3 Prostate Cancer
Prostate cancer is the most widespread cancer among men in the US. Prostate cancer starts in prostate gland tissues that are located in front of the rectum and under the bladder. The prostate is responsible for semen production and transports sperm [2, 115]. An electrochemical biosensor is used to detect prostate cancer. Pal and Khan [116] reported electrochemical immunosensor based on gold nanoparticles tagged on graphene oxide surface to immobilize monoclonal anti-PSA antibody for the prostate-specific antigen (PSA) detection in prostate cancer. Immunofluorescence staining was performed to confirm the antibody functionalization by using prostate adenocarcinoma cells (LNCaP). Also, Scanning Electron Microscopy (SEM) and cyclic voltammetry (CV) were performed to read out and analyze the results. The detection limit of PSA is 0.24 fg/mL. The immunosensor gave recovery 97.67% of the precise detection of PSA in the serum of the human. Moreover, Wei et al. [117] have developed electrochemical immunosensor. They used a sandwich assay to detect prostate-specific antigen (PSA). This biosensor based on Au–CoS with graphene and CeO2 with ionic liquids that doped with CMC/ILs carboxymethyl chitosan complex. The electrode was modified with Au–CoS/graphene to immobilize Anti-PSA on the electrode surface. Differential pulse voltammetry (DPV) and amperometric were performed to analyze the results. The linear range of detection for immunosensor was 0.5–50 ng/mL, and the detection limit was 0.16 pg/mL of PSA. Quenching electrochemiluminescence (ECL) immunosensor based on resonance energy transfer was reported for PSA detection. Nitrogen-graphene quantum dots (NGQDs) were loaded at Ni(OH)2 to produce good emission for electrochemiluminescence (ECL). Also, the Fe3O4@MnO2 was combined with microspheres. The sandwich immunoassay was performed based on the mechanism of the quenching between NGQDs/ECL (donor) and Fe3O4@MnO2/ECL (acceptor). ECL immunosensor can detect PSA with the concentration linear range of 10−5–10 ng/mL, and the detection limit was 5 fg/mL. This technique was applied in a real sample of serum with the recovery of 94.0–102% [118].
Furthermore, different types of optical biosensors have been developed for prostate cancer detection. An optical fiber SPR based sensor is developed to detect prostate-specific antigen by using a sandwich assay. The sensitivity of the optical fiber SPR sensor was 2.5 × 10–6 refractive index unit (RIU). The optical fiber SPR biosensor is a potential tool to detect PSA biomarker [119]. Yang et al. reported SERS based magnetic aptasensor that uses magnetic nanoparticles (MNPs) core-Au nanoparticles (AuNPs) for detection of prostate-specific antigen (PSA) from the serum. The biosensor shows that the limit of detection was 5.0 pg/mL. Thus, SERS based biosensor is a potential tool that can be used to detect PSA with high sensitive [120].
In addition, a chemiluminescence biosensor was developed, called electrogenerated chemiluminescence biosensor to detect prostate PC-3 cancer cells. The antibody was used as a capture probe and it was immobilized on the graphene electrode. PC-3 cells were captured on the biosensor, and signal probe bound with the captured PC-3 cells result in forming a sandwich. This biosensor can detect this linear range from 7.0 × 102 to 3.0 × 104 cells/mL, with the limit of detection at 2.6 × 102 cells/mL [121].
Another biosensor that is based on the piezoelectric ceramic resonator with a high resonance frequency that serves as transducer was reported by Su et al. for cancer biomarkers detection which is prostate Specific Antigen (PSA) and α-Fetoprotein (AFP). They immobilized the antibodies of the PSA and AFP on the ceramic resonator transducers. The detection of PSA and AFP was obtained from frequency change. This biosensor showed a high sensitivity of detection that can detect 0.25 ng/mL of biomarkers within 30 min [122].
Table 2 illustrates the studies done so far using different types of the biosensor to detect different kinds of cancer biomarkers like breast cancer, lung cancer, and prostate cancer. An electrochemical biosensor is widely used in the medical field and it is the best option and method for cancer detection over the other types of the biosensor for many reasons. it is high sensitivity and specificity, low cost, ease of use, portability, fast response, simple preparation [18, 22, 123]. Thus, electrochemical biosensors are most commonly preferred.
4 Limitations of Current Biosensors
Biosensors are effective tools to diagnose different kinds of cancers in the early stages. However, Biosensors for cancer detection have some challenges. The main limitation is that the complication of cancer cells, that one biomarker can evolve differently in many kinds of cancers and many cellular processes [150]. In other words, many biomarkers have low specificity. Thus, it is significant to understand the cancer progression and molecular changes and to develop highly sensitive techniques. Other challenges of the biosensors are the size of the target especially when it is too small, the level of the cancer biomarkers, the non-specific binding, improving sensitivity and selectivity of the biosensor, the cost reduction, and the real-time result and analysis [6].
5 Future Scope
The biosensors for cancer detection are still immature techniques. Future work should more focus on understanding the complexity of the cancer cells and the molecular change for cancer progression, also understanding the mechanism of interaction between the biomarkers and the nanomaterials on the electrode surface to increase the sensitivity and selectivity of the biosensors for cancers detection [46]. In addition, researchers should work on designing multiplex detection for multiple cancer biomarkers. Although biosensors have been developed to detect several biomarkers, the efficiency of these biosensors is still limited; furthermore, in the future, the biosensor could be potential tools for monitoring the effect of the treatment in the cancers such as chemotherapy or radiation, so patients can monitor the level of the biomarkers during or after the treatments. Biosensors tools promise a bright future for detecting and monitoring cancers.
6 Conclusions
It is important to diagnose and detect cancers in the early stages for successful treatment and rapid cure for cancer patients. Recently, there are various techniques for cancer detection in the early stages, but they are low sensitivity, time-consuming, expensive, unable to detect some types of cancers, and painful for patients. These days, Biosensors are new techniques in the medical field that promises early cancer diagnosis, because they play a crucial role in cancer detection in the early stages. They are highly sensitive, cost-effective, easy to use, and rapid methods that can be an efficient alternative for clinical serological diagnosis of cancers and can be conducted at home by patients. In the last decade, Electrochemical, optical and piezoelectric biosensors and other types of biosensors have been developed for cancer detection, including breast cancer, lung cancer, and prostate cancer. The results from studies showed that these biosensors can detect the cancers successfully with a low range concentration of biomarkers. Although biosensors are still immature techniques, they will be an effective and potential point of care devices in the future.
References
WHO (2020) Cancer, WHO. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 5 Nov 2020
What Is Cancer? (2018) National Cancer Institute. https://www.cancer.gov/about-cancer/understanding/what-is-cancer. Accessed 24 Mar 2018
Cancer—National Library of Medicine (2018) PubMed health. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0015630/. Accessed 24 Mar 2018
Bohunicky B, Mousa SA (2011) Biosensors: the new wave in cancer diagnosis. Nanotechnol Sci Appl 4:1. https://doi.org/10.2147/NSA.S13465
Rasooly A, Jacobson J (2006) Development of biosensors for cancer clinical testing. Biosens Bioelectron 21(10):1851–1858. https://doi.org/10.1016/j.bios.2006.01.003
Wang L (2017) Screening and biosensor-based approaches for lung cancer detection. Sensors 17(10):2420. https://doi.org/10.3390/s17102420
Altintas Z, Tothill I (2013) Biomarkers and biosensors for the early diagnosis of lung cancer. Sens Actuators B: Chem 188:988–998. https://doi.org/10.1016/j.snb.2013.07.078
Ståhlberg A, Zoric N, Åman P, Kubista M (2005) Quantitative real-time PCR for cancer detection: the lymphoma case. Expert Rev Mol Diagn 5(2):221–230. https://doi.org/10.1586/14737159.5.2.221
Ying L, Wang Q (2013) Microfluidic chip-based technologies: emerging platforms for cancer diagnosis. BMC Biotechnol 13(1):1–10
Li Y, Li Y, Zhao J, Zheng X, Mao Q, Xia H (2016) Development of a sensitive luciferase-based sandwich ELISA system for the detection of human extracellular matrix 1 protein. Monoclon Antibodies Immunodiagn Immunother 35(6):273–279. https://doi.org/10.1089/mab.2016.0033
Tothill IE (2009) Biosensors for cancer markers diagnosis. In: Higson SPJ, Parichy DM (eds) Seminars in cell & developmental biology, vol 20. Academic Press, Cambridge, pp 55–62. https://doi.org/10.1016/j.semcdb.2009.01.015
de Gonzalez AB, Darby S (2004) Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet 363(9406):345–351
Journy N, Rehel JL, Le Pointe HD, Lee C, Brisse H, Chateil JF et al (2015) Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer 112(1):185–193
Ghosal R, Kloer P, Lewis KE (2009) A review of novel biological tools used in screening for the early detection of lung cancer. Postgrad Med J 85(1005):358–363. https://doi.org/10.1136/pgmj.2008.076307
MARIBS Study Group (2005) Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 365(9473):1769–1778. https://doi.org/10.1016/S0140-6736(05)66481-1
Onega T, Goldman LE, Walker RL, Miglioretti DL, Buist DS, Taplin S et al (2016) Facility mammography volume in relation to breast cancer screening outcomes. J Med Screen 23(1):31–37. https://doi.org/10.1177/0969141315595254
Kronz JD, Allan CH, Shaikh AA, Epstein JI (2001) Predicting cancer following a diagnosis of high-grade prostatic intraepithelial neoplasia on needle biopsy: data on men with more than one follow-up biopsy. Am J Surg Pathol 25(8):1079–1085
Mittal S, Kaur H, Gautam N, Mantha AK (2017) Biosensors for breast cancer diagnosis: a review of bioreceptors, biotransducers and signal amplification strategies. Biosens Bioelectron 88:217–231. https://doi.org/10.1016/j.bios.2016.08.028
NCI Dictionary of Cancer Terms (2018). National Cancer Institute. https://www.cancer.gov/publications/dictionaries/cancer-terms. Accessed 24 Mar 2018
Van der Vaart M, Pretorius PJ (2010) Is the role of circulating DNA as a biomarker of cancer being prematurely overrated? Clin Biochem 43(1–2):26–36. https://doi.org/10.1016/j.clinbiochem.2009.08.027
Perfézou M, Turner A, Merkoçi A (2012) Cancer detection using nanoparticle-based sensors. Chem Soc Rev 41(7):2606–2622. https://doi.org/10.1039/C1CS15134G
Topkaya SN, Azimzadeh M, Ozsoz M (2016) Electrochemical biosensors for cancer biomarkers detection: recent advances and challenges. Electroanalysis 28(7):1402–1419. https://doi.org/10.1002/elan.201501174
Dong Y, Zheng X, Yang Z, Sun M, Zhang G, An X et al (2016) Serum carcinoembryonic antigen, neuron-specific enolase as biomarkers for diagnosis of nonsmall cell lung cancer. J Cancer Res Ther 12(5):34
Ibau C, Arshad MM, Gopinath SC (2017) Current advances and future visions on bioelectronic immunosensing for prostate-specific antigen. Biosens Bioelectron 98:267–284. https://doi.org/10.1016/j.bios.2017.06.049
Huang Z, Jiang Z, Zhao C, Han W, Lin L, Liu A et al (2017) Simple and effective label-free electrochemical immunoassay for carbohydrate antigen 19–9 based on polythionine-Au composites as enhanced sensing signals for detecting different clinical samples. Int J Nanomed 12:3049. https://doi.org/10.2147/IJN.S131805
Wang T, Zhang L, Tian P, Tian S (2017) Identification of differentially-expressed genes between early-stage adenocarcinoma and squamous cell carcinoma lung cancer using meta-analysis methods. Oncol Lett 13(5):3314–3322. https://doi.org/10.3892/ol.2017.5838
Porto-Mascarenhas EC, Assad DX, Chardin H, Gozal D, Canto GDL, Acevedo AC, Guerra ENS (2017) Salivary biomarkers in the diagnosis of breast cancer: a review. Crit Rev Oncol/Hematol 110:62–73. https://doi.org/10.1016/j.critrevonc.2016.12.009
Chatterjee SK, Zetter BR (2005) Cancer biomarkers: knowing the present and predicting the future. Future Oncol. https://doi.org/10.1517/14796694.1.1.37
Zhang Y, Yang D, Weng L, Wang L (2013) Early lung cancer diagnosis by biosensors. Int J Mol Sci 14(8):15479–15509. https://doi.org/10.3390/ijms140815479
Moelans CB, De Weger RA, Van der Wall E, Van Diest PJ (2011) Current technologies for HER2 testing in breast cancer. Crit Rev Oncol/Hematol 80(3):380–392. https://doi.org/10.1016/j.critrevonc.2010.12.005
Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JWW (2008) An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat 107(3):309–330. https://doi.org/10.1007/s10549-007-9556-1
Opstal-van Winden AW, Vermeulen RC, Peeters PH, Beijnen JH, van Gils CH (2012) Early diagnostic protein biomarkers for breast cancer: how far have we come? Breast Cancer Res Treat 134(1):1–12. https://doi.org/10.1007/s10549-011-1907-2
Molina R, Escudero JM, Muñoz M, Augé JM, Filella X (2012) Circulating levels of HER-2/neu oncoprotein in breast cancer. Clin Chem Lab Med (CCLM) 50(1):5–21. https://doi.org/10.1515/cclm.2011.822
Wu Y, Xue P, Hui KM, Kang Y (2014) A paper-based microfluidic electrochemical immunodevice integrated with amplification-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens Bioelectron 52:180–187. https://doi.org/10.1016/j.bios.2013.08.039
Lee JS, Park S, Park JM, Cho JH, Kim SI, Park BW (2013) Elevated levels of serum tumor markers CA 15–3 and CEA are prognostic factors for diagnosis of metastatic breast cancers. Breast Cancer Res Treat 141(3):477–484. https://doi.org/10.1007/s10549-013-2695-7
Jung JH, Park HY, Lee YH (2001) Clinical value of CEA, CA15-3 and TPS in breast cancer. J Korean Breast Cancer Soc 4(2):136–143. https://doi.org/10.4048/jkbcs.2001.4.2.136
Duffy MJ (1999) CA 15–3 and related mucins as circulating markers in breast cancer. Ann Clin Biochem 36(5):579–586. https://doi.org/10.1177/000456329903600503
Orlandi A, Di Dio C, Calegari MA, Barone C (2016) Paradox CA 15–3 increase in metastatic breast cancer patients treated with everolimus: a change of paradigm in a case series. Biomark Med 10(11):1191–1195. https://doi.org/10.2217/bmm-2016-0142
Cui JW, Li WH, Wang J, Li AL, Li HY, Wang HX et al (2005) Proteomics-based identification of human acute leukemia antigens that induce humoral immune response. Mol Cell Proteomics 4(11):1718–1724. https://doi.org/10.1074/mcp.M400165-MCP200
Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L et al (2014) Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol 32(15):1547
Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA (2015) Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem Rev 115(19):10530–10574. https://doi.org/10.1021/acs.chemrev.5b00321
Bennett WP, Hussain SP, Vahakangas KH, Khan MA, Shields PG, Harris CC (1999) Molecular epidemiology of human cancer risk: gene–environment interactions and p53 mutation spectrum in human lung cancer. J Pathol 187(1):8–18. https://doi.org/10.1002/(SICI)1096-9896(199901)187:1%3c8::AID-PATH232%3e3.0.CO;2-Y
Indovina P, Marcelli E, Pentimalli F, Tanganelli P, Tarro G, Giordano A (2013) Mass spectrometry-based proteomics: the road to lung cancer biomarker discovery. Mass Spectrom Rev 32(2):129–142. https://doi.org/10.1002/mas.21355
Sakao Y, Tomimitsu S, Takeda Y, Natsuaki M, Itoh T (2004) Carcinoembryonic antigen as a predictive factor for postoperative tumor relapse in early-stage lung adenocarcinoma. Eur J Cardiothorac Surg 25(4):520–522. https://doi.org/10.1016/j.ejcts.2004.01.029
Barlési F et al (2004) Prognostic value of combination of Cyfra 21–1, CEA and NSE in patients with advanced non-small cell lung cancer. Respir Med 98(4):357–362
Mehrotra P (2016) Biosensors and their applications—A review. J Oral Biol Craniofac Res 6(2):153–159. https://doi.org/10.1016/j.jobcr.2015.12.002
Bahadır EB, Sezgintürk MK (2015) Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal Biochem 478:107–120. https://doi.org/10.1016/j.ab.2015.03.011
Scognamiglio V, Pezzotti G, Pezzotti I, Cano J, Buonasera K, Giannini D, Giardi MT (2010) Biosensors for effective environmental and agrifood protection and commercialization: from research to market. Microchim Acta 170(3–4):215–225. https://doi.org/10.1007/s00604-010-0313-5
Selvam AP, Wangzhou A, Jacobs M, Wu T, Mohan C, Prasad S (2017) Development and validation of an impedance biosensor for point-of-care detection of vascular cell adhesion molecule-1 toward lupus diagnostics. Future Sci OA 3(3):0047. https://doi.org/10.4155/fsoa-2017-0047
Cesewski E, Johnson BN (2020) Electrochemical biosensors for pathogen detection. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2020.112214
Villalonga R, Díez P, Yáñez-Sedeño P, Pingarrón JM (2011) Wiring horseradish peroxidase on gold nanoparticles-based nanostructured polymeric network for the construction of mediatorless hydrogen peroxide biosensor. Electrochim Acta 56(12):4672–4677. https://doi.org/10.1016/j.electacta.2011.02.108
Wei N, Xin X, Du J, Li J (2011) A novel hydrogen peroxide biosensor based on the immobilization of hemoglobin on three-dimensionally ordered macroporous (3DOM) gold-nanoparticle-doped titanium dioxide (GTD) film. Biosens Bioelectron 26(8):3602–3607. https://doi.org/10.1016/j.bios.2011.02.010
Amine A, Mohammadi H, Bourais I, Palleschi G (2006) Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens Bioelectron 21(8):1405–1423. https://doi.org/10.1016/j.bios.2005.07.012
WHO’s first ever global estimates of foodborne diseases find children under 5 account for almost one third of deaths (2020). https://www.who.int/news/item/03-12-2015-who-s-first-ever-global-estimates-of-foodborne-diseases-find-children-under-5-account-for-almost-one-third-of-deaths. Accessed 26 Oct 2020
Jain S, Singh SR, Horn DW, Davis VA, Ram MJ, Pillai SR (2012) Development of an antibody functionalized carbon nanotube biosensor for foodborne bacterial pathogens. J Biosens Bioelectron 11:002. https://doi.org/10.4172/2155-6210.S11-002
Li Y, Liu X, Lin Z (2012) Recent developments and applications of surface plasmon resonance biosensors for the detection of mycotoxins in foodstuffs. Food Chem 132(3):1549–1554. https://doi.org/10.1016/j.foodchem.2011.10.109
Magrisso S, Erel Y, Belkin S (2008) Microbial reporters of metal bioavailability. Microb Biotechnol 1(4):320–330. https://doi.org/10.1111/j.1751-7915.2008.00022.x
Audrey S, Beatriz PS, Jean-Louis M (2012) Biosensors for pesticide detection: new trends. Am J Anal Chem. https://doi.org/10.4236/ajac.2012.33030
Zhang W, Asiri AM, Liu D, Du D, Lin Y (2014) Nanomaterial-based biosensors for environmental and biological monitoring of organophosphorus pesticides and nerve agents. TrAC Trends Anal Chem 54:1–10. https://doi.org/10.1016/j.trac.2013.10.007
Ali J, Najeeb J, Ali MA, Aslam MF, Raza AJJBB (2017) Biosensors: their fundamentals, designs, types and most recent impactful applications: a review. J Biosens Bioelectron 8(1):1–9. https://doi.org/10.4172/2155-6210.1000235
Grieshaber D, MacKenzie R, Vörös J, Reimhult E (2008) Electrochemical biosensors-sensor principles and architectures. Sensors 8(3):1400–1458. https://doi.org/10.3390/s80314000
Gauglitz G, Proll G (2007) Strategies for label-free optical detection. In: Renneberg R, Lisdat F (eds) Biosensing for the 21st Century. Springer, Berlin, pp 395–432
Pohanka M (2018) Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Materials 11(3):448. https://doi.org/10.3390/ma11030448
Medley CD, Smith JE, Tang Z, Wu Y, Bamrungsap S, Tan W (2008) Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal Chem 80(4):1067–1072. https://doi.org/10.1021/ac702037y
Chikkaveeraiah BV, Bhirde AA, Morgan NY, Eden HS, Chen X (2012) Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 6(8):6546–6561. https://doi.org/10.1021/nn3023969
Ranjan R, Esimbekova EN, Kratasyuk VA (2017) Rapid biosensing tools for cancer biomarkers. Biosens Bioelectron 87:918–930. https://doi.org/10.1016/j.bios.2016.09.061
Zhou T, Zhang B, Wei P, Du Y, Zhou H, Yu M et al (2014) Energy metabolism analysis reveals the mechanism of inhibition of breast cancer cell metastasis by PEG-modified graphene oxide nanosheets. Biomaterials 35(37):9833–9843. https://doi.org/10.1016/j.biomaterials.2014.08.033
Arora S, Kumar R, Kaur H, Rayat CS, Kaur I, Arora SK et al (2014) Translocation and toxicity of docetaxel multi-walled carbon nanotube conjugates in mammalian breast cancer cells. J Biomed Nanotechnol 10(12):3601–3609. https://doi.org/10.1166/jbn.2014.1875
Arya N, Arora A, Vasu KS, Sood AK, Katti DS (2013) Combination of single walled carbon nanotubes/graphene oxide with paclitaxel: a reactive oxygen species mediated synergism for treatment of lung cancer. Nanoscale 5(7):2818–2829. https://doi.org/10.1039/c3nr33190c
Liu X, Xie L, Li H (2012) Electrochemical biosensor based on reduced graphene oxide and Au nanoparticles entrapped in chitosan/silica sol–gel hybrid membranes for determination of dopamine and uric acid. J Electroanal Chem 682:158–163. https://doi.org/10.1016/j.jelechem.2012.07.031
Myung S, Solanki A, Kim C, Park J, Kim KS, Lee KB (2011) Graphene-encapsulated nanoparticle-based biosensor for the selective detection of cancer biomarkers. Adv Mater 23(19):2221–2225. https://doi.org/10.1002/adma.201100014
Zhang LN, Deng HH, Lin FL, Xu XW, Weng SH, Liu AL et al (2014) In situ growth of porous platinum nanoparticles on graphene oxide for colorimetric detection of cancer cells. Anal Chem 86(5):2711–2718. https://doi.org/10.1021/ac404104j
Chung YK, Reboud J, Lee KC, Lim HM, Lim PY, Wang KY et al (2011) An electrical biosensor for the detection of circulating tumor cells. Biosens Bioelectron 26(5):2520–2526. https://doi.org/10.1016/j.bios.2010.10.048
Cai HH, Pi J, Lin X, Li B, Li A, Yang PH, Cai J (2015) Gold nanoprobes-based resonance Rayleigh scattering assay platform: sensitive cytosensing of breast cancer cells and facile monitoring of folate receptor expression. Biosens Bioelectron 74:165–169. https://doi.org/10.1016/j.bios.2015.06.012
Chen S, Zhao Q, Zhang L, Wang L, Zeng Y, Huang H (2015) Combined detection of breast cancer biomarkers based on plasmonic sensor of gold nanorods. Sens Actuators B: Chem 221:1391–1397. https://doi.org/10.1016/j.snb.2015.08.023
Manikandan M, Abdelhamid HN, Talib A, Wu HF (2014) Facile synthesis of gold nanohexagons on graphene templates in Raman spectroscopy for biosensing cancer and cancer stem cells. Biosens Bioelectron 55:180–186. https://doi.org/10.1016/j.bios.2013.11.037
Sato Y, Fujimoto K, Kawaguchi H (2003) Detection of a K-ras point mutation employing peptide nucleic acid at the surface of a SPR biosensor. Colloids Surf B 27(1):23–31. https://doi.org/10.1016/S0927-7765(02)00027-9
Guo X (2012) Surface plasmon resonance based biosensor technique: a review. J Biophotonics 5(7):483–501. https://doi.org/10.1002/jbio.201200015
Mayer KM, Hafner JH (2011) Localized surface plasmon resonance sensors. Chem Rev 111(6):3828–3857. https://doi.org/10.1021/cr100313v
Thakur MS, Ranjan R, Vinayaka AC, Abhijith KS, Sharma R (2013) Nanoparticles and biophotonics as efficient tools in resonance energy transfer-based biosensing for monitoring food toxins and pesticides. In: Park B, Appell M (eds) Advances in applied nanotechnology for agriculture. American Chemical Society, Washington, pp 55–84
Lu S, Wang Y (2010) Fluorescence resonance energy transfer biosensors for cancer detection and evaluation of drug efficacy. Clin Cancer Res 16(15):3822–3824. https://doi.org/10.1158/1078-0432.CCR-10-1333
Algar WR, Tavares AJ, Krull UJ (2010) Beyond labels: a review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal Chim Acta 673(1):1–25. https://doi.org/10.1016/j.aca.2010.05.026
Li J, Xu M, Huang H, Zhou J, Abdel-Halimb ES, Zhang JR, Zhu JJ (2011) Aptamer-quantum dots conjugates-based ultrasensitive competitive electrochemical cytosensor for the detection of tumor cell. Talanta 85(4):2113–2120. https://doi.org/10.1016/j.talanta.2011.07.055
Chen NT, Cheng SH, Liu CP, Souris JS, Chen CT, Mou CY, Lo LW (2012) Recent advances in nanoparticle-based Förster resonance energy transfer for biosensing, molecular imaging and drug release profiling. Int J Mol Sci 13(12):16598–16623. https://doi.org/10.3390/ijms131216598
Wagner MK, Li F, Li J, Li XF, Le XC (2010) Use of quantum dots in the development of assays for cancer biomarkers. Anal Bioanal Chem 397(8):3213–3224. https://doi.org/10.1007/s00216-010-3847-9
Wang LW, Peng CW, Chen C, Li Y (2015) Quantum dots-based tissue and in vivo imaging in breast cancer researches: current status and future perspectives. Breast Cancer Res Treat 151(1):7–17. https://doi.org/10.1007/s10549-015-3363-x
Xiao L, Zhu A, Xu Q, Chen Y, Xu J, Weng J (2017) Colorimetric biosensor for detection of cancer biomarker by au nanoparticle-decorated Bi2Se3 nanosheets. ACS Appl Mater Interfaces 9(8):6931–6940. https://doi.org/10.1021/acsami.6b15750
Kuroda K, Ishida T, Haruta M (2009) Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J Mol Catal A: Chem 298(1–2):7–11. https://doi.org/10.1016/j.molcata.2008.09.009
Okumura M, Haruta M (2016) Interplay of theoretical calculations and experiments for a study of catalysis by gold. Catal Today 259:81–86. https://doi.org/10.1016/j.cattod.2015.05.006
Abad A, Concepción P, Corma A, García H (2005) A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew Chem Int Ed 44(26):4066–4069
Liu M, Zhao H, Chen S, Yu H, Quan X (2012) Interface engineering catalytic graphene for smart colorimetric biosensing. ACS Nano 6(4):3142–3151
Blum LJ, Marquette CA (2006) Chemiluminescence-based sensors. In: Baldini F, Chester A, Homola J, Martellucci S (eds) Optical chemical sensors. Springer, Dordrecht, pp 157–178
Roda A, Mirasoli M, Michelini E, Di Fusco M, Zangheri M, Cevenini L et al (2016) Progress in chemical luminescence-based biosensors: a critical review. Biosens Bioelectron 76:164–179. https://doi.org/10.1016/j.bios.2015.06.017
Laschitsch A, Johannsmann D (1999) High frequency tribological investigations on quartz resonator surfaces. J Appl Phys 85(7):3759–3765. https://doi.org/10.1063/1.369745
Sun W, Song W, Guo X, Wang Z (2017) Ultrasensitive detection of nucleic acids and proteins using quartz crystal microbalance and surface plasmon resonance sensors based on target-triggering multiple signal amplification strategy. Anal Chim Acta 978:42–47. https://doi.org/10.1016/j.aca.2017.04.047
Becker B, Cooper MA (2011) A survey of the 2006–2009 quartz crystal microbalance biosensor literature. J Mol Recognit 24(5):754–787. https://doi.org/10.1002/jmr.1117
Breast cancer—Symptoms and causes (2019) Mayo clinic. https://www.mayoclinic.org/diseases-conditions/breast-cancer/symptoms-causes/syc-20352470. Accessed 16 Jan 2019
Selwyna PGC, Loganathan PR, Begam KH (2013) Development of electrochemical biosensor for breast cancer detection using gold nanoparticle doped CA 15–3 antibody and antigen interaction. In 2013 International Conference on signal processing, image processing & pattern recognition. IEEE, pp 75–81. https://doi.org/10.1109/ICSIPR.2013.6497963
Ge S, Sun M, Liu W, Li S, Wang X, Chu C et al (2014) Disposable electrochemical immunosensor based on peroxidase-like magnetic silica–graphene oxide composites for detection of cancer antigen 153. Sens Actuators B: Chem 192:317–326. https://doi.org/10.1016/j.snb.2013.10.127
Zhu Y, Chandra P, Shim YB (2013) Ultrasensitive and selective electrochemical diagnosis of breast cancer based on a hydrazine–Au nanoparticle–aptamer bioconjugate. Anal Chem 85(2):1058–1064. https://doi.org/10.1021/ac302923k
Wang K, He MQ, Zhai FH, He RH, Yu YL (2017) A novel electrochemical biosensor based on polyadenine modified aptamer for label-free and ultrasensitive detection of human breast cancer cells. Talanta 166:87–92. https://doi.org/10.1016/j.talanta.2017.01.052
Azimzadeh M, Rahaie M, Nasirizadeh N, Ashtari K, Naderi-Manesh H (2016) An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens Bioelectron 77:99–106. https://doi.org/10.1016/j.bios.2015.09.020
Hayes J, Peruzzi PP, Lawler S (2014) MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20(8):460–469. https://doi.org/10.1016/j.molmed.2014.06.005
Liang YH, Chang CC, Chen CC, Chu-Su Y, Lin CW (2012) Development of an Au/ZnO thin film surface plasmon resonance-based biosensor immunoassay for the detection of carbohydrate antigen 15–3 in human saliva. Clin Biochem 45(18):1689–1693. https://doi.org/10.1016/j.clinbiochem.2012.09.001
Shi J, Lyu J, Tian F, Yang M (2017) A fluorescence turn-on biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheets for epithelial cell adhesion molecule (EpCAM) detection. Biosens Bioelectron 93:182–188. https://doi.org/10.1016/j.bios.2016.09.012
Rasheed PA, Sandhyarani N (2016) Quartz crystal microbalance genosensor for sequence specific detection of attomolar DNA targets. Anal Chim Acta 905:134–139. https://doi.org/10.1016/j.aca.2015.11.033
Loo L, Capobianco JA, Wu W, Gao X, Shih WY, Shih WH et al (2011) Highly sensitive detection of HER2 extracellular domain in the serum of breast cancer patients by piezoelectric microcantilevers. Anal Chem 83(9):3392–3397. https://doi.org/10.1021/ac103301r
Nanavaty P, Alvarez MS, Alberts WM (2014) Lung cancer screening: advantages, controversies, and applications. Cancer control 21(1):9–14. https://doi.org/10.1177/107327481402100102
Singh VK, Kumar S, Pandey SK, Srivastava S, Mishra M, Gupta G et al (2018) Fabrication of sensitive bioelectrode based on atomically thin CVD grown graphene for cancer biomarker detection. Biosens Bioelectron 105:173–181. https://doi.org/10.1016/j.bios.2018.01.014
Altintas Z, Kallempudi SS, Sezerman U, Gurbuz Y (2012) A novel magnetic particle-modified electrochemical sensor for immunosensor applications. Sens Actuators B: Chem 174:187–194. https://doi.org/10.1016/j.snb.2012.08.052
Tabrizi MA, Shamsipur M, Farzin L (2015) A high sensitive electrochemical aptasensor for the determination of VEGF165 in serum of lung cancer patient. Biosens Bioelectron 74:764–769. https://doi.org/10.1016/j.bios.2015.07.032
Altintas Z, Uludag Y, Gurbuz Y, Tothill IE (2011) Surface plasmon resonance based immunosensor for the detection of the cancer biomarker carcinoembryonic antigen. Talanta 86:377–383. https://doi.org/10.1016/j.talanta.2011.09.031
Li H, Shi L, Sun DE, Li P, Liu Z (2016) Fluorescence resonance energy transfer biosensor between upconverting nanoparticles and palladium nanoparticles for ultrasensitive CEA detection. Biosens Bioelectron 86:791–798. https://doi.org/10.1016/j.bios.2016.07.070
Qu S, Liu J, Luo J, Huang Y, Shi W, Wang B, Cai X (2013) A rapid and highly sensitive portable chemiluminescent immunosensor of carcinoembryonic antigen based on immunomagnetic separation in human serum. Anal Chim Acta 766:94–99. https://doi.org/10.1016/j.aca.2012.12.043
“What Is Prostate Cancer?” (2018). https://www.cancer.org/cancer/prostate-cancer/about/what-is-prostate-cancer.html. Accessed 01 Oct 2018
Pal M, Khan R (2017) Detection of prostate cancer risk factor immunosensor based deposition of graphene layer decorated gold nanoparticles. Anal Biochem. https://doi.org/10.1016/j.ab.2017.08.001
Wei Y, Li X, Sun X, Ma H, Zhang Y, Wei Q (2017) Dual-responsive electrochemical immunosensor for prostate specific antigen detection based on Au-CoS/graphene and CeO2/ionic liquids doped with carboxymethyl chitosan complex. Biosens Bioelectron 94:141–147. https://doi.org/10.1016/j.bios.2017.03.001
Zhu W, Khan MS, Cao W, Sun X, Ma H, Zhang Y, Wei Q (2018) Ni (OH) 2/NGQDs-based electrochemiluminescence immunosensor for prostate specific antigen detection by coupling resonance energy transfer with Fe3O4@ MnO2 composites. Biosens Bioelectron 99:346–352. https://doi.org/10.1016/j.bios.2017.08.005
Jang HS, Park KN, Kang CD, Kim JP, Sim SJ, Lee KS (2009) Optical fiber SPR biosensor with sandwich assay for the detection of prostate specific antigen. Opt Commun 282(14):2827–2830. https://doi.org/10.1016/j.optcom.2009.03.078
Yang K, Hu Y, Dong N, Zhu G, Zhu T, Jiang N (2017) A novel SERS-based magnetic aptasensor for prostate specific antigen assay with high sensitivity. Biosens Bioelectron 94:286–291. https://doi.org/10.1016/j.bios.2017.02.048
Yang H, Li Z, Shan M, Li C, Qi H, Gao Q et al (2015) Electrogenerated chemiluminescence biosensing for the detection of prostate PC-3 cancer cells incorporating antibody as capture probe and ruthenium complex-labelled wheat germ agglutinin as signal probe. Anal Chim Acta 863:1–8. https://doi.org/10.1016/j.aca.2014.09.001
Su L, Fong CC, Cheung PY, Yang M (2017) Development of novel piezoelectric biosensor using pzt ceramic resonator for detection of cancer markers. In: Prickril B, Rasooly A (eds) Biosensors and biodetection. Humana Press, New York, NY, pp 277–291. https://doi.org/10.1007/978-1-4939-6911-1_19
Jainish P, Prittesh P (2017) Biosensors and biomarkers: promising tools for cancer diagnosis. Int J Biosens Bioelectron 3(4):00072. https://doi.org/10.15406/ijbsbe.2017.03.00072
Qureshi A, Gurbuz Y, Niazi JH (2015) Label-free capacitance based aptasensor platform for the detection of HER2/ErbB2 cancer biomarker in serum. Sens Actuators B: Chem 220:1145–1151. https://doi.org/10.1016/j.snb.2015.06.094
Chun L, Kim SE, Cho M, Choe WS, Nam J, Lee DW, Lee Y (2013) Electrochemical detection of HER2 using single stranded DNA aptamer modified gold nanoparticles electrode. Sens Actuators B: Chem 186:446–450. https://doi.org/10.1016/j.snb.2013.06.046
Arkan E, Saber R, Karimi Z, Shamsipur M (2015) A novel antibody–antigen based impedimetric immunosensor for low level detection of HER2 in serum samples of breast cancer patients via modification of a gold nanoparticles decorated multiwall carbon nanotube-ionic liquid electrode. Anal Chim Acta 874:66–74. https://doi.org/10.1016/j.aca.2015.03.022
Salahandish R, Ghaffarinejad A, Naghib SM, Majidzadeh-A K, Zargartalebi H, Sanati-Nezhad A (2018) Nano-biosensor for highly sensitive detection of HER2 positive breast cancer. Biosens Bioelectron 117:104–111. https://doi.org/10.1016/j.bios.2018.05.043
Ravalli A, da Rocha CG, Yamanaka H, Marrazza G (2015) A label-free electrochemical affisensor for cancer marker detection: the case of HER2. Bioelectrochemistry 106:268–275. https://doi.org/10.1016/j.bioelechem.2015.07.010
Marques RC, Costa-Rama E, Viswanathan S, Nouws HP, Costa-García A, Delerue-Matos C, González-García MB (2018) Voltammetric immunosensor for the simultaneous analysis of the breast cancer biomarkers CA 15–3 and HER2-ECD. Sens Actuators B: Chem 255:918–925. https://doi.org/10.1016/j.snb.2017.08.107
Jiang X, Wang H, Yuan R, Chai Y (2015) Sensitive electrochemiluminescence detection for CA15-3 based on immobilizing luminol on dendrimer functionalized ZnO nanorods. Biosens Bioelectron 63:33–38. https://doi.org/10.1016/j.bios.2014.07.009
Li H, He J, Li S, Turner AP (2013) Electrochemical immunosensor with N-doped graphene-modified electrode for label-free detection of the breast cancer biomarker CA 15–3. Biosens Bioelectron 43:25–29. https://doi.org/10.1016/j.bios.2012.11.037
Li W, Yuan R, Chai Y, Chen S (2010) Reagentless amperometric cancer antigen 15–3 immunosensor based on enzyme-mediated direct electrochemistry. Biosens Bioelectron 25(11):2548–2552. https://doi.org/10.1016/j.bios.2010.04.011
Benvidi A, Tezerjani MD, Jahanbani S, Ardakani MM, Moshtaghioun SM (2016) Comparison of impedimetric detection of DNA hybridization on the various biosensors based on modified glassy carbon electrodes with PANHS and nanomaterials of RGO and MWCNTs. Talanta 147:621–627. https://doi.org/10.1016/j.talanta.2015.10.043
Wang W, Fan X, Xu S, Davis JJ, Luo X (2015) Low fouling label-free DNA sensor based on polyethylene glycols decorated with gold nanoparticles for the detection of breast cancer biomarkers. Biosens Bioelectron 71:51–56. https://doi.org/10.1016/j.bios.2015.04.018
Cui M, Wang Y, Wang H, Wu Y, Luo X (2017) A label-free electrochemical DNA biosensor for breast cancer marker BRCA1 based on self-assembled antifouling peptide monolayer. Sens Actuators B: Chem 244:742–749. https://doi.org/10.1016/j.snb.2017.01.060
Tian L, Qian K, Qi J, Liu Q, Yao C, Song W, Wang Y (2018) Gold nanoparticles superlattices assembly for electrochemical biosensor detection of microRNA-21. Biosens Bioelectron 99:564–570. https://doi.org/10.1016/j.bios.2017.08.035
Rafiee-Pour HA, Behpour M, Keshavarz M (2016) A novel label-free electrochemical miRNA biosensor using methylene blue as redox indicator: application to breast cancer biomarker miRNA-21. Biosens Bioelectron 77:202–207. https://doi.org/10.1016/j.bios.2015.09.025
Salahandish R, Ghaffarinejad A, Omidinia E, Zargartalebi H, Majidzadeh-A K, Naghib SM, Sanati-Nezhad A (2018) Label-free ultrasensitive detection of breast cancer miRNA-21 biomarker employing electrochemical nano-genosensor based on sandwiched AgNPs in PANI and N-doped graphene. Biosens Bioelectron 120:129–136. https://doi.org/10.1016/j.bios.2018.08.025
Jolly P, Tamboli V, Harniman RL, Estrela P, Allender CJ, Bowen JL (2016) Aptamer–MIP hybrid receptor for highly sensitive electrochemical detection of prostate specific antigen. Biosens Bioelectron 75:188–195. https://doi.org/10.1016/j.bios.2015.08.043
Liu S, Su W, Li Y, Zhang L, Ding X (2018) Manufacturing of an electrochemical biosensing platform based on hybrid DNA hydrogel: Taking lung cancer-specific miR-21 as an example. Biosens Bioelectron 103:1–5. https://doi.org/10.1016/j.bios.2017.12.021
Heydari-Bafrooei E, Shamszadeh NS (2017) Electrochemical bioassay development for ultrasensitive aptasensing of prostate specific antigen. Biosens Bioelectron 91:284–292. https://doi.org/10.1016/j.bios.2016.12.048
Mao K, Wu D, Li Y, Ma H, Ni Z, Yu H et al (2012) Label-free electrochemical immunosensor based on graphene/methylene blue nanocomposite. Anal Biochem 422(1):22–27. https://doi.org/10.1016/j.ab.2011.12.047
Gohring JT, Dale PS, Fan X (2010) Detection of HER2 breast cancer biomarker using the opto-fluidic ring resonator biosensor. Sens Actuators B: Chem 146(1):226–230. https://doi.org/10.1016/j.snb.2010.01.067
Li J, Wang J, Zhang X, Chang H, Wei W (2018) Highly selective detection of epidermal growth factor receptor by multifunctional gold-nanoparticle-based resonance Rayleigh scattering method. Sens Actuators B: Chem 273:1300–1306. https://doi.org/10.1016/j.snb.2018.07.046
Park YM, Kim SJ, Kim K, Han YD, Yang SS, Yoon HC (2013) Lectin-based optical sensing for quantitative analysis of cancer antigen CA15-3 as a breast cancer marker. Sens Actuators B: Chem 186:571–579. https://doi.org/10.1016/j.snb.2013.06.060
Ertürk G, Özen H, Tümer MA, Mattiasson B, Denizli A (2016) Microcontact imprinting based surface plasmon resonance (SPR) biosensor for real-time and ultrasensitive detection of prostate specific antigen (PSA) from clinical samples. Sens Actuators B: Chem 224:823–832. https://doi.org/10.1016/j.snb.2015.10.093
Wang X, Yu H, Lu D, Zhang J, Deng W (2014) Label free detection of the breast cancer biomarker CA15. 3 using ZnO nanorods coated quartz crystal microbalance. Sens Actuators B: Chem 195:630–634. https://doi.org/10.1016/j.snb.2014.01.027
Chen JC, Sadhasivam S, Lin FH (2011) Label free gravimetric detection of epidermal growth factor receptor by antibody immobilization on quartz crystal microbalance. Process Biochem 46(2):543–550. https://doi.org/10.1016/j.procbio.2010.10.006
Uludağ Y, Tothill IE (2010) Development of a sensitive detection method of cancer biomarkers in human serum (75%) using a quartz crystal microbalance sensor and nanoparticles amplification system. Talanta 82(1):277–282. https://doi.org/10.1016/j.talanta.2010.04.034
Jayanthi VSA, Das AB, Saxena U (2017) Recent advances in biosensor development for the detection of cancer biomarkers. Biosens Bioelectron 91:15–23. https://doi.org/10.1016/j.bios.2016.12.014
Acknowledgements
The authors also would like to thank Robert M. Urban and Hannah J. Lundberg for valuable comments and discussions.
Funding
The authors acknowledge financial support from NIH R01 AR070181 and the Blazer foundation for the Regenerative Medicine and Disability Research (RMDR) lab and Blazer Nanomedicine Lab at the Department of Biomedical Sciences, UIC College of Medicine at Rockford.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alharthi, S.D., Bijukumar, D., Prasad, S. et al. Evolution in Biosensors for Cancers Biomarkers Detection: A Review. J Bio Tribo Corros 7, 42 (2021). https://doi.org/10.1007/s40735-020-00463-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00463-7