Abstract
Sugarcane (Saccharum officinarum L.) is an effective crop for biomass production and is used mainly for sugar production and bio-fuel. Irrigation water has become less available in many regions due to global climate change and domestic. To confront the challenge of water utilization, there is a need to develop water-saving irrigation techniques in order to maximize crop water use efficiency. Partial rootzone drying (PRD) is a water-saving irrigation strategy that involves irrigating only part of the rootzone while leaving the other portion to dry to a predetermined level before the next irrigation. The objective of this study was to examine whether PRD affects photosynthetic capacity and growth in young sugarcane plants (48-day old). The experiment was conducted from January to April, 2011 in a greenhouse. Sugarcane mini-stalks were transplanted with divided root systems at 20 days of age into two pots. The pots were filled with soil (oxisol), sand and manure (1:1:1). The sugarcane plants were well-watered in the first 10 days after transplanting. Thereafter, the plants were exposed to three irrigation regimes: (1) Full irrigation (FI) (control); in which both soil compartments were watered to 100 % field capacity; (2) PRD; in which one soil compartment was watered to the field capacity while the other was allowed to dry for 17 days, then the plants were re-irrigated; (3) no irrigation (NI); in which both compartments were allowed to dry for 17 days, then re-irrigated. Net photosynthetic rate, stomatal conductance, transpiration, predawn leaf water potential, leaf area, shoot dry weight, root dry weight, root volume, and intrinsic water use efficiency were measured. PRD did not significantly reduce growth and gas exchange in comparison to FI, yet there was a 17.6 % reduction in water application. In addition, the agronomic water use efficiency was higher in PRD (4.1 g l−1) and FI (3.6 g l−1) than NI (2.9 g l−1). In this study, PRD irrigation reduced water consumption by 17.6 % with a total biomass reduction of 11.3 % as compared with fully watered plants. In conclusion, PRD may be an efficient irrigation strategy and promising for application in drought-prone regions for saving water where sugarcane is produced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In Brazil most sugarcane is cultivated in the dry season. However it is known that well-designed irrigation for this crop can generate significant yield increases. Currently, the demand for increased biofuel resources is accelerating the need for increase sugar cane productivity. In order to achieve greater productivity, an understanding of water management effects on productivity are needed, especially the effect of water availability on the photosynthetic process.
According to UNICA (2014), sugarcane production will have a lower harvest (2014/2015) compared to the previous year (2013/2014). The projection indicates a cane crush of 580.00 million tons, a 16.94 million ton reduction from the 2013/2014 crush of 596.94 million tons. The reasons were various but the climate was the main factor for the reduction in production primarily due to the occurrence of drought. The projection reflects an increase in the production area available for harvesting but a significant drop in agricultural yields stemming from a long period of drought stretching from the end of 2013 into early 2014. The reduction in sugarcane production shows the importance of irrigation to increase productivity. Nevertheless, irrigated agriculture is responsible for the use of 69 % of the water consumed in the world, and the limited water resources in the context of global warming has made it necessary to increase water use efficiency (WUE) for crops and optimize irrigation systems (Sampaio et al. 2010).
One of the tools to increase WUE is partial rootzone drying (PRD). This technique irrigates only one side of the root system while the remaining root system is exposed to water restriction. In this type of management, water is either applied to only one side of the root system or it is applied alternately to the dry and irrigated sides of the rootzone (Kang and Zhang 2004). It is important that one portion of the rootzone receives a moderate water shortage while the other portion maintains an adequate water balance in the plant. Liu et al. (2006) and Sobeih et al. (2004) verified that in the PRD treatment, the leaf water potential should remain constant.
Dry and Loveys (1998) using PRD demonstrated in grape that it is possible to increase the water use efficiency and restrict plant growth but maintain production, although lower, at a satisfactory level. The theoretical base of the technique is that the rootzone under moderate stress produces a greater quantity of chemical signals (Wilkinson 1999; Stoll et al. 2000). The hypothesis is that plant hormones translocate via the xylem to the shoot and thus causes partial stomata closing. This fact results in reduced water loss to the atmosphere (Davies and Zhang 1991; Davies et al. 2002), because under constant vapor pressure deficit between the leaf and the air, transpiration is proportional to the stomata conductance. In this way the partial reduction in the stomata opening can decrease water loss with a minimum effect on the photosynthetic process (Jones 1992), and thus does not reduce plant productivity. Partial reduction in stomata conductance at the start of water stress reduces transpiration more significantly than reducing the internal CO2 concentration (Morison et al. 2008). This response is based on the non-linear relationship between carbon assimilation and stomata conductance (gs) and a reduction in gs from 1.6 to 0.6 mol m−2 s−1 does not modify the net photosynthetic rate (A), resulting in an increase in the intrinsic water use efficiency [iWUE (A/gs)] (Morison et al. 2008, Sepaskhah and Ahmadi 2010). In potato plants, A was less sensitive in the PRD treatment than gs when compared to completely irrigated treatment. Consequently, iWUE increased in the PRD treatment with a linear increase in iWUE when gs decreased (Liu et al. 2006).
The effects of PRD on photosynthetic assimilation depends on the genotype, climatic conditions (Zegbe and Behboudian 2008), root volume and substrate drying rate. The reports in the literature on the effects of PRD on photosynthetic carbon assimilation are contradictory, which illustrates that the effects of PRD on the photosynthetic capacity vary according to the experimental model applied.
There was no reduction in the net photosynthetic rate due to PRD treatment in cotton (Du et al. 2006), sweet pepper (Kang et al. 2001), corn (Du et al. 2010), grapes (Du et al. 2008), potato (Liu et al. 2008), tomato (Campos et al. 2009) and apple (Zegbe and Behboudian 2008), when compared with the treatment where the rootzone was irrigated to field capacity (FI). However, other studies have shown that plants under PRD conditions present lower A values compared to plants cultivated in the FI treatment (Liu et al. 2006; Kirda et al. 2005, Yuan et al. 2013).
In addition to the effects of PRD on the photosynthetic process, this technique may reduce the shoot growth in some species by chemical signaling associated with abscisic acid (ABA) in the leaf or between the root and the shoot (Davies et al. 2002; Saeed et al. 2005; Liu et al. 2006; Kim et al. 2010). Other studies have shown that PRD reduces leaf expansion in vines (Stoll et al. 2000), cotton (Tang et al. 2005) and other species (Kang and Zhang 2004). However, this irrigation technique had a beneficial effect on root growth, in corn (Kang et al. 1998) and in tomato (Mingo et al. 2004) in which PRD stimulated lateral root growth. Wang et al. (2012) showed that, compared to the deficit irrigation (DI) and full irrigation (FI) treatments, the PRD treatment increased growth in the rootzone and this greater increase may have contributed to a greater nitrogen concentration observed in the leaves of corn plants.
There is insufficient literature on the effects of the PRD technique in sugarcane and the effects of PRD on the photosynthetic process and water relationships. The objective of the present study was to measure the initial growth, photosynthetic capacity and water use efficiency in sugarcane in response to PRD. These data will be the basis for future studies to use PRD in commercial plantations in an attempt to maintaining productivity while increasing water use efficiency in sugarcane.
2 Materials and methods
2.1 Experimental setup and irrigation treatments
The experiment was performed in a greenhouse between January and April 2011 on the campus of the State University of Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes-RJ (Lat: 21°45′15″S; Long:41°19′28″W). Sugarcane mini-stalks (RB867515) with a shoot were transplanted to trays and 20 days later the seedlings were transferred to 12 l pots, joined one to another so that the root system was equally divided but with no moisture exchange between pots.
The sugarcane plants were well-watered (100 % field capacity) and each plant received ≈0.587 l water per day (0.294 l each side of the rootzone) during 48 days after planting. Thereafter, the plants were exposed to three irrigations regimes: (1) FI: in which both sides of the rootzone were maintained at 100 % field capacity for 22 days (soil water potential ≈ 0 kPa); (2) PRD: one component of the rootzone was maintained at field capacity while the other portion of the rootzone was maintained without water for 19 days and then re-irrigated); and (3) NI: where irrigation was suspended for 19 days on both portions of the rootzone, and thereafter, the plants were re-irrigated. The 19th day without water in the soil was considered the day with maximum stress (soil water potential ≈−100 kPa) in which the net photosynthetic rate of the leaves of the NI treatment was not detectable.
The quantity of water applied to each treatment was controlled using a 500 ml cylinder. In the FI and PRD treatments, water was applied until drainage was observed from the pot. At this point, the volume was recorded for calculation of consumption, water saving and water use efficiency.
2.2 Microclimatological variables
The microclimatological variables (minimum, mean and maximum values) of air temperature (T, °C), relative air humidity (RH, %) and the photosynthetic photon flux (PPF) in the greenhouse were monitored using a mini meteorological station model 450 (Spectrum Technologies, Inc., Illinois, USA). The vapor pressure deficit of the air (VPD) was calculated from the air temperature and RH data, according to Jones (1992) (Fig. 1).
Air temperature (T, °C), air relative humidity (UR %), maximum photosynthetic photon flow (PPF), and air vapor pressure deficit (VPDair) inside the greenhouse. The sugarcane (Saccharum spp.) plants were grown in splitroot pots under three different water regimes: full irrigated (FI), non-irrigated (NI), and partial rootzone drying (PRD). The arrows indicate the start of the treatments
2.3 Soil moisture
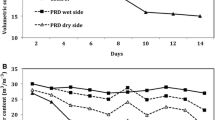
Soil water potential (kPa) was measured with sensors (model 6450 WD; Watermark Soil Moisture Sensor, Spectrum Technologies, USA) installed 15 cm deep and about 13 cm from the edge of the pot. The sensors were attached to a Watchdog 200 data collector (Spectrum Technologies, Inc., Illinois, USA) and the information was recorded at 30 min intervals (Fig. 2).
Soil water potential in each side of splitroot pots with greenhouse-grown sugarcane (Saccharum spp.) under partial rootzone drying (PRD) treatment during the experimental period. The arrow indicates the time of re-irrigation. The FI and NI treatment had ≈0 and ≈−100 kPa of soil water potential in maximum water stress, respectively. The soil water potential of NI treatment had the same decline of PRD dry side
2.4 Growth and gas exchange characteristics
The growth parameters were measured at the end of the experimental period [(22 days after applying the treatments (DAAT)]. The leaf area was determined using a bench meter LI-3100 area meter (Li-Cor, USA). The shoot dry mass (SDM) and root dry mass (RDM) were determined by weighing these components after drying in a oven at 70 °C until constant weight. The root volume was measured on each side of the pot using a 2 L cylinder. For this, the cylinder was filled with a known volume of water (V1), after inserting the roots, the new volume was observed (V2), and the difference was calculated as the root volume in ml (Volroot = V2 − V1).
Leaf gas exchange was measured every 2 days after applying the treatments using leaves +1 and +2, following the classification system by Kujiper (Dillewijn 1952; Gallo et al. 1962), until the end of the experiment.
The net photosynthetic rate (A), stomatal conductance (gs), transpiration (E) were determined with an infrared gas analyzer (IRGA), model LI-6400 (LI-COR, Lincoln, NE, USA) at 8:00–10:00 h. A 1,200 µmol m−2 s−1 light intensity was used on a 6 cm2 leaf surface, using an artificial lighting system composed of a mix of blue and red light-emitting diodes coupled to the equipment. The CO2 concentration inside the chamber was 314 ± 46 μmol mol−1, T and RH were 31 ± 2.3 °C and 39 ± 3 %, respectively. The A, gs and E data were used to obtain the intrinsic water use efficiency (iWUE) and the water use efficiency (WUE). The variables iWUE and WUE were obtained from the slope of the linear curve between A versus gs and A versus E, respectively.
The agronomic water use efficiency (AWUE) was estimated by dividing the total dry matter of the plant (SDM + RDM) by the quantity of water applied in each treatment. To calculate the water saving in the PRD and WI treatments compared to the control, the total water applied to the plants of the control treatment was considered 100 %. Water applied to the treatment = X (%); thus: X = water applied to the treatment * 100/water applied to the control.
Growth and gas exchange data were analyzed using ASSISTAT 7.6 beta software in a completely randomized design with 7 replications. An analysis of variance followed by Tukey’s test, at p < 0.05, was used to compare the treatments. Regression analysis used SAS PROC REG (SAS, Inc. Cary, NC) to test slope differences.
3 Results
3.1 Growth measurements
The sugarcane plants in the PRD treatment were not significant different from FI in growth parameters (Tables 1, 2). Conversely, plants in the NI treatment were significantly lower than FI and PRD, with a 59 % reduction compared to the FI (Table 1). Plants in the NI treatment had reduced shoot dry mass, root dry mass and root/shoot dry mass ratio, and root volume compared to the FI and PRD treatments (Tables 1, 2). The PRD root volume without irrigation was 48.8 % of the root volume of the irrigated rootzone (Table 2).
3.2 Gas-exchange and water use efficiency measurements
The PRD treatment did not reduce the A, gs or E compared to FI. The NI treatment decreased net photosynthetic rates, stomatal conductance and transpiration until 19 DAAT (days after applying the treatments). This reduction was associated with stomata closure on 13 DAAT. After re-irrigation, the non-irrigated plants recovered stomatal conductance and transpiration to the same values as in the other treatments (Figs 3A–C).
Net photosynthetic rate (A), stomatal conductance (B), transpiration (C) of greenhouse-grown sugarcane (Saccharum spp.) in splitroot pots under three different water regimes: full irrigated (FI), non-irrigated (NI), and partial rootzone drying (PRD). Each symbol represents the mean of seven replications Means followed by the same letters do not differ statistically by the Tukey test at 5 % probability. The arrows indicate the time of re-irrigation
The NI treatment had the lowest water use (35.6 l), with a 29.3 % reduction compared to the FI treatment (50.4 l) while the PRD treatment had a 17.6 % reduction in water use (Table 3). AWUE of PRD (4.1 g DM l−1) was 12.2 % greater than in the FI treatment (3.6 g DM l−1) and significantly greater than NI.
The water use efficiency (WUE) derived from the slope of the A/E curve was significantly higher (p < 0.05) for the NI treatments (4.36 µmol CO2 mmol H2O−1) compared to the FI treatment (3.05 µmol CO2 mmol H2O−1) and PRD (2.9 µmol CO2 mmol H2O−1) (data not showed). iWUE (A/gs), of the NI treatment (103.8 µmol CO2 mol H2O−1) and PRD treatment (89.03 µmol CO2 mol H2O−1) did not differ (p < 0.05) from FI treatment (83.06 µmol CO2 mol H2O−1)(Fig. 4).
Net photosynthetic rate dispersion (A) versus transpiration (E) (water use efficiency—WUE) and stomata conductance (gs) (intrinsic water use efficiency—iWUE) of greenhouse-grown sugarcane (Saccharum spp.) in splitroot pots under three different water regimes: full irrigated (FI), non-irrigated (NI), and partial rootzone drying (PRD). Each point represents one unit of data collected over the experimental period
3.3 Leaf water potential measurement
Leaf water potential was affected by the NI treatment (Table 4). At 0 DAAT, there were no treatment differences; at DAAT 19 the leaf water potential on the day on which the net photosynthetic rate of the NI treatment reached value equal to 0 µmol m−2 s−1 and one day after re-irrigation was significantly different from FI and PRD.
3.4 Water use efficiency and biomass production
Analysis of covariance for total water use during the treatment period, using final leaf area as the covariate, indicated that the FI and NI treatments were not different but the PRD treatment was significantly lower (P = 0.05) than either FI or NI (Fig. 5). These results demonstrate that the PRD treatment had reduced water use at equivalent leaf areas to the FI based on slope values of 11.3 vs 29.3, respectively. While there were no treatment differences, total dry mass production (shoot + root) was linearly related to water consumption during the study period and there was a production of 4.7 g of dry mass per liter of water consumed by the plant (Fig. 6). The maximum and minimum production of total dry mass was 225 and 90 g for FI and NI treatments, respectively.
4 Discussion
In this present study, we have shown that localized application of water to the root system of young plants of sugarcane can be effective in maintaining the productivity of plants, without significant effects on gas exchange in leaf water potential, and agronomic efficiency water usage. However, irrigation in only a part of the root system did not affect the intrinsic water use efficiency. This fact shows that the technique can be an alternative to saving water and can used in young plants of sugarcane.
Sugarcane in the PRD treatment did not have a significant reduction in leaf area or transpiration but used less water (41.8 l) than the FI treatment (50.4 l) (Table 4). Actually, when there is a water deficit, the reduction in turgor pressure and ABA signaling from the root (Davies et al. 2002) or the leaves (Kim et al. 2010) can cause a reduction in the leaf area. Munns and Cramer (1996) have showed that under certain conditions, ABA can cause decreases in shoot growth, and with a smaller leaf area, a decrease can occur in whole-canopy transpiration that effectively conserves the water supply in the soil for a longer period (Wakrim et al. 2005). However, Similar results were obtained by Huitziméngari et al. (2009) with tomatoes in which 30 % field capacity was maintained on one side of the rootzone and 90 % on the other, resulting in a 15 % decrease in leaf area compared with the control treatment.
According to Arias et al. (1996), dry weight production and water consumption in the sugarcane crop are highly correlated. The results found in the present study for the total dry mass (shoot + root) support this conclusion (Fig. 6). However, there was no significant difference between the PRD and FI treatments for the total dry weight (Table 1) or the ratio of dry weight: water consumption (Fig. 6).
Other studies have shown that reduction in shoot dry mass production with PRD is minimal in corn (Yazar et al. 2009; Wang et al. 2012) and sweet pepper (Dorji et al. 2005). When plants are under stress, root growth is of fundamental importance for plant survival (Burkart et al. 2004). According to Tardieu (1997), rootzone growth can be maintained under moderate water shortage. In the present research, similar to the shoot dry mass, the total root dry mass production (sum both sides of the rootzone) was not affected in the plants in the PRD treatment, although the root volume in the dry side was reduced (Table 2). However, the component of the rootzone maintained at field capacity in the PRD treatment had 20.6 g with 223.8 ml root volume, while the other portion of the rootzone was maintained without water had 10.4 g and 103 ml root volume, i.e. two times more in irrigated rootzone part. The FI treatment had 12.7 g and 160 ml (side A) and 15.8 g and 163 ml (side B). In addition, the NI treatment had 7.1 g and 81.3 ml (side A) and 6.7 g and 75 ml (side B) (data not showed). This fact shows that the PRD treatment can increase the component of the rootzone maintained at field capacity counteracting the negative effects of water stress located in the rootzone that was maintained without water. Tang et al. (2010) have the same results with cotton in which the root number and length per plant increased in wet part due to the fact that a decrease in water supply can reduce the root development in dry part.
Roots also send chemical signals (ABA) to the shoot (Stoll et al. 2000; Kang and Zhang 2004) and this ABA response is linked to PRD and the increase in water use efficiency. However, in cultivated plants in pots, this root growth cannot be expressed due to the confinement of root volume. Another factor, related to greater rootzone growth, is associated with re-irrigation of previously dry roots, that may stimulate biomass accumulation in this organ (Mingo et al. 2004). Studies where the technique was applied alternately (Tang et al. 2010; Wang et al. 2012) showed secondary root production is stimulated due to these dry–wet cycles (Kang and Zhang 2004). Plants exposed to alternating dry-wet cycles maybe more robust and able to resist possible situations of moderate stress in the field. However, in this experiment, irrigation was not alternated on the root sides and the experiment was carried out in 12 l pots. Thus, the roots may not have expressed the potential growth.
Root dry mass and volume are influenced by the dry–wet cycles (Kang and Zhang 2004) of the substrate in response to the strategy of the partial irrigation system adopted. In the present study, the non-irrigated portion of the PRD treatment had a 48.8 % reduction in volume compared to the irrigated portion. Under this condition, the roots of the plants under PRD (not alternated), on the non-irrigated side, can die and can represent possible productivity losses such as reduced water and nutrient absorption. However, it has been observed in wood species that water and mineral nutrients can be translocated from the parts of the root grown in the wet soil zone to the part of the root that are in the dry area of the soil (Caldwell and Richards 1989; Ferrier and Alexander 1991; Glenn and Welder 1993). In the present experiment, reduction in root growth on the non-irrigated site in the PRD treatment did not result in significant effects to shoot growth or on any alteration between the root and shoot dry biomass.
Despite reduced root growth on the non-irrigated side in the PRD treatment, localized irrigation of the roots maintained the net photosynthetic rate when compared with the FI treatment. Other studies have demonstrated similar results (Kang et al. 2001; Liu et al. 2008; Du et al. 2010). In contrast, stomata closure was observed in the plants in the PRD treatment 19 DAAT but not on the subsequent sampling at 21 DAAT. PRD reduced water application over a 22 day period (108.8 l), when compared to the FI treatment (177.2 l), but did not significantly alter gas exchange (Fig. 3). The different gs between PRD and FI on 19 DAAT may be associated with a chemical signal because there was no alteration in the value of the leaf water potential (Table 4). Hartung et al. (2002) and Zhang et al. (2001) showed that stomata closure occurred when the leaf water potential had not yet been affected, supporting the theory of chemical signals from the root to the shoot. Jones (1992) reported that a small reduction in the stomata opening can substantially reduce water loss with a minimum effect on CO2 absorption. According to Comstock (2002), using this knowledge in practice can result in a significant decrease in water use, because transpiration consumes more than 95 % of the water absorbed by the plant.
In spite of the reduction in the gs values of the PRD treatment 19 DAAT, no significant decrease in transpiration was observed between the FI and PRD treatments.
After re-irrigation, the non-irrigated plants recovered stomata conductance to the same level as the other treatments. However, the values of the photosynthetic rate of these plants (6.04 μmol CO2 m−2 s−1) remained 50 % lower than the plants in the FI and PRD treatments (12.1 μmol CO2 m−2 s−1). Water stress affects photosynthesis of the plants due to the stomatal and non-stomatal limitations. The stomatal effect would be the primary event and would lead to lower CO2 availability in the intercellular spaces, reducing assimilation at the Rubisco carboxylation sites (Faria et al. 1996; He and Lee 2001). The non-stomatal effects are related to disturbances in the photochemical process (reduction in electron transport and decrease in ATP and NADPH formation) and in the biochemical processes mainly associated with the Calvin–Benson cycle (Kanechi et al. 1996; Lu and Zhang 1999). Thus, it can be concluded that the NI treatment resulted in irreversible and non-stomatal effects that prevented the net photosynthetic rate values from returning to the level of the control treatment. Actually, we have shown in this experiment that SPAD readings measured in the leaves of young plants of sugarcane in NI treatment decreased from 45 to 15 from 15 days after treatment application. However, the SPAD values measured in the leaves of PRD and FI treatments did not decrease and were similar (≈45) (data not showed). In addition, Du et al. (1996) demonstrated that severe drought stress in sugarcane inhibited the activities of Rubisco, fructose bisphosphatase (FBPase), phosphoenolpyruvate carboxylase (PEPcase), NADP-ME, and pyruvate, orthophosphate dikinase (PPDK). In particular, PPDK activity correlated very closely with CO2 assimilation rates during the latter stages of drought stress, and it was concluded that PPDK was very likely be the limiting factor in non-stomatal components in sugarcane during drought stress (Du et al. 1996).
Based on the concept that 50 % of the root system is irrigated in PRD, there is a potential water saving of nearly 50 % compared to the control treatment, without a negative impact on the product quality or crop yield (Santos et al. 2003). Thus, the water use efficiency can be raised due to the plant’s response to ABA resulting in the reduction in water loss through transpiration (Senyigit and Ozdemir 2011). In the present study, it was observed that the greatest water saving (29.3 %) and the greatest water use efficiency (4.1 g DM l−1) were in the PRD treatment, and the PRD reduced water use 17.6 %. However, during the experiment, there were negative effects in all the variables analyzed in the NI treatment because of the severe water shortage. Certainly during the commercial crop cycle, if the crop were submitted to a similar water shortage, productivity would be significantly damaged.
Water uptake of plants in the PRD treatment was reduced 17.6 % compared to FI. However, the dry mass gain per liter water applied was greater (4.1 vs 3.6 g DM l−1), a 12.2 % gain per gram dry weight per liter of water applied. In environmental and agronomic terms, these data can be relevant because the PRD treatment may be an important technique in juvenile phase of sugarcane and was an efficient alternative in water saving while maintaining crop productivity.
Similar water use efficiency results were observed in apple, without reduction in fruit quality (Senyigit and Ozdemir 2011). In corn a 35 % reduction in the irrigation water was associated with a decrease in biomass of only 11 % compared to irrigated plants at field capacity (Kang and Zhang 2004). PRD was used in peach orchards in China, with a spray irrigation system (Gong et al. 2001), and in a pear orchard in Victoria, Australia, with a flood irrigation system (Kang et al. 2002) resulting in a 52 and 23 % water saving, respectively. As in previous studies, the present experiment demonstrated that PRD can be used at the juvenile phase of sugarcane plants to reduce water use by 38.5 % without a significant reduction in biomass.
While there were no treatment differences in the relationship between total biomass production and water consumption, there was a significantly lower rate of water consumption in the PRD treatment based on leaf area, compared to NI and FI. This suggests that greater dry matter partitioning to the stem may occur in the PRD treatment to account for the difference in biomass. NI had the highest shoot/leaf (g/m2) ratio while FI had the lowest and PRD was intermediate. This may have value as the sugarcane plant develops since the stem is the storage organ for sucrose.
5 Conclusion
The present research demonstrated that PRD can be efficiently applied at the juvenile phase of sugarcane plants based on the fact that in the PRD treatment, compared to the FI treatment, the plants had similar gas exchange, leaf water potential and growth characteristics. The efficacy of this technique, compared to the FI treatment, was confirmed with a 17.6 % reduction in water application, a 12.2 % increase in the value of the agronomic water use efficiency (4.1 g DM l−1). These results will be validated by field experiments, with the goal of evaluating whether PRD can affect the productivity and water use of field-grown sugarcane.
Abbreviations
- A:
-
Net photosynthetic rate
- AWUE:
-
Agronomic water use efficiency
- ABA:
-
Abscisic acid
- DI:
-
Deficit irrigation
- DAAT:
-
Days after applying the treatments
- E:
-
Transpiration
- FI:
-
Full irrigation
- gs :
-
Stomatal conductance
- iWUE:
-
Intrinsic water use efficiency
- NI:
-
No irrigation
- PPF:
-
Photosynthetic photon flux
- PRD:
-
Partial rootzone drying
- RDM:
-
Root dry mass
- SDM:
-
Shoot dry mass
- VPD:
-
Vapor pressure deficit of the air
- WUE:
-
Water use efficiency
References
Arias MIB, Delgado EO, Carmenate RV (1996) Cambios fisiológicos de la caña de azúcar ante el déficit hídrico, 1st edn. Universidad Autónoma Chapingo, México, p. 135
Burkart S, Manderscheid R, Weigel HJ (2004) Interactive effects of elevated atmospheric CO2 concentrations and plant available soil water content on shoot evapotranspiration and conductance of spring wheat. Eur J Agron 21:401–417
Caldwell MM, Richards JH (1989) Hydraulic lift: water efflux from upper roots improves effectiveness of water uptake by deep roots. Oecologia 79:1–5
Campos H, Trejo C, Pena Valdivia BC, Ramirez-Ayala C, Sanchez-Garcia P (2009) Effect of partial rootzone drying on growth, gas exchange, and yield of tomato (Solanum lycopersicum L.). Sci Hortic 120:493–499
Comstock JP (2002) Hydraulic and chemical signaling in the control of stomatal conductance and transpiration. J Exp Bot 53:195–200
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Mol Biol 42:55–76
Davies WJ, Wilkinson S, Loveys B (2002) Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytol 153:449–460
Dillewijn C Van (1952) Botany of sugarcane. The Cronica Botanica Co., Waltham, p. 371
Dorji K, Behboudiana MH, Zegbe-Domınguez JA (2005) Water relations, growth, yield, and fruit quality of hot pepper under deficit irrigation and partial rootzone drying. Sci Hortic 104:137–149
Dry PR, Loveys BR (1998) Factors influencing grapevine vigour and the potential for control with partial rootzone drying. Aust J Grape Wine Res 4:140–148
Du YC, Kawamitsu Y, Nose A, Hiyane S, Murayama S, Muraya S, Wasano K, Uchida Y (1996) Effects of water stress on carbon exchange and activities of photosynthetic enzyme in leaves of sugarcane (Saccharum sp.). Aust J Plant Physiol 23:719–726
Du T, Kang S, Zhang J, Li F, Hu X (2006) Yield and physiological responses of cotton to partial root-zone irrigation in the oasis field of northwest China. Agric Water Manag 84:41–52
Du T, Kang S, Zhang J, Li F, Yan B (2008) Water use efficiency and fruit quality of table grape under alternate partial root-zone drip irrigation. Agric Water Manag 95:659–668
Du T, Kang S, Sun J, Zhang X, Zhang J (2010) An improved water use efficiency of cereals under temporal and spatial deficit irrigation in north China. Agric Water Manag 97:66–74
Faria T, Garcia-Plazaola JI, Abadia A, Cerasoli S, Pereira JS, Chaves MM (1996) Diurnal changes in photoprotective mechanisms in leaves of cork oak (Quercus suber) during summer. Tree Physiol 16:115–123
Ferrier RC, Alexander IJ (1991) Internal redistribution of N in Sitka spruce seedlings with partly drought root systems. For Sci 37:860–870
Gallo JR, Alvarez R, Abramides E (1962) Amostragem em cana-de-açúcar, para fins de análise foliar. Bragantia 21:899–921
Glenn DM, Welker WV (1993) Water transfer diminishes root competition between peach and tall fescue. J Am Soc Hortic Sci 118:570–574
Gong DZ, Wang JP, Kang SZ (2001) Variation of root and trunk sap flow rate under different soil water wetting patterns. Trans Chin Soc Agric Eng 17:34–38
Hartung W, Sauter A, Hose E (2002) Abscisic acid in the xylem: Where does it come from, where does it go to? J Exp Bot 53:27–32
He J, Lee SK (2001) Relationship among photosynthesis, ribulose-1,5-bisphosphate carboxylase (Rubisco) and water relations of the subtropical vegetable Chinese broccoli grown in the tropics by manipulation of root-zone temperature. Environ Exp Bot 46:119–128
Huitziméngari C, Trejo C, Peña-Valdivia CB, Ramírez-Ayala C, Sánchez-Garcia P (2009) Effect of partial rootzone drying on growth, gas exchange, and yield of tomato (Solanum lycopersicum L.). Sci Hortic 120:493–499
Jones HG (1992) Plant and microclimate: a quantitative approach to environmental plant physiology, 2nd edn. Cambridge University Press, Cambridge, pp 19–46
Kanechi M, Uchida N, Yasuda T, Yamaguchi T (1996) Non-stomatal inhibition associated with inactivation of Rubisco in dehydrated coffee leaves under unshaded and shaded conditions. Plant Cell Physiol 37:455–460
Kang S, Zhang J (2004) Controlled alternate partial rootzone irrigation: its physiological consequences and impact on water use efficiency. J Exp Bot 55:2437–2446
Kang SZ, Liang ZS, Hu W, Zhang JH (1998) Water use efficiency of controlled alternate irrigation on rootdivided maize plants. Agric Water Manag 38:69–76
Kang S, Zhang L, Hu X, Li Z, Jerie P (2001) An improved water use efficiency for hot pepper grown under controlled alternate drip irrigation on partial roots. Sci Hortic 89:257–267
Kang S, Hu XT, Goodwin I, Jerie P, Zhang J (2002) Soil water distribution, water use, and yield response to partial rootzone drying under a shallow groundwater table condition in a pear orchard. Sci Hortic 92:277–291
Kim TH, Boehmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ Signaling. Annu Rev Plant Biol 61:561–591
Kirda C, Topcu S, Kaman H, Ulger AC, Yazici A, Cetin M, Derici MR (2005) Grain yield response and N-fertiliser recovery of maize under deficit irrigation. Field Crops Res 93:132–141
Liu F, Shahnazari A, Andersen MN, Jacobsen SE, Jensen CR (2006) Physiological responses of potato (Solanum tubersum L.) to partial root-zone drying: ABA signaling, leaf gas exchange, and water use efficiency. J Exp Bot 57:3727–3735
Liu F, Song R, Zhang X, Shahnazari A, Andersen MN, Plauborg F, Jacobsen SE, Jensen CR (2008) Measurement and modeling of ABA signaling in potato (Solanum tuberosum L.) during partial root-zone drying. Environ Exp Bot 63:385–391
Lu C, Zhang J (1999) Effects of water stress on photosystem II photochemistry and its thermo stability in wheat plants. J Exp Bot 50:1199–1206
Mingo DM, Theobald JC, Bacon MA, Davies WJ, Dodd IC (2004) Biomass allocation in tomato (Lycopersicon esculentum) plants grown under partial rootzone drying: enhancement of root growth. Funct Plant Biol 31:971–978
Morison JI, Baker NR, Mullineaux PM, Davies WJ (2008) Improving water use in crop production. Philos Trans R Soc Lond B 12:639–658
Munns R, Cramer GR (1996) Is coordination of leaf and root growth mediated by abscisic acid? Opinion. Plant Soil 185:33–49
Saeed H, Grove IG, Kettlewell PS, Hall NW (2005) Potato root and shoot growth under different water management strategies. Asp Appl Biol 73:85–91
Sampaio HRA, Coelho Filho MA, Coelho EF, Rossini D, Machado VV, Carvalho GC, Santana Júnior EBS (2010) Déficit hídrico e secamento parcial do sistema radicular em pomar de lima ácida. Pesqui Agropecu Bras 45:1141–1148
Santos TPD, Lopes CM, Rodrigues ML, Souza CRD, Maroco JP, Pereira JS, Silva JR, Chaves MM (2003) Partial rootzone drying, effects on growth and fruit quality of field-grown grapevines (Vitis vinifera). Funct Plant Biol 30:663–671
Senyigit U, Ozdemir FO (2011) Effects of partial rootzone drying and conventional deficit irrigation on yield and quality parameters of “Willians Pride” apple cultivar drafted on M9 rootstock. Sci Res Essay 6:3776–3783
Sepaskhah AR, Ahmadi SH (2010) A review on partial root-zone drying irrigation. Int J Plant Prod 4:241–258
Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ (2004) Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial rootzone drying. J Exp Bot 55:2353–2363
Stoll M, Loveys B, Dry P (2000) Hormonal changes induced by partial rootzone drying of irrigated grapevine. J Exp Bot 51:1627–1634
Tang LS, Li Y, Zhang JH (2005) Physiological and yield responses of cotton under partial rootzone irrigation. Field Crops Res 94:214–223
Tang LS, Li Y, Zhang J (2010) Biomass allocation and yield formation of cotton under partial rootzone irrigation in arid zone. Plant Soil 337:413–423
Tardieu F (1997) Drought perception by plants. Do cells of droughted plants experience water stress. In: Belhassen E (ed) Drought tolerance in higher plants: genetical, physiological and molecular biological analysis. Kluwer, New York, pp 15–26
UNICA (2014) Brazilian Sugarcane Industry Association. http://www.unica.com.br/index.php?idioma=2. Accessed 9 Jul 2014
Wakrim S, Wahbi S, Tahi H, Aganchich B, Serraj R (2005) Comparative effects of partial root drying (PRD) and redulated deficit irrigation (RDI) on water relations and water use efficiency in common bean (Phaseolus vulgaris L.). Agric Ecosyst Environ 106:275–287
Wang Z, Liu F, Kang S, Jensen CR (2012) Alternate partial root-zone drying irrigation improves nitrogen nutrition in maize (Zea mays L.) leaves. Environ Exp Bot 75:36–40
Wilkinson S (1999) pH as a stress signal. Plant Growth Regul 29:87–99
Yazar A, Gökçel F, Sezen MS (2009) Corn yield response do partial rootzone drying and deficit irrigation strategies applied with drip system. Plant Soil Environ 55:494–503
Yuan J, Xu M, Duan W, Fan P, Li S (2013) Effects of whole-root and half-root water stress on gas exchange and chlorophyll fluorescence parameters in apple trees. J Am Soc Hortic Sci 138:395–402
Zegbe JA, Behboudian MH (2008) Plant water status, CO2 assimilation, yield, and fruit quality of ‘Pacific Rose’ apple under partial rootzone drying. Adv Hortic Sci 22:27–32
Zhang J, Jia WS, Kang S (2001) Partial rootzone irrigation: its physiological consequences and impact on water use efficiency. Acta Bot Boreal 21:191–197
Acknowledgments
The authors thank the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the study grant and to the Santa Tereza sugar mill for collaborating in the research. The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the fellowships awarded to E. Campostrini.
Disclaimer
Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the U.S. Dept. of Agriculture and does not imply its approval to the exclusion of other products or vendors that also may be suitable. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribeiro, M.S., Netto, A.T., do Couto, T.R. et al. Partial rootzone drying in sugarcane (Saccharum officinarum L.): effects on gas exchange, growth and water use efficiency. Theor. Exp. Plant Physiol. 26, 251–262 (2014). https://doi.org/10.1007/s40626-014-0024-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-014-0024-0