Abstract
Purpose of Review
The present topical review aims to summarize the literature to give an overview of the clinical utility of non-invasive brain stimulation (NIBS) techniques to manage insomnia symptoms and other sleep disturbances.
Recent Findings
Repetitive transcranial magnetic stimulation and transcranial electric stimulation techniques have shown potential to manage sleep disturbances, although studies with robust double-blind controlled designs and larger samples are warranted to better support their use. In addition, other techniques such as transcranial random noise stimulation or transcutaneous vagal nerve stimulation can be promising and merit attention in future research.
Summary
The application of NIBS in sleep disorders is promising, but it is still an emerging research domain which requires exhaustive characterization with state-of-the-art sleep architecture investigation techniques such as in-laboratory or at-home polysomnography.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last few years, the use of non-invasive neurostimulation and neuromodulation techniques has become extremely popular for clinical and research purposes across a series of different conditions [1••, 2]. Given their safety and potential for precision, techniques such as transcranial magnetic stimulation (TMS) or transcranial electrical stimulation (TES) are being tested in a wide variety of neuropsychiatric conditions, as they can affect and change neural networks and, if applied repeatedly, even induce long-term depression (LTD) and long-term potentiation (LTP) changes [1••, 2]. Non-invasive brain stimulation (NIBS) has been successfully used for conditions such as depression, epilepsy, motor stroke, or chronic pain, among others, mainly as an alternative or as an add-on treatment if the typical management options fail. Although the ideal methodologies to acquire the most optimal results still remain to be identified, different protocols and guidelines based on clinically significant results have been established and approved by experts [1••, 2].
Among other neuropsychiatric conditions where NIBS could be beneficial are sleep disorders, given the number of neural pathways that could be stimulated to modulate wakefulness or sleepiness [3••]. Indeed, in the last years, evidence is accumulating regarding the clinical utility of NIBS in sleep disorders, especially concerning repetitive transcranial magnetic stimulation (rTMS) and insomnia. Considering the popularity of studies applying NIBS techniques to sleep disorders, the present topical review aimed to summarize the literature to give an overview of such techniques and describe their use and therapeutic potential in the most common sleep disorders. To this end, a comprehensive search combining keywords and MESH terms for “NIBS” and “sleep” and their derivatives, with the specific terms for each sleep disorder and NIBS type, was performed in Medline (PubMed).
Transcranial Magnetic Stimulation (TMS)
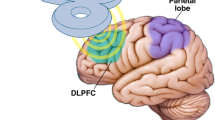
TMS is a stimulation technique based on magnetic pulses to create electric fields stimulating neurons mainly at the cortical level (although it can also stimulate subcortical regions). Simply put, when TMS pulses are administered repeatedly (rTMS); it can increase or decrease cortical excitability, depending on the protocol [1••]. For instance, the administration of rTMS at low frequencies (< 1 Hz) can induce neuronal inhibitory function, whereas at high frequencies (> 5 Hz), it can increase neuronal excitatory function. Its mechanism of action is based on the principle of inductance, where electrical energy is generated by passing a changing and powerful current through a coil positioned over the skull, producing a magnetic field that can depolarize or hyperpolarize neurons’ membranes [4]. These neuronal effects can produce different physiologic and behavioral responses, depending on the targeted area of the brain and the type of stimulation [5]. Even though their use is more established in the fields of depression and chronic pain, rTMS is gaining a lot of attention as an alternative management option for sleep disorders, as it can affect cortical and subcortical pathways by reducing cortical arousal levels and by balancing autonomic function via downregulation of hypothalamic–pituitary–adrenal (HPA) and hypothalamic–pituitary–thyroid (HPT) axes. rTMS has also been found to promote the release of melatonin, BDNF, and GABA, which are critical for the sleep–wake cycle [3••]. Therefore, the potential of rTMS is currently being thoroughly assessed especially in insomnia but also to improve poor sleep quality, restless leg syndrome, hypersomnia, and more conjecturally in sleep apnea and sleep bruxism.
Insomnia
A systematic review published in 2021 collected data on 28 studies among different neuropsychological conditions assessing the effects of rTMS on sleep quality and sleep disturbances [3••]. From the included studies, five were on insomnia (n = 303). Globally, these studies showed an overall improvement in objective and subjective measures of sleep quality. More specifically, improvements in sleep efficiency, non-rapid eye movement (NREM) stage 3, rapid eye movement (REM) sleep cycle, and also indirect sleep markers involving the HPA and HPT axes were present. Although not sham-controlled, one study also observed that improvement in subjective sleep quality was correlated with upregulated expression of brain-derived neurotrophic factor (BDNF) and gamma-aminobutyric acid (GABA) levels [6]. The stimulation site for most of these studies was the dorsolateral prefrontal cortex (mostly left hemisphere) with stimulation frequency protocols at lower frequencies (e.g., 1 Hz). In these studies, it was observed that the effects could last up to 1 month, and according to another study, relapse and recurrence rates of sleep disturbances were reported to be significantly reduced at 3 months in the active rTMS group when compared with sleep medication and psychotherapy groups [7]. Reported side effects were either minor (mild headaches and neck pain) or not reported.
Another systematic review including 5 more studies of rTMS applied among primary insomnia was recently published, gathering information on 211 more patients with insomnia [8••]. In general, reported studies stimulated the same cortical areas at the same frequencies as studies included in the previous review, but the number of sessions (e.g., 10 or 12) was higher, which yielded more durable results, as improvements lasted up to 1 month after the end of the treatment. In addition, the improvement of sleep variables was correlated with decreased functional connectivity between the right insula and the left medial frontal gyrus in one of the studies [9•]. A significant increase in GABA and creatine concentration in the left dorsolateral prefrontal cortex measured with 1H-magnetic resonance spectroscopy was also found to be associated with improvements in sleep [10•]. Other important findings from those reviews were that (a) for neuropsychiatric conditions like depression, chronic pain, anxiety, or Parkinson’s disease, stimulations applied over the dorsolateral prefrontal cortex (DLPFC) and motor cortex (M1 mainly) at higher frequencies improved sleep quality, perhaps secondarily to the improvement of the predominant condition, and that (b) in healthy subjects with sleep deprivation, stimulation over different cortical areas (medial frontal gyrus, medial occipital gyrus, upper part middle occipital gyrus) at higher frequencies (5 Hz and 10 Hz) and over the DLPFC promoted concentration and beneficial cognitive effects and improved tasks’ performances.
In summary, possible mechanisms involved in the improvement of insomnia would be related to the reduction of the hyperarousal state that many of these patients present, a regulation of the HPA and HPT axis that can induce dopamine and pineal melatonin release, increase concentrations of brain serotonin and noradrenaline, and also serum levels of GABA and BDNF, which are key neurotransmitters in the sleep–wake cycle [3••, 11].
Sleep Apnea
Results in sleep apnea are more inconsistent, partly because of the difficulty and difference in mechanisms when compared to insomnia. For instance, TMS has been used as a method to recruit upper airway dilator muscles (submental muscles) in patients with obstructive sleep apnea (OSA) to improve maximal inspiratory inflow during sleep while not waking the patient [12,13,14]. In general, a cortical motor facilitation over the genioglossus was noticed, as well as an augmented maximal inspiratory flow and inspiratory volume of flow-limited respiratory cycles. [14]. Thus, single TMS pulses increased inspiratory patterns without arousing subjects with OSA. However, the mechanical properties of the upper airways did not significantly change with rTMS, and high-frequency rTMS during sleep in other experiments did not improve upper airways mechanical properties [8••, 14, 15], thereby questioning the therapeutic potential of rTMS in sleep apnea. In addition, OSA is now an entity that could be subdivided into four different endotypes (i.e., anatomy, muscle tone, loop gain, and arousal) [16], and it is possible that TMS could more effectively remediate muscle tone and hyperarousal endotypes relative to other OSA subtypes.
Restless Leg Syndrome
Regarding restless leg syndrome (RLS), there is less but favorable evidence about the potential benefits of rTMS. Several TMS studies have demonstrated cortical excitability differences and altered pathways in RLS patients relative to healthy controls, such as intracortical and corticospinal imbalance, mostly concerning gamma-aminobutyric acid GABAergic and glutamatergic networks. Other TMS studies highlighted an impairment of the short-term mechanisms of cortical plasticity and lower amplitude of low-frequency fluctuations (ALFF) in spontaneous brain activity during asymptomatic periods [17,18,19,20]. Thus, hypothetically, rTMS could have the potential to restore those impaired cortical networks that involve reduced short-term plasticity by inducing, for example, dopamine release, and improving motor and sensory disturbances [18, 21]. Emerging data suggest that both high-frequency and low-frequency rTMS applied over the primary motor cortex or the supplementary motor cortex seem to have transient beneficial effects in patients with restless legs syndrome (RLS) [21, 22]. Besides improving subjective motor and sensory symptoms in these patients, emerging data suggest that rTMS could also “restore” some of the circuitries’ imbalances as measured by functional magnetic resonance imaging (fMRI) or motor-evoked potentials (MEP) [8••, 20, 23].
Sleep Bruxism
Evidence regarding rTMS and sleep bruxism (SB) is anecdotal, with only one open-label study showing a decrease in muscle soreness and night jaw-closing muscle electromyographic (EMG) activity during sleep recorded with a portable EMG when M1 of the masseter muscles was stimulated [24]. There is reason to believe that differences in the neural pathways related to the control of the jaw-closing muscles exist in these patients [15, 25, 26] and that rTMS could be able to impact those pathways. However, a recent meta-analysis including nine studies showed no statistically significant differences between patients and controls, possibly due to the variability in terms of classification of bruxism and assessment of neuroplasticity [25]. Therefore, replication of rTMS benefits on SB patients is needed, hopefully with more standardized outcome measures and controlled double-blind studies to understand the putative actions as well, over arousal of muscle tone, for example.
Transcranial Direct Current Stimulation (tDCS)
tDCS is another non-invasive neuromodulation technique based on the injection of a weak and prolonged electrical current through the scalp [2]. This technique is considered sub-threshold because of its indirect mechanism of action [27, 28]. tDCS main effect resides in its capacity to alter neuronal membrane potentials and its propensity to fire action potentials [29]. Anodal tDCS has been associated with the amplification of cortical excitability (increased neuronal firing), whereas cathodal tDCS has been associated with cortical inhibition (decreased neuronal firing) [2]. tDCS has also the potential to contribute to the management of several conditions such as chronic pain, depression, and cognitive dysfunction. Moreover, tDCS has some advantages over rTMS, including lower costs, ease of use, minimal side effects, and the availability of affordable home-based portable devices [30, 31], thus improving the feasibility of multisession stimulation protocols.
Insomnia
A systematic review previously mentioned identified two tDCS studies including insomnia patients with small samples and mixed results and 11 with other predominant conditions where sleep disturbances were present. In general, favorable results were observed in most studies (n = 8/11), with anodal stimulations over the DLPFC, M1, and premotor cortex, as these stimulations improved significantly subjective sleep quality and sleep parameters such as sleep efficiency or number of awakenings [32•]. Overall, less durable and impactful benefits were qualitatively observed than with rTMS. However, several papers have been published since then. For example, a new randomized controlled trial (RCT) performed on 60 eligible TBI-induced insomnia patients who were assigned to real and sham tDCS groups and were treated for 3 weeks reported durable subjective sleep quality and insomnia symptom improvements beyond 6 weeks post-treatment onset in the anodal tDCS group. These effects were found to be more pronounced in young men [33]. The authors argue that these results may be due to men having cancellous parietal bone and females having denser parietal bone or, rather, neuroplasticity differences [33]. In patients with major depression (n = 37), another RCT showed that 10 sessions of daytime tDCS with the anode positioned over F3 and the cathode over F4, at 2 mA current for 30 min, changed the complexity of sleep in the rapid eye movement (REM) stage [34]. In addition, bifrontal tDCS where anode and cathode were placed over the left and right dorsolateral prefrontal cortices, respectively, improved daytime sleepiness but not objective sleep measures in a pilot study among patients with multiple sclerosis [35]. In addition, tDCS significantly prolonged total sleep time (TST) and NREM sleep stage 2 of post-stroke unresponsive wakefulness syndrome in patients that were experiencing sleep cycles [36].
There is research on tDCS application to enhance memory and performance in sleep-deprived patients, yet this does not fall within the scope if this review. For more information on this issue, we refer the reader to another more specific review [37•].
Other Sleep Disorders
Regarding restless leg syndrome (RLS), a 2-week, double-blind, randomized, sham-controlled pilot trial did not show any benefit of either cathodal or anodal tDCS on sleep quality in drug-naïve RLS patients, when compared to sham [38].
No other intervention study using tDCS in sleep apnea or sleep bruxism was found.
Transcranial Alternating Current Stimulation
Transcranial alternating current stimulation (tACS) is of particular interest among TES techniques for its singular ability to modulate endogenous brain oscillations [29, 39, 40]. Similar to tDCS, tACS implicates an injection of an electrical current at low intensity. However, it is rather an alternating sinusoidal current that is administered through the scalp between two or more electrodes. Two main mechanisms are associated with tACS’ neuromodulation effects: entrainment and neuroplasticity. The online effects of tACS have been attributed to an entrainment phenomenon, resulting from the synchronization of the targeted endogenous oscillation with the frequency of the applied external current [41]. The electric field resulting from the injected current is thought to polarize the membrane potential of cortical neurons [29, 39, 40, 42•]. What distinguishes this technique from other NIBS is that it enables the modulation of brain activity in a frequency-specific manner [29, 39, 43, 44]. Long-lasting effects which correspond to the sustained modulation of brain activity observed via offline recording of electroencephalography (EEG) have rather been hypothesized to implicate neuroplastic phenomena [42•, 45, 46].
Given the known role of theta rhythm during early phases of spontaneous sleep onset and as a marker of sleepiness [47, 48], several tACS studies have stimulated at a 5 Hz frequency and showed an acceleration of the sleep onset [49] and an increase in sleep propensity [50] and subjective sleepiness [50]. Additionally, a 2-week at-home crossover experiment studied the effect of a fixed 0.6 mA tACS stimulation at 5 Hz or 10 Hz versus a personalized tACS stimulation. The personalized tACS stimulation was programmed based on the identification of individual power peaks within the alpha and theta bands of each subject in a preliminary EEG session. The personalized stimulation before sleep resulted in an increase in sleep duration and a faster sleep onset, particularly in the insomnia subgroup, which offers an appealing alternative intervention for the treatment of insomnia [51]. Regarding sleepiness, a pilot study by D’Atri and al. showed that a 30-Hz tACS stimulation over the frontal cortex blocked the increase of sleepiness reported by the sham group [52]. This is consistent with a smaller increase of low-frequency activity and an increase in high-frequency activity in the active group compared to the sham group.
Some researchers have been interested in the effects of tACS on dream awareness, though mixed results have been found. More studies are therefore warranted. One group administered tACS over fronto-temporal brain regions at various frequencies (from 2 to 100 Hz) during REM sleep in inexperienced lucid dreamers (n = 27) and found that lower gamma frequency band activity and lucid dreams increased with 40 Hz and 25 Hz stimulation [53]. However, in a single-blind study where experienced lucid dreamers (n = 20) and non-experienced lucid dreamers (n = 13) received a 40 Hz tACS stimulation over the frontal brain region or a sham stimulation during a nap, results did not support an increase of dream self-awareness following tACS stimulation [54].
Regarding memory consolidation, 8 subjects received either sham stimulation or 0.75 Hz tACS at a maximum of 0.55 mA over the frontal lobes during NREM sleep. tACS stimulation resulted in disrupted generation of slow and delta oscillations of slow wave sleep (SWS), thus altering declarative memory consolidation [55]. Additionally, motor memory consolidation was improved by tACS set at 1-s epochs of alternating current at 12 Hz applied bi-frontally with a current of 1 mA. The epochs were applied during a night of sleep upon detection of sleep spindles. No effect was observed on sleep architecture, although an increase in post-stimulation spindle activity was detected [56].
Closed-Loop tACS
Considering inter-individual differences of endogenous brain oscillations, closed-loop protocols have been developed to allow the adjustment of the stimulation frequency and the phase of the exogenous activity in a synchronous manner to those of the endogenous activity [57].
In a study, 36 participants underwent a protocol of 8 h of uninterrupted sleep during which closed-loop tACS (CL-tACS) was delivered at 1.5 mA over bilateral frontal electrodes to investigate the effect of tACS on memory. The stimulation parameters were set to match one’s dominant endogenous slow wave band (0.5–1.2 Hz). Length and quality of sleep were not affected by the stimulation. However, the sham group outperformed the stimulation group on a memory test suggesting that stimulation over a full sleeping period may be detrimental to declarative learning. Ketz et al. [58] also targeted the modulation of slow wave (SW) activity by matching the phase and the frequency of tACS to positive half phases of the endogenous SW activity to improve long-term memory consolidation [58]. Two conditions were compared: [1••] closed-loop tACS, which was applied at 1.5 mA via electrodes placed on F3 and F4 (international EEG system) and administered for five cycles according to the detected endogenous frequency, and [2] sham (no current administered). The closed-loop stimulation protocol was initiated once a persistent N2/N3 sleep stages (greater occurrences of SW events) for 4 min was detected and pursued for the total duration of sleep. Results showed that the active tACS condition induced an increase in SW spectral power at the targeted frequency during sleep, as well as a coupling augmentation with high spindle power. This modulation of SW activity by tACS during sleep was also correlated with an increase in long-term memory performance [59]. As part of this study, Robinson et al. (2018) also found an increase in subjective sleep quality and efficiency after the stimulation protocol [59].
Transcranial Random Noise Stimulation (tRNS)
Stimulation techniques using “white noise” or “pink noise” have been studied for years to induce sleepiness and improve sleep [60,61,62]. However, other varieties of sounds have also been employed with similar purposes [63]. In summary, these sleep-inducing noises can be divided in (a) noises such as white noise and pink noise; (b) autonomous sensory meridian response sounds such as natural sounds like rain and firewood burning, sounds of whispers, or rubbing various objects with a brush; and (c) classical music or a preferred type of music. For instance, pink noise is usually performed to amplify slow oscillatory (0.5–1 Hz) and delta frequency and thus induce or “protect” deep sleep or even to manipulate sleep spindles [64]. In a pilot study with a small sample (n = 8) stimulation with pink noise during sleep, the active “enhancing” stimuli increased restorative sleep and preserved sleep continuity [65]. The administration of rhythmic acoustic stimulation (RAS) during slow wave sleep (SWS) has been shown to be a cost-effective method to enhance slow wave activity. Thirty-five participants received 12-s-long rhythmic bursts of pink noise (at a rate of 1 Hz) during SWS that alternated with non-stimulated, silent periods. These bursts were unilaterally delivered into one of the ears of the participants. As expected, RAS enhanced delta spectral power, especially in its low-frequency range (0.75 and 2.25 Hz). Interestingly, oscillatory activity was observed in both hemispheres regardless of the stimulation site. The most robust increase in slow oscillatory activity appeared during the first 3–4 s of the stimulation period. Furthermore, a short-lasting increase in theta and sigma power was evidenced immediately after the first pulse of the stimulation sequences [66].
Another technique is autonomous sensory meridian response (ASMR), which in essence is a pleasant physiological tingling sensation induced by certain visual and auditory triggers. ASMR has been shown to reduce stress and increase positive mood, among others. In an online study with 1037 participants (18–66 years), participants showed significantly increased relaxation and improved mood after watching relaxing videos featuring beneficial clinical effects in other participants experiencing ASMR. The latter technique was shown to improve mood and reduce arousal, therefore having potential implications for alleviating symptoms of insomnia and depression [67•]. Other studies partly replicated the latter findings, as classical music and ASMR produced sensations of comfort and relaxation and showed significant activation in common cortical areas, while ASMR only showed activation in more cortical areas, with the medial prefrontal cortex being the main area of activation [68]. Moreover, very recent study showed a noticeable difference between auditory and audiovisual stimulation in regard to the different areas of activation in the brain [69]. Whereas audiovisual stimulation showed activation of the middle frontal gyrus and the nucleus accumbens, auditory stimulation showed activation of the insular cortex, suggesting different pathways and encouraging more research with different stimuli.
Transcutaneous Vagal Nerve Stimulation (ta-VNS)
ta-VNS is another non-invasive neurostimulation technique applied to different central nervous system areas innervated by the vagus nerve, such as to the afferent auricular branch of the vagus nerve in the auricular concha, especially cymba concha [70], or at the neck (cervical branch of the vagus nerve) [71], to reduce hyperarousal and sympathetic tone. Regarding sleep, an RCT in patients with insomnia (n = 30 divided into two groups) revealed that after 1 month of daily treatment, Pittsburgh Sleep Quality Index (PSQI) scores decreased significantly in the treatment group. Moreover, treatment response in the treatment group was significantly higher than that of the control group, suggesting that this technique may be safe and effective for at least some insomnia cases [72]. Another study with 24 chronic insomnia patients and 18 healthy controls revealed significant improvements at the PSQI after 4 treatment weeks, which were associated with activity changes in the prefrontal cortex in patients with chronic insomnia [73]. However, another recent study showed that although a 2-week course of ta-VNS improved global sleep scores in community-dwelling adults, the change in sleep was not significantly different from that of the control groups [74]. Interestingly, in another study with participants above 55 years old, it was shown that improvements in measures of autonomic balance were more pronounced in participants with greater baseline sympathetic prevalence [75], perhaps suggesting that this treatment could be more effective when the sympathetic drive is augmented.
Importantly, there is reason to believe that ta-VNS can aggravate or induce sleep apnea episodes in adults and children [76,77,78], likely due to the increase of airway resistance due to increased lateral laryngeal muscle tone and vocal cord paresis or subglottic spasm during the stimulation, which could also increase respiratory efforts [77].
Regarding other sleep disorders, there is a small open trial in sleep bruxism with 10 patients undergoing four sessions of ta-VNS in auricular areas [79]. Moreover, the patients were advised to manually stimulate the same areas between sessions. Masticatory muscle activity and sleep parameters were measured by polysomnography (PSG) before and after the treatment, as well as heart rate variability (HRV) parameters during each stimulation. PSG analysis revealed a statistically significant reduction in tonic SB index and tonic contraction time. Heart rate variability (HRV) parameters showed a statistically significant increase in the mean values of the vagal tone after each stimulation session, and no side effect was reported. Thus, as with rTMS, results seem to be promising, but replication with more accurate and reliable methods is needed.
Another pilot study was done on 15 patients with severe pharmaco-resistant RLS, who underwent 1-h weekly sessions of ta-VNS in the left cymba concha, for 8 weeks [80]. It appears that in general, RLS symptoms improved by the end of the study, such as quality of life, anxiety, or depression. Of these participants, according to the Restless Legs Syndrome Rating Scale (IRLS), 27% (4/15) had a total response, 40% (6/15) had a partial response, and 33% (5/15) were non-responders, which may suggest the possibility of different phenotypes/subgroups of responders. This needs to be investigated more exhaustively, with controlled designs and larger samples.
Conclusions and Future Directions
The application of NIBS in sleep disorders is promising, but it is still an emerging research domain which requires exhaustive characterization with state-of-the-art sleep architecture investigation techniques such as in-laboratory or at-home PSG. For that purpose, studies with robust double-blind controlled designs and larger samples are warranted. Furthermore, the importance of systematically contrasting effectiveness of stimulation methods to try to delineate which one maximizes the balance between treatment response and side effects is emphasized. For example, the comparison of standardized treatment duration, the number of sessions, stimulation intensity, and frequency are basic parameters to compare in order to assess treatment efficacy properly. Finally, the study of NIBS with objective (e.g., PSG, actigraphy) and subjective (e.g., questionnaires) sleep assessments could help to characterize better the possible changes in sleep architecture with the application of each stimulation technique.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol: Off J Int Fed Clin Neurophysiol. 2020;131(2):474–528. Experts in the field synthesized evidence and produce guidelines for the therapeutic use of rTMS.
Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol: Off J Int Fed Clin Neurophysiol. 2017;128(1):56–92.
•• Herrero Babiloni A, Bellemare A, Beetz G, Vinet SA, Martel MO, Lavigne GJ, et al. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: a systematic review. Sleep Med Rev. 2021;55:101381. Systematic review summarizing the evidence of rTMS and tDCS for the treatment of sleep disturbances, which is not limited to primary sleep disorders but also other neuropsychiatric conditions associated with sleep problems.
Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16.
Klein MM, Treister R, Raij T, Pascual-Leone A, Park L, Nurmikko T, et al. Transcranial magnetic stimulation of the brain: guidelines for pain treatment research. Pain. 2015;156(9):1601–14.
Feng J, Zhang Q, Zhang C, Wen Z, Zhou X. The effect of sequential bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum level of BDNF and GABA in patients with primary insomnia. 2019;9(2):e01206.
Jiang CG, Zhang T, Yue FG, Yi ML, Gao D. Efficacy of repetitive transcranial magnetic stimulation in the treatment of patients with chronic primary insomnia. Cell Biochem Biophys. 2013;67(1):169–73.
•• Lanza G, Fisicaro F, Cantone M, Pennisi M, Cosentino FII, Lanuzza B, et al. Repetitive transcranial magnetic stimulation in primary sleep disorders. Sleep medicine reviews. 2022;67:101735. More recent systematic review focused on the use of rTMS in primary sleep disorders.
• Li M, Zhu Y, Zhang X, Yang H, Zhang S, Liu J, et al. 1Hz rTMS over left DLPFC rewired the coordination with hippocampus in insomnia patients: A pilot study. Brain Stimulation. 2022;15(2):437–40. Neuroimaging study evaluating correlates of sleep symptoms improvement.
• Zhang H, Huang X, Wang C, Liang K. Alteration of gamma-aminobutyric acid in the left dorsolateral prefrontal cortex of individuals with chronic insomnia: a combined transcranial magnetic stimulation-magnetic resonance spectroscopy study. Sleep Med. 2022;92:34–40. Another study evaluating correlates of sleep symptoms improvement with a different analytical tool.
Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain : J Neurol. 2003;126(Pt 12):2609–15.
Melo-Silva CA, Borel JC, Gakwaya S, Series F. Acute upper airway muscle and inspiratory flow responses to transcranial magnetic stimulation during sleep in apnoeic patients. Exp Physiol. 2013;98(4):946–56.
Melo-Silva CA, Gakwaya S, Rousseau E, Series F. Consecutive transcranial magnetic stimulation twitches reduce flow limitation during sleep in apnoeic patients. Exp Physiol. 2013;98(9):1366–75.
Rousseau E, Melo-Silva CA, Gakwaya S, Series F. Effects of repetitive transcranial magnetic stimulation of upper airway muscles during sleep in obstructive sleep apnea patients. J Appl Physiol. 2016;121(5):1217–25.
Herrero Babiloni A, De Beaumont L, Lavigne GJ. Transcranial magnetic stimulation: potential use in obstructive sleep apnea and sleep bruxism. Sleep Med Clin. 2018;13(4):571–82.
Lavigne GJ, Herrero Babiloni A, Beetz G, Dal Fabbro C, Sutherland K, Huynh N, et al. Critical issues in dental and medical management of obstructive sleep apnea. J Dent Res. 2020;99(1):26–35.
Lanza G, Lanuzza B, Arico D, Cantone M, Cosentino FII, Bella R, et al. Impaired short-term plasticity in restless legs syndrome: a pilot rTMS study. Sleep Med. 2018;46:1–4.
Lanza G, Cantone M, Arico D, Lanuzza B, Cosentino FII, Paci D, et al. Clinical and electrophysiological impact of repetitive low-frequency transcranial magnetic stimulation on the sensory-motor network in patients with restless legs syndrome. Ther Adv Neurol Disord. 2018;11:1756286418759973.
Nardone R, Sebastianelli L, Versace V, Orioli A, Saltuari L, Trinka E, et al. Involvement of central sensory pathways in subjects with restless legs syndrome: a neurophysiological study. Brain Res. 2021;1772:147673.
Liu C, Dai Z, Zhang R, Zhang M, Hou Y, Qi Z, et al. Mapping intrinsic functional brain changes and repetitive transcranial magnetic stimulation neuromodulation in idiopathic restless legs syndrome: a resting-state functional magnetic resonance imaging study. Sleep Med. 2015;16(6):785–91.
Nardone R, Sebastianelli L, Versace V, Brigo F, Golaszewski S, Pucks-Faes E, et al. Contribution of transcranial magnetic stimulation in restless legs syndrome: pathophysiological insights and therapeutical approaches. Sleep Med. 2020;71:124–34.
Nardone R, Sebastianelli L, Versace V, Brigo F, Golaszewski S, Pucks-Faes E, et al. Effects of repetitive transcranial magnetic stimulation in subjects with sleep disorders. Sleep Med. 2020.
Rizzo V, Arico I, Mastroeni C, Morgante F, Liotta G, Girlanda P, et al. Dopamine agonists restore cortical plasticity in patients with idiopathic restless legs syndrome. Mov Disord. 2009;24(5):710–5.
Zhou WN, Fu HY, Du YF, Sun JH, Zhang JL, Wang C, et al. Short-term effects of repetitive transcranial magnetic stimulation on sleep bruxism - a pilot study. Int J Oral Sci. 2016;8(1):61–5.
Boscato N, Exposto F, Nascimento GG, Svensson P, Costa YM. Is bruxism associated with changes in neural pathways? A systematic review and meta-analysis of clinical studies using neurophysiological techniques. Brain Imaging Behav. 2022;16(5):2268–80.
Ikuta M, Iida T, Kothari M, Shimada A, Komiyama O, Svensson P. Impact of sleep bruxism on training-induced cortical plasticity. J Prosthodont Res. 2019;63(3):277–82.
Vosskuhl J, Struber D, Herrmann CS. Non-invasive brain stimulation: a paradigm shift in understanding brain oscillations. Front Hum Neurosci. 2018;12:211.
Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–99.
Antal A, Alekseichuk I, Paulus W. The new modalities of transcranial electric stimulation: tACS, tRNS, and other approaches. In: Brunoni A, Nitsche M, Loo C, editors. Transcranial direct current stimulation in neuropsychiatric disorders: clinical principles and management. Cham: Springer International Publishing; 2016. p. 21–8.
Ahn H, Sorkpor S, Miao H, Zhong C, Jorge R, Park L, et al. Home-based self-administered transcranial direct current stimulation in older adults with knee osteoarthritis pain: An open-label study. J Neuroeng Rehabil. 2019;66:61–5.
Brietzke AP, Zortea M, Carvalho F, Sanches PRS, Silva DPJ, Torres I, et al. Large treatment effect with extended home-based transcranial direct current stimulation over dorsolateral prefrontal cortex in fibromyalgia: a proof of concept sham-randomized clinical study. J Pain. 2019.
• Herrero Babiloni A, Beetz G, Tang NKY, Heinzer R, Nijs J, Martel MO, et al. Towards the endotyping of the sleep-pain interaction: a topical review on multitarget strategies based on phenotypic vulnerabilities and putative pathways. Pain. 2021;162(5):1281–8. Topical review proposing a theoretical framework for future assessment and management of patients with sleep and pain problems.
BakhshayeshEghbali B, Ramezani S, SedaghatHerfeh S, Emir Alavi C, Najafi K, EsmaeeliLipaei P, et al. Not transcranial direct current stimulation improves sleep quality in patients with insomnia after traumatic brain injury. Brain Inj. 2023;37(1):63–73.
Li Z, Zhao X, Feng L, Zhao Y, Pan W, Liu Y, et al. Can daytime transcranial direct current stimulation treatment change the sleep electroencephalogram complexity of REM sleep in depressed patients? A double-blinded, randomized, placebo-controlled trial. Front Psych. 2022;13:851908.
Chalah MA, Grigorescu C, Kumpfel T, Lefaucheur JP, Padberg F, Palm U, et al. The effects of transcranial direct current stimulation on sleep in patients with multiple sclerosis-a pilot study. Neurophysiol Clin= Clin Neurophysiol. 2022;52(1):28–32.
Yu J, Wu Y, Wu B, Xu C, Cai J, Wen X, et al. Sleep patterns correlates with the efficacy of tDCS on post-stroke patients with prolonged disorders of consciousness. J Transl Med. 2022;20(1):601.
• McIntire LK, McKinley RA, Goodyear C, McIntire JP. The effects of anodal transcranial direct current stimulation on sleep time and efficiency. Front Human Neurosci. 2020;14:357. Study finding that tACS may induce faster recovery from fatigue caused by acute periods of sleep deprivation
Koo YS, Kim SM, Lee C, Lee BU, Moon YJ, Cho YW, et al. Transcranial direct current stimulation on primary sensorimotor area has no effect in patients with drug-naive restless legs syndrome: a proof-of-concept clinical trial. Sleep Med. 2015;16(2):280–7.
Tavakoli AV, Yun K. Transcranial Alternating Current Stimulation (tACS) Mechanisms and protocols. Front Cell Neurosci. 2017;11:214.
Gundlach C, Muller MM, Nierhaus T, Villringer A, Sehm B. Modulation of somatosensory alpha rhythm by transcranial alternating current stimulation at Mu-frequency. Front Hum Neurosci. 2017;11:432.
Pikovsky A, Rosenblum M. Synchronization: a general phenomenon in an oscillatory world. Nova Acta Leopoldina NF88. 2003;332:255–68.
• De Koninck BP, Brazeau D, Guay S, Herrero Babiloni A, De Beaumont L. Transcranial alternating current stimulation to modulate alpha activity: a systematic review. Neuromodulation. 2023. Recent systematic review assessing tACS methodology and potential to modulate alpha activity.
Antal A, Herrmann CS. Transcranial alternating current and random noise stimulation: possible mechanisms. Neural Plast. 2016;2016:3616807.
Paulus W. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol Rehabil. 2011;21(5):602–17.
Vossen A, Gross J, Thut G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (alpha-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 2015;8(3):499–508.
Zaehle T, Rach S, Herrmann CS. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One. 2010;5(11):e13766.
Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101(3):523–9.
De Gennaro L, Ferrara M, Curcio G, Cristiani R. Antero-posterior EEG changes during the wakefulness-sleep transition. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol. 2001;112(10):1901–11.
Xie J, Wang L, Xiao C, Ying S, Ren J, Chen Z, et al. Low frequency transcranial alternating current stimulation accelerates sleep onset process. IEEE Trans Neural Syst Rehabil Eng. 2021;29:2540–9.
D’Atri A, Scarpelli S, Gorgoni M, Alfonsi V, Annarumma L, Giannini AM, et al. Bilateral theta transcranial alternating current stimulation (tACS) modulates EEG activity: when tACS works awake it also works asleep. Nature Sci Sleep. 2019;11:343–56.
Ayanampudi V, Kumar V, Krishnan A, Walker MP, Ivry RB, Knight RT, et al. Personalized transcranial alternating current stimulation improves sleep quality: initial findings. Front Hum Neurosci. 2022;16:1066453.
D’Atri A, Romano C, Gorgoni M, Scarpelli S, Alfonsi V, Ferrara M, et al. Bilateral 5 Hz transcranial alternating current stimulation on fronto-temporal areas modulates resting-state EEG. Sci Rep. 2017;7(1):15672.
Voss U, Holzmann R, Hobson A, Paulus W, Koppehele-Gossel J, Klimke A, et al. Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nat Neurosci. 2014;17(6):810–2.
Blanchette-Carriere C, Julien SH, Picard-Deland C, Bouchard M, Carrier J, Paquette T, et al. Attempted induction of signalled lucid dreaming by transcranial alternating current stimulation. Conscious Cogn. 2020;83:102957.
Garside P, Arizpe J, Lau CI, Goh C, Walsh V. Cross-hemispheric alternating current stimulation during a nap disrupts slow wave activity and associated memory consolidation. Brain Stimul. 2015;8(3):520–7.
Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Frohlich F. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Biol. 2016;26(16):2127–36.
Bergmann TO, Karabanov A, Hartwigsen G, Thielscher A, Siebner HR. Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: current approaches and future perspectives. Neuroimage. 2016;140:4–19.
Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK. Closed-loop slow-wave tACS improves sleep-dependent long-term memory generalization by modulating endogenous oscillations. J Neurosci: Off J Soc Neurosci. 2018;38(33):7314–26.
Robinson CSH, Bryant NB, Maxwell JW, Jones AP, Robert B, Lamphere M, et al. The benefits of closed-loop transcranial alternating current stimulation on subjective sleep quality. Brain Sci. 2018;8(12).
Spencer JA, Moran DJ, Lee A, Talbert D. White noise and sleep induction. Arch Dis Child. 1990;65(1):135–7.
Kawada T, Suzuki S. Sleep induction effects of steady 60 dB (A) pink noise. Ind Health. 1993;31(1):35–8.
Warjri E, Dsilva F, Sanal TS, Kumar A. Impact of a white noise app on sleep quality among critically ill patients. Nurs Crit Care. 2022;27(6):815–23.
Yoon H, Baek HJ. External auditory stimulation as a non-pharmacological sleep aid. Sensors (Basel). 2022;22(3).
Antony JW, Paller KA. Using oscillating sounds to manipulate sleep spindles. Sleep. 2017;40(3).
Schade MM, Mathew GM, Roberts DM, Gartenberg D, Buxton OM. Enhancing slow oscillations and increasing N3 sleep proportion with supervised, non-phase-locked pink noise and other non-standard auditory stimulation during NREM sleep. Nature Sci Sleep. 2020;12:411–29.
Simor P, Steinbach E, Nagy T, Gilson M, Farthouat J, Schmitz R, et al. Lateralized rhythmic acoustic stimulation during daytime NREM sleep enhances slow waves. Sleep. 2018;41(12).
• Smejka T, Wiggs L. The effects of autonomous sensory meridian response (ASMR) videos on arousal and mood in adults with and without depression and insomnia. J Affect Disord. 2022;301:60–7. Online survey based study showing ASMR's potential to improve mood, reduce arousal, and alleviate symptoms of insomnia and depression.
Sakurai N, Ohno K, Kasai S, Nagasaka K, Onishi H, Kodama N. Induction of relaxation by autonomous sensory meridian response. Front Behav Neurosci. 2021;15:761621.
Sakurai N, Nagasaka K, Takahashi S, Kasai S, Onishi H, Kodama N. Brain function effects of autonomous sensory meridian response (ASMR) video viewing. Front Neurosci. 2023;17:1025745.
Kreuzer PM, Landgrebe M, Husser O, Resch M, Schecklmann M, Geisreiter F, et al. Transcutaneous vagus nerve stimulation: retrospective assessment of cardiac safety in a pilot study. Front Psych. 2012;3:70.
Butt MF, Albusoda A, Farmer AD, Aziz Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat. 2020;236(4):588–611.
Wu Y, Song L, Wang X, Li N, Zhan S, Rong P, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10).
He JK, Jia BH, Wang Y, Li SY, Zhao B, Zhou ZG, et al. Transcutaneous auricular vagus nerve stimulation modulates the prefrontal cortex in chronic insomnia patients: fMRI study in the first session. Front Neurol. 2022;13:827749.
Jackowska M, Koenig J, Vasendova V, Jandackova VK. A two-week course of transcutaneous vagal nerve stimulation improves global sleep: findings from a randomised trial in community-dwelling adults. Auton Neurosci. 2022;240:102972.
Bretherton B, Atkinson L, Murray A, Clancy J, Deuchars S, Deuchars J. Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: potential benefits of daily stimulation. Aging (Albany NY). 2019;11(14):4836–57.
Oh DM, Johnson J, Shah B, Bhat S, Nuoman R, Ming X. Treatment of vagus nerve stimulator-induced sleep-disordered breathing: a case series. Epilepsy Behav Rep. 2019;12:100325.
Dye TJ, Hantragool S, Carosella C, Huang G, Hossain MM, Simakajornboon N. Sleep disordered breathing in children receiving vagus nerve stimulation therapy. Sleep Med. 2021;79:101–6.
Parhizgar F, Nugent K, Raj R. Obstructive sleep apnea and respiratory complications associated with vagus nerve stimulators. J Clin Sleep Med. 2011;7(4):401–7.
Polini F, Budai R. Multimodal transcutaneous auricular vagus nerve stimulation: an option in the treatment of sleep bruxism in a "polyvagal" context. Cranio. 2022:1–9.
Hartley S, Bao G, Zagdoun M, Chevallier S, Lofaso F, Leotard A, et al. Noninvasive vagus nerve stimulation: a new therapeutic approach for pharmacoresistant restless legs syndrome. Neuromodulation. 2022.
Funding
Vanier Scholarship (AHB), the Foundation Caroline Durand Research Chair in Acute Traumatology of Université de Montréal (LDB), and Canada Research Chairs (GL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
None applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herrero Babiloni, A., Brazeau, D., De Koninck, B.P. et al. The Utility of Non-invasive Brain Stimulation in Relieving Insomnia Symptoms and Sleep Disturbances Across Different Sleep Disorders: a Topical Review. Curr Sleep Medicine Rep 9, 124–132 (2023). https://doi.org/10.1007/s40675-023-00254-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-023-00254-9