Abstract
Sensitivity of photosynthetic processes towards environmental stress is used as a bioanalytical tool to evaluate the responses of aquatic plants to a changing environment. In this paper, change of biomass density, chlorophyll a fluorescence and photosynthetic parameters during growth phases of two microalgae Chlorella vulgaris and Scenedesmus obliquus were studied. The photosynthetic growth behaviour changed significantly with cell age and algae species. During the exponential phase of growth, the photosynthesis capacity reached its maximum and decreased in ageing algal culture during stationary phase. In conclusion, the chlorophyll a fluorescence OJIP method and the derived fluorescence parameters would be an accurate method for obtaining information on maximum photosynthetic capacities and monitoring algal cell growth. This will contribute to more understanding, for example, of toxic actions of pollutants in microalgae test.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae receive presently a lot of attention because of their potential to be used in biotechnology [13, 19]. Likewise, the assessment of risks of chemical compounds towards microalgae is one of the major concerns of environmental studies [5], because algal biomass accounts for the first step of the aquatic food chain [9]. The use of algal bioassays becomes increasingly required by authorities for the management of pollutants in discharges. Therefore, microalgae bioassays are performed in the laboratory on small populations and these ecotoxicological tests provide useful information on the effect of pollutants on aquatic plants [4].
The use of unicellular microalgae in the assessment of risks of chemical compounds has advantages compared to higher plant since algae cells exhibit high growth rates and have bigger surface contact to pollutants. Furthermore, response to different toxic pollutants varies between algae cell species [3]. In microalgae bioassay, the toxicity tests are simple and economical. However, it is decisive to have a strict and standard procedure in laboratory methods, for use in general environmental hazard evaluations.
Knowledge on time-related metabolic responses of an organism to chemical compounds may significantly contribute to understanding of toxic actions [15]. Physiological tests provide a prospective quantification of the effects of a toxic substance on algal cell communities [20]. However, photosynthesis can be considered the most important physiological process for natural ecosystems, as it provides the primary biomass production upon which all the trophic chain is based.
Photosynthesis is the process by which light energy is absorbed by light-harvesting complexes and transferred as excitation energy from water to NADPH, via the oxygen-evolving complex, Photosystem II (PSII) and Photosystem I (PSI) [12]. However, photosynthesis can be affected by environmental stress and is one of the most sensitive processes for pollutants’ effect. The sensitivity of photosynthetic processes towards environmental stress can be used for the development of bioanalytical tools to evaluate the toxicological risk of contaminants in the environment. Indeed, the analysis of changes in Chlorophyll (Chl) a fluorescence kinetics provides detailed information on the structure and function of the PSII [1, 29, 32].
Chl a fluorescence is emitted by photosynthetic organisms on a dark-to-light transition and is characterized by a polyphasic fluorescence increase. Chl a fluorescence transient depends on the redox state of the PSII reaction centres. The fluorescence intensity increases quickly from a minimum fluorescence intensity (F o) to a maximum fluorescence intensity (F M) and with two intermediate steps labelled as J (F J) and I (F I). This OJIP transient was divided into three phases (O–J, J–I and I–P) and these phases reflect three different reduction processes of the electron transport chain [25]. The O to J rise is called the photochemical phase [25] and contains information on antenna size and connectivity between PSII reaction centres [33]. The J to P rise is called the thermal phase [8]. The J to I rise can be associated with the reduction of the PQ pool [34] and I to P rise is associated with electron flow through PSI [25–27].
Microalgae play an important role in aquatic ecology as primary producers. They display high growth rates compared to macroalgae and higher plants, and furthermore they are able to acclimatize to different culture factors [ 7 ]. However, their growth rates are affected by several environmental parameters, e.g. light intensity, temperature and the nutrient composition of the culture medium. Indeed, each species has optimal conditions that must be specified to optimize their productivity. It has been reported that in limited-volume culture, ageing algal cells showed a decrease in chlorophyll content and carbohydrate, and also a decline in photosynthesis and respiration [7]. Ageing algal cells are of interest as a model system for studying physiological changes occurred in many species of algae following their growth. For instance, in algal bioassay, it is necessary to target the responses of algal cells in control condition to obtain a high reproducibility. However, it is of interest to take into account the importance of the exponential phase of algal growth in the assessment of risks of chemical compounds when photosynthesis capacity is used as a physiological parameter.
With the aim of developing a rapid method for determination of the maximum photosynthetic capacity of algal populations, this paper work was carried out to study the changes on the photosynthetic electron transport in ageing algal cells of Scenedesmus obliquus (S. obliquus) compared with the better understood Chlorella vulgaris (C. vulgaris) [18].
Materials and Methods
Biological Material
The microalga Chlorella vulgaris and Scenedesmus obliquus were obtained from the Canadian Phycological Culture Center (CPCC, University of Waterloo, Canada). The algal cells were cultured in sterilized TAP medium (Tris-Acetate-Phosphate). The algal cells were grown under continuous and constant light intensity (100 µmol m−2 s−1, SYLVANIA GRO-LUX Wide Spectrum light F40/GRQ/AQ/WS) at 20 ± 2 °C. The stock culture was aerated with bubbling air. In this experiment, the growth of each algal species was assessed over 20 days. The algal biomass was measured by changes in optical density at 750 nm using a Perkin-Elmer Lambda 40 UV/VIS spectrophotometer.
Chlorophyll a Fluorescence Transient
Chl a fluorescence OJIP measurements were conducted at room temperature with a portable fluorimeter (Plant Efficiency Analyser, built by Hansatech Instruments Ltd. King’s Lynn Norfolk, UK). The algal cells in Erlenmeyer flask were kept in dark for 15 min before the Chl a fluorescence measurements were started. This to allow the PSII reaction centres to open (re-oxidize) and the electron transport chain to be fully oxidized [32]. Then 5 ml of algal culture was uniformly placed on glass fibre filter (Millipore #AP20 013 00) using low-pressure filtration to avoid physiological stress effects. The measurement consisted of a single strong 1 s light pulse (3500 µmol photons m−2 s−1, peak 650 nm) provided by an array of six light-emitting diodes in the Plant Efficiency Analyser instrument [32].
Chl a Fluorescence Parameters
From the OJIP transient measured during the 1 s of illumination of dark adapted samples, several expressions and fluorescence parameters leading to a description of a photosynthetic sample in a given physiological state were calculated [33]. In this study, F V/F o = k P/k N, φ Po = F V/F M = k P/(k P + k N), ψ Eo (= 1−V J), the antenna size of PS II as ABS/RC, the performance index PIabs and the quantum yield of reduction of end acceptors (RE) of PSI as φ Ro = REo/ABS parameters were analysed [22].
φ Po corresponds to the maximum yield of primary photochemistry of PSII, or in other words it corresponds to the efficiency by which an absorbed photon will be trapped by PSII reaction centres [16]. The F V/F o parameter corresponds to the relative contribution of the trapping flux to the total de-excitation fluxes of excited chlorophyll [14]. The absorption of photons (ABS) per active reaction centre (RC) (ABS/RC ratio) parameter showed change in antenna size.
ψ Eo (=1−V J) is the fraction of electrons transported beyond Q −A per exciton trapped by the open reaction centres (RC) of PSII. It is the probability that the energy of a trapped exciton is used for electron transport beyond Q A.
PIabs is defined as the capability or potential to do work and it provides quantitative information about the vitality state of the photosynthetic organisms [33].
PIABS is expressed as [γ o/(1−γ o)].[φ Po/(1−φ Po)].[ψ Eo/(1−ψ Eo)] and γ o/(1−γ o) is estimated by the JIP test as equal to the ratio of reaction centres and the light absorbance flux (RC/ABS).
Finally, the quantum yield of reduction of end acceptors (RE) of photosystem I (PSI) is expressed as
V t is defined as (F t − F o)/(F M − F o) and this expression can be taken as a measure of the fraction of the primary quinone electron acceptor of PS II in its reduced state [Q −A /Q A (total)].
δ Ro is the efficiency with which an electron can move from the reduced intersystem electron acceptors to the PS I end electron acceptors.
All fluorescence parameters derived from Chl a fluorescence transient were analysed by JIP test model. This JIP test uses the theory of energy fluxes in biomembranes to calculate several phenomenological and biophysical expressions for a given physiological state [30].
The experiments were done in five replicates for each measurement. Means and standard deviations were calculated for each measure.
Results and Discussion
Figure 1 shows the growth rate of C. vulgaris and S. obliquus population. The time biomass growth consists of an exponential phase followed by a stationary phase. C. vulgaris cells reached the stationary phase in 15 days from inoculation, while S. obliquus grew exponentially 10 days to reach stationary phase.
Optical density at 750 nm of C. vulgaris and S. obliquus cultured in sterilized TAP medium (Tris-Acetate-Phosphate) under 100 µmol m−2 s−1 continuous and constant light intensity and at 20 ± 2 °C. The arrows represent the time when the Chl a fluorescence measurements were performed. Experiments were carried out five times
The measurement of in vivo Chl a fluorescence transient was used to evaluate the overall photosynthetic performance in different growth phases of C. vulgaris and S. obliquus (Fig. 2). The shape of the OJIP fluorescence transients recorded in ageing algal cells during stationary phase (15 and 20 days after inoculation) of both species differed from those recorded in the exponential phase of cell growth (3 and 6 days after inoculation). The decrease of fluorescence levels at phases J and I is explained by the inhibition of the electron transport at the donor side of PSII, which results in the accumulation of P680+, a strong fluorescence quencher [12]. It has been recognized that Chl a fluorescence transitions F J (fluorescence intensity at 2 ms), F I (fluorescence intensity at 30 ms) and F M (maximal chlorophyll fluorescence intensity) are dependent on photosynthetic electron transport of PSII reaction centres, reduction of PSII primary electron acceptor Q A and PQ pool [27]. Therefore, fluorescence yield of the rapid fluorescence induction was quenched and the photosynthetic electron transport appeared to be affected. However, growth phases of algal cells may affect the conversion of light energy into photosynthetic electron transport and change the rapid rise of Chl a fluorescence OJIP [33]. Further, a decrease in F M and an increase in F o (level of fluorescence when all the primary quinone acceptors Q A are in the oxidized state) were observed in the OJIP transients of the two algal species in ageing algal cells and then a decrease in F V/F o = k P/k N (Fig. 2). In C. vulgaris, ageing algal cells affect the conversion of light energy into photosynthetic electron transport and then change the Chl a fluorescence rise. Indeed, transitions J, I and P were distinguishable for C. vulgaris at 5, 6 and 9 days after inoculation, respectively. However, the fluorescence yield of the rapid fluorescence induction was quenched at 15 and 20 days after inoculation (Fig. 2). The suppression of the J–I phase of fluorescence rise kinetic showed a weakened electron donation from the oxidizing side [11]. On the other hand, in ageing algal cells of S. obliquus the suppression of the J–I phase occurred at 9 days after inoculation. These change in S. obliquus indicated a strong inhibition of electron transport from water splitting system toward PQ pool [23].
The increase of F o observed in both algae species in Fig. 2 has been attributed to physical separation of the PSII from associated pigment antennae, resulting in an inhibition of electron transfer on the reductant side of PSII [6]. Furthermore, an increase of F o has been reported to phosphorylation of light-harvesting complexes of PSII (LHCII) that caused dissociation of LHC from PSII in order to protect plants from excessive light [21]. In a previous study, Tóth et al. [34] showed that an increase of F o was related to an accumulation of reduced plastoquinone (PQ) and this accumulation of reduced PQ was found to induce LHCII phosphorylation in response to abiotic stresses [21]. Indeed, LHCII are important in energy redistribution between PSII and PSI for performing optimal photosynthesis.
The decrease of F M observed in C. vulgaris at 15 and 20 days after inoculation (Fig. 2) could be due to a strong inhibition of the PSI acceptor side [25]. However, the state transitions could represent a process that can explain this decrease of F M. Schansker et al. [26] reported that a smaller PSII antenna size could slow down the reduction of the PQ pool and also would result in a lower F M level. However, a lower F M level was not observed in S. obliquus compared to C. vulgaris. But this can be explained by a significant increase of F o. Furthermore, the suppression of the I–P phase of fluorescence rise kinetic already from the 9 days after inoculation indicates a decrease in PSI activity [25].
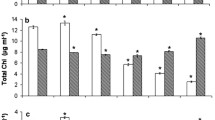
Changes in the fluorescence parameters derived from OJIP transient in ageing cultures are presented in Fig. 3. During ageing algal cells, a decrease of the maximum yield of primary photochemistry of PSII (F V/F M) was observed. F V/F M declined by 62 and 71 % after 20 days, respectively, in C. vulgaris and S. obliquus compared to the values of the first measurement (3 days after inoculation). As seen in Fig. 3, after 20 days, F V/F o decreased by 84 and 89 %, respectively, in C. vulgaris and S. obliquus compared to the values calculated on the third day after inoculation. The decrease in F V/F o reflects a decrease in the relative contribution of the trapping flux to the total de-excitation fluxes of excited chlorophyll and may indicate as well a blocking of electron transport after Q −A [14]. According to the data presented in Fig. 3, the decrease of the fraction of active reaction centres (manifested as an increase of ABS/RC) was observed over the growth phases of algal cells. For example, active reaction centres decreased by 3.6 and 3.4 times, respectively, in C. vulgaris and S. obliquus compared to the values in the first measurement. The change ψ Eo (1 − V J) parameter which is related to the balance between efficiency and inefficiency of the dark reactions after Q −A significantly declined by 37 and 65 %, respectively, in C. vulgaris and S. obliquus after 20 days. This result indicated that in ageing algal cells for both species the redox reaction after Q A was inhibited due to interruption of electron flow from Q A to Q B. The performance index PI is used to quantify the PSII behaviour and as measures of the performance up to the reduction of PSI end electron acceptors. Here, PI parameter deceased later in stationary phase (after 20 days) by 98 and 99 %, respectively, in C. vulgaris and S. obliquus compared to PI calculated during the exponential phase (after 3 days). The change in the quantum yield with which electrons reduce the PSI end electron acceptors (φ Ro) (fluorescence parameters related to PSI activity) is shown in Fig. 3. This fluorescence parameter decreased in ageing algal culture. Such results indicated that in ageing algal cells electron flow at the PSI acceptor side was affected. φ Ro parameter decreased later in stationary phase (after 20 days) by 72 and 83 %, respectively, in C. vulgaris and S. obliquus.
Changes in the relative contribution of the trapping flux to the total de-excitation fluxes of excited chlorophyll (F V/F o), the maximum quantum yield of PSII (F v/F m), the absorption of photons (ABS) per active reaction centre (RC) (ABS/RC), electrons transported beyond Q A per exciton trapped by the reaction centres (RC) of PSII (ψ Eo), the performance index (PI) and the quantum yield of reduction of end acceptors (φ Ro) of photosystem I. The mean and SD from five samples are given
The growth phase of algal cells also has been found to have a significant impact on photosynthetic response of both algae species. This is consistent with the work of Kulandaivelu and Senger [17]. These authors have reported that S. obliquus cultures when grown heterotrophically for 10 or 30 days showed 85 and 98 % loss of their photosynthetic capacity, respectively. Earlier, Samuelsson and Öquist [24] reported that when the algal culture gradually reached the stationary growth phase, the rate of photosynthesis decreased. The loss of activity in ageing cultures might be caused by the depletion of certain of the nutrient substances and the increase of metabolic toxins in the culture medium [28]. Moreover with increasing algal cell biomass, light availability may become a limiting factor, causing changes in photosynthetic parameters. Indeed, the small availability of light might cause a decrease of the electron flow in photosynthesis [28]. It is worth to note that the results presented here show that the sensitivity of Chl a fluorescence change depends on growth phase for both algae species and the maximum photosynthetic performance was reached during the exponential growth phase and then gradually decreased during stationary growth phase. In a previous study, fingerprints of excitation spectra of Chl fluorescence were used to differentiate ‘spectral groups’ of different groups of microalgae in vivo and in situ [2]. Here, comparison of the growth phase in both algae species was also described by dynamic energy pipeline of the photosynthetic apparatus [31, 33] (Fig. 4). This energy flux model deals with the phenomenological energy fluxes (per cross-section). ETo/CS indicates electron transport in a PS II cross-section and is related to the re-oxidation of reduced Q A. A decrease in ETo/CS due to inactivation of reaction centre was observed in both species [10]. Indeed, the number of active reaction centres (RC/CS) decreased (indicated as open circles in Fig. 4) and the damage, for example, at 15 days after inoculation was drastic in C. vulgaris compared to S. obliquus. This is evident from the larger changes in the sizes of DIo/ABS and ETo/CS and an increase in inactive centres. In fact, an increase in the number of inactive RCs is associated with the increased efficiency of dissipation energy as heat (DIo/ABS). This increase in DIo/ABS indicates the efficiency of non-photochemical de-excitation processes. It is of interest to mention that while the number of active RCs was higher in S. obliquus, the efficiency of ETo/CS was efficient in C. vulgaris. In other words, regulation of PSII in C. vulgaris is accomplished by lower active RCs.
Energy pipeline model of phenomenological fluxes (per cross-section, CS) in ageing algal cells. The value of each parameter is presented with relative changes in width of each arrow. TR/CS, trapped energy flux per cross-section. ET/CS, electron transport flux per cross-section. DI/CS, dissipated energy flux per cross-section. Active reactions centres (RCs) are shown as open circles and inactive RCs are closed circles
In conclusion, change in PSII photochemistry during algal growth phases differed among both species in ageing algal cells. Significant reduction of the studied fluorescence parameters and the change in the Chl a fluorescence rise were observed in S. obliquus compared to C. vulgaris. In limited-volume culture, C. vulgaris presents the best photosynthetic growth behaviour. However, different mechanisms might be attributed to the reduction of the photosynthetic growth behaviour.
Abbreviations
- ABS:
-
Absorption
- Chl a :
-
Chlorophyll a
- ETo :
-
Energy flux for electron transport
- F o (F 50μs) and F M :
-
Initial and maximum Chl a fluorescence
- F 2ms :
-
Chl a fluorescence measured at 2 ms
- F V :
-
Maximum variable Chl fluorescence
- PIABS :
-
Photosynthetic performance index
- PS II:
-
Photosystem II
- k P :
-
Photochemical de-excitation rate constant in the excited antennae of energy fluxes for photochemistry
- k N :
-
Non-photochemical de-excitation rate constant in the excited antennae for non-photochemistry
- φ Ro :
-
Quantum yield with which electrons reduce the PSI end electron acceptors
- δ Ro :
-
Efficiency with which an electron can move from the reduced intersystem electron acceptors to the PSI end electron acceptors
- ψ Eo :
-
Efficiency with which a trapped exciton can move an electron into the electron transport chain
- Q A and Q B :
-
Primary and secondary quinone electron acceptors of photosystem II, respectively
- RC:
-
Reaction centre
- TRo :
-
Energy flux for trapping
- OJIP transient:
-
Fluorescence induction transient defined by the names of its intermediate steps
- P700:
-
primary electron donor of photosystems I
- PQ:
-
Plastoquinone
- V I :
-
Relative variable Chl a fluorescence at the I-step
- V J :
-
Relative variable Chl a fluorescence at the J-step
- V t :
-
Relative variable Chl a fluorescence at time t
References
Antal TK, Matorin DN, Ilyash LV, Volgusheva AA, Osipov V, Konyuhov IV, Krendeleva TE, Rubin AB (2009) Probing of photosynthetic reactions in four phytoplanktonic algae with a PEA fluorometer. Photosynth Res 102:67–76
Beutler M, Wiltshire KH, Meyer B, Moldaenke C, Lüring C, Meyerhöfer M, Hansen UP, Dau H (2002) A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth Res 72:39–53
Boyle TP (1984) The effect of environmental contaminants on aquatic algae. In: Shubert LE (ed) In algae as ecological indicators. Academic Press, New York, pp 237–256
Cairns J Jr, Niederlehner BR (1995) Predictive ecotoxicology. In: Hoffman DJ, Rattner BA, Burton GA, Cairns J Jr (eds) Handbook of ecotoxicology. CRC Press, Boca Ratón, pp 667–680
Christensen ER, Nyholm N (1984) Ecotoxicological assays with algae: Weibull dose-response curves. Environ Sci Technol 18:713–718
Costa ES, Smith RB, Oliveira JG, Campostrini E, Pimentel C (2002) Photochemical efficiency in bean plants (Phaseolus vulgaris L. and Vigna unguiculata L. Walp) during recovery from high temperature stress. Braz J Plant Physiol 14:105–110
Costache TA, Fernández FGA, Morales MM, Fernández-Sevilla JM, Stamatin I, Molina E (2013) Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Appl Microbiol Biotechnol 97:7627–7637
Delosme R (1967) Étude de l’induction de fluorescence des algues vertes et des chloroplastes au début d’une illumination intense. Biochim Biophys Acta 143:108–128
Field CB, Behrenfeld MJ, Randenon JT, Fakowski PG (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Force L, Critchley C, van Rensen JS (2003) New fluorescence parameters for monitoring photosynthesis in plants. Photosynth Res 78:17–33
Govindachary S, Bukhov NG, Joly D, Carpentier R (2004) Photosystem II inhibition by moderate light under low temperature in intact leaves of chilling-sensitive and –tolerant plants. Physiol Plant 121:322–333
Govindjee S (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 34:1073–1079
Harun R, Singh M, Forde GM, Danquah MK (2010) Bioprocess engineering of microalgae to produce a variety of consumer products. Renew Sustain Energ Rev 14:1037–1047
Havaux M, Strasser RJ, Greppin H (1991) A theoretical and experimental analysis of the qP and qN coefficients of chlorophyll fluorescence quenching and their relation to photochemical and nonphotochemical events. Photosynth Res 27:41–55
Kluender C, Sans-Piché F, Riedl J, Altenburger R, Härtig C, Laue G, Schmitt-Jansen M (2009) A metabolomics approach to assessing phytotoxic effects on the green alga Scenedesmus vacuolatus. Metabolomics 5:59–71
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kulandaivelu G, Senger H (1976) Changes in the reactivity of the photosynthetic apparatus in heterotrophic ageing cultures of Scenedesmus obliquus. I. Changes in the Photochemical Activities. Physiol Plant 36:157–164
Kumar MS, Miao ZH, Wyatt SK (2010) Influence of nutrient loads, feeding frequency and inoculum source on growth of Chlorella vulgaris in digested piggery effluent culture medium. Bioresour Technol 101:6012–6018
McGinn PJ, Dickinson K, Park KC, Whitney CG, MacQuarrie SP, Black FJ, Frigon JC et al (2012) Assessment of the bioenergy and bioremediation potentials of the microalga Scenedesmus sp. AMDD cultivated in municipal wastewater effluent in batch and continuous mode. Algal Res 1:155–165
Navarro E, Guasch H, Sabater S (2002) Use of microbenthic algal communities in ecotoxicological tests for the assessment of water quality: the Ter river case study. J Appl Phycol 14:41–48
Nellaepalli S, Kodru S, Malavath T, Subramanyam R (2012) Anaerobiosis induced state transition: a non-photochemical reduction of PQ pool mediated by NDH in Arabidopsis thaliana. PLoS ONE 7:e49839
Oukarroum A, Bussotti F, Goltsev V, Kalaji HM (2015) Correlation between reactive oxygen species production and photochemistry of photosystem I and II in Lemna gibba L. plants under salt stress. Environ Exp Bot 109:80–88
Oukarroum A, Schansker G, Strasser RJ (2009) Drought stress effects on photosystem I content and photosystem II thermotolerance analyzed using Chl a fluorescence kinetics in barley varieties differing in their drought tolerance. Physiol Plant 137:188–199
Samuelsson G, Öquist G (1977) A method for studying photosynthetic capacities of unicellular algae based on in vivo chlorophyll fluorescence. Physiol Plant 40:315–319
Schansker G, Srivastava A, Govindjee S, Strasser RJ (2003) Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct Plant Biol 30:785–796
Schansker G, Tóth SZ, Strasser RJ (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim Biophys Acta 1706:250–261
Schreiber U, Neubauer C, Klughammer C (1989) Devices and methods for room temperature fluorescence analysis. Philos Trans R Soc Lond B 323:241–251
Sigaud-Kutner TCS, Pinto E, Okamoto OK, Latorre LR, Colepicolo P (2002) Changes in superoxide dismutase activity and photosynthetic pigment content during growth of marine phytoplankters in batch-cultures. Physiol Plant 114:566–571
Stirbet A, Govindjee S (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B 104:236–257
Strasser RJ (1986) Mono-bi-tri and polypartite models in photosynthesis. Photosynth Res 10:255–276
Strasser RJ (1987) Energy pipeline model of the photosynthetic apparatus. In: Biggins J (ed) Progress in photosynthesis research, vol 2. Maritinus Publishers, Dordrecht, pp 717–720
Strasser RJ, Srivastava A, Govindjee S (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:32–42
Strasser RJ, Srivastava A, Tsimilli-Michael M (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee S (eds) Chlorophyll fluorescence a signature of photosynthesis, advances in photosynthesis and respiration series. Kluwer Academic Publishers, Dordrecht, pp 321–362
Tóth SZ, Schansker G, Strasser RJ (2007) A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynth Res 93:193–203
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he have no conflict of interest.
Ethical standard
This article does not contain any studies with human participants or animals performed by the author.
Rights and permissions
About this article
Cite this article
Oukarroum, A. Change in Photosystem II Photochemistry During Algal Growth Phases of Chlorella vulgaris and Scenedesmus obliquus . Curr Microbiol 72, 692–699 (2016). https://doi.org/10.1007/s00284-016-1004-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-016-1004-1