Abstract
Background
Severe skin reactions, mostly following medication use, are rare and can be associated with high mortality. A suitable treatment approach that is able to reduce mortality is needed.
Methods
Recent publications on this topic were reviewed and evaluated.
Results
In the case of the self-limiting diseases acute generalized exanthematous pustulosis (AGEP) and generalized bullous fixed drug eruption (GBFDE), there is no clear indication for systemic immunomodulating treatment, and supportive care remains the gold standard. The situation is less clear in the case of drug reaction with eosinophilia and systemic symptoms (DRESS); nevertheless, primarily in the case of severe organ involvement, systemic glucocorticosteroids are recommended. This is associated with complications and often also with virus reactivation, which may delay healing. The evidence on various immunomodulating therapies in Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) is controversial. Recent publications favor steroid pulse treatment, the tumor necrosis factor (TNF)-α inhibitor etanercept, as well as the calcineurin inhibitor cyclosporine A, with the latter representing the most promising approach.
Conclusion
The rarity and unpredictability of the reactions make a randomized double-blind therapeutic trial extremely difficult. Using meta-analyses, it is possible to trace a trend in the use of systemic treatment options. Supportive care remains the most important treatment strategy for all clinical entities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe skin reactions generally occur as a result of medication use, but can also have other causes. While they differ in terms of clinical picture, they share their rarity. Some types of reaction are life-threatening and carry a high mortality rate [1]. With an incidence of between one and two cases per million inhabitants per year, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are the most feared types of reaction [2]. Differentiating between SJS/TEN and generalized bullous fixed drug eruption (GBFDE) is often challenging, particularly in the case of GBFDE with extensive skin detachment. In addition to the above-mentioned bullous skin reactions, there are also reactions that are primarily non-bullous: acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophila and systemic symptoms (DRESS), formerly referred to as hypersensitivity syndrome. Although these reactions appear to be less dangerous, organ involvement, particularly in DRESS, should not be underestimated [3, 4]. This article provides an overview of reaction types, their diagnostic work-up, and their treatment.
Clinical picture, diagnostic work-up, and differential diagnoses
AGEP
Acute-onset erythema, which may cause an itchy or burning sensation and is accompanied by fever, is characteristic for AGEP. This is followed by the appearance of multiple, non-follicular pustules, which are often more pronounced in the flexures, whereas the erythema is usually more generalized (Fig. 1). In addition to widespread erythema, there may be macular, target-like and patchy redness. In a small number of cases, confluent pustules may mimic a positive Nikolsky sign, often resulting in misdiagnosis as TEN. The reaction resolves within a few days with the typical post-pustular desquamation [3].
Approximately 20% of AGEP patients exhibit mild, non-erosive mucosal involvement. Organ involvement is seen in less than 20% of cases, with this usually resolving within a few days after discontinuation of the trigger. The liver, kidneys, lungs, and bone marrow are the organs most frequently affected [5].
Histology typically reveals spongiosis, subcorneal and/or intraepidermal pustules with a perivascular infiltrate containing neutrophils, as well as edema of the papillary dermis [6, 7].
To improve diagnostics, an AGEP validation score was formulated as part of a multinational case control study [3], enabling cases to be classified as “definite,” “probable,” “possible,” and “no AGEP” (Table 1).
In addition to the laboratory tests included in the score (in particular a differential blood count to detect neutrophilia), a swab of the pustules should be taken, which is a typically sterile.
Numerous diseases are associated with pustular skin reactions. Follicular diseases such as acne, acne-form dermatitis, bacterial/fungal folliculitis, furunculosis, as well as impetiginized eczema, impetigo, localized pustular contact dermatitis, pemphigus foliaceus, immunoglobulin (Ig) A pemphigus, Sweet syndrome, and varicella infection can be readily differentiated from AGEP on the basis of laboratory parameters and histology. In contrast, differentiation from generalized pustular psoriasis (von Zumbusch type) is often challenging [3, 7]. In a small number of cases, AGEP may be mistaken for SJS/TEN, erythema necrolyticum migrans, or staphylococcal scalded skin syndrome (SSSS). This often happens in cases with confluent pustules that imitate a positive Nikolsky sign [3].
DRESS
The clinical picture of DRESS—referred to as drug-induced hypersensitivity syndrome (DIHS) in Japan—is heterogeneous and often varies over its course, meaning that the diagnosis is primarily one of exclusion [4]. The initial manifestation is usually a maculopapular exanthema, spreading from the face to the rest of the body, sometimes progressing to erythroderma (Fig. 2). The eruptions may also be urticarial, target-like, or purpuriform. Pustules are also described. The primary skin lesions develop over the disease course from infiltrated plaques to exfoliative dermatitis. The exanthema is accompanied by fever, facial edema, and frequently lymphadenopathy. Cheilitis, discrete oral mucosal erosions, or pharyngeal redness may also be seen [8, 9]. In terms of organ involvement, interstitial inflammation of the liver, kidneys, and lungs occur most often, but arthralgia, myositis, and cardiac involvement are also observed [8, 10].
Due to the heterogeneous clinical picture, histological findings are also non-specific. There may be predominantly perivascular lymphocytic infiltration and spongiotic, pustular lesions with an inflammatory infiltrate, dyskeratosis, single-cell necrosis, or interface dermatitis [11, 12].
A DRESS validation score, which was formulated as part of a multinational registry study [4], enables cases to be classified as “definite,” “probable,” “possible,” and “no DRESS” (Table 2).
Laboratory values should be checked carefully and regularly, since pathological values persist for several days. For example, liver involvement can only be deemed positive if liver transaminases are elevated by a factor of two on at least 2 days, and kidney involvement is only positive if creatinine values are at least 1.5 times above the patient’s normal values. Furthermore, the characteristic features often do not appear simultaneously, but rather in a delayed manner. For example, eosinophilia may develop several days after the appearance of skin lesions, while elevated liver or kidney values become evident several days after changes in blood count. Serological analysis for human herpesvirus 6 (HHV-6) should also be carried out in order to identify prognostically relevant reactivation. If this is negative, repeating the investigation after 2–3 weeks is recommended. HHV‑6 has been associated with a protracted course as well as flare-ups of fever and hepatitis [13].
Maculopapular drug eruption is often suspected at the onset of the reaction, and AGEP if pustules are present. Depending on infiltration of the skin, lymphoma and pseudolymphoma need to be considered in the differential diagnosis. Other differential diagnoses primarily include hematological disorders, angioimmunoblastic lymphadenopathy, hypereosinophilic syndrome, as well as adult-onset Still’s disease, viral infections, and SJS/TEN if there are edema-related tension blisters [14].
SJS/TEN
Clinically, one sees erythematous exanthema comprising atypical flat target lesions (these lack the typical three-zone, target-like constellation of so-called typical target lesions seen in erythema multiforme (EM)) and/or macules that frequently spread from cranial in a caudal direction, with the eruptions becoming confluent. Blisters form on the erythema and coalesce (Fig. 3). At least one mucous membrane is affected by erosion in addition to the skin. Fever and a marked deterioration in general condition are very common [1]. SJS/TEN is assigned to the spectrum of EM, since the eruptions are similar and the histology barely distinguishable from one another, particularly when biopsies are taken from bullous lesions. Nevertheless, it was shown that SJS/TEN and EM majus (EMM; EM with mucosal involvement) are two distinct entities with differing clinical picture and etiology [15].
EMM and SJS/TEN are classified according to the skin lesions and the extent of blister formation relative to body surface area (BSA) [14, 16]. Hemorrhagic erosions affecting one or more mucous membranes (in particular oral, ocular, and genital, but also nasal, anal, and tracheobronchial) are seen in virtually all cases (Fig. 4; [15]). Skin detachment is limited to <10% BSA in SJS, while it is 10–30% BSA in SJS/TEN overlap and >30% BSA in TEN with macules. In case of the rare TEN on widespread erythema, detachment barely exceeds 10% BSA (Table 3; [1, 16]). There is a discussion among experts as to whether the latter cases are potentially severe forms of GBFDE.
Histologically, one sees necrotic (dyskeratotic or apoptotic) keratinocytes in a disseminated distribution or as complete epidermal necrosis with subepidermal fissure formation. The localization and timing of sample collection play an important role, since, if the sample is taken from the central blister of an EMM target, complete epidermal necrosis may also be visible here. One also sees a sparse superficial lymphocytic infiltrate in the dermis, often in a perivascular location [17, 18].
GBFDE
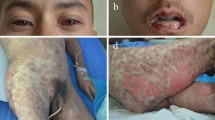
There are two variants of GBFDE: one reveals oval, egg-sized livid patches that are distributed over the body in a generalized manner (Fig. 5a; [14, 19,20,21]); the other manifests as diffuse erythema that only becomes demarcated from healthy skin in the further course (Fig. 5b; [14, 20]). In both variants, flaccid blisters form on the erythema, whereby the skin remains intact between the areas of blistering. Therefore, blister formation often affects less than 10% BSA (Fig. 5). There may be mild erosive mucous membrane involvement, with the genital and/or oral mucosa often affected, but not the ocular mucous membrane [14, 19,20,21].
Histologically, one sees the same features as in SJS/TEN, with a distinction sometimes being possible in the course of the disease. If a biopsy is taken at a later stage, a deep perivascular infiltrate containing neutrophils and eosinophils may be seen, and potentially also pigment deposits [14].
Diagnosis and differential diagnoses of SJS/TEN and GBFDE
There is no published score system yet to distinguish SJS/TEN and GBFDE. To supplement the consensus definition for SJS/TEN described above [16], the RegiSCAR group developed a score for the diagnostic differentiation of GBFDE; this score is currently in the validation phase and has not yet been published. There are no specific laboratory parameters to differentiate between the various types of blistering reactions.
Diagnosis is based on the clinical picture, histology, immunofluorescence where appropriate, and patient history (e.g., known previous reactions). SJS/TEN and GBFDE need to be differentiated from staphylococcal scalded skin syndrome (SSSS), which shows intraepidermal, subcorneal separation on histology. Bullous autoimmune dermatoses such as bullous pemphigoid, linear IgA dermatosis, pemphigus vulgaris, and paraneoplastic pemphigus should be included in the differential diagnosis. Therefore, if one of these is suspected, performing an immunofluorescence test is recommended. Other disorders that should be considered in the differential diagnosis include generalized drug eruptions, erythroderma, exfoliative dermatitis, and subacute cutaneous lupus erythematosus [1, 14]. EMM is an important differential diagnosis to SJS and can generally be well differentiated based on the consensus definition (Table 3; [16]). However, differentiation may be challenging in the case of untypical EMM involving atypical “giant targets” in addition to the mainly truncal and generalized distribution of typical target lesions, since these lesions sometimes coalesce. Moreover, due to their demarcation to intact skin, older “giant targets” that are already resolving have the appearance of resolving patches in GBFDE [14].
Epidemiology and etiology
AGEP
The incidence of AGEP cannot be reliably determined due to the lack of population-based data and the use of non-standardized nomenclature. The EuroSCAR study estimated it to be 1–5 cases per million inhabitants/year [22]. AGEP can occur at any age, with women being more commonly affected [22,23,24,25]. Mortality is estimated to be less than 5% [5, 22, 26].
More than 90% of cases are drug-related, with antibiotics representing the most frequent trigger [3, 22]. High-risk drugs include aminopenicillins, antibacterial sulfonamides, quinolones, pristinamycin (a macrolide antibiotic not approved in Germany), (hydroxy-)chloroquine, terbinafine, as well as diltiazem. Other substances with a lower but nevertheless significant risk include antiepileptic agents, glucocorticosteroids, macrolides, and non-steroidal anti-inflammatory drugs (NSAIDs) of the oxicam type [22]. Furthermore, there are numerous case reports in the literature on other medications, including topical, systemic, and plant-based substances, while contact with mercury, viral infections, spider bites, and pre-existing underlying diseases have also been described as AGEP triggers [5, 24, 25, 27,28,29].
The reaction typically occurs in the first cycle of use, and the latency period between the start of drug use and the onset of the skin reaction depends on the triggering agent: median latency is 1 day for antibiotics and 11 days for other medications [22]. However, it has been suggested that previous use of, e.g., penicillin, could cause a reaction to aminopenicillins, for instance, of more rapid onset.
DRESS
The term “hypersensitivity syndrome” was used for a long time, but it encompassed a number of disorders due to a lack of criteria. Therefore, criteria were defined in 1996 and the term “drug rash with eosinophilia and systemic symptoms” (DRESS) was coined, which was later modified by the authors to “drug reaction with eosinophilia and systemic symptoms” [9]. In Japan, the term “drug-induced hypersensitivity syndrome” (DIHS) is used with slightly different definition criteria [30].
As with AGEP, it is virtually impossible to determine the incidence of DRESS, since the disease often goes unidentified and the definition has not yet found its way into all textbooks. DRESS can occur at any age, with women more frequently affected. An analysis of 117 strictly validated cases revealed a mortality of less than 2% compared to earlier observations of 10% [8, 31]. The same analysis showed that a drug was identified as the definite or probable trigger in 79% of all cases. Drugs that bear a high risk for DRESS include: allopurinol, carbamazepine, lamotrigine, oxcarbazepine, phenobarbital, phenytoin, dapsone, antimicrobial sulfonamides, minocycline, nevirapine, and vancomycin [8]. The reaction occurs in the first cycle of use and, depending on the trigger, two latency periods may be seen: on average, 30 days for antiepileptic drugs and 20 days for allopurinol and antimicrobial drugs. However, in certain cases, the latency between initiation of use and reaction onset can extend over up to 8 weeks [8, 31].
SJS/TEN
The incidence of the rare SJS/TEN reaction is 1.5–1.8 per million inhabitants/year [2]. Women are more frequently affected and the reaction can occur at any age, with children, adolescents, and young adults being less often affected compared to older individuals [1, 15]. The age of the patient and the severity of the reaction both affect mortality. Approximately 9% of SJS patients, 29% of patients with SJS/TEN overlap, and 48% of those with TEN die [1].
Although SJS/TEN is considered a severe adverse drug reaction, two case control studies showed that only 65% of cases were associated with a high- or moderate-risk drug. Medications previously not known to carry a risk, as well as new substances, are suspected in around another 10% of cases, and there is no drug cause in approximately 25% of all cases [32]. Allopurinol, antimicrobial sulfonamides (but not sulfonamide diuretics and antidiabetic drugs), as well as antiepileptic agents are the commonest triggers; Table 4 provides an overview of SJS/TEN triggers. The reaction occurs during the first cycle of use and the latent period is generally 4 days to 4 weeks [33]. Especially in children and adolescents, only around 50% of cases can be explained by a medication, with antiepileptic drugs and antimicrobial sufonamides, including sulfasalazine, most commonly detected as the cause [33,34,35]. The severe skin and mucous membrane reaction is often preceded by an infection that may be causal, while other cases need to be regarded as idiopathic [1, 32].
GBFDE
It is currently not possible to determine the incidence of GBFDE, since there are no population-based data yet. As with most types of severe skin reactions, GBFDE affects women more frequently and patients are older than 70 years in 70% of cases. Approximately 22% of patients die due to advanced age and disease severity [21]. There are numerous case reports in the literature providing information on possible drug triggers; however, no analyses have been conducted on large patient numbers so far.
The range of triggers includes antimicrobial sulfonamides (especially cotrimoxazole), analgesics (especially metamizole), rarer antibiotics, allopurinol, and antiepileptic drugs (especially carbamazepine) [14, 19, 20, 36,37,38]. The latency between the start of drug use and reaction onset is between a few hours to a few days and, in contrast to the reactions described above, the triggering agent has often been used and tolerated in the past [14]. Sensitization occurs over time, meaning that a reaction consistent with a fixed drug eruption rapidly occurs upon renewed use of the drug. One or more previous events are often seen in the patient history [14, 20, 21, 39]. As such, GBFDE is a classic allergic reaction—in contrast to the forms of severe skin reaction discussed above.
An overview of the skin reactions in relation to their clinical characteristics and most frequent inducers is shown in Tables 5 and 6.
Pathogenesis
The severe skin reactions described above are generally regarded as T cell-mediated reactions, although the different T cell populations vary considerably depending on the type of reaction.
AGEP
It is assumed in AGEP that substance-specific cytotoxic T cells and cytotoxic proteins, such as granzyme B and perforin, induce keratinocyte apoptosis and that the migration of neutrophil granulocytes causes subcorneal pustules [40]. In addition, the specific T cells produce interleukin (IL)-8 (CXCL8), which, as a chemokine, plays a central role in recruiting neutrophils. Other proinflammatory cytokines and chemokines are induced besides IL‑8 (e.g., IL-17, IL-22, and tumor necrosis factor (TNF)-α), which lead to further neutrophil recruitment [41, 42].
Genetic investigations in AGEP have shown that there are variations in the IL36RN gene, which encodes the IL-36 receptor antagonist IL-36Ra, as also found in generalized pustular psoriasis and other pustular skin reactions. Therefore, it is postulated that a proportion of AGEP cases are associated with IL-36Ra dysfunction and a subsequently stronger IL-36 signal [41].
DRESS
A number of immunological mechanisms are involved in the development of DRESS. Activation of CD 4+ and CD 8 + T cells results in the release of various cytokines that have cytotoxic potential and cause inflammation. The release of IL‑5 is also important, since it promotes eosinophil activation, one of the essential characteristics of DRESS [43].
The pathogenesis of DRESS has not been definitively elucidated, but there are various hypotheses regarding regulatory T cells (Tregs) and effector T cells (Teffs). Tregs expand in the acute phase of DRESS, which might promote herpes reactivation. The cell count normalizes again in the resolution phase and T helper (Th)-17 cell differentiation appears to take place, which could possibly explain the development of autoimmune diseases following DRESS [44]. However, the extent to which the reactivation of viruses in the herpes group is actually involved in the pathogenesis of DRESS is a subject of controversy, since, on one hand, immunostimulation that occurs as part of the disease could be causal in the reactivation of the lymphotropic viruses, while on the other, the virus reactivation itself might be an additional stimulus for the immune system, leading to a chronic disease course [43].
Furthermore, a link has been observed between HLA subtypes and the occurrence of DRESS. For example, a Taiwanese study showed that HLA-B*5801 is a genetic marker for allopurinol-induced cases of DRESS in the Han Chinese population [45]. This is the same allele for which a link between allopurinol and SJS/TEN was identified (see below) [45, 46].
SJS/TEN
Since immunohistochemical investigations in SJS/TEN detected primarily CD4+ cells in the dermis and CD8+ cells in the epidermis, a T cell reaction comparable to graft-versus-host disease is assumed. The acute necrosis of keratinocytes in SJS/TEN is attributed to an extensive process of apoptosis. Cytotoxic T cells are able to initiate apoptosis, enhanced by the release of perforin and cytokines such as TNF‑α or granzyme B. It is also assumed that proteins such as Fas antigen (CD 95) and the P55 TNF-α receptor enhance apoptosis in keratinocytes [46]. However, it was shown that Fas and Fas ligand are not the most important cytokines in the acute phase of SJS/TEN, but rather the cationic protein granulysin. This showed the strongest cytotoxicity in the blister fluids of SJS/TEN patients compared to other blistering diseases, with its concentration correlating to the severity of the clinical picture [47]. From this, one can conclude that granulysin is a marker for SJS/TEN severity and provides a target for possible immunomodulating treatments. It has also been shown that IL-15 is associated with the severity of the reaction as well as the risk of mortality [48].
Genetic analyses revealed a predisposition to SJS/TEN that is specific not only for particular drugs but also ethnic factors [46]. For example, the highly significant link between carbamazepine-induced SJS/TEN cases and HLA-B*1502 in Han Chinese patients was not confirmed in European patients [49, 50]. In allopurinol-induced SJS/TEN cases, on the other hand, HLA-B*5801 was demonstrated in Han Chinese (100%) as well as European subjects (55%) [45, 51]. To date there have been no systematic investigations into the genetic pattern of infection-induced SJS/TEN cases. However, some reports on specific HLA alleles in cases presumed to be triggered by antipyretics and secretolytics appear to be ultimately associated with infection-induced reactions [52]. Although a large genome-wide association study in European SJS/TEN patients demonstrated that the relevant alleles/genetic variants are all located in the HLA locus on chromosome 6 [53], the variability in the European population seems to be too large to deploy a medication-specific predictive test to prevent SJS/TEN. In South East Asians,in contrast, this is possible at least in the case of carbamazepine, which has led to a substantial reduction in carbamazepine-induced SJS/TEN cases [46, 54].
GBFDE
Although systematic investigations into the pathogenesis of GBFDE are lacking so far, there are analyses on the T cell population in fixed drug eruption. T cells play an important role here, since they remain in the affected areas of skin as “memory cells,” which explains why a reaction re-occurs at the same site. The name “fixed drug eruption” takes this fact into account, although the reaction may expand if it re-occurs [55, 56]. The cases of GBFDE studied to date in the RegiSCAR study appear to fall into two groups: in the first, GBFDE is preceded by one or more localized or less extensive events, while in the second, GBFDE onset is sudden and without previous event (data not yet published).
Treatment
Identifying the triggering agent
The first important step in all severe skin reactions is to discontinue the inducing drug, assuming one is dealing with a drug cause rather than an infectious trigger. In order to identify the trigger, it is crucial to know the latency period from the start of drug use up to the onset of the reaction, as well as the drugs that have a high to moderate risk for the type of reaction in question. Taking a detailed and thorough medication history is essential. This includes information on: (1) start of use, (2) discontinuation, (3) frequency of use, (4) prior use and whether this was tolerated. Creating a timeline diagram may be helpful here, where the chronological sequence of clinical symptoms is entered on the x‑axis and the drugs used or applied are listed on the y‑axis (Fig. 6). Based on the diagram and the information on duration of use (start and discontinuation), it is possible to narrow down, and in the best case identify, the trigger, meaning that not all drugs—some of which may be vital for life—need to be discontinued. Substances administered to treat prodromal symptoms and often suspected of being the trigger for severe skin reactions (propathic bias) can also be excluded as such (Fig. 6, medication 5).
Differentiation in the case of antibiotics used to treat infections can be challenging, since either the drug or the infection may be causal [1, 14]. Whereas AGEP and DRESS are virtually always drug-induced, one must bear in mind that around 25% of all cases of SJS/TEN and as much as 50% of all cases in children and adolescents are not drug-induced. If an infection is identified as the trigger in such cases, patients should receive adequate antibiotic or antimicrobial treatment [32].
AGEP
AGEP is a self-limiting disease, i.e., progression ceases shortly after discontinuation of the triggering factor. Although the use of systemic steroids is often recommended in AGEP patients, a number of case series demonstrate that the use of topical glucocorticosteroids in addition to symptomatic antipruritic and antipyretic treatment is often sufficient and that systemic administration confers no benefit in terms of resolution [1, 5, 23, 25].
DRESS
There are to date no controlled clinical studies on the treatment of DRESS. Nevertheless, pruritus and fever should always prompt antipruritic and antipyretic treatment. If exfoliative dermatitis develops in the further course, the ambient temperature should be raised, electrolytes administered, and sepsis prevention undertaken [14, 57]. In a handful of case series, patients were treated purely symptomatically, in some cases in comparison to patients that were treated with highly potent topical steroids or systemic steroids. There were no differences between the groups that revealed one approach to be superior to the other [58, 59]. The use of systemic glucocorticosteroids at a dose of 0.5–2.0 mg/kg body weight (BW) is often recommended, particularly in the case of severe involvement of internal organs [14, 60, 61]. The steroid dose should be reduced gradually over a period of weeks, sometimes even months, since abrupt or very rapid discontinuation can promote immune reconstitution syndrome with excessive inflammation and cause a flare-up of the reaction [9, 14, 60]. However, patients receiving systemic steroids appear to develop infections, septicemia, disease relapses, and HHV‑6 reactivation more frequently [61]. Case series show that HHV‑6 reactivation can be associated with a prolonged course [61, 62]. Individual case reports and smaller case series on the treatment of DRESS patients have also used other treatment options, such as intravenous immunoglobulins (IVIG), cyclosporine A, cyclophosphamide, and rituximab; however, no clearly positive effect could be demonstrated [57, 60].
SJS/TEN
Topical treatment plays a special role in bullous reactions. Antiseptic solutions or gels, as well as non-medicated and non-adhesive gauze dressings are used. Some experts recommend leaving the blister roof in situ as a “natural plaster” to protect the dermis, while others recommend complete removal of detached skin in order to protect against infection, as well as the use of biosynthetic dressings [63, 64]. In addition to wound treatment and adequate pain management, it is important to monitor fluid requirements, nutrition, electrolyte balance, as well as kidney and liver function and make appropriate adjustments where required [60]. It may be necessary to increase room temperature to 25–28 °C in order to compensate for loss of thermoregulation in the case of extensive skin detachment [63].
Intravenous fluids need to be adjusted to the individual case, with daily monitoring of urine output serving as a basis for this [63, 65]. Depending on the extent of detachment, nutrients should also be provided. Enteral feeding (oral, transnasal) should always be preferred over parenteral in order to prevent gastric ulcers and the translocation of gut bacteria [63, 66].
In the case of erosive mucous membrane involvement, local antiseptic treatment is recommended and the relevant medical specialist should be consulted. Severe ocular involvement should prompt daily consultation of an ophthalmologist, since symblepharon prophylaxis is often required. In the worst case, amniotic membranes need to be used. Anti-inflammatory and/or antibiotic eye drops should be administered as a general rule in the case of ocular involvement [63, 67].
Due to the rarity of SJS/TEN and the resulting low patient numbers, as well as the unexpected onset and rapid progression of the reaction, conducting a randomized controlled trial on treatment efficacy is challenging. Nevertheless, there are a number of case reports and case series in which the use of systemic and/or immunomodulating substances in SJS/TEN is discussed controversially.
SCORTEN
SCORTEN (severity-of-illness score for TEN) makes it possible, at the onset of a reaction, to determine a patient’s chances of survival. Seven independent but equally significant factors are used in the calculation: (a) age (≥40 years), (b) heart rate (≥120/min), (c) malignancy, (d) percentage of detachment relative to BSA on day 1 (≥10%), (e) serum urea (>10 mmol/l), (f) serum bicarbonate (<20 mmol/l), (g) serum glucose (>14 mmol/l) [68]. SCORTEN is often used as a factor of comparison to assess therapeutic effect.
Glucocorticosteroids
Glucocorticosteroids are the most frequently used form of systemic treatment in SJS/TEN patients [60]. Having said that, their use is controversial, since they increase the risk of infection and sepsis and may slow down healing [69, 70]. However, a recently published meta-analysis on the treatment of SJS/TEN that investigated publications in the period 1990–2012 showed that the administration of systemic glucocorticosteroids conferred a survival benefit compared to supportive care (odds ratio (OR) 0.54; 95% confidence interval (CI) 0.29–1.01) [71]. A number of smaller case series on the administration of glucocorticosteroid pulse therapy with methylprednisolone or dexamethasone (100 mg/day for 3 days) demonstrated a benefit on the basis of the SCORTEN [70, 72]. A case series with five patients reported on the positive effect of methylprednisolone pulse therapy (500 mg/day for 3 days) in massive eye involvement on the development of ocular sequelae; this effect could not be confirmed in larger observational studies [73]. For this reason, individual case reports and small case series should be viewed with caution. Nevertheless, if administered short-term at a medium dose (50–250 mg) for several days, glucocorticosteroids represent a treatment option that, although having little impact in terms of arresting the progression of skin detachment, often has a positive effect on swollen and painful mucous membranes [60, 71, 74].
IVIG
The potential effect of intravenous immunoglobulin (IVIG) therapy is based on the assumption that Fas-induced keratinocyte apoptosis is blocked by antibodies present in human IVIG [75]. But here too, its use is the subject of controversy, given that some reports describe a positive effect [75, 76], while others were unable to show any benefit for patients [74, 77, 78]. However, a number of methodological weaknesses were found in the studies showing a positive effect for IVIG [79]. Moreover, the effect of IVIG dose is often the subject of discussion. In studies that showed a disadvantage for IVIG, the dose was mostly ≤2 g/kg BW, whereas it was at least 2.8 g/kg BW in positive studies [60]. However, using the SCORTEN, a recent retrospective study on 64 patients showed that the administration of IVIG generally did not positively affect survival, not even at a higher dose [80]. Two extensive meta-analyses also found no survival benefit for patients undergoing IVIG treatment compared to supportive therapy [71, 81].
Cyclosporine A
Due to its immunosuppressive properties, cyclosporine A is used to inhibit cytotoxic T cells, which play a role in SJS/TEN [82]. The first larger retrospective case series, in which 11 patients were treated with 2 × 3 mg/kg BW/day, was published as early as 2000. The progression in skin detachment ceased earlier in the patient group receiving cyclosporine A treatment and wound healing was faster compared to the control group, which received cyclophosphamide and glucocorticosteroids [83]. In the years that followed, individual case reports and case series were published, all showing a survival benefit in patients treated with cyclosporine A compared to SCORTEN values and/or other systemic therapies [82, 84, 85]. A recently published study conducted in Madrid used three different approaches in order to assess the effect of cyclosporine A. Here again, re-epithelialization began earlier than in the comparison group (IVIG, glucocorticosteroids, supportive care only), and the observed mortality was below that expected according to SCORTEN, whereas more patients than anticipated died in the comparison group [86]. Children and adolescents were not included in many of these studies, but cyclosporine A has been used successfully in children with SJS/TEN in smaller case series [87].
The meta-analyses mentioned above come to the conclusion that cyclosporine A is a very promising treatment: firstly, re-epithelialization begins earlier and, secondly, the observed mortality is below the expected rate [71, 86]. The recommended dose is 3–5 mg/kg BW/day for a total of 10 days, whereby a dose adjustment based on kidney function may need to be made. Therefore, it is necessary to monitor creatinine levels during treatment. The determination of cyclosporine A levels is advisable in case of higher doses and renal insufficiency, but not mandatory in other cases. Strict contraindications to short-term treatment with cyclosporine A at the dose mentioned here are rare, but there are barely any reports on the treatment of older patients with SJS/TEN (>70 years) [88].
TNF-α inhibitors
Elevated levels of TNF‑α were found in the blister fluids, serum, and skin samples from SJS/TEN patients, with the level correlating to the severity of the reaction [60, 89]. Therefore, the use of TNF-α inhibitors appeared to represent a possible treatment approach in SJS/TEN. At the end of the 1990s, a randomized double-blind placebo-controlled treatment study using thalidomide was conducted in patients with SJS/TEN. However, it was necessary to stop the study early, since significantly more patients died in the thalidomide group compared to the placebo group [89]. Paradoxically high levels of TNF‑α were found in the serum of patients in the treatment arm of the study. Nevertheless, later studies used other TNF-α inhibitors, e.g., infliximab and etanercept, for the treatment of SJS/TEN, but only scant reports of treatment success have been published [90, 91].
A recently published randomized treatment study showed lower mortality for etanercept compared to the achieved SCORTEN values. Wound healing started earlier with etanercept, but the administration of steroids for 2–3 weeks may have been responsible for the delayed wound healing in the comparison group. In the in-vitro investigation, the inhibitor reduced the levels of TNF‑α and granulysin in serum and blister fluids compared to the glucocorticosteroid-treated control group [92]. The prospective randomized study design can be viewed positively, since treatment studies of this kind in the area of severe skin reactions are lacking. However, the results are mostly non-significant and this study too has a number of methodological problems.
Other immunomodulating treatment options
Although other therapies have been used for the treatment of SJS/TEN, the reliability of findings is extremely low due to the small number of patients treated. In some cases, these options are no longer—or only rarely—used, as in the case of cyclophosphamide [71, 83]. Other therapies such as plasmapheresis, which is based on the removal of cytokines involved in apoptosis, are still used despite not having shown verifiable success [93, 94].
GBFDE
Much like AGEP, GBFDE is also a self-limiting disease that ceases to progress shortly after discontinuation of the inducing drug. Therefore, supportive care alone is adequate, particularly since there are no data on the benefit of systemic immunomodulating therapy in the treatment of GBFDE [20]. However, complications requiring intensive care can occur, especially in older patients and patients with very extensive skin detachment. Topical treatment is the same as in SJS/TEN. Since the mucous membranes are generally unaffected, interdisciplinary consultations are not mandatory, but can be helpful in some cases. Systemic glucocorticosteroids are also used in some patients, whereby their short-term use does no harm, nor does it result in faster healing [1, 14].
Complications and sequelae
Whereas AGEP generally resolves without complications, protracted courses may be seen in DRESS, with recurrent flare-ups of the reaction on the skin and internal organs. Late sequelae involving the development of autoimmune reactions such as thyroiditis have been described in DRESS [10].
Over the disease course, SJS/TEN may be accompanied by hepatitis, tubular nephritis, or tracheobronchial mucosal involvement; however, these resolve relatively fast [14, 57]. The most common complications include nosocomial infection and sepsis, not infrequently caused by central venous catheters. For this reason, peripheral catheters should be preferred wherever possible and specific hygiene measures undertaken, e.g., reverse isolation, etc. [14].
The majority of patients that survive SJS/TEN suffer long-term sequelae of varying severity, affecting primarily the skin and mucous membranes [95, 96]. Whereas skin lesions generally heal without scarring, hyper- and hypopigmentation of the skin as a result of the inflammatory reaction often persist for months to years. Reversible effluvium, nail loss, and nail growth impairment have also been observed. Mucosal adhesions that can cause strictures in, e.g., the urethra or esophagus, represent a greater problem. The by far the most hazardous—and for the patient dramatic—sequela is symblepharon formation with entropium and trichiasis, which can cause blindness [1, 14, 67, 95, 96].
Many patients still suffer somatic as well as psychologic sequelae years after their reaction, with the latter ranging from symptoms of post-traumatic stress, sleep disorders, and nightmares to fear of using any medications. Many patients and their relatives are inadequately informed about their reaction, its sequelae, and how to behave in the future [96].
Allergy testing
The severe skin reactions SJS/TEN, DRESS, and AGEP are not allergic reactions in the strictest sense, since there is no classic sensitization as in delayed allergic reactions. Here, initial exposure to the substance is well tolerated, with a reaction only developing upon renewed use. The severe reactions discussed here differ in that they typically occur during the first course of treatment with a drug [33]. However, it is possible in the case of AGEP induced, e.g., by commonly prescribed antibiotics such as penicillin, that the substance has been used previously, which could explain the rapid onset of AGEP upon renewed exposure [22]. On the other hand, the half-life of a substance also appears to play a role in the temporal latency between beginning of use and reaction onset. For example, the half-life as well as the latency period from start of use to the onset of a reaction are very short for aminopenicillins in AGEP, whereas both are significantly longer for hydroxychloroquine [22, 26].
GBFDE, on the other hand, is a true allergic reaction, since previous exposure to the triggering drug has usually taken place and renewed use often causes localized fixed drug eruptions. While renewed administration of a triggering drug in GBFDE patients can be expected to cause a rapid-onset and possibly even more extensive repeat reaction, SJS/TEN were rarely observed following similar re-exposure [97].
Skin tests such as the patch test are generally safe, but not always helpful in terms of confirming the suspected triggering agent in severe skin reactions. The success of testing depends to a great extent on the type of reaction and the T cell populations involved, as well as on the drug to be tested. For example, the triggering agent was confirmed by patch testing in a high percentage of AGEP and DRESS cases (up to 58% in AGEP; 32–64% in DRESS depending on the drug), but in only less than 25% of SJS/TEN cases [98]. One should also bear in mind that allopurinol, a very common trigger of SJS/TEN and DRESS, is not suitable for skin testing due to the lack of lipophilicity and skin penetration [26, 98, 99].
Although in vitro tests were the most suitable instrument to identify the inducing drug in severe skin reactions, their use has been more in an experimental vein to date and has not yet found its way into routine diagnostics. This may be due in part to the fact that the specificity of the various tests, e.g., the lymphocyte proliferation test, the lymphocyte stimulation test, and cytokine assays, is high, while their sensitivity is much lower [99].
Conclusion
Severe skin reactions such as AGEP, DRESS, SJS/TEN, and GBFDE, although rare, are associated with a high mortality rate. Therefore, it is important to diagnose these disorders promptly in order to initiate the necessary treatment steps. To this end, other diseases need to be excluded and the suspected diagnosis confirmed by means of a targeted clinical, histological, and laboratory diagnostic work-up. Diagnostic scoring systems, like those available for AGEP and DRESS, as well as a consensus definition in the case of bullous reactions, are helpful here. Once the correct diagnosis has been made, it is important to assess which drugs or, where appropriate, other factors have induced the reaction. Data from epidemiological studies that have calculated the risk of particular substances for a specific type of reaction and the relevant time window of use are helpful. If a drug is identified as the triggering factor, it should be discontinued immediately. Should infectious triggers, e.g., in the case of SJS/TEN, be a possibility, appropriate anti-infective treatment needs to be initiated. Symptomatic supportive care plays a particularly important role. This includes simultaneous ophthalmological care, particularly in SJS/TEN; in general, severe skin reactions require interdisciplinary care by a multiprofessional team.
Immunomodulating therapies can also be considered: these consist primarily of the systemic administration of glucocorticosteroids in the case of AGEP and DRESS and the systemic administration of cyclosporine A in the case of SJS/TEN. Since the frequency of long-term sequelae is extremely high in surviving patients, follow-up examinations should be carried out.
Abbreviations
- AGEP:
-
Acute generalized exanthematous pustulosis
- ANA:
-
Antinuclear antibodies
- BSA:
-
Body surface area
- BW:
-
Body weight
- CI:
-
Confidence interval
- CMV:
-
Cytomegalovirus
- DIHS:
-
Drug-induced hypersensitivity syndrome
- DRESS:
-
Drug reaction with eosinophilia and systemic symptoms
- EBV:
-
Epstein-Barr virus
- EM:
-
Erythema multiforme
- EMM:
-
Erythema multiforme majus
- GBFDE:
-
Generalized bullous fixed drug eruption
- HHV‑6:
-
Human herpesvirus 6
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- IVIG:
-
Intravenous immunoglobulins
- NSAID:
-
Non-steroidal anti-inflammatory drugs
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- SCORTEN:
-
Severity-of-illness score for TEN
- SJS:
-
Stevens-Johnson syndrome
- SSSS:
-
Staphylococcal scalded skin syndrome
- Teffs:
-
Effector T cells
- TEN:
-
Toxic epidermal necrolysis
- Th:
-
T helper cells
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cell
References
Mockenhaupt M, Roujeau JC. Epidermal Necrolysis (Stevens-Johnson syndrome and toxic epidermal Necrolysis). In: Kang S, Amagai M, Bruckner A, Enk AH, Margolis DJ, McMichael AJ, Orringer JS, editors. Fitzpatrick’s dermatology. 9th ed. New York: McGraw-Hill; 2019. pp. 33–48.
Rzany B, Mockenhaupt M, Baur S, Schröder W, Stocker U, Mueller J, et al. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population-based registry. J Clin Epidemiol. 1996;49:769–73.
Sidoroff A, Halevy S, Bouwes Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113–9.
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609–11.
Hotz C, Valeyrie-Allanore L, Haddad C, Bouvresse S, Ortonne N, Duong TA, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223–32.
Burrows NP, Russell Jones RR. Pustular drug eruptions: a histopathological spectrum. Histopathology. 1993;22:569–73.
Halevy S, Kardaun SH, Davidovici B, Wechsler J, EuroSCAR, RegiSCAR study group. The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. Br J Dermatol. 2010;163:1245–52.
Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80.
Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250–7.
Kano Y, Ishida T, Hirahara K, Shiohara T. Visceral involvements and long-term sequelae in drug-induced hypersensitivity syndrome. Med Clin North Am. 2010;94:743–59.
Chi MH, Hui RCY, Yang CH, Lin JY, Lin YT, Ho HC, et al. Histopathological analysis and clinical correlation of drug reaction with eosinophilia and systemic symptoms (DRESS). Br J Dermatol. 2014;170:866–73.
Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, Wechsler J, de Feraudy S, Duong TA, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50–8.
Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007;156:1083–4.
Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625–45.
Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schröder W, Roujeau J‑C, et al. Severe cutaneous adverse reactions. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002;138:1019–24.
Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–6.
Rzany B, Hering O, Mockenhaupt M, Schröder W, Goerttler E, Ring J, et al. Histopathological and epidemiological characteristics of patients with erythema exudativum multiforme majus, Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 1996;135:6–11.
Ziemer M, Mockenhaupt M. Severe drug-induced skin reactions: clinical pattern, diagnostics and therapy. Skin Biopsy Perspect. 2011; https://doi.org/10.5772/22335.
Cho YT, Lin JW, Chen YC, Chang CY, Hsiao CH, Chung WH, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539–48.
Paulmann M, Mockenhaupt M. Unintended rechallenge: Generalized bullous fixed drug eruption in two elderly women. Hautarzt. 2017;68:59–63.
Lipowicz S, Sekula P, Ingen-Housz-Oro S, Liss Y, Sassolas B, Dunant A, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726–32.
Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JNB, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989–96.
Chang SL, Huang YH, Yang CH, Hu S, Hong HS. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363–5.
Davidovici B, Dodiuk-Gad R, Rozenman D, Halevy S, Israeli RegiSCAR Network. Profile of acute generalized exanthematous pustulosis in Israel during 2002–2005: results of the RegiSCAR Study. Isr Med Assoc J. 2008;10:410–2.
Alniemi DT, Wetter DA, Bridges AG, El-Azhary RA, Davis MDP, Camilleri MJ, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996–2013. Int J Dermatol. 2017;56:405–14.
Brockow K, Ardern-Jones MR, Mockenhaupt M, Aberer W, Barbaud A, Caubet JC, et al. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy. 2019;74:14–27.
Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333–8.
Davidovici BB, Pavel D, Cagnano E, Rozenman D, Halevy S, EuroSCAR, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525–9.
Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583–4.
Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55:1–8.
Chen YC, Chiu HC, Chu CY. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373–9.
Paulmann M, Mockenhaupt M. Fever in Stevens-johnson syndrome and toxic epidermal Necrolysis in pediatric cases: laboratory work-up and antibiotic therapy. Pediatr Infect Dis J. 2017;36:513–5.
Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128:35–44.
Quirke KP, Beck A, Gamelli RL, Mosier MJ. A 15-year review of pediatric toxic epidermal necrolysis. J Burn Care Res. 2015;36:130–6.
Levi N, Bastuji-Garin S, Mockenhaupt M, Roujeau JC, Flahault A, Kelly JP, et al. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatr Electron Pages. 2009;123:e297–e304.
Kauppinen K, Stubb S. Fixed eruptions: causative drugs and challenge tests. Br J Dermatol. 1985;112:575–8.
de Argila D, Angeles Gonzalo M, Rovira I. Carbamazepine-induced fixed drug eruption. Allergy. 1997;52:1039.
Baird BJ, De Villez RL. Widespread bullous fixed drug eruption mimicking toxic epidermal necrolysis. Int J Dermatol. 1988;27:170–4.
Dharamsi FM, Michener MD, Dharamsi JW. Bullous fixed drug eruption masquerading as recurrent Stevens-Johnson syndrome. J Emerg Med. 2015;48:551–4.
Schmid S, Kuechler PC, Britschgi M, Steiner UC, Yawalkar N, Limat A, et al. Acute generalized exanthematous pustulosis: role of cytotoxic T cells in pustule formation. Am J Pathol. 2002;161:2079–86.
Navarini AA, Valeyrie-Allanore L, Setta-Kaffetzi N, Barker JN, Capon F, Creamer D, et al. Rare variations in IL36RN in severe adverse drug reactions manifesting as acute generalized exanthematous pustulosis. J Invest Dermatol. 2013;133:1904–7.
Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized Exanthematous Pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;27(17):8–E1214.
Musette P, Janela B. New insights into drug reaction with Eosinophilia and systemic symptoms Pathophysiology. Front Med. 2017;4:179.
Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DIHS) / drug reactionwith eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol Int. 2019;68:301–8.
Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–9.
White KD, Abe R, Ardern-Jones M, Beachkofsky T, Bouchard C, Carleton B, et al. SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract. 2018;6:38–69.
Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343–50.
Su SC, Mockenhaupt M, Wolkenstein P, Dunant A, Le Gouvello S, Chen CB, et al. Interleukin-15 is associated with severity and mortality in Stevens-Johnson syndrome/toxic epidermal Necrolysis. J Invest Dermatol. 2017;137:1065–73.
Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486.
Lonjou C, Thomas L, Borot N, Ledger N, de Toma C, LeLouet H, et al. A marker for Stevens-Johnson syndrome …: ethnicity matters. Pharmacogenomics J. 2006;6:265–8.
Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA‑B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmaco Genom. 2008;18:99–107.
Roujeau JC, Dunant A, Mockenhaupt M. Epidermal necrolysis, ocular complications, and “cold medicines”. J Allergy Clin Immunol Pract. 2018;6:703–4.
Genin E, Schumacher M, Roujeau J, Naldi L, Liß Y, Kazma R, et al. Genome-wide association study of Stevens-Johnson syndrome and toxic epidermal Necrolysis in europe. Orphan J Rare Dis. 2011;6:52.
Chen Z, Liew D, Kwan P. Effects of a HLA-B*15:02 screening policy on antiepileptic drug use and severe skin reactions. Neurology. 2014;83(22):2077–84.
Shiohara T, Mizukawa Y, Teraki Y. Pathophysiology of fixed drug eruption: the role of skin-resident T cells. Curr Opin Allergy Clin Immunol. 2002;2:317–23.
Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by selfinflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201–8.
Ghislain P‑D, Roujeau J‑C. Treatment of severe drug reactions: Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity syndrome. Dermatol Online J. 2002;8:5.
Uhara H, Saiki M, Kawachi S, Ashida A, Oguchi S, Okuyama R. Clinical course of drug-induced hypersensitivity syndrome treated without systemic corticosteroids. J Eur Acad Dermatol Venereol. 2013;27:722–6.
Ushigome Y, Kano Y, Ishida T, Hirahara K, Shiohara T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721–8.
Cho YT, Chu CY. Treatments for severe cutaneous adverse reactions. J Immunol Res. 2017. https://doi.org/10.1155/2017/1503709.
Funck-Brentano E, Duong T‑A, Bouvresse S, Bagot M, Wolkenstein P, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246–52.
Tohyama M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157:934–40.
Creamer D, Walsh SA, Dziewulski P, Exton LS, Lee HY, Dart JK, et al. U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol. 2016;174:1194–227.
Dorafshar AH, Dickie SR, Cohn AB, Aycock JK, O’Connor A, Tung A, et al. Antishear therapy for toxic epidermal necrolysis: an alternative treatment approach. Plast Reconstr Surg. 2008;122:154–60.
Shiga S, Cartotto R. What are the fluid requirements in toxic epidermal necrolysis? J Burn Care Res. 2010;31:100–4.
Kreymann KG, Berger MM, Deutz NEP, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on Enteral nutrition: intensive care. Clin Nutr. 2006;25:210–23.
Chronopoulos A, Pleyer U, Mockenhaupt M. Ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Klin Monatsbl Augenheilkd. 2012;229:534–9.
Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–53.
Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg. 1986;204:503–12.
Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2007;87:144–8.
Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic Immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal Necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017;153:514–22.
Hirahara K, Kano Y, Sato Y, Horie C, Okazaki A, Ishida T, et al. Methylprednisolone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis: clinical evaluation and analysis of biomarkers. J Am Acad Dermatol. 2013;69:496–8.
Araki Y, Sotozono C, Inatomi T, Ueta M, Yokoi N, Ueda E, et al. Successful treatment of Stevens-Johnson syndrome with steroid pulse therapy at disease onset. Am J Ophthalmol. 2009;147:1004–11.
Schneck J, Fagot J‑P, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58:33–40.
Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–3.
Trent JT, Kirsner RS, Romanelli P, Kerdel FA. Analysis of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis using SCORTEN: The University of Miami Experience. Arch Dermatol. 2003;139:39–43.
Bachot N, Revuz J, Roujeau JC. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol. 2003;139:33–6.
Shortt R, Gomez M, Mittman N, Cartotto R. Intravenous immunoglobulin does not improve outcome in toxic epidermal necrolysis. J Burn Care Rehabil. 2004;25:246–55.
Faye O, Roujeau JC. Treatment of epidermal necrolysis with high-dose intravenous immunoglobulins (IVIG). Clinical experience to date. Drugs. 2005;65:2085–90.
Lee HY, Lim YL, Thirumoorthy T, Pang SM. The role of intravenous immunoglobulin in toxic epidermal necrolysis: a retrospective analysis of 64 patients managed in a specialized centre. Br J Dermatol. 2013;169:1304–9.
Huang YC, Li YC, Chen TJ. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol. 2012;167:424–32.
Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maître B, Revuz J, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163:847–53.
Arévalo JM, Lorente JA, González-Herrada C, Jiménez-Reyes J. Treatment of toxic epidermal necrolysis with Cyclosporin A. J Trauma. 2000;48:473–8.
Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. 2014;71:941–7.
Singh GK, Chatterjee M, Verma R. Cyclosporine in Stevens-Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol. 2013;79:686–2.
González-Herrada C, Rodríguez-Martín S, Cachafeiro L, Lerma V, González O, Lorente JA, et al. Cyclosporine use in epidermal Necrolysis is associated with an important mortality reduction: evidence from three different approaches. J Invest Dermatol. 2017;137:2092–100.
St John J, Ratushny V, Liu KJ, Bach DQ, Badri O, Gracey LE, et al. Successful use of Cyclosporin A for Stevens-Johnson syndrome and toxic epidermal Necrolysis in three children. Pediatr Dermatol. 2017;34:540–6.
Roujeau JC, Mockenhaupt M, Guillaume JC, Revuz J. New evidence supporting cyclosporine efficacy in epidermal necrolysis. J Invest Dermatol. 2017;137:2047–9.
Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586–9.
Kreft B, Wohlrab J, Bramsiepe I, Eismann R, Winkler M, Marsch WC. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904–6.
Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278–83.
Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, et al. Randomized, controlled trial of TNF‑α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985–6.
Narita YM, Hirahara K, Mizukawa Y, Kano Y, Shiohara T. Efficacy of plasmapheresis for the treatment of severe toxic epidermal necrolysis: Is cytokine expression analysis useful in predicting its therapeutic efficacy? J Dermatol. 2011;38:236–45.
Giudice G, Maggio G, Bufano L, Memeo G, Vestita M. Management of toxic epidermal Necrolysis with plasmapheresis and Cyclosporine A: our 10 years’ experience. Plast Reconstr Surg Glob Open. 2017;5:e1221.
Yang CW, Cho YT, Chen KL, Chen YC, Song HL, Chu CY. Long-term sequelae of Stevens-Johnson syndrome/toxic epidermal Necrolysis. Acta Derm Venereol. 2016;96:525–9.
Paulmann M, Kremmler C, Sekula P, Valeyrie-Allanore L, Naldi L, Kardaun S, et al. Long-term sequelae in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis: a 5-year analysis. Clin Transl Allergy. 2016;6(Suppl 3):31.
Kauppinen K. Cutaneous reactions to drugs. With special reference to severe bullous mucocutaneous eruptions and sulphonamides. A clinical study. Acta Derm Venereol Suppl. 1972;68:1–89.
Barbaud A, Collet E, Milpied B. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;3:555–62.
Ardern-Jones M, Mockenhaupt M. Making a diagnosis in severe cutaneous drug reactions. Curr Opin All Clin Immnunol. 2019;19:283–393.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Paulmann and M. Mockenhaupt declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Paulmann, M., Mockenhaupt, M. Severe skin reactions: clinical picture, epidemiology, etiology, pathogenesis, and treatment. Allergo J Int 28, 311–326 (2019). https://doi.org/10.1007/s40629-019-00111-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-019-00111-8