Abstract
The common bean (Phaseolus vulgaris L.) is a major food legume cultivated by smallholder farmers in the tropics of Latin America and eastern and southern Africa. Acid soil and high temperature limit its production. Here we quantify differences in agronomical, phenological and physiological performance of common bean lines in order to identify promising lines with adaptation to acid soils and high temperatures in the Amazon region of Colombia. A field study was conducted using a 6 × 6 Alfa Lattice design with four replications consisting of 30 genotypes and one control genotype (Calima) which was repeated six times in each block (with a total of 36 plots) to cope with the spatial variability within the experimental site. During the growing period of the crop, maximum and minimum average temperatures were 32 and 23 °C, respectively while the total precipitation was 933 mm and the soil was acidic with aluminum toxicity. Differences in agronomical (grain yield, canopy biomass, number of seeds and pods per area), phenological (days to flowering and days to physiological maturity) and physiological (biomass partitioning, photosynthetic ability and pollen viability) attributes among the genotypes tested were quantified. The results obtained on the relationships between different plant attributes indicated that adaptation to acid soils and high temperatures is related to pollen viability, earliness, greater values of canopy biomass and better mobilization of plant reserves to the formation of pods and grains. Two common bean lines (BFS 10 and NCB 280) were identified as better adapted to the combined conditions of acidic soil and high temperatures in the Amazon region of Colombia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Common bean (Phaseolus vulgaris L.) is the most important food legume cultivated by smallholder farmers in the tropics of Latin America and eastern and southern Africa (Butare et al. 2011a; Polania et al. 2016a, b). Being the main protein source for 400 million people, it provides fiber, carbohydrates and vitamins, meeting the nutritional needs of the human diet (Polania et al. 2012; Araujo et al. 2015). It is produced in developing countries by smallholders, where abiotic factors such as drought, high temperature, phosphorus (P) deficiency and aluminum (Al) toxicity could cause marked yield losses in bean growing areas, particularly in the tropics (Rangel et al. 2005; Polanía et al. 2012; Rao 2014; Polania et al. 2016a, b, c; Rao et al. 2016, 2017). In low fertility acidic soils, Al toxicity limits root growth and development (Rao et al. 2016).
In South America, especially in the tropics, the soils that predominate are Oxisols and Ultisols where Al toxicity is a major production constraint (Rao 2014). Although the use of amendments such as lime is practiced to amend the arable layer, the roots are exposed to the toxic Al in lower soil layers inhibiting root elongation, limiting the growth of the plant and affecting the absorption of nutrients thereby impacting on grain yield (Rangel et al. 2007; Blair et al. 2009; Butare et al. 2011a; Polania et al. 2016b; Rao et al. 2016). Sensitivity to Al is localized specifically at the apex of the root (Butare et al. 2011b) especially in the transition zone, situated at 1–2 mm behind the root tip (Rangel et al. 2007), generating modifications in the cell wall and plasma membranes, affecting the mechanical properties of the cell wall and playing a prominent role in the inhibition of the root elongation by Al (Yang et al. 2013).
Smallholder agriculture is often affected by heat stress and heat sensitivity, and in common bean these factors are major limiting factors that can reduce yield, quality, and could lead to restricted geographic adaptation (Beebe et al. 2011). Yield losses occur under high night temperatures, which negatively affect all stages of reproductive development (Hall 2004). Heat stress during the reproductive phase in legumes adversely affects pollen viability, fertilization, pod set and seed development leading to abscission of flowers and pods and substantial losses in grain yield (Hall 2004). Even in current climates, high temperature is the climatic factor which is most widely limiting the common bean yields, and breeding for heat tolerance could benefit 7.2 million ha of common bean, and could highly increase the areas suitable for this culture by some 54% (Beebe et al. 2011). Reports show that day temperatures > 30 °C and night temperatures > 20 °C can result in yield reductions in common bean (Rainey and Griffiths 2005). This mainly affects the reproductive growth phase by reducing pollen viability, causing an increase in bud production and promoting the detachment of flower buds during pre-flowering, and causing the fall or decrease of pod number during post-flowering, which consequently affects the fertility, performance, and quality of the seed (Porch and Jahn 2001; Beebe et al. 2011, 2013; Rao 2014). High temperatures also affect physiological processes such as photosynthesis or the redistribution of assimilates toward the reproductive organs; they also cause an increase in the rate of photoinhibition and decrease in the amount of water found in buds and leaf tissue (Daniel 1997; Rao 2014). Sugar metabolism also responds to increase in heat stress by decreasing photosynthesis (Traub et al. 2017) leading to a lower supply of photosynthates towards tissue development thereby causing abortion of the embryo (Traub et al. 2017).
Improved understanding of the genetic, physiological, and morphological mechanisms of beans in response to environmental changes contributes to the development of bean genotypes that are adapted to both heat and acidic soil conditions. This increased knowledge is needed to design breeding strategies for the development of stress-adapted cultivars to improve food and nutrition security in the face of climate change in bean growing regions (Beebe et al. 2011; Butare et al. 2011a; Rao 2014) while facilitating the geographical expansion of the crop. One of the key adaptation mechanisms to improve performance under both drought and high temperature conditions is the development of a vigorous root system to obtain water and nutrients (Beebe et al. 2013), since in many acidic soils in the tropics, variability in rainfall distribution and longer dry periods are considered to be increasingly important yield-limiting factors for the development of crops, particularly during the early stages of the growing period (Yang et al. 2013; Rao et al. 2017).
In the Department of Caquetá (Colombia) of the Amazon region, many of the lowlands are currently used for extensive livestock farming, while family agriculture tends to be located on hillsides in conditions that present challenges for their development and economic sustainability, such as high acidity of the soil and high temperatures. These are the areas where bean cultivation can be fundamental for improving food and nutrition security for local communities, if genotypes adapted to these conditions could be identified. Thus the two main objectives of the present study were to: (i) identify advanced lines of the common bean with adaptations to acidic soils and high temperatures; and (ii) identify the physiological mechanisms contributing to improved adaptation based on the agronomical, phenological, and physiological performance of 31 bean lines that were evaluated under high temperature and acidic soils of the Colombian Amazon.
2 Material and methods
2.1 Experimental site and meteorological conditions
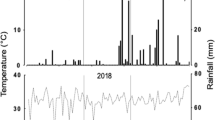
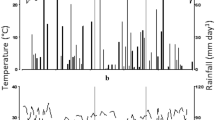
The field evaluation was conducted between the months of January and April 2016, at the Macagual Research Center of the University of the Amazon, Colombia (1°37′N and 75°36′W). Located in the moist climate of the tropical rainforest ecosystem, it exhibits an average annual precipitation of 3800 mm, 1700 h of sunshine year−1, an average temperature of 25.5 °C, and a relative humidity of 84%. During the growing period of the crop, maximum and minimum average temperatures were 32 °C and 23 °C, respectively (Fig. 1). Total precipitation during crop growth was 933 mm (Fig. 1). Soil samples were collected at 20 cm soil depth and the samples were pooled and analyzed to quantify physicochemical characteristics. It is a clay loam soil with bulk density values that ranged between 1 and 1.3 g cm−3, pH values that ranged from pH 4.1 to 5.2, with a mean organic carbon content of 1.35%, available P content (Bray- II) of 2.58 mg kg−1, saturation of total bases of 7.1% (Ca: 0.38 meq 100 g−1, Mg: 0.1 meq 100 g−1, K: 0.14 meq 100 g−1, Na: 0.1 meq 100 g−1, total bases: 0.8 meq 100 g−1), a cation exchange capacity of 11.3 cmol(−) kg−1, and an exchangeable Al content of 6.3 cmol(+) kg−1 with 73.4% of Al saturation.
2.2 Plant material and experimental design
A total of 31 genotypes of bean from the Mesoamerican and Andean gene pools were used: 17 advanced lines of the common bean (Phaseolus vulgaris) (A 774, A 801, MAM 38, VAX 1, BFS 10, BFS 35, BFS 81, BFS 142, BFS 143, EMP 507, EMP 509, NCB 280, SAB 618, SAB 659, SAB 686, SAP 1–15, SER 118), two interspecific lines resulting from a combination of three species Phaseolus acutifolius, Phaseolus vulgaris, and Phaseolus coccineus (SEF 10, SEF 60), six interspecific lines resulting from a combination of two species Phaseolus vulgaris and Phaseolus coccineus (ALB 60, ALB 91, ALB 191, ALB 210, ALB 213, ALB 214), four commercial Andean varieties (CAL 143, CALIMA, ICA QUIMBAYA, RADICAL), one advanced biofortified line (SMR 139), and one germplasm accession (G 21212). These lines constitute potential parental material, due to their resistance to environmental factors and diseases, improved grain quality (micronutrient content), and production components. The BFS lines (small red) have enhanced adaptation to low soil fertility. NCB (small black) and SER (small red) lines have enhanced adaptation to drought. The ALB (small red kidney, black kidney) lines have enhanced adaptation to drought and Al toxicity. SEF (red) lines have enhanced adaptation to drought and heat. CAL (red mottled), CALIMA (red mottled of cream), and ICA QUIMBAYA (red) are resistant commercial varieties to Al toxicity. VAX-1 (cream mottled) is sensitive to Al toxicity. SAB (red mottled, speckled brown) lines of the Andean gene pool are resistant to drought. SMR (red) lines are resistant to drought, with a high mineral (Fe) content in seed with cream or cream mottled seed color. MAM (cream mottled or purple) is part of the Durango race of the Mesoamerican gene pool. G 21212 (opaque black) is an accession of Mesoamerican germplasm, and is tolerant to low phosphorus levels in soil. RADICAL (bright solid red) is a commercial variety of the Andean Nueva Granada gene pool (Table 1).
An Alpha Lattice design was used, consisting of 6 × 6 complete superblocks with rows and columns with four replicates (blocks), and each block comprised of 30 genotypes and one check (Calima) replicated six times per block (for a total of 36 plots) so as to cope with spatial variability within the experimental site. Each experimental unit consisted of 3 rows with 2 m row length with a row to row distance of 0.6 m and plant to plant spacing of 15 cm (equivalent to 11 plants m−2).
2.3 Phenological characteristics and pollen viability
Phenological traits such as days to flowering and days to physiological maturity were determined. The number of days to flowering (DF) was determined for each genotype, this being the number of days after sowing when 50% of plants had developed at least one flower. Likewise, the number of days to physiological maturity (DPM) was determined for each genotype, this being the number of days after sowing when 50% of plants had at least one pod that was losing its green pigmentation.
To determine adaptation to high temperatures for the different lines of bean, viability of pollen (VP) was evaluated following the methodology of Porch and Jahn (2001) and Suzuki et al. (2001). Flower buds were collected one day before anthesis and stored in plastic jars with a solution of 1:3 glacial acetic acid-96% alcohol at 4 °C. The grains of pollen were removed from the anthers, adding one drop of 1% acetocarmine. In order to determine the viability of each flower bud, 100 grams of pollen were analyzed per repetition. The pollen grains that were dyed red were considered viable (fertile pollen), while grains without dye indicated pollen that was infertile or unviable. After the tally, the percentage of viability was calculated as a proportion of dyed grains to total grains.
2.4 Photosynthetic measurements
The net rate of photosynthesis (A) was determined using the infrared gas analyzer CIRAS-3 Portable Photosynthesis System (PP Systems Inc. Amesbury, MA, USA). Environmental conditions in the leaf cuvette were of a vapor pressure deficit (VPD) between a range of 1.0 to 1.5 kPa and a temperature of 25 °C. Measurements were taken between 08:00 and 11:00 h at room temperature, at a partial pressure of 400 μmol mol−1 of CO2, and under artificial radiation provided by the LED light source in the leaf cuvette (PP Systems Inc. Amesbury, MA, USA). Photosynthesis response curves to light intensity (A/PAR) were generated by increasing PAR in ten steps from 0 to 2000 µmol m−2 s−1. Initially, the leaves were exposed to a partial pressure of 50 μmol mol−1 of CO2 for 5 min to allow the stomata to open. Next, A/PAR curves were recorded at a partial pressure of 400 μmol mol−1of CO2. In order to determine the photosynthetic performance of different genotypes of common bean as affected by the microclimatic conditions in which they were grown, the above data collected were adjusted to the hyperbolic Michaelis–Menten model to determine different parameters derived from the A/PAR curves as light-saturated A (Amax), light compensation point (LCP), dark respiration rates (Rd), and light saturation point (LSP). The quantum yield of photosynthesis (ΦPAR) was determined from the slope of the initial linear portion of the A/PAR curve.
2.5 Biomass, grain yield, yield components and indices of partitioning of dry matter
To evaluate performance, destructive sampling was conducted in the central part of each plot, the pods of the harvested plants were threshed, and the grains were cleaned and dried to determine grain yield (kg ha−1). The weight (g) of 100 seeds was quantified with a random homogenized sample. During mid-pod filling, a destructive sample was collected on a 0.5 m row segment (equivalent to an area of 0.3 m2) per plot. A total of three plants were sampled to determine total canopy biomass (CB). At harvest time, another destructive sampling was conducted (0.5 m row segment, three plants), and the dry weight of leaves, stems, pods and seeds were recorded. The seed number per area (SNA) and pod number per area (PNA) were recorded. The following indices were determined (Beebe et al. 2013): pod partitioning index (PPI), pod biomass dry weight at harvest/total canopy biomass dry weight at mid-pod filling × 100; pod harvest index (PHI), seed biomass dry weight at harvest/pod biomass dry weight at harvest × 100; and harvest index (HI), seed biomass dry weight at harvest/total canopy biomass dry weight at mid-pod filling × 100. According to Polania et al. (2016b), HI and PPI are calculated using the CB value at mid-pod filling because, supposedly, this is the time that reflects the genotype’s maximum shoot vigor.
2.6 Data analysis
Fisher’s LSD test (p < 0.05) was used to conduct a variance analysis using the determined variables. Pearson’s correlation coefficient and Spearman’s rank correlation coefficient were calculated in order to determine significant relationships between variables. The genotypes that responded best to acidic soil conditions were determined using a scatterplot. Variables that affect grain production (pollen viability, total canopy biomass, pod partitioning index, and seed number per area) were plotted on the X axis. As the dependent or response variable, grain yield was plotted on the Y axis. Four quadrants were produced by plotting the corresponding averages on each axis. Genotypes in Quadrant 1 (top right box) responded best to the conditions evaluated in the study. Genotypes in Quadrant 3 (bottom left box) responded poorly to the conditions evaluated in the study. All analyses were performed using the statistical software InfoStat (Di Rienzo et al. 2017).
3 Results
3.1 Genotypic differences in agronomic performance (yield and yield components)
Both soil and climatic conditions impacted the agronomic performance of bean lines tested. Low soil pH and Al toxicity impacted plant growth and development and higher temperatures, particularly during the night, impacted reproductive development of most of the genotypes. Grain yield (GY) for the 31 genotypes ranged between 359 and 1832 kg ha−1, with an average value of 849 kg ha−1 (Table 2). Five lines (SEF 10, NCB 280, BFS 143, ALB 213, SAB 686) showed the greater values of GY under acidic soils and high temperatures (Table 2), while the line SMR 139 was very sensitive with the lowest GY value. Total canopy biomass (CB) was between 900 and 4822 kg ha−1, with an average value of 2423 kg ha−1. Four lines (ALB 191, ALB 91, EMP 509 and BFS 81) presented the greater values of CB under the conditions evaluated in this study (Table 2). It was found that some lines with higher values of GY did not show greater values of CB (BFS 10 and NCB 280), while lines such as ALB 191 and ALB 91 were contrasting with greater values of CB but lower values of GY (Fig. 2).
A negative relationship was observed between CB and GY (Fig. 2). The values of seed number per area (SNA) oscillated between 220 and 790 seeds, and the lines ALB 213, BFS 10, BFS 143, and NCB 28 presented greater values of SNA than the other genotypes under acidic soil and high temperatures conditions. These lines also presented higher values of pod number per area (PNA) (Table 2). Additionally, these genotypes exhibited a positive relationship between GY and SNA (Fig. 3). The line SAB 659 showed the highest value for the 100 seeds weight under high temperatures and acid soils, followed by ICA QUIMBAYA, SAB 686, CALIMA, and SAP 1–15. Genotypes ALB 191 and SMR 139 produced the fewest pods (Table 2). The negative relationship between CB and GY, allowed to identify lines with poor adaptation to acidic soils and high temperature conditions, despite having greater values of CB.
3.2 Phenological differences (days to flowering and days to physiological maturity) among genotypes
Days to flowering (DF) values ranged between 41 and 59 days after sowing. The lines A 801, ALB 214, ALB 60, EMP 507, EMP 509, SMR 139 and VAX 1 showed the shorter time to flowering under acidic soil and high temperature than the other lines evaluated. While the lines CALIMA, CAL 143, ICA QUIMBAYA, RADICAL, SAB 618, SAB 686, SAB 659 and SAP 1–15 showed the values of DF under the conditions evaluated. Regarding days to physiological maturity (DPM) the lines BFS 10, NBC 280, SEF 60, A 801, SEF 10 and BFS 35 showed lower values of DPM while the lines CAL 143, SAB 686, ALB 191 and EMP 509 needed more number of days to reach maturity. An analysis of the relationship between DPM and GY showed that genotypes with a greater GY displayed lower values of DPM (Fig. 4).
Relationship between grain yield (GY) and days to physiological maturity (DPM). Genotypes with greater values of GY and DPM under acidic soil and high temperature conditions are shown in the upper right quadrant. Two very early maturing and high yielding genotypes are shown in the upper left hand quadrant
3.3 Genotypic differences in physiological attributes (biomass partitioning, photosynthetic characteristics and viability of pollen)
Light response curves are indicative of the impact of light on the 31 genotypes tested, as there were significant differences (p < 0.05) in photosynthetic characteristics among genotypes tested. In this aspect, the line SAP 1–15 exhibited the best level of physiological adaptation under the conditions of this study, based on its photosynthetic capacity (Amax), its lowest light compensation point (LCP), its highest light saturation point (LSP), and lowest dark respiration rate (Rd) (Table 3, Fig. 5).
The pod partitioning index (PPI) exhibited a distribution between 3 and 70%; the line SAB 659 being the most efficient at mobilizing its CB towards pod formation (Table 2, Fig. 6). Other lines such as BFS 10, BFS 143, ALB 213 and NCB 280 also showed greater values of PPI (Fig. 6). The pod harvest index (PHI) values ranged between 56 and 81%. All the lines except ALB 191 mobilized more than 60% of their pod biomass towards seed formation (Table 2, Fig. 7). The HI values oscillated between 0.6 and 16%. The lines SAB 686, SAB 659, SAB 618, ICA QUIMBAYA, RADICAL, and SAP 1–15 mobilized over 10% of their CB toward seed formation (Table 2).
An average value of viability of pollen of 85.7% was observed for the 31 bean lines tested (Table 2). The SEF lines showed 95% viability of pollen under high temperature and acidic soil conditions. A total of 22 lines showed values greater than 80% for viability of pollen. Genotypes such as SMR 139, SAB 618, and ICA QUIMBAYA showed values of viability of pollen below 75%, the line SMR 139 showed the lowest value of 68.2% (Table 2).
3.4 Correlation analysis
Viability of pollen was negatively correlated with the 100 seed weight (SW) (Table 4). Total value of CB was negatively correlated with PPI, HI and SW (Table 4). GY was positively correlated with PPI, PHI, HI < SW and SNA (Table 4).
4 Discussion
4.1 Identifying high performing lines that are adapted to acidic soil
Canopy biomass (CB) may indicate a higher growth rate for a genotype, due to greater CO2 fixation, nutrient assimilation, and efficient use of water (Polania et al. 2016a), where greater accumulation of assimilates will be reflected in a higher growth rate of the crop (Bringham 2001; Polania et al. 2016a). For the genotypes tested, it was found that those (BFS 10 and NCB 280) that exhibited adaptation to acidic soils and high temperatures (Assefa et al. 2013; Polania et al. 2016b) also exhibited lower values of CB, probably due to the water deficit caused by the dry period at the beginning of the crop’s development (Fig. 2). This effect causes not only reduced root elongation due to Al toxicity but also a decrease in leaf water potential, thereby inducing the closure of stomata and reducing the rate of photosynthesis, and accumulation of dry matter (Blum 2005, 2009; Rao 2014; Polania et al. 2016c) (Fig. 2). However, two lines (ALB 91 and ALB 191) were found to be inefficient in remobilizing photosynthates toward the reproductive organs of the plant (Beebe et al. 2014) (Fig. 2). These lines showed higher values of CB but lower values of PPI and PHI. The negative relationship between CB and GY (Fig. 2) showed that several lines were not adapted to acidic soil and high temperature conditions. These lines presented good values of canopy biomass production but no pod and seed formation, reflecting the negative effect of acidic soil and high temperature on reproductive development and photosynthate remobilization. In other crops, such as cereals, it has been reported that HI becomes limited, which causes that genetic gains in yield may depend on whether there is an increase in CB (Bingham 2001; Polania et al. 2017). However, it should be taken into account that CB values are underestimated because these are taken at the time when the plant’s greatest vigor is seen and that an additional increase in dry matter can occur from mid-pod fill to physiological maturity, depending on the presence or not of rain (Polania et al. 2016c, 2017). The effect of heat was evident in the majority of the genotypes, affecting grain filling. The genotype BFS 10, was outstanding in the capacity of assimilate mobilization to pod and seed formation as reflected by its greater values of SNA and PNA (Omae et al. 2006) which, in turn, is correlated with a higher grain yield (Fig. 3).
4.2 The importance of earliness for escaping stress induced by high temperatures
The number of days to flowering (DF) were high in some genotypes because of poor adaptation to these conditions (Polania et al. 2016b). Under abiotic stress conditions the plants tend to show phenological plasticity by adjusting the days to physiological maturity (DPM) as an escape mechanism (Porch and Jahn 2001; Suzuki et al. 2001). This was evident for some genotypes (BFS 10, NCB 280, SEF 10, SEF 60, and BFS 35), that showed a negative correlation between GY and DPM under acidic soil and high temperature conditions (Table 4). Likewise, recent studies also showed that some early maturing common bean genotypes are very efficient in mobilizing photosynthates from vegetative structures to developing grain thereby resulting in greater grain yield per day (Rao et al. 2013; Beebe et al. 2014). With respect to the negative relationship between GY and DPM, these studies also suggested that a rapid accumulation of CB combined with greater values of water use efficiency due to greater mobilization of photosynthates to grain make these early maturing genotypes resistant to drought stress (Polania et al. 2016b). According to Assefa et al. (2015, 2017), DPM values generally decrease under conditions of stress, reducing the growth cycle of the crop and increasing yield per day. The lines BFS 10 and NCB 280 showed earliness, with the highest GY values (Fig. 4). In this light, the adaptation of DPM to environmental factors is considered as an important trait for improving the resistance of the common bean to both stresses of acidic soil and high temperature (Assefa et al. 2015).
4.3 The role of net photosynthesis and photosynthate remobilization in improving adaptation to stress
High radiation levels allow genotypes to perform at their maximum photosynthetic capacity. According to Bingham (2001), when the supply of water and nutrients is adequate, CB accumulation is determined by both the amount of solar radiation intercepted by the canopy and the efficiency of its conversion into dry matter. Furthermore, a 33% increase in leaf photosynthesis can translate into an 18% increase in biomass and only a 5% increase in grain yield, or a − 6% change in grain yield in the absence of additional nitrogen (Long et al. 2006; Traub et al. 2017). Different studies (Polania et al. 2016a; Rao et al. 2013, 2017) have reported that CB accumulation has a certain sensitivity to stress over time, which can reduce transpiration and photosynthesis. We found evidence for this observation in the present study since most of the lines showed higher degree of sensitivity to stress conditions. When CB values were correlated with Amax values, two lines ALB 191 and ALB 91 showed very high positive association (Fig. 5). The response of line SAP 1–15 in its CB production (Fig. 5) was outstanding, possibly because of its ability to alter its biomass partitioning in favor of root growth to acquire more water and nutrients (Polania et al. 2017), which could be an exceptional physiological response to adapt to Al toxicity and acidic soil conditions (Table 3).
It has been indicated that the efficient remobilization of photosynthates or greater dry matter partitioning with respect to grain yield could be an important physiological mechanism for tolerating high temperatures (Assefa et al. 2015). Likewise, indices such as PHI, PPI, and HI are important to analyze changes in dry matter partitioning into pods and grains (Assefa et al. 2017), since they are crucial to the varietal development of the selected genotypes that are resistant to stress (Beebe et al. 2013; Polanía et al. 2016a; Assefa et al. 2013, 2017). When PPI values were plotted against GY values, lines such as BFS 10, BFS 143, ALB 213, and NCB 280 exhibited a positive relationship (Table 3), indicating their improved capacity to partition greater quantities of biomass into the formation of pods from vegetative plant organs such as leaves and stems thereby resulting in greater values of HI and PPI (Omae et al. 2006, 2012; Rao et al. 2017) (Fig. 6). However, genotypes with a higher values of PPI did not always resulted in higher values of GY due to poor grain filling. In this regard, other researchers also suggested that under field conditions, this observed decrease in GY is due to a decrease in formation of pods and grains, which itself is caused by high temperatures, among other factors (Porch and Jahn 2001; Omae et al. 2006, 2007, 2012). We found similar response for the lines SAB 659, SAB 618, and SAB 686 (Fig. 6).
The capacity to mobilize photosynthates from the podwall to the seed has been evaluated using PHI as a measure (Polanía et al. 2016c). When the mean values of PHI were plotted against GY values, five lines BFS 10, NCB 280, BF 143, ALB 213, and SAB 686 showed a combination of greater values of PHI and GY (Fig. 7), confirming the importance of the contribution of the mobilization of photosynthates from the pod wall to the grain (Rao et al. 2013; Beebe et al. 2014; Assefa et al. 2015; Polania et al. 2016c, 2017). Assefa et al. (2017) indicated that greater partitioning of biomass towards reproductive growth is an adaptive response of stress resistant genotypes. Similar observations were made by Polania et al. (2016b), where under stress PHI and GY values showed a highly significant correlation. Four lines (ALB 191, EMP 509, SMR 139) exhibited a phenomenon known as “lazy pod syndrome” due to their limited performance in mobilizing the photosynthates from podwall to seed (Beebe et al. 2009, 2013; Assefa et al. 2017; Polania et al. 2016a) (Fig. 7). Regarding the two SAB lines (SAB 686 and SAB 618) although they did not stand out, it is worth noting that these lines could be further improved in their GY values under stress by improving their PHI values (Polania et al. 2016b). These results agree with previous studies where it was suggested that PHI (Assefa et al. 2017) could serve as a criterion to improve bean resistance to stress by its simple measurement and significant correlation with GY (Assefa et al. 2013; Rao et al. 2013; Beebe et al. 2014; Polania et al. 2016a, 2017; Rao et al. 2017).
In general, the indices of photosynthate remobilization (HI, PHI, and PPI) were positively and significantly correlated under conditions of acidic soil and high temperature, indicating their usefulness for improving stress resistance in common bean. This is because of the simplicity of measuring them, their significant correlation with GY, and their high heritability (Assefa et al. 2013, 2017; Rao et al. 2013, 2017; Assefa et al. 2017). The poor performance observed with some lines was due to the presence of the angular leaf spot disease together with higher values of relative humidity, precipitation and temperature during the experimental period. The yield losses due to this disease can be 40–80% under humid conditions that favor the development of the fungus (León 2009).
4.4 Identification of lines that combine desirable attributes for tolerance to acidic soil and high temperature
Studies on viability of pollen under tropical moist climactic conditions allow for the identification of genotypes that are adapted to stress induced by high temperatures (Suzuki et al. 2001). Two lines (SEF 10 and SEF 60) exhibited very high values of viability of pollen but their grain yield was not that high (Fig. 8), possibly due to the abscission of flowers and detachment of fertilized ovules caused by high temperatures or due to their poor adaptation to acidic soils (Porch and Jahn 2001).
Once a high viability of pollen and good grain yield are achieved at high temperatures, other mechanisms that are directly correlated with production become important, including biomass production, partitioning of assimilates, and formation of pods and grains per area (Suzuki et al. 2001; Hernández et al. 2007; Omae et al. 2007). The impact of high average nocturnal temperatures on pollen viability is due to damage in the structure of the endoplasmic membrane that impedes the function of the anther’s tapetum layer (Suzuki et al. 2001; Omae et al. 2012). This provokes degeneration and infertility in the pollen grain, limiting the number of grains per pod and, hence, its weight (Suzuki et al. 2001).
Our results on the correlation analyses and the relationships between different plant attributes tested indicate that adaptation to acidic soil and high temperature conditions is related to viability of pollen, earliness, greater plant growth, and better mobilization of plant reserves toward pod and grain formation. Two genotypes, BFS 10 and NCB 280 were outstanding in combining the desitable characteristics under combined stress conditions of acidic soil and high temperature.
5 Conclusions
Based on an evaluation of the agronomical, phenological, and physiological performance, lines such as BFS 10, BFS 143, NBC 280, and ALB 213 demonstrated greater level of adaptation to acidic soil and high temperature with a desirable combination of plant attributes such as CB, GY, Amax, PPI, PHI, SNA and PNA. A few promising lines identified from this study could serve as parents in bean breeding programs designed to further improve the adaptation of the common bean to stress conditions induced by a combination of acidic soil and high temperature in the humid regions of the tropics. Also the lines identified could contribute to improve food and nutritional security in the humid tropical regions of Colombia.
References
Araujo S, Beebe S, Crespi M, Delbreil B, González E, Gruber V, Lejeune-Henaut I, Link W, Monteros M, Prats E, Rao I, Valdez V, Vaz Patto M (2015) Abiotic stress responses in legumes: strategies used to cope with environmental challenges. Crit Rev Plant Sci 34:237–280. https://doi.org/10.1080/07352689.898450
Assefa T, Sperling L, Dgne B, Argaw W, Tessema D, Rubryogo J (2013) Participatory plant breeding with traders and farmers for white pea bean in Ethiopia. J Agric Educ Ext 20:497–512. https://doi.org/10.1080/1389224X.2013.824385
Assefa T, Wu J, Beebe S, Rao I, Marcomin D, Claude R (2015) Improving adaptation to drought stress in small red common bean: phenotypic differences and predicted genotypic effects on grain yield, yield components and harvest index. Euphytica 203:477–489. https://doi.org/10.1007/s10681-014-1242-x
Assefa T, Rao I, Cannon S, Wu J, Guatema Z, Blair M, Otyama P, Alemayehu F, Dagne B (2017) Improving adaptation to drought stress in white pea bean (Phaseolus vulgaris L.): genotypic effects on grain yield, yield components and pod harvest index. Plant Breed. https://doi.org/10.1111/pbr.12496
Beebe S, Ramirez J, Jarvis A, Rao I, Mosquera G, Bueno M, Blair M (2011) Genetic improvement of common beans and the challenges of climate change. Crop Adapt Clim Change. https://doi.org/10.1002/9780470960929.ch25
Beebe S, Rao I, Blair M, Acosta-Gallegos J (2013) Phenotyping common beans for adaptation to drought. Front Physiol 4:1–20. https://doi.org/10.3389/fphys.2013.00035
Beebe S, Rao I, Devi J, Polanía J (2014) Common beans, biodiversity, and multiple stresses: challenges of drought resistance in tropical soils. Crop Pasture Sci 65:667–675. https://doi.org/10.1071/CP13303
Bingham J (2001) Soil root canopy interactions. Ann Appl Biol 138:243–251. https://doi.org/10.1111/j.1744-7348.2001.tb00108.x
Blair M, Lopez-Marin H, Rao I (2009) Identification of aluminium resistant Andean common bean (Phaseolus vulgaris L.) genotypes. Braz J Plant Physiol 21:291–300. https://doi.org/10.1590/S1677-04202009000400005
Blum A (2005) Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust J Agric Res 56:1159–1168. https://doi.org/10.1071/AR05069
Blum A (2009) Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crop Res 112:119–123. https://doi.org/10.1016/j.fcr.2009.03.009
Butare L, Rao I, Lepoivre P, Polania J, Cajiao C, Cuasquer J, Beebe S (2011a) New genetic sources of resistance in the genus Phaseolus to individual and combined aluminium toxicity and progressive soil drying stresses. Euphytica 181:385–404. https://doi.org/10.1007/s10681-011-0468-0
Butare L, Rao I, Lepoivre P, Cajiao C, Polania J, Cuasquer J, Beebe S (2011b) Phenotypic evaluation of interspecific recombinant inbred lines (RILs) of Phaseolus species for aluminium resistance and root growth response to aluminium-toxic acid soil. Euphytica 186:715. https://doi.org/10.1007/s10681-011-0564-1
Daniel E (1997) The temperature dependence of photoinhibition in leaves of Phaseolus vulgaris (L.) Influence of CO2 and O2 concentrations. Plant Sci 124:1–8. https://doi.org/10.1016/S0168-9452(96)04574-8
Di Rienzo A, Casanoves F, Balzarini G, Gonzalez L, Tablada M, Robledo W (2017) InfoStat versión (2017). Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
Hall E (2004) Comparative ecophysiology of cowpea, common bean, and peanut. In: Nguyen HT, Blum A (eds) Physiology and biotechnology integration for plant breeding. Marcel Dekker Inc, New York, pp 271–325
León I (2009) La antracnosis y la mancha angular del frijol común (Phaseolus vulgaris L.). Temas de Ciencia y Tecnología 13:45–54
Long S, Zhu X, Naidu S, Ort D (2006) Can improvement in photosynthesis increase crop yields? Plant, Cell Environ 29:315–330. https://doi.org/10.1111/j.1365-3040.2005.01493.x
Omae H, Kumar A, Kashiwaba K, Shono M (2006) Influence of high temperature on morphological characters, biomass allocation, and yield components in snap bean (Phaseolus vulgaris L.). Plant Prod Sci 9:200–205. https://doi.org/10.1626/pps.9.200
Omae H, Kumar A, Kashiwaba K, Shono M (2007) Influence of temperature shift after flowering on dry matter partitioning in two cultivars of snap bean (Phaseolus vulgaris L.) that differ in heat tolerance. Plant Prod Sci 10:14–19. https://doi.org/10.1626/pps.10.14
Omae H, Kumar A, Kashiwaba K, Shono M (2012) Adaptation to high temperature and water deficit in the common bean (Phaseolus vulgaris L.) during the reproductive period. J Bot 1:6. https://doi.org/10.1155/2012/803413
Polania J, Rao I, Mejía S, Beebe S, Cajiao C (2012) Características morfo-fisiológicas de frijol común (Phaseolus vulgaris L.) relacionados con la adaptación a sequía. Acta Agron 61:197–206
Polania J, Poschenrieder C, Rao I, Beebe S (2016a) Estimation of phenotypic variability in symbiotic nitrogen fixation ability of common bean under drought stress using 15 N natural abundance in grain. Eur J Agron 79:66–73. https://doi.org/10.1016/j.eja.2016.05.014
Polania J, Poschenrieder C, Rao I, Beebe S (2016b) Estimation of phenotypic variability in symbiotic nitrogen fixation ability of common bean under drought stress using 15 N natural abundance in grain. Eur J Agron 79:66–73. https://doi.org/10.1016/j.eja.2016.05.014
Polania J, Poschenrieder C, Rao I, Beebe S (2017) Root traits and their potential links to plant ideotypes to improve drought resistance in common bean. Theor Exp Plant Physiol 29(3):143–154
Polanía J, Rao I, Cajiao C, Rivera M, Bodo R, Beebe S (2016) Physiological traits associated with drought resistance in andean and mesoamerican genotypes of common bean (Phaseolus vulgaris L.). Euphytica 210:17–29. https://doi.org/10.1007/s10681-016-1691-5
Porch G, Jahn M (2001) Effects of high-temperature stress on microsporogenesis in heat-sensitive and heat-tolerant genotypes of Phaseolus vulgaris. Plant Cell Environ 24:723–731. https://doi.org/10.1046/j.1365-3040.2001.00716.x
Rainey M, Griffiths D (2005) Identification of heat tolerant Phaseolus acutifolius A. Gray plant introductions following exposure to high temperatures in a controlled environment. Genet Resour Crop Evol 52:117–120
Rangel A, Mohammad M, Rao I, Horst W (2005) Proton toxicity interferes with the screening of common bean (Phaseolus vulgaris L.) genotypes for aluminium resistance in nutrient solution. J Plant Nutr Soil Sci 168:607–616. https://doi.org/10.1002/jpln.200520509
Rangel A, Rao I, Horst W (2007) Spatial aluminium sensitivity of root apices of two common bean (Phaseolus vulgaris L.) genotypes with contrasting aluminium resistance. J Exp Bot 58:3895–3904. https://doi.org/10.1093/jxb/erm241
Rao I (2014) Advances in improving adaptation of common bean and Brachiaria forage to abiotic stresses in the tropic. In: Pessarakli M (ed) Handbook of plant and crop physiology. CRC Press, Boca Raton, pp 847–889. https://doi.org/10.1201/b16675-49
Rao I, Beebe S, Polania J, Ricaurte J, Cajiao C, Garcia R et al (2013) Can tepary bean be a model for improvement of drought resistance in common bean? Afr Crop Sci J 21:265–281
Rao I, Miles J, Beebe S, Horst W (2016) Root adaptations to soils with low fertility and aluminium toxicity. Ann Bot 118:593–605. https://doi.org/10.1093/aob/mcw073
Rao I, Beebe S, Polania J, Grajales M, Cajiao C, Ricaurte J, García R, Rivera M (2017) Evidence for genotypic differences among elite lines of common bean in their ability to remobilize photosynthate to increase yield under drought. J Agric Sci 155:857–875. https://doi.org/10.1017/S0021859616000915
Suzuki K, Tsukaguchi T, Takeda H, Egawa Y (2001) Decrease of pollen stainability of green bean at high temperatures and relationship to heat tolerance. J Am Soc Hortic Sci 126:571–574
Traub J, Kelly J, Loescher W (2017) Early metabolic and photosynthetic responses to drought stress in common and Tepary bean. Crop Sci 57:1670–1686. https://doi.org/10.2135/cropsci2016.09.0746
Yang Z, Rao I, Horst W (2013) Interaction of aluminium and drought stress on root growth and crop yield on acid soils. Plant Soil 372:3–25. https://doi.org/10.1007/s11104-012-1580-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salazar, J.C.S., Polanía, J.A., Bastidas, A.T.C. et al. Agronomical, phenological and physiological performance of common bean lines in the Amazon region of Colombia. Theor. Exp. Plant Physiol. 30, 303–320 (2018). https://doi.org/10.1007/s40626-018-0125-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-018-0125-2