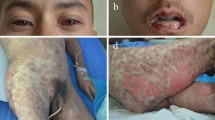
Abstract
Background
In contrast to the classic frequently seen drug eruptions, the rare severe drug reactions such as acute generalized exanthematous pustulosis (AGEP), erythema multiforme (EM), Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN, Lyell’s syndrome), and drug reaction with eosinophilia and systemic symptoms (DRESS) are frequently associated with increased mortality. Neither their acute management nor their further allergy diagnostic testing to avoid re-exposure and enable restriction of substances prohibited due to the event are standardized.
Materials and methods
The management of severe adverse drug reactions was investigated in a 10-year monocentric retrospective study.
Results
TEN (43.5%) and EM (29.0%) were the two most common subtypes of severe adverse drug reactions, while AGEP (3.2%), SJS (6.5%), and DRESS (17.7%) were less frequent. The acute management of 62 patients with severe adverse drug reactions was generally performed using systemic glucocorticoids (58.1%) or as a combination therapy consisting of glucocorticoids and intravenous immunoglobulins (IVIG, 41.9%), which were usually used in severe clinical courses. The most commonly suspected triggers were beta-lactam antibiotics (28.8%), followed by metamizole (19.4%) and sulfonamide antibiotics (17.7%).
Conclusion
Due to the rarity and heterogeneity of this patient population, there is scant reliable data on the systemic treatment of SJS/TEN. Therefore, whether it confers an evident benefit remains unclear. Although the allergy diagnostic testing of severe adverse drug reactions is complex, it is often able to yield important insights and should be performed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Adverse drug reactions (ADR) are defined as harmful reactions in which a link between the drug effect and the adverse reaction is suspected. These are divided into type A and type B reactions. Type A reactions are predictable, dose-dependent, non-immunological, mostly pharmacologically toxic reactions. These type A reactions account for the majority of ADR. Type B reactions are unusual, strictly individual, and unpredictable reactions. They can be further subdivided into immunological and non-immunological reactions. Non-immunological type B reactions may cause symptoms similar to those of immunological type B reactions, but are based on metabolic disorders or non-specific mast cell activation [1]. In clinical routine, one speaks of intolerance or pseudoallergies, for example analgesic intolerance.
Four types of immunological type B reactions are distinguished according to Coombs and Gell (1963) [2]. The most common immunologically mediated ADR are IgE-mediated type I allergies, induced by the immediate-type reaction, and the cellularly mediated type IV allergy, induced by the late-type reaction.
In addition to anaphylactic shock, which is the most severe form of type I allergy, severe ADR of the skin are especially feared. These include:
-
AGEP (acute generalized exanthematous pustulosis)
-
SJS (Stevens–Johnson syndrome)
-
TEN (toxic epidermal necrolysis, Lyell’s syndrome)
-
DRESS syndrome (drug reaction with eosinophilia and systemic symptoms)
-
Drug-induced EM (erythema multiforme)
AGEP causes generalized, non-follicular pustulosis on livid red erythema a few hours to several days following allergen exposure. The exanthema is often accompanied by fever and pronounced leukocytosis and neutrophilia. The most common triggers include antibiotics such as aminopenicillins, quinolones, and sulfonamides, as well as the antimalarial drug chloroquine and the calcium antagonist diltiazem [3]. Its clinical and histological differentiation from generalized pustular psoriasis is often challenging.
SJS and TEN are regarded as entities with different forms. SJS is a common truncal exanthema with atypical rosette-like lesions, as well as macular and hemorrhagic, erosive mucosal involvement. By definition, less than 10% of the body surface area is affected by epidermal detachment [4]. TEN presents as generalized, confluent exanthema accompanied by fever and a worsening of the patient’s general condition, with blistering, epidermal detachment, and mucosal erosion. Epidermal detachment affects more than 30% of the body surface area. In the case of 10–30% epidermal detachment, one refers to overlap SJS/TEN [4]. Sulfonamides, in particular cotrimoxazole and allopurinol, as well as various antiepileptic drugs [5], often with extremely long latency from first exposure, are the most common triggers.
DRESS syndrome is a generalized cutaneous reaction occurring 2–6 weeks following first use of a drug. In contrast to usual drug eruptions, hematological symptoms are also seen (eosinophilia, atypical lymphocytosis), as well as a systemic reaction (lymphadenopathy, fever, fatigue, and organ involvement). The most common triggers include allopurinol and antiepileptic drugs [6].
EM is a pronounced cutaneous reaction of acute onset comprising typical rosette-like lesions, some exhibiting central pallor and, in the majus type, mucosal involvement (erythema multiforme majus, EMM). It is usually triggered by viral infections, in particular herpes simplex type 1, but may also be triggered by medication use.
Severe ADR are rare but often life-threatening events. In most cases, affected patients require acute inpatient systemic therapy. Since not only the acute management but also the allergy diagnostic work-up of patients with ADR are the subject of controversy, the acute management, as well as the value of allergy testing in patients with histologically confirmed EM, TEN, SJS, DRESS, or AGEP were evaluated retrospectively.
Materials and methods
Histologically confirmed severe ADR treated between 1 January 2006 and 31 December 2016 at the Department of Dermatology, Tübingen University Hospital, Germany, were included in this retrospective statistical analysis. To this end, the histological database was searched for the terms “EM,” “SJS,” “TEN,” “AGEP,” “DRESS,” and “bullous drug reaction.” The parameters age, gender, secondary diagnoses (classified into “no disease,” “immunosuppression/cancer,” “cardiovascular diseases,” “autoimmune diseases,” “other diseases”), primary treatment, substitution of suspected drugs, herpes simplex virus diagnosis, prohibited drugs or substance classes, further allergy testing, and the issuing of an allergy pass were determined from electronic medical records. The diagnosis of histopathological findings was compared with the clinical diagnosis in the medical report and a final diagnosis was established. Data analysis was performed using Microsoft Excel 2016.
Histologically, EM is identical to SJS and is often difficult to distinguish from TEN. It usually occurs para-infectiously. However, in order to avoid overlooking drug-related EM, it is advisable to perform allergy testing in those patients in whom a drug could be causal.
Results
Patient collective
During the study period, 62 severe ADR were histopathologically confirmed in the Department of Dermatology, Tübingen University Hospital, Germany. Patient ages ranged from 21 to 93 years (mean, 60.6 years; standard deviation 20.8 years). Of these 62 patients, 35 were female (56.5%) and 27 male (43.5%). Seven patients had no other secondary diagnoses (11.3%) and 18 patients were immunosuppressed, e. g., due to cancer or pharmacological immunosuppressive therapy (29.0%). In all, 22 patients (35.5%) had cardiovascular diseases, 9 patients (14.5%) had autoimmune disorders, and 50 patients (80.6%) had disorders that could not be attributed to the aforementioned entities, such as mental illness and orthopedic or infectious diseases.
Distribution of severe ADR
TEN was diagnosed in 27 patients (43.5%), EM in 18 (29.0%), and DRESS syndrome in 2 (3.2%) of the 62 histopathologically confirmed severe ADR. Severe pustular reactions consistent with AGEP occurred eleven times (17.7%) and SJS four times (6.5%) (Fig. 1). Since biopsies corresponded histologically to EM rather than a drug eruption, drug-induced EM could not be classified as an EM-like eruption, since this corresponds histologically to an eruption involving a perivascular inflammatory reaction and only minor intraepithelial changes.
The distribution of severe drug reactions according to individual diagnoses in absolute numbers. AGEP acute generalized exanthematous pustulosis, DRESS drug reaction with eosinophilia and systemic symptoms, EM erythema multiforme, SJS Stevens–Johnson syndrome, TEN toxic epidermal necrolysis (Lyell’s syndrome); n = 62
Acute management and clinical course
All 62 patients received systemic treatment. Monotherapy with glucocorticoids (GC) was used in 36 cases (58.1%), and as combination treatment with high-dose intravenous immunoglobulins (IVIG) in 26 cases (41.9%; Fig. 2).
Systemic treatment of the different severe adverse drug reactions. AGEP acute generalized exanthematous pustulosis, DRESS drug reaction with eosinophilia and systemic symptoms, EM erythema multiforme, IVIG intravenous immunoglobulins, SJS Stevens–Johnson syndrome, TEN toxic epidermal necrolysis (Lyell’s syndrome)
A total of 41 (66.1%) patients were treated in the Department of Dermatology, 10 (16.1%) patients were treated in another department, and 3 (4.8%) patients were treated in the outpatient setting. Eight patients (12.9%) were admitted or transferred in the further course to an intensive care unit. Ten patients (16.1%) died in the inpatient setting.
Of the 27 TEN patients, 16 (59.3%) were treated in the dermatology unit, 3 (11.1%) on a medical unit, and 8 (29.6%) on an intensive care unit. Only 3 patients appeared to receive adequate steroid monotherapy (11.1%), while 24 patients were treated with a combination of GC and IVIG (88.9%). Seven patients (25.9%) died in the inpatient setting in this TEN treatment group.
Of the 18 patients with EM, 12 (66.7%) were inpatients on the dermatology unit, 3 (16.7%) were treated as outpatients, and 3 patients (16.7%) were treated on another unit. In all, 17 patients (94.4%) were treated with cortisone alone and 1 patient (5.6%) with steroids plus IVIG. One patient (5.6%) died. Both patients with DRESS syndrome were treated as inpatients on the dermatology unit with systemic monotherapy comprising GC (100%). Of the 11 AGEP patients, 10 (90.9%) were treated on the dermatology unit and 1 (9.1%) on another unit. All 11 patients received systemic steroid therapy (100%). One patient died in the inpatient setting (9.1%). Of the 4 SJS patients, 2 (50.0%) were treated on the dermatology unit and 2 (50.0%) on another unit. Systemic therapy with GC was performed in three cases (75.0%) and with additional IVIG in one case (25.0%). The mortality rate was 25%.
Switching and prohibiting medications, as well as further diagnostic testing
Due to their temporal relationship to severe ADR, drugs were suspected to be the trigger in all 62 cases. One or more drugs were switched for alternative drugs in 34 cases (54.8%). The dermatology unit undertook no change of medication in 28 cases (45.2%), since the suspected drugs had already been discontinued prior to presentation. The inpatient medical report or transfer report recommended that one active substance be strictly avoided in 35 cases (56.5%), two active substances in 10 cases (16.1%), and three or more active substances in nine cases (14.5%). It was recommended that a group of substances be avoided in eight cases (12.9%).
Altogether, 63 different active substances and five different substance groups were considered as possible elicitors of ADR in the 62 cases. The drugs most commonly prohibited in the discharge letter included beta-lactam antibiotics, e. g., penicillins or cephalosporins (15 cases), metamizole (12 cases), cotrimoxazole or substances in the sulfonamide group (11 cases), and allopurinol (five cases).
Allergy diagnostic testing was carried out in 21 of the 52 surviving patients (40.4%). In 19 further cases of ADR, allergy diagnostic testing was recommended in the discharge letter; however, these patients did not present to the authors’ allergy unit (36.5%). No allergy diagnostic testing was explicitly recommended in 12 cases (23.1%), and hence not carried out. Skin testing was carried out upon presentation of the 21 patients in the authors’ outpatient allergy unit in all cases by means of patch testing and skin prick testing (with late readings; Fig. 3). Of these tested patients, 10 had EM, 4 patients had TEN, 4 patients had AGEP, 2 patients had SJS, and 1 patient had DRESS syndrome. Altogether, patch testing was positive for the suspected substance in eight cases (38.1%), including all 4 patients with AGEP (100%), 1 DRESS patient (100%), 1 TEN patient (25%), 1 SJS patient (50%), and 1 EM patient (10%; Fig. 4). AGEP was triggered by an aminolcillin in three cases and by metamizole in one case. Carbamazepine was the trigger in DRESS syndrome, the contrast agent iomeprol in TEN, hydrochlorothiazide in SJS, and azathioprine was found to be the trigger in EM. Altogether, beta-lactam antibiotics were tested six times in patch tests; five tests were positive (83.3%). In the case of negative skin tests, oral provocation testing with the suspected substance was additionally performed in 4 patients who had developed EM. Tests were positive in three cases (75%). The reactive substances included metamizole, cotrimoxazole (trimethoprim + sulfamethoxazole), and phenoxymethylpenicillin. Oral provocation testing to find an alternative drug was performed with cefaclor in one case following a positive patch test for aminopenicillins. The lymphocyte transformation test (LTT) was performed in three cases: this was negative in two cases and positive in one case for hydrochlorothiazide.
The results of allergy testing in the different severe adverse drug reactions. AGEP acute generalized exanthematous pustulosis, DRESS drug reaction with eosinophilia and systemic symptoms, EM erythema multiforme, HCT hydrochlorothiazide, SJS Stevens-Johnson syndrome, TEN toxic epidermal necrolysis (Lyell’s syndrome)
Discussion
Severe ADR are rare but frequently life-threatening events. This is confirmed by the occurrence of 62 cases in 10 years at the Department of Dermatology of the Tübingen University Hospital, and a mortality rate of 16.1% in a retrospective monocentric analysis. The most common severe ADR were EM, TEN, and AGEP; SJS and DRESS syndrome were less common. Determining the precise incidence of severe ADR is hampered by its rarity and the fact that it is often challenging to diagnose. Incorrect diagnoses can occur particularly in cases where the suspected diagnosis is not established by a dermatologist and bioptic confirmation is not obtained. The Documentation Center for Severe Skin Reactions (“Dokumentationszentrum schwerer Hautreaktionen”, dZh) at the University Department of Dermatology and Venerology in Freiburg has been recording hospitalized cases of TEN, SJS, and EMM in Germany for more than 25 years. The incidence of EMM, SJS, and TEN in Germany ranges from 1.17 to 1.89 cases per million persons per year [7]. Studies in the USA and France report similar incidence rates [8, 9]. It is striking that in the present study, the diagnosis of TEN was made far more frequently than was the diagnosis of SJS. This does not correspond to the population-based data reported in the literature. Unfortunately, it is no longer possible to determine retrospectively whether epidermal detachment was clinically overestimated in some cases.
Besides symptomatic treatment, there is still no clear recommendation for the systemic treatment of severe ADR. A survival analysis of a cohort of 460 TEN patients [10] found that none of the immunomodulatory systemic therapies were able to achieve a significant improvement in survival compared to purely supportive treatment. Systemic reviews and meta-analyses have confirmed this observation [11, 12]. Whereas in France it is not uncommon for systemic therapy not to be administered, systemic treatment with GC is comparatively common in Germany in severe ADR [10]. The present study shows that systemic treatment with GC or a combination of GC plus IVIG was performed in all 62 cases. In the 27 cases in which TEN was present, systemic combination treatment consisting of GC and IVIG was performed in 24 cases and monotherapy with GC was performed in three cases. The European guideline (S1) on the use of high-dose IVIG in dermatological applications recommends the early use of high-dose IVIG as long as the possible benefits outweigh the risks of this medication and the natural course of the disease [13]. The combination of IVIG and GC is the subject of controversy: in this study, 7 of 24 TEN patients treated with GC and IVIG died (29.2%). This mortality rate is thus comparable to that found by Mahar et al. in a 2014 systematic review covering 20 studies and 708 TEN patients [14]. However, the severe cases of TEN preferentially required the combination of steroids and IVIG, meaning that the risk and side effects of the treatment were not the cause of mortality, but rather the severity of the disease. If one takes into account those patients treated with steroids only, the mortality rate for TEN in this study drops to 25.9%, since this group includes less severely affected patients. A recent meta-analysis showed that the use of GC and IVIG reduces the duration of stay by 3.19 days compared with GC monotherapy in the clinical setting of TEN/SJS, but yields no significant difference in mortality [15]. A meta-analysis conducted by Zimmermann et al. of 3248 patients also demonstrates a benefit for GC monotherapy only, but no positive effect for IVIG or combination therapies [12]. Interestingly, however, cyclosporine A (CsA) was found to be beneficial in that particular meta-analysis. Zimmermann refers primarily to an interventional study of 29 patients in France with SJS, overlap SJS/TEN, and TEN conducted by Valeyrie-Allanore et al. in 2010 [16]. A recent study by González-Herrada et al. also shows a survival benefit for CsA over other treatment options [17]. CsA has not been used as yet in TEN in our collective, since cardiac and liver side effects were feared. No randomized controlled trials on the use of IVIG or CsA have been conducted to date and would undoubtedly be challenging due to the rarity of the disease and the heterogeneity of the patient population. Thus, the benefit of the extremely costly—but in our collective extremely frequently used—IVIG treatment for SJS/TEN remains unclear.
A Canadian cohort study of 581 patients treated on an inpatient basis for SJS/TEN observed the group over an average period of 1283 days for SJS/TEN recurrence. It was shown that 7.2% of patients were admitted to hospital during this period due SJS/TEN recurrence [18], meaning that there is a more than 1000-fold increased risk for developing SJS/TEN if SJS/TEN has already occurred. In all, 86% of AGEP patients reported an ADR in their patient history prior to developing AGEP [19].
As a result, closer analysis of the triggering substance appears essential in order to avoid recurrence. In the present study, the discharge report recommended strict avoidance of two or more active substances or entire substance groups in 44.9% of cases. However, the triggering substance often remains unknown until inpatient discharge: firstly, medical history taking is often hampered by the severity of the clinical picture; secondly, precise knowledge of the latency period between first ingestion and disease manifestation is important. Since knowledge of the likelihood of triggering the disorder with the individual drugs is needed, experience with allergies or the keeping of a drug log is also helpful in the acute situation [20, 21].
The mean age of patients in the group studied here was 60.6 years. Onset at the beginning of the seventh decade of life is presumably caused by frequent drug use in middle to older age groups, and warrants allergy diagnostic testing in order to avoid the risk of accidental re-exposure. In all, 63 different active substances and five different groups of substances were considered as possible triggers in the 62 cases. This demonstrates the variety of drugs considered culprits in severe ADR. Beta-lactam antibiotics were the most frequently suspected group of substances. This substance group is extremely important and is often prescribed. This produces a dilemma, since prohibiting this drug group would lead to considerable limitations on future treatment options on the one hand, while the enormous frequency with which they are prescribed means, on the other, that the risk of accidental re-exposure is extremely high.
There are significant discrepancies—even within centers—between the allergy diagnostic testing performed and recommended following severe ADR, ranging from a non-explicit recommendation on allergy diagnostic testing in the medical report, to presentation at an allergy center, including skin testing, LTT, and oral provocation testing.
Although the literature describes a number of cases in which systemic reactions occurred as a result of skin testing [22, 23], skin tests such as the skin prick or patch test are regarded as relatively safe. However, since skin testing is often negative in the case of severe ADR, the procedure is frequently not performed due to the limited validity of its results.
Skin tests were performed in 21 cases in the present study; patch testing was positive in nine cases. It was striking that it was positive in all four cases of AGEP and in the one case of DRESS, whereas tests were positive in only one of the 10 EM cases and only four of the TEN cases. The lack of evidence in EM can undoubtedly be explained by the fact that the disorder usually occurs para-infectiously and drugs are rarely causal. Other studies have also demonstrated a tendency for the sensitivity of patch testing to depend on the type of severe ADR. For example, Barbaud et al. showed that patch testing was positive in 64% of DRESS cases and 58% of AGEP cases, but only in 24% of TEN/SJS cases [24]. The sensitivity of patch testing also appears to depend on the suspected substance. While patch testing was positive in only 38.1% of cases overall, it was positive in 83.4% of cases when beta-lactam antibiotics were tested. This dependence on individual substances was also demonstrated in a study on DRESS syndrome: patch testing was positive in 72.2% of cases when carbamazepine was the suspected substance, whereas all 19 patch tests were negative in the 19 cases where allopurinol was the suspected substance [25]. This may be due to a reaction to metabolites, which renders the original substance non-reactive [26].
Thus, if appropriately selected by an experienced allergist, skin tests can yield important insights and prevent the avoidance of harmless substances. However, numerous other factors need to be taken into account in skin testing besides appropriate selection. For example, according to the guideline, allergy diagnostic testing should be carried out between 4 weeks and 6 months after the occurrence of the event, since the likelihood of detecting a hypersensitivity reaction decreases thereafter [27,28,29]. Other circumstances surrounding the reaction also need to be taken into account, such as concomitant infections, cofactors for allergic reactions such as stress, exertion, food intake, alcohol intake, and UV exposure, as well as the selection of the correct test concentration [27]. In cases where skin testing is not possible or fails to yield valid results, in vitro techniques such as LTT are available in some centers [27]. LTT is a complex in vitro method for the detection of type IV reactions involving the incubation of patient lymphocytes with the antigen to be tested. Only antigen-specific lymphocytes proliferate more strongly, enabling sensitization to be detected here. However, LTT analysis is complex, its sensitivity is limited, and it should always be performed while taking other parameters and clinical findings into account.
In summary, one needs to carefully weigh up the gain in knowledge against the risk posed by any skin and provocation tests performed. However, whenever a drug is the confirmed or suspected elicitor, patients should present to an allergy center, provide a detailed patient history, receive patient education, be considered for in vitro and in vivo testing to establish the indication, and be issued with an allergy passport. Only in this way can the risk of a repeat, possibly lethal severe ADR be minimized, while at the same time keeping the restrictions on future treatment options as low as possible.
Abbreviations
- AGEP:
-
Acute generalized exanthematous pustulosis
- CsA:
-
Cyclosporine A
- DRESS:
-
Drug reaction with eosinophilia and systemic symptoms
- EM:
-
Erythema multiforme
- GC:
-
Glucocorticoids
- IgE:
-
Immunoglobulin E
- IVIG:
-
Intravenous immunoglobulins
- LTT:
-
Lymphocyte transformation test
- SJS:
-
Stevens–Johnson syndrome
- TEN:
-
Toxic epidermal necrolysis (Lyell’s syndrome)
- ADR:
-
Adverse drug reactions
References
Bork K. Arzneinebenwirkungen. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC, Landthaler M, editors. Dermatologie und Venerologie. 5th ed. Berlin-Heidelberg: Springer; 2005. pp. 425–6.
Coombs RR. Immunopathology. Br Med J. 1968;1:597–602.
Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP) – results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989–96.
Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–6.
Mockenhaupt M. Severe drug-induced skin reactions. Stevens-Johnson syndrome and toxic epidermal necrolysis. Hautarzt. 2014;65:415–23.
Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80.
Rzany B, Mockenhaupt M, Baur S, Schröder W, Stocker U, Mueller J, et al. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population-based registry. J Clin Epidemiol. 1996;49:769–73.
Chan HL, Stern RS, Arndt KA, Langlois J, Jick SS, Jick H, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126:43–7.
Roujeau JC, Guillaume JC, Fabre JP, Penso D, Fléchet ML, Girre JP. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981–1985. Arch Dermatol. 1990;126:37–42.
Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133:1197–204.
Roujeau JC, Bastuji-Garin S. Systematic review of treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis using the SCORTEN score as a tool for evaluating mortality. Ther Adv Drug Saf. 2011;2:87–94.
Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic Immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. Jama Dermatol. 2017;153:514–22.
Enk AH, Hadaschik EN, Eming R, Fierlbeck G, French LE, Girolomoni G, et al. European Guidelines (S1) on the use of high-dose intravenous immunoglobulin in dermatology. J Eur Acad Dermatol Venereol. 2016;30:1657–69.
Mahar PD, Wasiak J, Hii B, Cleland H, Watters DA, Gin D, et al. A systematic review of the management and outcome of toxic epidermal necrolysis treated in burns centres. Burns. 2014;40:1245–54.
Ye LP, Zhang C, Zhu QX. The effect of intravenous immunoglobulin combined with corticosteroid on the progression of Stevens-Johnson syndrome and toxic epidermal necrolysis: a meta-analysis. PLoS ONE. 2016;11:e167120.
Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maitre B, Revuz J, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163:847–53.
González-Herrada C, Rodríguez-Martín S, Cachafeiro L, Lerma V, González O, Lorente JA, et al. Cyclosporine use in epidermal necrolysis is associated with an important mortality reduction: evidence from three different approaches. J Invest Dermatol. 2017;137:2092–100.
Finkelstein Y, Macdonald EM, Li P, Hutson JR, Juurlink DN. Recurrence and mortality following severe cutaneous adverse reactions. JAMA. 2014;311:2231–2.
Alniemi DT, Wetter DA, Bridges AG, El-Azhary RA, Davis MD, Camilleri MJ, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996–2013. Int J Dermatol. 2017;56:405–14.
Möbs C, Pfützner W. Diagnostics of drug hypersensitivity reactions. Hautarzt. 2017;68:19–28.
Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625–45.
Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181–3.
Vaillant L, Camenen I, Lorette G. Patch testing with carbamazepine: reinduction of an exfoliative dermatitis. Arch Dermatol. 1989;125:299.
Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555–62.
Santiago F, Gonçalo M, Vieira R, Coelho S, Figueiredo A. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Derm. 2010;62:47–53.
Yaylacı S, Demir MV, Temiz T, Tamer A, Uslan MI. Allopurinol-induced DRESS syndrome. Indian J Pharmacol. 2012;44:412–4.
Brockow K, Przybilla B, Aberer W, Bircher AJ, Brehler R, Dickel H, et al. Guideline for the diagnosis of drug hypersensitivity reactions: S2K-guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Dermatological Society (DDG) in collaboration with the Association of German Allergologists (AeDA), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Contact Dermatitis Research Group (DKG), the Swiss Society for Allergy and Immunology (SGAI), the Austrian Society for Allergology and Immunology (ÖGAI), the German Academy of Allergology and Environmental Medicine (DAAU), the German Center for Documentation of Severe Skin Reactions and the German Federal Institute for Drugs and Medical Products (BfArM). Allergo J Int. 2015;24:94–105.
Brockow K, Ring J. Anaphylaxis to radiographic contrast media. Curr Opin Allergy Clin Immunol. 2011;11:326–31.
Fernández TD, Torres MJ, Blanca-López N, Rodríguez-Bada JL, Gomez E, Canto G, et al. Negativization rates of IgE radioimmunoassay and basophil activation test in immediate reactions to penicillins. Allergy. 2009;64:242–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Ali, B. Schoch, G. Glatthaar, J. Fischer, and A.S. Yazdi declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ali, J., Schoch, B., Glatthaar, G. et al. Management of severe drug reactions: a retrospective monocentric analysis. Allergo J Int 27, 49–55 (2018). https://doi.org/10.1007/s40629-017-0047-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-017-0047-6