Abstract
The common bean (Phaseolus vulgaris L.) is a food crop that provides fiber, vitamins and carbohydrates and it is produced by smallholders in developing countries under nitrogen (N)-depleted and low-fertility soil conditions. Soil acidity and the associated aluminum toxic and phosphorus deficient conditions are known to reduce symbiotic N-fixing ability of common bean. Breeding lines of common bean have been developed to adapt to acid-soil and high-temperature conditions in the Amazon region but the response of these lines to N application was not determined. The present study was conducted with an objective to identify genotypic differences among ten common bean breeding lines (4 Andean, 5 Mesoamerican, 1 interspecific) in their physiological characteristics (gas exchange, chlorophyll fluorescence and photosynthetic efficiency) and their contribution to superior agronomic performance under three different levels of N (0, 60 and 120 kg N ha−1; N0, N60 and N120) supply. We tested the hypothesis that common bean genotypes that combine greater capacity to assimilate carbon with improved ability to mobilize photosynthates to developing pods and seeds could enhance their agronomic performance at different levels of N supply under high-temperature and acid soil-stress conditions in the field. A randomized complete block design with factorial arrangement (3 levels of N × 10 genotypes) in three replications was used for the study. Measurements included photosynthetic gas exchange characteristics (light and CO2-saturated photosynthesis), chlorophyll fluorescence, viability of pollen, leaf N concentration, photosynthetic N use efficiency and grain yield. The increase in supply of N had a significant effect on grain yield and on different photosynthetic characteristics. Results showed that lines with indeterminate growth habit responded better to increase in N supply through their physiological response in improving grain yield. Among the genotypes evaluated, two lines (BFS 10 and SEF 10) were outstanding in their agronomic performance due to greater photosynthetic capacity combined with greater ability to mobilize photosynthates to grain with increase in N supply. These two lines can serve as parents for further improvement in resource use efficiency and multiple-stress resistance of common bean in the tropics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The common bean (Phaseolus vulgaris L.) is an important food for more than 400 million people in the tropics of Latin America and Africa with a high content of fiber, vitamins and carbohydrates (Beebe 2012). Smallholders contribute to most of the bean production in developing countries and most bean producing regions are affected by different abiotic stress factors (Rao 2014). These include variations in rainfall (Beebe et al. 2013) and temperature (Beebe 2012; Suárez et al. 2020); and soil conditions, such as acidity, and low phosphorus (P) (Rao 2014; Suárez et al. 2018a) and nitrogen (N) availability (Guo et al. 2002). These abiotic stress factors limit grain production, causing losses of 10–100% of the crop in 30–73% of the areas planted worldwide (Rao et al. 2016; Polania et al. 2016b).
As bean cultivation is of short cycle, the crop requires that nutrients are readily available in the soil (Guimarães et al. 2017). Nitrogen being the nutrient most absorbed by the bean plant, it plays a key role in chlorophyll production, vegetative growth, flowering, and pod and seed filling (Toso et al. 2017). This element (N) can be supplied to the soil by symbiotic nitrogen fixation (SNF), due to its high fixation capacity compared to other leguminous species (Ramaekers et al. 2012; Polania et al. 2016a). Additionally, the SNF process is highly sensitive to abiotic stress factors (drought, heat, soil acidity, low P among others) that affect both the plant and the bacteria, limiting the absorption and utilization of N in marginal environments (Polania et al. 2016b). The N requirement for crop growth is estimated to be around 2.5 kg ha−1 day−1 during flowering and 3.5 kg ha−1 day−1 during grain filling (Peres et al. 2004). However, inadequate supply of N has significant effects on the CO2 assimilation capacity of photosynthesis (Jin et al. 2015), quantum yield of photosystem II (PSII) electron transport and the maximum efficiency of PSII photochemistry (Fv/Fm) (Dai et al. 2003) that are associated with decreases in rubisco content and activity which influence the regulation of Calvin cycle (Jin et al. 2015).
The increase in supply of N enhances physiological processes, such as the photosynthetic rate of the plant, since the dry matter produced depends entirely on the N status of the plant (Saberali et al. 2015). Increase in supply of N to the plant increases the specific leaf area of the plant, the efficiency of interception of solar radiation, the rate of photosynthesis, and the amounts of both total chlorophyll (Chlt) and rubisco (Seepaul et al. 2016). It also generates an increase in the efficiency of the excitation energy captured by the PSII open centers (Jin et al. 2015). However, a low supply of N to the plant affects the function of both PSII and PSI, inducing a decrease and degradation in protein content mainly in the PSII reaction center resulting in decreased rate of net photosynthesis (Dai et al. 2003; Jin et al. 2015).
Most of the N used by plants establishes and maintains the photosynthetic apparatus that can contain up to 75% of the total leaf N content in C3 plants (Seepaul et al. 2016). As a result, primary processes that determine plant growth, such as gas exchange, photosynthesis, respiration and transpiration (Zhu et al. 2012), influence biomass production, biomass distribution and overall grain yield depending on different limiting factors, such as light intensity, CO2 concentration and N availability (Schulze et al. 2005). Thus, it is important to determine the influence of N supply on genotypic variation in plant physiological responses, such as gas exchange, photosynthetic efficiency, chlorophyll fluorescence and their impact on agronomic performance. The chlorophyll fluorescence is a widely used parameter related to the efficiency of PSII of the plant. Fluorescence is considered as one of the main routes of absorbed light energy and it is indicative of PSII performance (Jiménez-Suancha et al. 2015).
The analysis of genotypic differences in physiological responses in relation to N supply helps to understand not only how physiological characteristics influence dry matter partitioning and superior agronomic performance but also it helps to identify superior genotypes that can be used in genetic improvement programs targeted to marginal environments. This knowledge is also useful to design breeding strategies for the development of cultivars that can use the applied N efficiently in photosynthesis and yield formation (Mi et al. 2007; Long et al. 2013).
Currently, the main use in the department of Caquetá (Colombian Amazon) is extensive cattle ranching, with the development of family farming taking place in hillside areas. In addition, the soils are characterized by low pH, toxic levels of aluminum (Al) and manganese (Mn), along with deficiencies of essential nutrients, such as N and P. These are the areas where the cultivation of beans can be fundamental to improve food security and nutrition of local communities. Therefore, identifying genotypes that are adapted to these conditions will improve nutritional security and income generation in the region. Previous research resulted in identification of a few common bean lines that are adapted to acid soils (Suárez et al. 2018a) but their response to N application is not known. The main objective this study is to identify genotypic differences in physiological response (gas exchange, chlorophyll fluorescence and photosynthetic efficiency) and their contribution to superior agronomic performance under three different levels of N (0, 60 and 120 kg N ha−1; referred as N0, N60 and N120) supply. We tested the hypothesis that common bean genotypes that combine greater capacity to assimilate carbon with improved ability to mobilize photosynthates to developing pods and seeds could enhance their agronomic performance at different levels of N supply under high-temperature and acid soil-stress conditions in the Colombian Amazon.
Materials and methods
Experimental site and environmental conditions
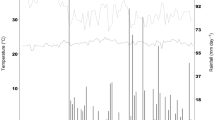
The trial was conducted during two seasons (February to May in 2017; and November 2018 to February 2019), at the Centro de Investigaciones Amazonicas (CIMAZ) Macagual—Universidad de la Amazonía-Colombia (1°37′ N and 75°36′ W) under a warm humid climate, which is characteristic of the humid tropical forest ecosystem, with an average annual rainfall of 3,800 mm, a sunshine of 1,700 h per year, an average temperature of 25.5 °C and relative humidity of 84%. During the growing season, the average maximum and minimum temperatures for 2017 were 35 °C and 20 °C and for 2018–2019 were 36 °C and 19 °C, respectively (Fig. 1). The total precipitation during crop growth over the two seasons was 1,281 mm (2017) and 587 mm (2018–2019) (Fig. 1). The soil is a clay loam Oxisol with bulk density values that ranged between 1.0 and 1.3 g cm−3, pH values that ranged from 4.1 to 5.2, with a mean soil organic carbon content of 1.35%, available P content (Bray-II) of 2.58 mg kg−1, total base saturation of 7.1% (Ca: 0.38 cmol kg−1, Mg: 0.1 cmol kg−1, K: 0.14 cmol kg−1, Na: 0.1 cmol kg−1, total bases: 0.8 cmol kg−1), a cation exchange capacity of 11.3 cmol kg−1, and an exchangeable Al content of 6.3 cmol kg−1 with 73.4% of Al saturation.
Distribution of rainfall and maximum/minimum temperatures during the cultivation period at the Centro de Investigaciones Amazonicas (CIMAZ), Macagual, Colombia from February to May 2017 (a) and from November 2018 to February 2019 (b). Vertical lines with light black color refer to the average number of days to flowering (43) and physiological maturity (73), respectively, during the two seasons
Plant material and experimental design
Ten genotypes of common bean developed by CIAT Bean Improvement Program were used in this study and these were selected based on their higher values of grain yield and better physiological response to soil and climatic conditions in the Colombian Amazon (Suárez et al. 2018a). These genotypes include 9 advanced lines of common bean (Phaseolus vulgaris L.) (4 from Andean genepool, growth habit Type I: SAB 618, SAB 659, SAP 1–15, SAB 686; 5 from Mesoamerican genepool, growth habit Type II: NCB 280, BFS 10, BFS 35, BFS 81, BFS 143) and an interspecific line resulting from the combination between the species of Phaseolus acutifolius, P. vulgaris and P. coccineus (growth habit Type II: SEF 10). Growth habit type I is an erect determinate bush while growth habit type II is an erect indeterminate bush (Kornegay et al. 1992). These bean lines represent potential parental materials for improving resistance to abiotic and biotic stress factors, grain yield and grain quality (micronutrient content). The BFS lines (small red) have a better adaptation to low soil fertility. The SAB lines (mottled red, mottled brown) are adapted to drought. NCB (small black) line is improved for drought adaptation. SEF (red) line is improved for adaptation to both drought and heat. The SAP (red mottled) line is adapted to drought.
A randomized complete block (RCB) design with factorial arrangement (3 levels of N × 10 genotypes) in three replications was used for the study. Each replication had three blocks corresponding to three levels of N fertilizer applied (kg ha−1: N0, N60, N120), where for each level of N treatment, 10 genotypes were randomly assigned for each plot. Each experimental unit consisted of three rows, and each row was six meters long with a distance between rows of 0.5 m and a distance between plants of 15 cm (equivalent to 11 plants m−2). The N supply (application) rates used in the study were based on those recommended for tropical regions with soils that are very poor in N availability (Rosas 2014). The three treatments of N supply: N0, N60 and N120 represent N-deficient, low N and high N supply, respectively, for a grain legume crop with N-fixing ability. The total dose of N for each treatment was supplied in the form of urea as split applications with equal amounts at 14, 29 and 44 days after sowing (DAS) and incorporated into soil after each application. Those N applications correspond to three developmental stages: 3rd true leaf (first trifoliate leaf) unfolded, first flower buds visible, and at the end of flowering/first pods visible, respectively.
Photosynthetic light- and CO2-response curves of common bean lines grown under different levels of N supply
The cuvette methodology and the specific environmental conditions that were described by Suárez et al. (2018a, b) were used to measure gas exchange characteristics of three fully expanded leaves (located between the 7th and 9th leaf developed from the base of the plant) of each plant with three independent replicates per genotype. Gas exchange measurements were performed between 08:00 and 11:00 h (solar time) during the pod-filling growth phase (R8), which corresponded to a period of 70 to 80 days after planting. Using the information derived from the A/PAR curves that were obtained from the CIRAS-3 Portable Photosynthesis System infrared gas analyzer (PP Systems Inc., Amesbury, MA, USA), different gas exchange parameters were determined, such as light-saturated maximum net carbon assimilation rate (Amax), light compensation point (LCP), dark respiration rates (Rd), light saturation point (LSP), and apparent quantum efficiency (ΦPAR), which is the slope of the initial linear portion of the A/PAR curve (Bauerle et al. 2006). Likewise, using the recommendations of Long and Bernacchi (2003), different gas exchange parameters, such as the maximum rate of ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO) carboxylation (Vcmax), maximum rate of electron transport driving regeneration of ribulose-1, 5-bisphosphate (RuBP) (Jmax), and leaf respiration under light conditions (RD) from photosynthetic assimilation response to internal CO2 concentration (hereafter A/Ci), were determined. A/Ci curves were determined according to Martins et al. (2013) and the leakage corrections were made according to Flexas et al. (2007).
Chla fluorescence and imaging for the chlorophyll (Chla) parameters under different levels of N supply
Using the chlorophyll fluorescence module (CFM-3) adapted to the CIRAS-3 infrared gas analyzer (PP Systems Inc. Amesbury, MA, USA), different chlorophyll fluorescence measurements were performed following the methodology of Suárez et al. (2018a, b). With the different data obtained, parameters, such as the maximum quantum yield of PSII (Fv/Fm), the apparent electron transport rate (ETR), the actual quantum efficiency of PSII (ΦPSII), the non-photochemical quenching of Chla fluorescence (NPQ), were determined. The equations described by Brooks and Niyogi (2011) and Genty et al. (1989) were used to determine these parameters. Fluorescence imaging parameters were determined using the Imaging-PAM M-Series chlorophyll fluorometer and the software version 2.32 Imaging WIN (both from Heinz Walz GmbH, Effeltrich, Germany) according to the methodology used by Rios et al. (2018). The software generated the image of the maximum PSII photochemical efficiency (Fv/Fm = (Fm − F0)/Fm) (Maxwell and Johnson 2000). The leaf tissues were exposed to actinic photon irradiance (110 μmol m−2 s−1) for 120 s to obtain the steady-state fluorescence yield (Fs), after which a saturating white light pulse (2,400 μmol m−2 s−1; 0.8 s) was applied to achieve the light-adapted maximum fluorescence (F′m). Following the calculations proposed by Kramer et al. (2004), the energy absorbed by the PSII for the following two yield components for dissipative processes was determined: the yield of photochemistry [(YII = (F′m − F)/F′m)], the yield for dissipation by downregulation [Y(NPQ) = (Fs/F′m) − (Fs/Fm)] and the yield for other non-photochemical (non-regulated) losses [Y(NO) = Fs/Fm].
Pollen viability, specific leaf area, total chlorophyll and photosynthetic nitrogen-use efficiency
To determine the effect of different levels of N supply on different lines of bean, viability of pollen (VP) was evaluated following the methodology of Porch and Jahn (2001). Flower buds in the middle of the plant were collected one day before anthesis and stored in plastic jars with a solution of 1:3 glacial acetic acid–96% alcohol at 4 ºC. The grains of pollen were removed from the anthers, and one drop of 1% acetocarmine was added on grains located on microscope glass. A total of hundred grains of pollen were analyzed per repetition to determine the viability of pollen from each flower bud. The pollen grains that were dyed red were considered viable (fertile pollen), while grains without dye indicated pollen that was infertile or non-viable. After the tally, the percentage of viability was calculated as a proportion of dyed grains to total grains.
Specific leaf area (SLA) was determined using six leaf discs (3.14 cm2) excluding the mid-vein for each leaf that had been previously used to measure gas exchange and Chla fluorescence [n = 4860 corresponding to 3 plants per genotype (10 genotypes) per treatment (3N levels), 3 leaves per plant and 18 discs per leaf]. The discs were dried to constant mass at 70 °C, and the SLA was determined as the ratio between leaf disc area and its respective dry mass (Cornelissen et al. 2003). We followed the protocol described by Lichtenthaler (1987) to determine the levels of total Chl (Chlt), Chla and Chlb, and carotenoids for each leaf by taking six leaf discs (3.14 cm2) from the same leaves that had been previously used to measure SLA. The Photosynthetic nitrogen-use efficiency (PNUE) was calculated by dividing the value of Amax by the N content of the leaf (Lynch et al. 1994; Yin et al. 2018).
Leaf of nitrogen, phenology and grain yield
The Kjeldahl method was used to determine total leaf nitrogen (Cornelissen et al. 2003). Days to flowering and physiological maturity were determined as described by Suárez et al. (2018a). To evaluate agronomic performance, destructive sampling was conducted on the central row of each plot, the pods of the harvested plants were threshed, and the grains were cleaned and dried to determine grain yield (kg ha−1).
Data analysis
The Michaelis–Menten hyperbolic constant was used to adjust the A/PAR curves; the parameters Amax, LSP, LCP, Rd, and ΦPAR were calculated following the equations described in Lobo et al. (2013). The model created by Farquhar et al. (1980) (the ‘FvCB model’) was used to evaluate the A/Ci curve and to estimate Vcmax, Jmax, and RD using the plantecophys package in R (Duursma 2015). A generalized linear model (GLM) was adjusted for the different parameters derived from the A/PAR and A/Ci curves for each common bean line at each level of N applied (fixed factor). The plant and leaf were included as random factors (n = 16). Likewise, a generalized linear model (GLM) was made for SLA, photosynthetic pigments, and leaf N content, including the level of N applied as the fixed factor. The season, the plant, leaf and leaf discs were included as random factors. The assumptions of normality and of homogeneity of variance were evaluated using an exploratory residual analysis. Differences between mean values of common bean lines’ plant responses with the level of N (fixed factor) were analyzed with the Fisher’s LSD post hoc test at a significance of α = 0.05. The association between agronomic, phenological and physiological variables was analyzed by calculating Pearson correlation coefficients. To visualize the correlations, heat maps and chord diagrams were elaborated using the corrplot (Wei et al. 2017) and circlize (Gu et al. 2014) packages. Analyses of GLM were performed using the lme function in the nlme package (Pinheiro et al. 2012) in R language software, version 3.6.1 (R Development Core Team 2017), and using the interface in InfoStat (Di Rienzo et al. 2019).
Results
Photosynthetic light- and CO2-response curve differences among common bean lines grown under different levels of N supply
Significant differences (p < 0.05) were observed in parameters obtained from the light- and CO2-response curves in the interaction between the applied N level and bean genotypes (Fig. 2). The increase in supply of N had a significant effect on different photosynthetic characteristics; genotypes with growth habit Type II (BFS 10, BFS 35, BFS 81) presented a greater response to the increase of N supply (Fig. 2a,b,d). However, the greatest efficiency in terms of electron transfer (ΦPAR Fig. 2f) (revealed as the highest maximum rate of regeneration of ribulose-1,5-bisphosphate controlled by electron transport) was observed with three genotypes of growth habit Type I (SAB 686, SAP 1–15 and SAB 659) at the N0 level of N application.
Photosynthetic gas exchange characteristics of ten genotypes of common bean grown at three levels of nitrogen (0, 60 and 120 kg N ha-1; N0, N60 and N120) supply. a, b Amax: Light-saturated maximum net carbon assimilation rate; DM Dry mass; c Rd: Dark respiration rate; d LSP: Light saturation point; e LCP: Light compensation point; f ΦPAR: Quantum efficiency; g Vcmax: Maximum carboxylation rate; h RD: Leaf respiration in light conditions; i Jmax: Maximum rate of regeneration of ribulose-1,5-bisphosphate (RuBP) controlled by electron transport
Chla fluorescence and imaging for the chlorophyll (Chla) parameters under different levels of N supply
The behavior of all fluorescence parameters as a function of PAR was different with respect to the N supply (p < 0.05) (Fig. 3). According to quantitative analysis and images of Chla fluorescence parameters, the maximum quantum yield of photosystem (PS) II (Fv/Fm) was between 0.81 and 0.80 at different N levels, the value being lower at the low N level. The magnitude of the changes in F0, Fm and Fv/Fm was differently proportional to the reduction in the N level, with the changes observed with the N120 level were more evident (Fig. 3, Supplementary 1).
Images of chlorophyll a fluorescence parameters taken in fully developed leaves at the onset of physiological maturity: Initial fluorescence (F0), maximal fluorescence (Fm), maximum PSII quantum efficiency (Fv/Fm) as influenced by three levels of nitrogen (0, 60 and 120 kg N ha-1; N0, N60 and N120) supply. *Does not differ statistically among the three nitrogen levels
Pollen viability, specific leaf area and chlorophyll and carotenoid contents
In terms of pollen viability, genotype BFS 35 had the highest values regardless of N level (p < 0.05, Fig. 4a). Only for genotype SAB 686, pollen viability increased as the supply of N increased (p < 0.05, Fig. 4a). SLA showed a significant effect on genotype interaction (p < 0.05, Fig. 4b). There was a greater response of genotype NCB 280 at the N60 level of N, followed by BFS 143 and BFS 35 for the N0 level of N and finally BFS 81 at the N120 level of N (p < 0.05, Fig. 4b).
Viability of pollen (VP) (a); and specific leaf area (SLA) (b) of different genotypes of common bean grown at three different levels of nitrogen (0, 60 and 120 kg N ha-1; N0, N60 and N120) supply. a %VP % Viability of pollen; b SLA Specific leaf area. *Does not differ statistically among the three nitrogen levels
The carotenoid content was higher in comparison to Chla and Chlb, responding positively when the N supply was zero (Fig. 5). Some genotypes, such as BFS 10 and NCB 280, had the ability to accumulate more pigments, such as Chla and Chlb, as well as carotenoids. However, genotypes, such as SAB 618 and SAB 659, had higher Chlt content at the N0 level. In terms of Chla/b ratio, genotype SAB 659 showed the highest value with N120. However, genotypes BFS 143, BFS 35 and SEF 10 showed higher values with N0. Four genotypes (BFS 10, BFS 143, SAP 1–15 and SEF 10) presented higher values of Chlt/car with N60 treatment (Fig. 5).
Photosynthetic pigment content of ten common bean genotypes as influenced by three levels of nitrogen (0, 60 and 120 kg N ha-1; N0, N60 and N120) supply. a Chla chlorophyll a; b Chlb chlorophyll b; c Car Carotenoid; d Chlt Total chlorophyll; e Chla/Chlb chlorophyll a /b ratio; f Chlt/Car Total chlorophyll/ carotenoids ratio. *Does not differ statistically among the three nitrogen levels
Leaf nitrogen and grain yield
Leaf N content was affected by the level of N applied to the soil (Fig. 6a, p < 0.05). Genotype BFS 35 presented the highest leaf N content with N0 and N120 levels, followed by three other genotypes (NBC 280, SAB 686 and SEF 10). However, genotype BFS 10 together with BFS 81 presented among others, lower values of N content. Surprisingly, only BFS 81 did not show any significant response to increase in N supply. The mean genotype value of grain yield under N0 conditions was 1,215 ± 40 kg ha−1, with an increase of 35% and 69% in yield compared to the N60 and N120 treatments, respectively (Fig. 6a b). Among the genotypes tested, regardless of the level of N applied, the highest value of grain yield was observed with BFS 10 (2,542 ± 105 kg ha−1). When analyzed for each genotype evaluated, a significant effect on grain yield was found with an increase in the level of N applied (Fig. 6a , p < 0.05). Genotypes, such as BFS 10, BFS 81 and NCB 280, presented the highest yields at N120, followed by genotypes BFS 143 and BFS 35. However, these two genotypes were different in their response compared to BFS 10, BFS 81 and NCB 280 (Fig. 6a , p < 0.05). On the other hand, genotypes, such as SAB 659 and SAB 686, presented higher values of grain yield with N0 than with N60 or N120. This particular response from these Andean lines needs further investigation.
Photosynthetic nitrogen-use efficiency (PNUE)
The SEF 10 line showed the highest value of PNUE with N120, followed by NBC 280, BFS 10, BFS 81 and BFS 35 (Fig. 7). However, SEF 10 showed the lowest PNUE values, among others, with N0 and N60 treatments. The BFS 81 genotype was the only one that showed the highest value of PNUE with N0 while the SAP 1–15 showed the highest value of PNUE with N60 treatment.
Relationships between phenological/physiological variables and grain yield
In general, grain production was found to correlate positively with N supply (r: 0.34), Chlb (r: 0.48), Chlt (r: 0.4), A (r: 0.47), LSP (r: 0.4) and NPQ (r: 0.49), and negatively with ΦPAR (r: − 0.35) (Fig. 8a, b). Between N supply treatments, it was found that with N0 grain production correlated positively with Vcmax (r: 0.82, Fig. 8c); while with N60 grain production correlated positively with VP (r: 0.66), Chlb (r: 0.71) and Chlt (r: 0.62 Fig. 8d). On the other hand, N120 treatment showed positive correlation with A (r: 0.67) and negative correlation with ΦPAR (r: − 0.65) (Fig. 8e).
Pearson correlation coefficients between agronomic, phenological and physiological variables. a Heat map of Pearson correlation coefficients between grain production and other tested variables. The colors represent the direction and strength of the correlation, *, ** and ***indicate significant correlations with 10, 5 and 1% probability level, respectively. b Chord diagram of correlation coefficients between agronomic, phenological and physiological variables in general. c Chord diagram of correlation coefficients between agronomic, phenological and physiological variables with N0 treatment. d String diagram of correlation coefficients between agronomic, phenological and physiological variables with N60 treatment. e String diagram of correlation coefficients between agronomic, phenological and physiological variables with N120 treatment. The ribbons within the circle correspond to correlations with coefficients > 0.45 and a p < 0.05, the red ribbons indicate positive coefficients and the blue ribbons indicate negative coefficients. Amax light-saturated maximum net carbon assimilation rate, LCP light compensation point, LSP light saturation point, Rd dark respiration rate, RD leaf respiration in light conditions, Vmax maximum carboxylation rate, Jmax maximum rate of regeneration of ribulose-1,5-bisphosphate (RuBP), ETR electron transport rate, qL Photochemical quenching, ΦPSII current PSII quantum yield, NPQ Non-photochemical quenching, YII Yield of photochemistry, ΦPAR quantum efficiency, Chla Chlorophyll a, Chlb Chlorophyll b, Car Carotenoid, Chlt total chlorophyll, Chla/Chlb Chlorophyll a/b ratio, DM dry mass, SLA specific leaf area, Fo initial fluorescence, Fm maximal fluorescence; Fv/Fm PSII quantum efficiency, N leaf nitrogen concentration; PNUE photosynthetic nitrogen-use efficiency, VP viability of pollen, PAR photosynthetically active radiation, GY grain yield
Discussion
In general, the trends found in this study for the genotypes studied, showed that the plant type (architecture) in terms of Type I or Type II had greater impact on the ability of genotypes to accumulate N in the leaf and improve its physiological function by increasing the supply of N to photosynthesis. This is achieved by improving the ability of its photosynthetic apparatus, influencing both gas exchange and fluorescence parameters thereby enhancing their physiological responses to increase in N supply. Two common bean genotypes (BFS 10 and SEF 10) were superior in their photosynthetic efficiency as well as agronomic performance as reflected by greater values of grain yield.
Photosynthetic light- and CO2-response curves differ among common bean lines grown under different levels of N supply
Our results showed a higher efficiency in the use of N due to the increase in photosynthetic capacity, specifically in growth habit Type II lines, such as BFS 10 and BFS 81. This is due to the increase in the carbon gain per unit of photosynthetic N, allowing N to be efficiently utilized (Evans and Clarke 2019), probably due to the way photosynthates are distributed in each of the sinks (Michelangeli et al. 2019) for the formation of new tissues. When analyzing C fixation, parameters, such as Vcmax and Jmax, were affected for some genotypes (Long and Bernacchi 2003), for example, SAB 686, BFS 81 and SAB 618, which presented the highest values of Jmax, when the level of N supply was zero (N0). This observation indicates that carboxylation is limiting photosynthesis due to the low N content (or morphological changes in tissues connected to low N) in the leaf (Gao et al. 2018). This generates a high investment in Jmax in relation to Vcmax which would lead to a transport of electrons that are not used in linear electron flow that would require the dissipation of electron energy to avoid photoinhibition (Krause et al. 2012) and this could result in decrease of the Amax with N0 treatment.
We found that the CO2 assimilation capacity was reduced mainly due to the supply of N, which affects the RubisCo content and the RuBPcase activity in the Calvin cycle (Lu and Zhang 2000). This resulted in a low maximum rate of RubisCo carboxylation (Vcmax) and in the maximum rate of RuBP regeneration which are being controlled by electron transport (Jmax) (Gao et al. 2018). However, the efficiency found in the BFS 10 genotype was higher due to high Vcmax rates with a lower investment of Jmax with N0 treatment. This phenomenon can be connected to extra excitation energy consumption by photorespiration (Rd), which contributed to sustain the photosynthetic rate with N0 treatment (Gao et al. 2018). This physiological response of BFS 10 with N0 treatment is consistent with previous observations made by Beebe (2012) and Suárez et al. (2020) on genotypic differences in improving adaptation to low-fertility soils.
Chla fluorescence and imaging for the chlorophyll (Chla) parameters are affected with the level of N supply
Under different levels of N supply, the photosynthetic efficiency (Fv/Fm > 0.8) of genotypes with Type II growth habit was mostly intact (Ribeiro et al. 2018; Jin et al. 2015) due to the opening of the reaction centers and no state of reduction of QA (Ribeiro et al. 2018; Long et al. 2013). The above observation is inferred by the relationship between Fv/Fm, which did not present drastic variations that led to photoinhibitory damage (Correia et al. 2006). As there is no damage to the reaction centers, this allowed the different bean genotypes to have a rapid recovery of photosynthetic activity due to the alleviation of stress induced by increase in the level of N supply (Correia et al. 2006). However, with N0 treatment, a reduction in ETR, qL and ΦPSII was found in bean genotypes due to decreased efficiency of excitation energy capture (Sun et al. 2019). These results indicate that ETR is influenced by both N level and light intensity (PAR), which may cause a different response to the change in N supply (Long et al. 2013). In response to a high PAR at a low N supply, the photochemical and non-photochemical quenching capacity could increase and thus may reduce photoinhibitory damage of PSII (Shrestha et al. 2012).
Genotypes differ in specific leaf area, photosynthetic pigment content and leaf nitrogen content with change in nitrogen supply
The BFS and NCB lines, compared to the other lines evaluated, presented higher values of SLA and leaf N, independent of the N supply. This shows the capacity of these lines in terms of efficient N use, fixing higher amounts of CO2 and increasing leaf area (Lawlor 2002). Michelangeli et al. (2019) and Saberali et al. (2015) have observed that leaf N content and leaf area are influenced by three factors: the growth habit of the bean plant, the canopy architecture and the plant's ability to intercept light. These growth characteristics could allow type II genotypes to increase N fixation, and biomass and seed production (Ramaekers et al. 2012).
We also found that the reduction in photosynthetic pigment content was due to N limitation and this can cause oxidative stress (Murtaza et al. 2016; Peres et al. 2004). For example, the Chla content of type II genotypes, such as NCB 280 and BFS 10, was higher, indicating the ability to invest N to have a higher chlorophyll content, where cells are in a more mature stage of development (Koning et al. 2015), in addition to assigning more N to RubisCO and the other enzymes of C fixation pathway that favor the assimilation of CO2 (Akram 2014). Regarding the carotenoid content, genotypes, such as BFS 10, BFS 143, NCB 280 and SEF 10, stood out with the highest values among the different levels of N supply, indicating that these genotypes could not only adapt to a low N supply but could also respond physiologically better to an increased N supply (Zhu et al. 2012).
Capacity to mobilize photosynthate to increase yield varied among genotypes under different levels of N supply
The supply of N had a significant effect on grain yield and specifically on the BFS lines, which were developed to improve adaptation to low soil fertility (Beebe 2012), and even more so with acid soils where the Al toxicity is one of the major limiting factors for the effective use of applied N (Yang et al. 2013). In general, our results confirm the adaptive responses found by Suárez et al. (2018a) for genotypes evaluated with N0 treatment, which increased yields proportionally to the increased dose of N applied. For example, the BFS 10 line showed a greater capacity for transport of photosynthates to seeds contributing to greater grain yield response with increase in N supply. Similar responses were reported before (Aires et al. 2019; Leal et al. 2019; Guimarães et al. 2017; Soratto et al. 2017). Specifically, for our study, 70% of genotypes increased grain yield as a function of N level, with a linear relationship (GY = 1188.3 + 6.71(kg N) R2 = 0.97) independent of genotype.
Viability of pollen was not markedly affected by N supply, thus relating positively with increase in grain yield. This behavior was more evident in the genotypes of type II growth habit, and this could be due to their better capacity to adapt to high temperatures (different plant strata have likely different capacity to withstand heat by evaporative cooling effect). This could contribute toward better crop development during the R5 growth stage (pre-flowering) during which pollen formation occurs (Porch and Jahn 2001) and demand for N increases (Atasay et al. 2013). However, four genotypes (BFS 143, NCB 280, SAB 618 and SAB 659) presented greater values of viability of pollen with N0 treatment, whose variation was inversely proportional to the level of N. This behavior is probably due to the influence of N supply on the activity of some of the metabolic pathways in plants (Lau and Stephenson 1993). For example, the levels of polyamines may increase in response to the increase in dose of N and these are involved in cell division and morphogenesis (Hayashi 2011), and these in turn have a direct effect on the pollen tube growth and development (Atasay et al. 2013). From the above, we can infer that a high or inadequate supply of N may influence the viability of the pollen and its germination thereby influencing the production of grain (Lau and Stephenson 1993).
Genotypes differ in photosynthetic nitrogen-use efficiency (PNUE) with increase in nitrogen supply
The PNUE increased significantly with the increase in the supply of N, the SEF 10 line being the most efficient. Genotypes, such as NCB 280, BFS 10, BFS 81 and BFS 35 (Type II), were also found to have similar behavior. These observations are consistent with those reported by Yin et al (2018) and Townsend et al. (2017) who described that PNUE may have an improvement depending on the canopy architecture, which in the case of our study favored genotypes with indeterminate growth habit of Type II.
The results obtained by Suárez et al. (2018a, b) indicated that genotypes, such as BFS 10 and NCB 280, present a tendency to obtain less biomass from the canopy with more CO2 fixation in the leaves that allows them greater accumulation of reproductive organs, thus producing greater amounts of grain. For example, such a process can occur in rice cultivation where increasing the photosynthesis process increases 18% of biomass and only 5% of grain yield, or a 6% change in grain yield in the absence of additional N (Long et al. 2013). BFS 10 was characterized as the lowest N accumulator in leaves and this behavior was also found by Polania et al. (2016a) who described it as a genotype with limited aerial biomass, low symbiotic nitrogen fixing (SNF) ability, earliness, but with superior ability to transport photosynthates that allows it to produce greater yield. BFS 10 is also known as a genotype that adapts better to high temperature-stress conditions (Suárez et al. 2020). In addition, total leaf N content and CO2 assimilation may also be influenced by leaf longevity (Lynch and Rodriguez 1994). Leaf N decline may be limited by the restricted ability of the plant to remove structural and metabolic proteins from the leaves (Harrison et al. 2009), which may be another reason why BFS 10 showed lower leaf N content. Contrary to this behavior, the SEF 10 line showed higher values of N accumulation and PNUE, but a lower ability to transport photosynthates, which resulted in a lower yield than that of BFS 10.
Conclusion
The amount of N supplied to soil had a significant positive effect on grain yield, viability of pollen and on different physiological traits (A, LSP, Vcmax, NPQ, Chlb and Chlt). At genotypic level, we found that lines with growth habit Type II responded better to improved N supply at both agronomic and physiological levels. Among the genotypes evaluated, the BFS 10 and SEF 10 were outstanding in their capacity to acclimate their photosynthetic apparatus (higher Amax and Vcmax to a lower inversion of Jmax), and their optimal response to excess energy (ETR, ΦPSII, NPQ) and pigment content that resulted in higher SLA values and leaf N content, and efficient photosynthetic use of N. Four other genotypes (BFS 35, BFS 81, NCB 280 and SAP 1–15) also showed relatively good performance in acidic Amazonian soil conditions. These promising lines also combined other desirable plant characteristics, such as a higher level of total N uptake in the leaves and a greater ability to partition photosynthates to grain. We suggest that BFS 10 and SEF 10 could serve as potential parents for breeding programs that are aiming to improve resource use efficiency and multiple-stress resistance in common bean.
Author contribution statement
JCS: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, roles/writing - original draft, writing - review and editing. JAP: Conceptualization, methodology, supervision, writing - review and editing. JAA: Conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, ATC: conceptualization, methodology, visualization, roles/writing - original draft, writing - review and editing. DLM: Conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization. JIV: Conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization. JEN: Conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization. LR: data curation, formal analysis, investigation, methodology validation, visualization. MOU: Conceptualization, visualization, writing - review and editing. SB: Conceptualization, investigation, methodology, supervision. IMR: Conceptualization, data curation, formal analysis, methodology, supervision, validation, visualization, roles/writing - original draft, writing - review and editing.
References
Aires BC, Soratto RP, Guidorizzi FVC (2019) Grain yield and quality of common bean cultivars in response to nitrogen. Científica 47(2):231–238
Akram M (2014) Effects of nitrogen application on chlorophyll content, water relations, and yield of maize hybrids under saline conditions. Commun Soil Sci Plant Anal 45(10):1336–1356
Atasay A, Akgül H, Uçgun K, Şan B (2013) Nitrogen fertilization affected the pollen production and quality in apple cultivars “Jerseymac” and “Golden Delicious.” Acta Agric Scand Sect B Soil Plant Sci 63(5):460–465
Baker N (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Bauerle W, Wang G, Bowden J, Hong C (2006) An analysis of ecophysiological responses to drought in American chestnut. Ann For Sci 63(8):833–842
Beebe S (2012) Common bean breeding in the tropics. Plant Breed Rev 36(36):357–426
Beebe S, Rao I, Blair M, Acosta J (2013) Phenotyping common beans for adaptation to drought. Front Physiol 4:35
Brooks MD, Niyogi KK (2011) Use of a pulse-amplitude modulated chlorophyll fluorometer to study the efficiency of photosynthesis in arabidopsis plants. In: Jarvis R (ed) Chloroplast research in Arabidopsis, methods in molecular biology (Methods and protocols). Humana Press , Totowa, pp 299–310
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Corrales-González M, Rada F, Jaimez R (2015) Efecto del nitrógeno en los parámetros fotosintéticos y de producción del cultivo de la gerbera (Gerbera jamesonii H. Bolus ex Hook. F.). Acta Agron 65(3):255–260
Correia MJ, Osório ML, Osório J, Barrote I, Martins M, David MM (2006) Influence of transient shade periods on the effects of drought on photosynthesis, carbohydrate accumulation and lipid peroxidation in sunflower leaves. Environ Exp Bot 58(1–3):75–84
da Ribeiro JES, Leite AP, Costa JE, de Albuquerque MB, Mielezrski F (2018) Development, physiology and productivity of the common bean under different nitrogen doses. J Agric Sci 10(6):171–183
Dai TB, Cao WX, Sun CF, Jiang D, Jing Q (2003) Effect of enhanced ammonium nutrition on photosynthesis and nitrate reductase and glutamine synthetase activities of winter wheat. Chin J Appl Ecol 14:1529–1532
de Lobo FA, de Barros MP, Dalmagro HJ, Dalmolin ÂC, Pereira WE, de Souza ÉC, Vourlitis G, Rodriguez C (2013) Fitting net photosynthetic light-response curves with Microsoft Excel—a critical look at the models. Photosynthetica 51(3):445–456
Di Rienzo J, Balzarini M, Gonzalez L, Casanoves F, Tablada M, Robledo CW (2019) InfoStat versión 2018. Grupo infostat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar. 2019
Duursma RA (2015) Plantecophys—an R package for analysing and modelling leaf gas exchange data. PLoS ONE 10(11):e0143346
Evans JR, Clarke VC (2019) The nitrogen cost of photosynthesis. J Exp Bot 70(1):7–15
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical-model of photosynthetic CO2 assimilation in eaves of C-3 species. Planta 149(1):78–90
Flexas J, Díaz-Espejo A, Berry JA, Cifre J, Galmés J, Cifre J, Kaldenhoff R, Medrano H, Ribas-Carbó M (2007) Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. J Exp Bot 58(6):1533–1543
Gao J, Wang F, Sun J, Tian Z, Hu H, Jiang S, Dai T (2018) Enhanced Rubisco activation associated with maintenance of electron transport alleviates inhibition of photosynthesis under low nitrogen conditions in winter wheat seedlings. J Exp Bot 69(22):5477–5488
Genty B, Briantais J, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990(1):87–92
Gu Z, Gu L, Eils R, Schlesner M, Brors B (2014) Circlize implements and enhances circular visualization in R. Bioinformatics 30(19):2811–2812
Guimarães RAM, Braz AJBP, Simon GA, Ferreira CJB, Braz GBP, da Silveira PM (2017) Resposta de cultivares de feijoeiro a adubação nitrogenada em diferentes estádios fenológicos. Glob Sci Technol 10(1):136–148
Guo S, Brück H, Sattelmacher B (2002) Effects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants. Plant Soil 239(2):267–275
Harrison M, Edwards E, Farquhar G, Nicotra A, Evans J (2009) Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen-use efficiency. Plant Cell Environ 32(3):259–270
Hayashi T (2011) Analysis of factors related to enhanced sterility due to coolness at the young microspore stage under high nitrogen supply in rice (Oryza sativa L.). Bull Natl Agric Res Cent Tohoku Reg 113:67–96
Jiménez-Suancha S, Alvarado O, Balaguera-López H (2015) Fluorescencia como indicador de estrés en Helianthus annuus L. Una revisión. Rev Colomb de Cienc Hortíc 9(1):149–160
Jin X, Yang G, Tan G, Zhoo C (2015) Effects of nitrogen stress on the photosynthetic CO2 assimilation, chlorophyll fluorescence, and sugar-nitrogen ratio in corn. Sci Rep 5(9311):1–9
Koning L, Veste M, Freese D, Lebzien S (2015) Effects of nitrogen and phosphate fertilization on leaf nutrient content, photosynthesis, and growth of the novel bioenergy crop Fallopia sachalinensis cv. ‘Igniscum Candy.’ J Appl Bot Food Qual 88:22–28
Kornegay J, White JW, de la Cruz OO (1992) Growth habit and gene pool effects on inheritance of yield in common bean. Euphytica 62(3):171–180
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79:209–218
Krause GH, Winter K, Matsubara S, Krause B, Jahns P, Virgo A, Aranda J, Garcia M (2012) Photosynthesis, photoprotection, and growth of shade-tolerant tropical tree seedlings under full sunlight. Photosynth Res 113:273–285
Lau TC, Stephenson AG (1993) Effects of soil nitrogen on pollen production, pollen grain size, and pollen performance in Cucurbita pepo (Cucurbitaceae). Am J Bot 80:763–768
Lawlor D (2002) Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J Exp Bot 53:773–787. https://doi.org/10.1093/jexbot/53.370.773
Leal FT, Filla VA, Bettiol JVT, Sandrini FDOT, Mingotte FLC, Lemos LB (2019) Use efficiency and responsivity to nitrogen of common bean cultivars. Ciênc e Agrotechnol 43:1–13
Lichtenthaler HK (1987) Chlorophyls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148(34):350–382
Long S, Bernacchi C (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54(392):2393–2401
Long L, Ma G, Wan Y, Song C, Sun J, Qin R (2013) Effects of nitrogen fertilizer level on chlorophyll fluorescence characteristics in flag leaf of super hybrid rice at late growth stage. Rice Sci 20(3):220–228
Lu C, Zhang J (2000) Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci 151:135–143
Lynch J, Nubia S, Rodriguez H (1994) Photosynthetic nitrogen-use efficiency in relation to leaf longevity in common bean. Crop Sci 34:1284–1290
Martins S, Detmann K, Reis J, Pereira L, Sanglard L, Rogalski M, DaMatta F (2013) Photosynthetic induction and activity of enzymes related to carbon metabolism: insights into the varying net photosynthesis rates of coffee sun and shade. Theor Exp Plant Physiol 25(1):62–69
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence, a practical guide. J Exp Bot 51:659–668
Mi G, Chen F, Zhang F (2007) Physiological and genetic mechanisms for nitrogen use efficiency in maize. J Crop Sci Biotechnol 10:57–63
Michelangeli J, Ricaute J, Sinclair T, Rao I, Beebe S (2019) Influence of plant density and growth habit of common bean on leaf area development and N accumulation. J Crop Improv. https://doi.org/10.1080/15427528.2019.1644694
Murtaza G, Rasool F, Habib R, Javed T, Sardar K, Ayub MM, Ayub MA, Rasool A (2016) A review of morphological, physiological and biochemical responses of plants under drought stress conditions. Imp J Interdiscip Res 2:1600–1606
Peres R, Camillo M, Arf O (2004) Teor de clorofila e produtividade do feijoeiro em razão da adubação nitrogenada. Pesq Agropec Bras Brasília 39(9):895–990
Pinheiro J, Bates D, Debroy S, Sarkar D (2012) Linear and nonlinear mixed effects models. R Package Version 3(1–104):31–74
Polania J, Poschenrieder C, Rao I, Beebe S (2016) Estimation of phenotypic variability in symbiotic nitrogen fixation ability of common bean under drought stress using 15N natural abundance in grain. Eur J Agron 79:66–73
Polanıa J, Rao I, Cajiao C, Rivera M, Bodo R, Beebe S (2016) Physiological traits associated with drought resistance in andean and mesoamerican genotypes of common bean (Phaseolus vulgaris L.). Euphytica 210:17–29
Porch G, Jahn M (2001) Effects of high-temperature stress on microsporogenesis in heat-sensitive and heat-tolerant genotypes of Phaseolus vulgaris. Plant Cell Environ 24:723–731
R Development Core Team (2017) R: A language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/
Ramaekers L, Galeano CH, Garzón N, Vanderleyden J, Blair MW (2012) Identifying quantitative trait loci for symbiotic nitrogen fixation capacity and related traits in common bean. Mol Breed 31(1):163–180
Rao I, Miles J, Beebe S, Horst W (2016) Root adaptations to soils with low fertility and aluminium toxicity. Ann Bot 118:593–605
Rios VS, Rios JA, Aucique-Pérez CE, Silveira PR, Barros AV, Rodrigues FÁ (2018) Leaf gas exchange and chlorophyll a fluorescence in soybean leaves infected by Phakopsora pachyrhizi. J Phytopathol 166(2):75–85
Rosas JC (2014) El cultivo del frijol común en América tropical. Escuela Agricola Panamericana, Zamorano, p 64
Saberali S, Modarres-Sanavy S, Bannayan M, Aghaalikhani M, Haghayegh G, Hoogenboom G (2015) Common bean canopy characteristics and N assimilation as affected by weed pressure and nitrogen rate. J Agric Sci 154(04):598–611
Schulze E, Beck E, Müller K (2005) Plant ecology. Springer, Berlin, p 702
Seepaul R, George S, Wright D (2016) Comparative response of Brassica carinata and B. napus vegetative growth, development and photosynthesis to nitrogen nutrition. Ind Crops Prod 94:872–883
Shrestha S, Brueck H, Asch F (2012) Chlorophyll index, photochemical reflectance index and chlorophyll fluorescence measurements of rice leaves supplied with different N levels. J Photochem Photobiol B Biol 113:7–13
Soratto RP, Catuchi TA, Souza EDFCD, Garcia JLN (2017) Plant density and nitrogen fertilization on common bean nutrition and yield. Rev Caatinga 30(3):670–678
Suárez JC, Polanía JA, Bastidas ATC, Suárez LR, Beebe S, Rao IM (2018a) Agronomical, phenological and physiological performance of common bean lines in the Amazon region of Colombia. Theor Exp Plant Physiol 30(4):303–320
Suárez JC, Melgarejo LM, Casanoves F, Di Rienzo JA, DaMatta FM, Armas C (2018b) Photosynthesis limitations in cacao leaves under different agroforestry systems in the Colombian Amazon. PLoS ONE 13(11):e0206149
Suárez JC, Polanía JA, Contreras AT, Rodríguez L, Machado L, Ordoñez C, Beebe S, Rao IM (2020) Adaptation of common bean lines to high temperature conditions: genotypic differences in phenological and agronomic performance. Euphytica 216:28. https://doi.org/10.1007/s10681-020-2565-4
Sun L, Xu H, Hao H, An S, Lu C, Wu R, Su W (2019) Effects of bensulfuron-methyl residue on photosynthesis and chlorophyll fluorescence in leaves of cucumber seedlings. PLoS ONE 14(4):e0215486
Toso V, Andriolo JL, Lerner MA, Schmitt OJ, Cardoso FL (2017) Nitrogen in plant growth and yield of common bean. J Plant Nutr 40(14):2006–2013
Towsend A, Retkute R, Chinnathambi K, Randall J, Foulkes J, Carmo-Silva E, Murchie E (2017) Suboptimal acclimation of photosynthesis to light in wheat canopies. Plant Physiol 176(2):1233–1246
Wei T, Simko V, Levy M, Xie Y, Jin Y, Zemla J (2017) Visualization of a correlation matrix. R Packag Version 0.73 230(231):11–15
Wong SC, Cowan IR, Farquhar GD (1985) Leaf conductance in relation to rate of CO2 assimilation. I. Influence of nitrogen nutrition, phosphorus nutrition, photon flux density, and ambient partial pressure of CO2 during ontogeny. Plant Physiol 78:821–825
Yang ZB, Rao IM, Horst WJ (2013) Interaction of aluminium and drought stress on root growth and crop yield on acid soils. Plant Soil 372(1–2):3–25
Yin L, Xu H, Dong S, Chu J, Dai X, He M (2018) Strategies to improve photosynthetic nitrogen use efficiency with no yield penalty: lessons from late-sown winter wheat. bioRxiv 379552
Zhu J, Liang Y, Zhu Y, Hao W, Lin X, Wu X, Luo A (2012) The interactive effects of water and fertilizer on photosynthetic capacity and yield in tomato plants. Aust J Crop Sci 6(2):200–209
Acknowledgements
We acknowledge the financial support from the CGIAR Research Program on Grain Legumes and Dryland Cereals for the development of breeding lines of common bean. We would also like to thank all donors who supported this work through their contributions to the CGIAR Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Bavaresco.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suárez, J.C., Polanía, J.A., Anzola, J.A. et al. Influence of nitrogen supply on gas exchange, chlorophyll fluorescence and grain yield of breeding lines of common bean evaluated in the Amazon region of Colombia. Acta Physiol Plant 43, 66 (2021). https://doi.org/10.1007/s11738-021-03233-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-021-03233-1