Abstract
Diabetic kidney disease (DKD) accounts for a large proportion of end-stage renal diseases that require renal replacement therapies including dialysis and transplantation. Therefore, it is critical to understand the occurrence and development of DKD. Podocytes are mainly injured during the development of DKD, ultimately leading to their extensive death and loss. In turn, the injury and death of glomerular podocytes are also the main culprits of DKD. This review introduces the characteristics of podocytes and summarizes the modes of their death in DKD, including apoptosis, autophagy, mitotic catastrophe (MC), anoikis, necroptosis, and pyroptosis. Apoptosis is characterized by nuclear condensation and the formation of apoptotic bodies, and it exerts a different effect from autophagy in mediating DKD-induced podocyte loss. MC mediates a faulty mitotic process while anoikis separates podocytes from the basement membrane. Moreover, pyroptosis activates inflammatory factors to aggravate podocyte injuries whilst necroptosis drives signaling cascades, such as receptor-interacting protein kinases 1 and 3 and mixed lineage kinase domain-like, ultimately promoting the death of podocytes. In conclusion, a thorough knowledge of the modes of podocyte death in DKD can help us understand the development of DKD and lay the foundation for strategies in DKD disease therapy.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
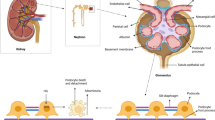
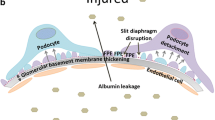
Diabetic kidney disease (DKD), a recognized microvascular complication of diabetes mellitus, is clinically characterized by albuminuria, elevated creatinine levels, and abnormal glomerular filtration rates, which eventually develops into end-stage renal disease after several years [1,2,3,4]. Pathologically, podocyte loss and foot process effacement, glomerulosclerosis, glomerular basement membrane (GBM) thickening, mesangial matrix expansion, interstitial fibrosis, and tubular atrophy contribute to the development of DKD [4, 5] (Fig. 1a). Among them, podocyte loss is an important early pathological marker of DKD, which accelerates the development of DKD [6, 7]. As terminally differentiated glomerular visceral epithelial cells, the complete structure of podocytes is essential for the glomerular filtration function. However, a variety of factors in the diabetic environment can damage podocytes, resulting in the disappearance of podocyte foot processes, phenotypic transformation, and even detachment or death.
Representative photomicrographs of podocytes in patients with DKD generated by transmission electron microscopy. Scale bars indicate 5 μm. a The foot process of podocytes fused, and the basement membrane to which the podocytes adhered was significantly thickened. b An autophagosome formed in the cytoplasm of a podocyte. c Abnormal mitotic podocytes with binuclear features. (d) A podocyte completely detached from the basement membrane. BMT, basement membrane thickening; FPF, foot process fusion; AU, autophagosome; MC, mitotic catastrophe; AK, anoikis
A discussion on the nature of podocyte loss events is required. Altintas and Reiser summarized the main modes of podocyte death in kidney diseases, including apoptosis, autophagy, mitotic catastrophe (MC), necroptosis, and anoikis [8]. Numerous studies have focused on the mechanism of podocyte apoptosis in DKD, indicating that it is the most common mode of podocyte death. Although autophagy is considered to be a protective mechanism in contrast to apoptosis, it also contributes to podocyte loss in some aspects. Interestingly, MC is defined as a failed process of multiplication and division that could result in podocyte injury. Additionally, anoikis implies that podocytes completely separate from the GBM. Besides, necroptosis and pyroptosis lead to lytic death of podocytes in DKD.
During the development of DKD, a variety of death events occur in podocytes that have lost their normal structure and function (Fig. 2). The loss of podocytes makes glomeruli lose their normal filtration function and promotes disease progression. A thorough discussion on the uniqueness and correlation among various podocyte death modes will lead to an understanding of podocyte death pathways that can be manipulated to achieve the prevention and treatment of DKD.
Modes of podocyte death in DKD. The process of apoptosis includes nuclear condensation, DNA fragmentation, the formation of apoptotic bodies, and finally phagocytosis by macrophages. Autophagy includes the formation of autophagosomes, fusion of autophagosomes and lysosomes, and degradation of autophagosomes. Meanwhile, mitotic catastrophe is characterized by the entry of podocytes into the cell cycle, chromosome replication, and the formation of large cells with multiple micronuclei and chromatin condensation. Besides, anoikis implies that the podocyte is completely detached from the GBM. In addition, the process of necroptosis includes cell swelling, membrane permeabilization, membrane breakdown and the release of cell contents. In the process of pyroptosis, inflammasomes activate caspase family proteins, and gasdermin proteins translocate to the membrane to form pores; then, cell swelling and cytoplasmic outflow occur, resulting in cell membrane rupture. DKD, diabetic kidney disease; GBM, glomerular basement membrane
Characteristics of podocytes
Structure and function of podocytes
Podocytes, endothelial cells, and GBM together constitute the glomerular filtration barrier. Podocytes are characteristic, terminally differentiated visceral epithelial cells in the kidney, which are composed of cell bodies, primary processes, and branched foot processes [9]. Podocytes adhere to GBM through α3β1 integrin [10]. Constitutively, intersecting foot processes always wrap capillaries [3]. And the space between adjacent foot processes is occupied by filtration slit, which plays an important role in establishing selective permeability of the glomerular filtration barrier and preserving macromolecules in the plasma [5, 11]. As highly differentiated cells, podocytes have a limited ability to proliferate. Thus, if the quantity of podocytes lessens in the glomeruli, the remaining podocytes will not be adequate to cover the surface of GBM unless they assume a more vulnerable hypertrophic form [12]. The detachment or death of podocytes from GBM and the disappearance of foot processes result in damage to the integrity of the filtration membrane and eventually lead to albuminuria [9, 13]. Therefore, podocytes are essential for maintaining the normal structure and function of the kidney.
Results and dangers of podocyte death in DKD development
Podocytes have a limited ability to repair and regenerate in the chemical and hemodynamic environment of diabetes, including in the presence of high glucose (HG), growth factors, fatty acids, angiotensin II (Ang II), transforming growth factor-β (TGF-β), hormones, and mechanical stretch [14,15,16]. When exposed to hyperfiltration and hyperperfusion, podocytes can undergo specific cellular responses, including signal transduction system activation, increase in the synthesis of cytokines, and extracellular matrix accumulation [17]. Besides, Wolf et al. [16] found that the local increase of Ang II concentration in DKD led to a series of podocyte injury events, which suppressed the expression of nephrin and activated TGF-β and vascular endothelial growth factor (VEGF) systems. Because nephrin is an important component of the slit diaphragm, its loss in podocytes leads to the broadening and effacement of the foot process [18]. The activation of TGF-β can lead to mesangial matrix deposition and GBM thickening. Additionally, overexpressed VEGF in podocytes could increase glomerular hemodynamic pressure, alter the composition of GBM, and inhibit the expression of nephrin [16]. Collectively, these events lead to the death and loss of podocytes and exacerbate albuminuria.
In the early stage of DKD, podocytes are present in a hypertrophy phenotype without change in the total number. Minakawa et al. [19] found that with the development of DKD, the number of podocytes decreased and the glomerular volume gradually increased, causing albuminuria and progressive glomerulosclerosis. Albuminuria itself exacerbates the development and progression of DKD. It is reported that podocytes have the ability to endocytose albumin [20], however, this process might be a potentially important molecular mechanism of podocyte injury during glomerular diseases [21]. Nephrin can be internalized by podocytes through clathrin-dependent pathways and raft-mediated endocytosis [22]. In DKD, both protein kinase C-alpha and regulator of ubiquitous kinase can mediate the endocytosis of nephrin by podocytes, further reducing the level of nephrin [23, 24]. In addition, excessive endocytosis of podocytes to plasma proteins triggers an inflammatory response that ultimately leads to the deterioration of podocyte function and induces cell death [25]. Loss and death of podocytes further increase the permeability of the glomerular filtration barrier to plasma proteins, thereby aggravating proteinuria and causing a vicious cycle [26]. Therefore, glomerular podocyte injury and death are important for the occurrence and early development of DKD.
Modes of podocyte death in DKD
Apoptosis of podocytes in DKD
Apoptosis is a highly regulated process of programmed cell death without an inflammatory response, which acts primarily through the action of the serine protease caspases and has also been conceptualized as a self-directed cell “suicide” [27,28,29]. In apoptosis, cells undergo lethal changes, such as blebbing of the cell membrane, rupture of mitochondria, and DNA fragmentation [30], followed by the formation of apoptotic bodies and their phagocytosis by macrophages [28]. As the most common mode of death, podocyte apoptosis and its underlying mechanisms have been widely reported in DKD.
Podocyte apoptosis induced by advanced glycation end products (AGEs)
AGEs and the corresponding receptor for AGEs (RAGE) display enhanced expressions in the kidney of diabetic db/db mice. RAGE is generally limited to glomerular podocytes [31]. Chuang et al. [32] confirmed that AGEs promoted podocyte apoptosis in DKD by combining with RAGE, thereby activating the forkhead box O4 (FOXO4) transcription factor and increasing the expression of the pro-apoptotic gene Bcl2l11. Using a DKD mouse model, Jing et al. [33] found that AGE-induced podocyte apoptosis was associated with the activation of the CXCL9-mediated JAK2/STAT3 signaling pathway. Besides, Tae et al. [34] suggested that diabetic conditions could down-regulate CD2-associated protein (CD2AP) expression by activating the phosphoinositide 3-kinase (PI3-K)/Akt signaling pathway, leading to AGE-induced podocyte injury. Thus, AGEs bind to their receptors to activate diverse signaling pathways, ultimately causing podocyte apoptosis.
HG-induced reactive oxygen species (ROS) production initiates podocyte apoptosis
Extracellular HG can rapidly stimulate the generation of intracellular ROS through NADPH oxidase and mitochondrial pathways, which ultimately results in the apoptosis of podocytes [35]. Moreover, the inactivation of AMP-activated protein kinase (AMPK)/tuberin protein in diabetes mice activated the mammalian target of rapamycin (mTOR) signaling pathway, which enhanced oxidative stress by upregulating the expressions of Nox4 and Nox1 and the activity of NADPH oxidase, subsequently leading to HG-induced podocyte apoptosis [36]. In addition, Chen et al. [37] have shown that FOXO3a played a vital role in mediating advanced oxidation protein product (AOPP)-induced podocyte apoptosis under oxidative stress in DKD. The accumulation and activation of FOXO3a in DKD accelerated podocyte injury induced by oxidative stress. Oxidative stress is known to be a very important part of diabetes. HG causes oxidative stress, which directly damages podocytes and contributes to their apoptosis.
Endoplasmic reticulum stress (ERS) is associated with podocyte apoptosis induced by HG
The process of unfolded or misfolded protein accumulation in the endoplasmic reticulum that triggers the signaling pathway dominated by an unfolded protein response is termed ERS. ERS induced by HG partially contributes to apoptosis in differentiated podocytes through various mechanisms [38, 39]. Yue et al. [40] reported that ERS mediated the upregulation of cyclin-dependent kinase 5 (Cdk5) in podocytes induced by HG, while the increase in Cdk5 promoted the phosphorylation of MEKK1 at Ser280 in podocytes, which indicated that Cdk5/MEKK1/JNK signaling axis was related to ERS-induced podocyte apoptosis. Besides, HG can also motivate Akt and NF-κB by activating cannabinoid receptor 1 (CB1R)-induced B1R and B2R [41]. This leads to stimulation of pro-apoptotic molecules by ERS, which ultimately triggers podocyte apoptosis [41]. Thus, CB1R/B1R, B2R/Akt, NF-κB signaling axis can promote ERS-mediated DKD podocyte apoptosis. In conclusion, podocyte apoptosis induced by ERS is an essential factor in the pathogenesis of DKD.
MicroRNAs (miRNAs) and podocyte apoptosis
Numerous studies have reported abundant expressions of miRNAs in DKD, indicating their significant roles in the pathogenesis of DKD. In the kidney tissue of patients with DKD, overexpression of miR-770-5p triggers podocyte apoptosis by targeting tissue inhibitors of metalloproteinase 3 and E2F transcription factor 3 [42, 43]. Meanwhile, HG stimulates the expression of miR-27a, which induces podocyte injury and apoptosis by activating β-catenin signaling through negative targeting of peroxisome proliferator-activated receptor gamma (PPARγ) [44]. Alternatively, miR-27a could also target FOXO1, subsequently triggering the ERS signaling pathway in podocytes and inducing DKD podocyte injury [45]. Other miRNAs including miRNA-337, miR-503, miR-218, miR195, and miR-134-5p could exert analogous effects in DKD podocyte apoptosis [30, 46,47,48,49]. These studies have shown that miRNAs play an important role in DKD podocyte apoptosis.
Apoptosis of DKD podocytes mediated by p53 pathway
Tumor suppressor p53-mediated podocyte apoptosis is a complex process involving many different genes. HG upregulates the Notch pathway of podocytes, which can mediate podocyte apoptosis through Bcl-2 and p53 pathways [50]. Furthermore, Benoit et al. [51] reported that under diabetic conditions, the downregulation of low-density lipoprotein receptor-related protein 6 (LRP6) would lead to the inactivation of the Wingless‐type (Wnt) pathway, thereby enhancing the interaction between glycogen synthase kinase-3β (GSK3β) and p53, and ultimately contributing to podocyte apoptosis. Interestingly, the expression of retinoic acid receptor responder protein 1 (RARRES1) was also positively correlated with the decline of renal function in DKD when overexpressed RARRES1 was endocytosed and interacted with RIO kinase 1(RIOK1), finally leading to p53 activation and podocyte apoptosis [52]. In addition, Zhang et al. [53] also reported that the expression of brain acid-soluble protein 1 (BASP1) was enhanced in patients with DKD and diabetic db/db mice, which promoted podocyte apoptosis by activating p53 apoptosis pathway via Wilms' tumor 1 transcription factor (WT1). In conclusion, if podocytes damaged in the diabetic environment cannot be repaired, the gene network involved in regulating p53 is activated, causing apoptosis of podocytes.
Apoptosis accounts for more than half of DKD podocyte death. Harmful irritants, such as AGEs, ROS, and miRNAs, accumulated in the diabetic environment can damage podocytes and induce podocyte apoptosis. Moreover, in podocytes exposed to the diabetic environment with hyperfiltration and hyperperfusion, p53, mTOR, Notch, and other classical signaling pathways are activated, which together lead to podocyte apoptosis (Fig. 3). Although apoptosis can remove diseased cells, excessive apoptosis not only fails to maintain cellular function, but also accelerates disease progression. Therefore, the massive apoptosis of podocytes in DKD causes excessive loss of podocytes and the normal structure and function of glomerular cannot be maintained.
Podocyte apoptosis signaling pathway network in DKD. In DKD, HG can induce functional events such as AGEs, ROS and ERS in podocytes while classical signaling pathways such as p53, mTOR and Notch are also activated, which jointly lead to podocyte apoptosis. Besides, the expression of a large number of miRNAs increases that participates in podocyte apoptosis. AGEs, advanced glycation end products; ROS, reactive oxygen species; ERS, endoplasmic reticulum stress; mTOR, mammalian target of rapamycin; miRNAs, microRNAs
Autophagy of podocytes in DKD
The role of autophagy in podocyte injury in DKD
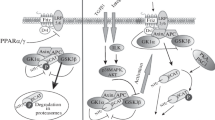
Autophagy, also known as cell self-digestion, is a conservative catabolic process that can degrade abnormal proteins, organelles, and macromolecules, and recover decomposition products to maintain cell homeostasis and survival [54,55,56]. Hence, abnormal autophagy can result in cell death. The process of autophagy involves autophagy induction, fusion of autophagic vacuoles with lysosomes, lysosomal degradation of autophagic vacuoles, etc. If any node is destroyed or blocked, autophagy will be devitalized [57]. Several types of autophagy exist, such as microautophagy, macroautophagy, and chaperone-mediated autophagy [55, 58].
Podocyte homeostasis is maintained by an adequate level of basal autophagy [58], which can protect podocytes from HG-induced damage by preventing insulin resistance [59]. However, the blockade of autophagy induction in DKD can cause the inhibition of lysosome degradation of autophagosomes, accumulation of damaged lysosomes, formation of large mitochondria in podocytes, and death of podocytes [56, 60] (Fig. 1b). Under HG exposure, the autophagy activity of podocytes was decreased and Sequestosome 1 expression was increased, suggesting a new mechanism of HG-induced podocyte injury [61].
Signaling pathway that mediates podocyte autophagy in DKD
Markus et al. [62] have demonstrated that the mTOR signal is significant for podocyte homeostasis and survival. However, excessive activation of mTOR eventually leads to abnormal kidney function, such as the development of DKD under the HG state. When podocytes are exposed to HG, the mTORC1 pathway is activated with a subdued level of protective autophagy [63]. Ji et al. [64] constructed the DKD rat model and proved that the expression of connexin 43 was upregulated after HG stimulation, which could lead to podocyte injury by activating the mTOR signaling pathway. Significantly, rapamycin can heighten the level of autophagy by inhibiting the mTOR pathway, thus reducing DKD induced podocyte injury [65].
AGEs not only induce apoptosis but are also closely associated with podocyte autophagy. The expression of AGEs is elevated in diabetic conditions, which inhibits the formation and turnover of podocyte autophagosomes by activating mTOR and inhibiting the nuclear translocation of the pro-autophagic transcription factor EB [66]. In addition, lysosomal membrane permeabilization induced by AGEs mediates lysosomal dysfunction, leading to insufficient autophagy and pathological changes in podocytes of patients with DKD [67].
Other defective autophagy pathways are also essential. For example, the increase of plasma apelin concentration in patients with diabetes has been shown to promote the development of DKD by inhibiting podocyte autophagy [68]. In addition, β-arrestin-1 and β-arrestin-2 are upregulated in the kidneys of diabetic db/db mice and patients with diabetes, and inhibit podocyte autophagy through the negative regulation of ATG12-ATG5 conjugation, resulting in podocyte injury [69]. Podocyte nucleotide-oligomerization domain-like receptor 3 (NLRP3) inflammasome can also negatively regulate the autophagy process of podocytes in DKD mice [70].
Autophagy exists as a protective mechanism in DKD. In the diabetic environment, a variety of signaling molecules mediate the inhibition of podocyte autophagy. Interestingly, the same signaling pathways that play a role in apoptosis also contribute to autophagy defects. As a defense and stress regulation mechanism, autophagy is impaired in the diabetic environment, which ultimately prevents the survival of podocytes. Therefore, autophagy defects in DKD facilitate podocyte death and renal insufficiency.
Podocyte MC in DKD
MC is defined as a type of cell death characterized by abnormal mitosis that commonly manifests in the form of large cells with multiple micronuclei and chromatin condensation [71]. The morphological characteristics of MC include binuclei, multiple nuclei (aneuploidy), micronuclei (round DNA aggregates close to the nucleus), irregularly shaped nuclei, and abnormal mitotic spindles [72,73,74,75]. Multiple molecules were involved in the control of MC, particularly, cell-cycle specific kinases (such as cyclin B1-dependent kinases Cdk1 and Aurora kinases), cell-cycle checkpoint proteins, survivin, p53, caspases, and the Bcl-2 family [72].
MC causes podocyte loss and death
Mature podocytes are considered resting cells in the G0 stage that lack the ability to proliferate [76, 77]. However, podocytes can reenter the cell cycle, although they cannot divide during kidney injury [78]. Owing to the absence of Aurora kinase B expression, mature podocytes cannot form effective mitotic spindles [79, 80]. In addition, cytokinesis requires the complete recombination of actin cytoskeleton, which is incompatible with maintaining the structure of the foot process and the slit diaphragm [81]. Because differentiated podocytes have inherent obstacles to mitosis, their proliferative response does not promote recovery from injury, but rather accelerates glomerulosclerosis and podocyte loss [76]. Academic studies by Masanori et al. [82] found that the exfoliated podocytes in the urine of patients with diabetes showed the morphological characteristics of MC, indicating that MC may be a major reason for the loss of podocytes in DKD. Multinucleation of podocytes is recognized as a feature of abnormal mitosis [71, 83] while binuclear, asymmetric nuclear, and multinuclear podocytes serve as markers of the cell cycle to raise podocyte sensitivity to cell death [78]. Tang et al. [84] discovered abnormal mitotic podocytes with binuclear characteristics, dot-like nuclear chromatin condensation and foot excess effacement in patients with DKD, which is the direct evidence of the occurrence of MC in podocytes (Fig. 1c). In addition, in vitro experiments also showed that HG-treated podocytes manifested multipolar mitotic spindles with irregularly distributed chromosomes, which are different from the formation of bipolar microtubule mitotic spindles in normal mitotic cells [84].
Molecular mechanism of podocyte MC in DKD
Many studies have reported various molecular mechanisms underlying podocyte MC in DKD. Su et al. [85] found that the overexpression of mitotic arrest deficiency (MAD)2B in patients with DKD and diabetic db/db mice inhibited the degradation of cyclin B1 and Skp2 driven by cadherin1-anaphase-promoting complex/cyclosome through the suppression of the expression of cadherin1, thereby causing the abnormal entry of podocytes into the cell cycle and aggravating podocyte injury. Meanwhile, HG exposure can enhance the expressions of Ki67, cyclinB1 and Aurora in podocytes, upregulate the level of murine double minute 2 (MDM2), and force podocytes to enter the S phase and bypass the G2/M checkpoint, thereby aggravating podocyte loss through MC [84]. MDM2 participates in HG-induced podocyte MC by activating Notch1 signal transduction in a p53-independent manner [84]. Interestingly, the activation of Notch signal can force podocytes to pass through the G2/M checkpoint, which induces MC and leads to podocyte loss [79]. It can be concluded that MAD2B, MDM2, and Notch are momentous molecular markers of podocyte MC in DKD.
During the development of DKD, podocytes stimulated by a harmful environment tend to renew themselves. However, podocytes do not have the ability to proliferate indefinitely like tumor cells. In fact, in vivo podocytes are quiescent cells that lack the ability to renew during adult life. Therefore, the increased expression of podocyte proliferation markers, the presence of multinuclear podocytes or even DNA replication observed in DKD do not provide evidence of local podocyte regeneration, however, these events may represent a paradoxical cell-cycle process that ultimately exacerbates podocyte loss, which is in fact MC.
Podocyte anoikis
Podocyte anoikis in DKD
Anoikis is defined as a cell deficiency caused by the loss of attachment or inappropriate adhesion to the extracellular matrix [86]. Podocyte anoikis implies that the foot process of the podocyte disappears completely along the basement membrane of the surrounding capillaries [87] (Fig. 1d). Foot processes connect podocytes to GBM through integrins and dystroglycans [88]. In patients with DKD and streptozotocin-induced diabetic rats, the expression of α3β1 integrin lessened and resulted in the focal detachment of podocytes from GBM [89, 90], which indicated that HG stimulation inhibited the expression of integrin in podocytes. However, some studies have also suggested that early podocyte detachment in DKD is mediated by the upregulation of α3β1 integrin [91].
Podocyte anoikis with epithelial-mesenchymal transition (EMT)
In DKD, podocytes can undergo the process of transformation from mature cells to mesenchymal cells. EMT is widely involved in the early stage of podocyte loss in diabetes by causing podocyte detachment or death [63, 92]. Podocytes undergoing EMT abandon their complex morphological structure and highly specialized functions, which impairs the integrity of the glomerular filtration barrier [15]. Interestingly, podocytes can be detected in the urine of patients with DKD [92], and they can be cultured and proliferated in vitro [93]. This suggests that although anoikis is a mode of death, detached podocytes do not die immediately unless they no longer get a normal living environment.
Yamaguchi et al. [94] indicated that podocytes in normal glomeruli rarely expressed fibroblast-specific protein 1 (FSP1). However, the upregulation of FSP1 expression was observed in podocytes of patients with diabetes, which may induce podocyte anoikis via EMT and is associated with more severe clinical and pathological manifestations of DKD. As a key event in DKD induction, overactivation of mTORC1 stimulates ERS and EMT-like phenotypes in podocytes, ultimately leading to podocyte anoikis [95]. Therefore, podocyte anoikis in DKD is closely connected with the level of α3β1 integrin and partly mediated by EMT. Podocytes detached from the GBM did not die immediately and were viable if given normal culture conditions.
Podocyte necroptosis
Necroptosis is a type of programmed cell death that occurs in the morphology of necrosis including swelling of organelles and plasma membrane rupture, which is different from that of apoptosis [96, 97]. Necroptosis can activate inflammatory responses by releasing cell contents from the ruptured plasma membrane [97, 98], which is driven by signaling cascades such as receptor-interacting protein kinase 1 (RIPK1), receptor-interacting protein kinase 3 (RIPK3), and mixed lineage kinase domain-like (MLKL) [99]. These signal molecules are not only the core regulatory factors of necroptosis, but also special markers that can be detected [100]. Necroptosis is an important model of cell death under numerous pathological conditions and shares multiple upstream signaling pathways with apoptosis [96].
Mechanisms underlying HG induced podocyte necroptosis
Studies have shown that necroptosis plays an important part in podocyte injury [101]. Xu et al. [96] found that UCHL1, a member of the group of deubiquitinating enzymes, was overexpressed in the podocytes of patients with DKD, which was consistent with the argument supporting podocyte necroptosis. Under the condition of DKD, HG stimulation induced podocyte necroptosis by activating RIPK1 and RIPK3 pathways, that were accompanied by the increased expression of UCHL1. Incremental UCHL1 further enhanced the activation of the RIPK3/MLKL pathway and promoted podocyte necroptosis. As a result, UCHL1 facilitates HG-induced podocyte necroptosis by regulating the ubiquitination status of the RIPK1/RIPK3 pathway [96].
As a new mode of podocyte death, necroptosis will be a preventative and therapeutic target for DKD. However, at present, only some studies focus on podocyte necroptosis in DKD. Thus, more efforts are required to explore the nature of necroptosis in DKD through fundamental and clinical studies.
Podocyte pyroptosis
Characteristics of podocyte pyroptosis
Pyroptosis is a form of lytic regulated cell death [102]. It is characterized as a proinflammatory response, which depends on the activation of caspase-1 and caspase-4/-5/-11 mediated by inflammasomes and the upregulation of gasdermin D (GSDMD) expression [97, 103].
Podocyte pyroptosis in DKD
Pyroptosis mediated by caspase-11/4 and GSDMD was activated in DKD and participated in podocyte loss. In the HG status, the expression of caspase-4, caspase-11, and the cleavage of GSDMD-N in podocytes increased significantly, accompanied by a decrease in the expressions of nephrin and podocin in podocytes [102]. Meanwhile, knockout of caspase-11 or GSDMD can inhibit the augmentation of inflammatory cytokines, indicating that the activation of pyroptosis is at least partly connected with the inflammatory response in DKD [102]. Thus, the suppression of podocyte pyroptosis may occlude the development of DKD by preventing the activation of local inflammation. In turn, inhibition of inflammatory factors can also reduce podocyte pyroptosis and ameliorate DKD [104]. Furthermore, a study by Ding et al. [105] found that miR-215p in macrophage-derived extracellular vesicles (EVs) mediated DKD podocyte pyroptosis by targeting A20. As reported, miR-215p was significantly elevated in the macrophage-derived EVs treated with HG. In addition, elevated miR-215p increased the inflammasomes NLRP3, caspases-1, and IL-1β related to pyroptosis by inhibiting A20, and thus, enhancing the production of ROS, ultimately resulting in podocyte pyroptosis [105]. The pyroptosis of podocytes in DKD is closely related to inflammatory factors. Thorough investigations on podocyte pyroptosis in DKD are few at present, and further research in this area is needed.
Modes of podocyte death in other podocyte-related diseases
Various modes of podocyte death are not unique to DKD; in fact, they also occur in other podocyte-related diseases. For example, RARRES1 gene, which induces podocyte apoptosis in DKD, has been proved to induce podocyte apoptosis and disease progression in focal segmental glomerulosclerosis (FSGS) as well [52]. In addition, adriamycin can promote podocytes to reenter the cell cycle in vivo and in vitro [106], while MDM2 drives the process of podocyte MC in FSGS [73]. Besides, autophagy is activated in lupus nephritis (LN), especially in podocytes. The increased level of autophagy has a protective effect on podocyte injury induced by antibody and interferon-α [107], which is similar to the protective effect of autophagy found in DKD. However, autophagy is not beneficial in all podocyte-related diseases. For instance, in patients with minimal change nephrotic syndrome (MCNS), podocyte autophagy is significantly associated with the effacement of the foot process and the occurrence of albuminuria [108]. Although the number of reports on podocyte necroptosis is insufficient, RIPK3 activation-mediated podocyte necroptosis has been found in LN and Fabry nephropathy [109, 110]. Interestingly, anoikis is a special form of progression in Alport syndrome because of the progressive detachment and death of glomerular podocytes in such patients [111]. In podocyte-related diseases, podocyte death is inevitable. Death mechanisms can be common in different podocyte diseases, such as RARRES1 in DKD and FSGS. Meanwhile, the same mode of death may play different roles in different podocyte-related diseases, such as autophagy in LN and MCNS. Moreover, kidney diseases such as DKD, LN, and FSGS obviously involve more than one mode of podocyte death, which shows that understanding the mode of podocyte death is conducive to the exploration and treatment of the diseases.
Conclusions
The occurrence and development of DKD, a main microvascular complication of diabetes, is closely associated with the injury of podocytes. Owing to the limited repair and proliferation capacity of mature podocytes, numerous harmful factors in the diabetic environment can lead to their loss and death. Podocyte loss is present in various modes in DKD, which in turn aggravates disease progression. Apoptosis mainly accounts for podocyte death in DKD via oxidative stress, ERS, AGEs, miRNAs, and other classic signaling pathways. Although autophagy plays an opposite role in regulating podocyte injury in DKD, it shares multiple signaling pathways with apoptosis-mediated podocyte loss. In DKD, MC encourages podocytes that lack the ability to proliferate to reenter the cell cycle, leading to wrong mitosis and podocyte death by various mechanisms. Moreover, anoikis emphasizes that podocytes detach from the GBM. In addition, necroptosis is driven by signaling cascades such as RIPK1, RIPK3, and MLKL while pyroptosis is closely relevant to the activation of inflammasomes. However, these death modes are present not only in DKD, but also in other podocyte-related diseases. Podocyte loss in DKD may present in one or several modes simultaneously, and we cannot always make accurate judgment on the foremost death mode. This review has its limitations, as most scholars have focused on the discussion of apoptosis, while few studies related to necroptosis and pyroptosis of podocytes in DKD have been reported. These novel modes of cell death, MC, necroptosis and pyroptosis, are expected to be the focus of future research. It is important to investigate the mechanism of podocyte death and identify the underlying signaling pathways. By comprehending the death mechanisms that mediate the loss of podocytes, it will be easier for us to identify novel targets for DKD treatment and the subsequent exploitation of new drugs.
Abbreviations
- AGEs:
-
Advanced glycation end products
- Ang II:
-
Angiotensin II
- AOPPs:
-
Advanced oxidation protein products
- AMPK:
-
AMP -activated protein kinase
- BASP1:
-
Brain acid-soluble protein 1
- CB1R:
-
Cannabinoid receptor 1
- Cdk5:
-
Cyclin-dependent kinase 5
- CD2AP:
-
CD2-associated protein
- DKD:
-
Diabetic kidney disease
- EMT:
-
Epithelial-mesenchymal transformation
- ERS:
-
Endoplasmic reticulum stress
- EVs:
-
Extracellular vesicles
- FOXO4:
-
Forkhead box O4
- FSGS:
-
Focal segmental glomerulosclerosis
- FSP1:
-
Fibroblast-specific protein 1
- GBM:
-
Glomerular basement membrane
- GSDMD:
-
Gasdermin D
- GSK3β:
-
Glycogen synthase kinase-3β
- HG:
-
High glucose
- LN:
-
Lupus nephritis
- LRP6:
-
Lipoprotein receptor-related protein 6
- MAD:
-
Mitotic arrest deficiency
- MC:
-
Mitotic catastrophe
- MCNS:
-
Minimal change nephrotic syndrome
- MDM2:
-
Murine double minute 2
- miRNAs:
-
MicroRNAs
- MLKL:
-
Mixed lineage kinase domain-like
- mTOR:
-
Mammalian target of rapamycin
- NLRP3:
-
Nucleotide-oligomerization domain-like receptor 3
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- PI3-K:
-
Phosphoinositide 3-kinase
- RAGE:
-
Receptors for AGEs
- RARRES1:
-
Retinoic acid receptor responder protein 1
- RIPK1:
-
Receptor-interacting protein kinase 1
- ROS:
-
Reactive oxygen species
- RIOK1:
-
RIO kinase 1
- TGF-β:
-
Transforming growth factor-β
- VEGF:
-
Vascular endothelial growth factor
- Wnt:
-
Wingless‐type
- WT1:
-
Wilms' tumor 1 transcription factor
References
Brosius FC, Tuttle KR, Kretzler M (2016) JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 59(8):1624–1627. https://doi.org/10.1007/s00125-016-4021-5
Zhou D, Zhou M, Wang Z et al (2019) PGRN acts as a novel regulator of mitochondrial homeostasis by facilitating mitophagy and mitochondrial biogenesis to prevent podocyte injury in diabetic nephropathy. Cell Death Dis 10(7):524. https://doi.org/10.1038/s41419-019-1754-3
Denhez B, Lizotte F, Guimond MO et al (2015) Increased SHP-1 protein expression by high glucose levels reduces nephrin phosphorylation in podocytes. J Biol Chem 290(1):350–358. https://doi.org/10.1074/jbc.M114.612721
Manda G, Checherita AI, Comanescu MV, Hinescu ME (2015) Redox signaling in diabetic nephropathy: hypertrophy versus death choices in mesangial cells and podocytes. Mediators Inflamm 2015:604208. https://doi.org/10.1155/2015/604208
Li JJ, Kwak SJ, Jung DS et al (2007) Podocyte biology in diabetic nephropathy. Kidney Int Suppl 106:S36-42. https://doi.org/10.1038/sj.ki.5002384
Wang X, Liu J, Zhen J et al (2014) Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int 86(4):712–725. https://doi.org/10.1038/ki.2014.111
Tuncdemir M, Ozturk M (2011) The effects of angiotensin-II receptor blockers on podocyte damage and glomerular apoptosis in a rat model of experimental streptozotocin-induced diabetic nephropathy. Acta Histochem 113(8):826–832. https://doi.org/10.1016/j.acthis.2010.12.003
Altintas MM, Reiser J (2019) Podocytes: way to go. Am J Pathol 189(2):226–228. https://doi.org/10.1016/j.ajpath.2018.11.003
Lin JS, Susztak K (2016) Podocytes: the weakest link in diabetic kidney disease? Curr Diab Rep 16(5):45. https://doi.org/10.1007/s11892-016-0735-5
Mathew S, Chen X, Pozzi A, Zent R (2012) Integrins in renal development. Pediatr Nephrol 27(6):891–900. https://doi.org/10.1007/s00467-011-1890-1
Pavenstadt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83(1):253–307. https://doi.org/10.1152/physrev.00020.2002
Saleem MA, O’Hare MJ, Reiser J et al (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13(3):630–638. https://doi.org/10.1681/ASN.V133630
Moreno JA, Sanchez-Nino MD, Sanz AB et al (2008) A slit in podocyte death. Curr Med Chem 15(16):1645–1654. https://doi.org/10.2174/092986708784911542
Liu M, Liang K, Zhen J et al (2017) Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun 8(1):413. https://doi.org/10.1038/s41467-017-00498-4
Anil Kumar P, Welsh GI, Saleem MA, Menon RK (2014) Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol (Lausanne) 5:151. https://doi.org/10.3389/fendo.2014.00151
Wolf G, Chen S, Ziyadeh FN (2005) From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54(6):1626–1634. https://doi.org/10.2337/diabetes.54.6.1626
Hostetter TH (2003) Hyperfiltration and glomerulosclerosis. Semin Nephrol 23(2):194–199. https://doi.org/10.1053/anep.2003.50017
Langham RG, Kelly DJ, Cox AJ et al (2002) Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition. Diabetologia 45(11):1572–1576. https://doi.org/10.1007/s00125-002-0946-y
Minakawa A, Fukuda A, Sato Y et al (2019) Podocyte hypertrophic stress and detachment precedes hyperglycemia or albuminuria in a rat model of obesity and type2 diabetes-associated nephropathy. Sci Rep 9(1):18485. https://doi.org/10.1038/s41598-019-54692-z
Dobrinskikh E, Okamura K, Kopp JB et al (2014) Human podocytes perform polarized, caveolae-dependent albumin endocytosis. Am J Physiol Renal Physiol 306(9):F941-951. https://doi.org/10.1152/ajprenal.00532.2013
Agrawal S, Smoyer WE (2017) Role of albumin and its modifications in glomerular injury. Pflugers Arch 469(7–8):975–982. https://doi.org/10.1007/s00424-017-2029-4
Qin XS, Tsukaguchi H, Shono A et al (2009) Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20(12):2534–2545. https://doi.org/10.1681/ASN.2009010011
Tossidou I, Teng B, Menne J et al (2010) Podocytic PKC-alpha is regulated in murine and human diabetes and mediates nephrin endocytosis. PLoS ONE 5(4):e10185. https://doi.org/10.1371/journal.pone.0010185
Teng B, Schroder P, Muller-Deile J et al (2016) CIN85 deficiency prevents nephrin endocytosis and proteinuria in diabetes. Diabetes 65(12):3667–3679. https://doi.org/10.2337/db16-0081
Okamura K, Dummer P, Kopp J et al (2013) Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PLoS ONE 8(1):e54817. https://doi.org/10.1371/journal.pone.0054817
Castrop H, Schiessl IM (2017) Novel routes of albumin passage across the glomerular filtration barrier. Acta Physiol (Oxf) 219(3):544–553. https://doi.org/10.1111/apha.12760
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592. https://doi.org/10.1002/cbin.11137
Xu X, Lai Y, Hua ZC (2019) Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep 39:1. 10.1042/BSR20180992
Hockenbery D (1995) Defining apoptosis. Am J Pathol 146(1):16–19
Chen YQ, Wang XX, Yao XM et al (2011) MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am J Nephrol 34(6):549–559. https://doi.org/10.1159/000333809
Chuang PY, Yu Q, Fang W et al (2007) Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int 72(8):965–976. https://doi.org/10.1038/sj.ki.5002456
Chuang PY, Dai Y, Liu R et al (2011) Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS ONE 6(8):e23566. https://doi.org/10.1371/journal.pone.0023566
Yu J, Wu H, Liu ZY et al (2017) Advanced glycation end products induce the apoptosis of and inflammation in mouse podocytes through CXCL9-mediated JAK2/STAT3 pathway activation. Int J Mol Med 40(4):1185–1193. https://doi.org/10.3892/ijmm.2017.3098
Ha TS, Hong EJ, Han GD (2015) Diabetic conditions downregulate the expression of CD2AP in podocytes via PI3-K/Akt signalling. Diabetes Metab Res Rev 31(1):50–60. https://doi.org/10.1002/dmrr.2562
Susztak K, Raff AC, Schiffer M, Böttinger EP (2006) Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55(1):225–233
Eid AA, Ford BM, Bhandary B et al (2013) Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes 62(8):2935–2947. https://doi.org/10.2337/db12-1504
Chen X, Liu W, Xiao J et al (2020) FOXO3a accumulation and activation accelerate oxidative stress-induced podocyte injury. FASEB J 34(10):13300–13316. https://doi.org/10.1096/fj.202000783R
Cao Y, Hao Y, Li H et al (2014) Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int J Mol Med 33(4):809–816. https://doi.org/10.3892/ijmm.2014.1642
Cao AL, Wang L, Chen X et al (2016) Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab Invest 96(6):610–622. https://doi.org/10.1038/labinvest.2016.44
Zhang Y, Gao X, Chen S et al (2017) Cyclin-dependent kinase 5 contributes to endoplasmic reticulum stress induced podocyte apoptosis via promoting MEKK1 phosphorylation at Ser280 in diabetic nephropathy. Cell Signal 31:31–40. https://doi.org/10.1016/j.cellsig.2016.12.009
Lim SK, Park SH (2012) The high glucose-induced stimulation of B1R and B2R expression via CB(1)R activation is involved in rat podocyte apoptosis. Life Sci 91(19–20):895–906. https://doi.org/10.1016/j.lfs.2012.07.020
Wang L, Li H (2020) MiR-770-5p facilitates podocyte apoptosis and inflammation in diabetic nephropathy by targeting TIMP3. Biosci Rep 40:4. 10.1042/BSR20193653
Guo J, Han J, Liu J, Wang S (2020) MicroRNA-770-5p contributes to podocyte injury via targeting E2F3 in diabetic nephropathy. Braz J Med Biol Res 53(9):e9360. https://doi.org/10.1590/1414-431x20209360
Zhou Z, Wan J, Hou X et al (2017) MicroRNA-27a promotes podocyte injury via PPARgamma-mediated beta-catenin activation in diabetic nephropathy. Cell Death Dis 8(3):e2658. https://doi.org/10.1038/cddis.2017.74
Bai X, Geng J, Li X et al (2018) Long noncoding RNA LINC01619 regulates MicroRNA-27a/Forkhead box protein O1 and endoplasmic reticulum stress-mediated podocyte injury in diabetic nephropathy. Antioxid Redox Signal 29(4):355–376. https://doi.org/10.1089/ars.2017.7278
Zhao SM, Zhang T, Qiu Q et al (2019) MiRNA-337 leads to podocyte injury in mice with diabetic nephropathy. Eur Rev Med Pharmacol Sci 23(19):8485–8492. https://doi.org/10.26355/eurrev_201910_19161
Zha F, Bai L, Tang B et al (2019) MicroRNA-503 contributes to podocyte injury via targeting E2F3 in diabetic nephropathy. J Cell Biochem 120(8):12574–12581. https://doi.org/10.1002/jcb.28524
Yang H, Wang Q, Li S (2016) MicroRNA-218 promotes high glucose-induced apoptosis in podocytes by targeting heme oxygenase-1. Biochem Biophys Res Commun 471(4):582–588. https://doi.org/10.1016/j.bbrc.2016.02.028
Qian X, Tan J, Liu L et al (2018) MicroRNA-134-5p promotes high glucose-induced podocyte apoptosis by targeting bcl-2. Am J Transl Res 10(3):989–997
Gao F, Yao M, Shi Y et al (2013) Notch pathway is involved in high glucose-induced apoptosis in podocytes via Bcl-2 and p53 pathways. J Cell Biochem 114(5):1029–1038. https://doi.org/10.1002/jcb.24442
Peixoto EB, Papadimitriou A, Teixeira DA et al (2015) Reduced LRP6 expression and increase in the interaction of GSK3beta with p53 contribute to podocyte apoptosis in diabetes mellitus and are prevented by green tea. J Nutr Biochem 26(4):416–430. https://doi.org/10.1016/j.jnutbio.2014.11.012
Chen A, Feng Y, Lai H et al (2020) Soluble RARRES1 induces podocyte apoptosis to promote glomerular disease progression. J Clin Invest 130(10):5523–5535. https://doi.org/10.1172/JCI140155
Zhang Y, Xu C, Ye Q et al (2021) Podocyte apoptosis in diabetic nephropathy by BASP1 activation of the p53 pathway via WT1. Acta Physiol (Oxf) 232(1):e13634. https://doi.org/10.1111/apha.13634
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075. https://doi.org/10.1038/nature06639
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721. https://doi.org/10.1126/science.290.5497.1717
Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20(3):460–473. https://doi.org/10.1089/ars.2013.5371
Yoshii SR, Mizushima N (2017) Monitoring and measuring autophagy. Int J Mol Sci 18:9. https://doi.org/10.3390/ijms18091865
Hartleben B, Gödel M, Meyer-Schwesinger C et al (2010) Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120(4):1084–1096. https://doi.org/10.1172/jci39492
Xin W, Li Z, Xu Y et al (2016) Autophagy protects human podocytes from high glucose-induced injury by preventing insulin resistance. Metabolism 65(9):1307–1315. https://doi.org/10.1016/j.metabol.2016.05.015
Woo CY, Kc R, Kim M et al (2020) Autophagic flux defect in diabetic kidney disease results in megamitochondria formation in podocytes. Biochem Biophys Res Commun 521(3):660–667. https://doi.org/10.1016/j.bbrc.2019.10.132
Li Z, Yuan Y, Meng Y et al (2017) Autophagy upregulation ameliorates cell injury in Sequestosome 1 knockout podocytes in vitro. Biochem Biophys Res Commun 490(2):98–103. https://doi.org/10.1016/j.bbrc.2017.05.102
Godel M, Hartleben B, Herbach N et al (2011) Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121(6):2197–2209. https://doi.org/10.1172/JCI44774
Dai H, Liu Q, Liu B (2017) Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res 2017:2615286. https://doi.org/10.1155/2017/2615286
Ji J, Zhao Y, Na C et al (2019) Connexin 43autophagy loop in the podocyte injury of diabetic nephropathy. Int J Mol Med 44(5):1781–1788. https://doi.org/10.3892/ijmm.2019.4335
Xiao T, Guan X, Nie L et al (2014) Rapamycin promotes podocyte autophagy and ameliorates renal injury in diabetic mice. Mol Cell Biochem 394(1–2):145–154. https://doi.org/10.1007/s11010-014-2090-7
Zhao X, Chen Y, Tan X et al (2018) Advanced glycation end-products suppress autophagic flux in podocytes by activating mammalian target of rapamycin and inhibiting nuclear translocation of transcription factor EB. J Pathol 245(2):235–248. https://doi.org/10.1002/path.5077
Liu WJ, Gan Y, Huang WF et al (2019) Lysosome restoration to activate podocyte autophagy: a new therapeutic strategy for diabetic kidney disease. Cell Death Dis 10(11):806. https://doi.org/10.1038/s41419-019-2002-6
Liu Y, Zhang J, Wang Y, Zeng X (2017) Apelin involved in progression of diabetic nephropathy by inhibiting autophagy in podocytes. Cell Death Dis 8(8):e3006. https://doi.org/10.1038/cddis.2017.414
Liu J, Li QX, Wang XJ et al (2016) beta-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis 7:e2183. https://doi.org/10.1038/cddis.2016.89
Hou Y, Lin S, Qiu J et al (2020) NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem Biophys Res Commun 521(3):791–798. https://doi.org/10.1016/j.bbrc.2019.10.194
Swanson PE, Carroll SB, Zhang XF, Mackey MA (1995) Spontaneous premature chromosome condensation, micronucleus formation, and non-apoptotic cell death in heated HeLa S3 cells. Ultrastructural observations Am J Pathol 146(4):963–971
Castedo M, Perfettini JL, Roumier T et al (2004) Cell death by mitotic catastrophe: a molecular definition. Oncogene 23(16):2825–2837. https://doi.org/10.1038/sj.onc.1207528
Mulay SR, Thomasova D, Ryu M et al (2013) Podocyte loss involves MDM2-driven mitotic catastrophe. J Pathol 230(3):322–335. https://doi.org/10.1002/path.4193
Galluzzi L, Vitale I, Aaronson SA et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3):486–541. https://doi.org/10.1038/s41418-017-0012-4
Migliorini A, Angelotti ML, Mulay SR et al (2013) The antiviral cytokines IFN-alpha and IFN-beta modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am J Pathol 183(2):431–440. https://doi.org/10.1016/j.ajpath.2013.04.017
Liapis H, Romagnani P, Anders HJ (2013) New insights into the pathology of podocyte loss: mitotic catastrophe. Am J Pathol 183(5):1364–1374. https://doi.org/10.1016/j.ajpath.2013.06.033
Thomasova D, Anders HJ (2015) Cell cycle control in the kidney. Nephrol Dial Transplant 30(10):1622–1630. https://doi.org/10.1093/ndt/gfu395
Hagen M, Pfister E, Kosel A et al (2016) Cell cycle re-entry sensitizes podocytes to injury induced death. Cell Cycle 15(14):1929–1937. https://doi.org/10.1080/15384101.2016.1191710
Lasagni L, Ballerini L, Angelotti ML et al (2010) Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells 28(9):1674–1685. https://doi.org/10.1002/stem.492
Lasagni L, Lazzeri E, Shankland SJ et al (2013) Podocyte mitosis - a catastrophe. Curr Mol Med 13(1):13–23. https://doi.org/10.2174/1566524011307010013
Shankland SJ (2006) The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69(12):2131–2147. https://doi.org/10.1038/sj.ki.5000410
Hara M, Oohara K, Dai DF, Liapis H (2019) Mitotic catastrophe causes podocyte loss in the urine of human diabetics. Am J Pathol 189(2):248–257. https://doi.org/10.1016/j.ajpath.2018.10.016
Nagata M, Nakayama K, Terada Y et al (1998) Cell cycle regulation and differentiation in the human podocyte lineage. Am J Pathol 153(5):1511–1520. https://doi.org/10.1016/s0002-9440(10)65739-2
Tang H, Lei CT, Ye C et al (2017) MDM2 is implicated in high-glucose-induced podocyte mitotic catastrophe via Notch1 signalling. J Cell Mol Med 21(12):3435–3444. https://doi.org/10.1111/jcmm.13253
Su H, Wan Q, Tian XJ et al (2015) MAD2B contributes to podocyte injury of diabetic nephropathy via inducing cyclin B1 and Skp2 accumulation. Am J Physiol Renal Physiol 308(7):F728-736. https://doi.org/10.1152/ajprenal.00409.2014
Gilmore AP (2005) Anoikis. Cell Death Differ 12(Suppl 2):1473–1477. https://doi.org/10.1038/sj.cdd.4401723
Weil EJ, Lemley KV, Yee B et al (2011) Podocyte detachment in type 2 diabetic nephropathy. Am J Nephrol 33(Suppl 1):21–24. https://doi.org/10.1159/000327047
Reddy GR, Kotlyarevska K, Ransom RF, Menon RK (2008) The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens 17(1):32–36. https://doi.org/10.1097/MNH.0b013e3282f2904d
Regoli M, Bendayan M (1997) Alterations in the expression of the alpha 3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia 40(1):15–22. https://doi.org/10.1007/s001250050637
Chen HC, Chen CA, Guh JY et al (2000) Altering expression of alpha3beta1 integrin on podocytes of human and rats with diabetes. Life Sci 67(19):2345–2353. https://doi.org/10.1016/s0024-3205(00)00815-8
Sawada K, Toyoda M, Kaneyama N et al (2016) Upregulation of α3β1-integrin in podocytes in early-stage diabetic nephropathy. J Diabetes Res 2016:9265074. https://doi.org/10.1155/2016/9265074
Nakamura T, Ushiyama C, Suzuki S et al (2000) Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 15(9):1379–1383. https://doi.org/10.1093/ndt/15.9.1379
Petermann AT, Krofft R, Blonski M et al (2003) Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int 64(4):1222–1231. https://doi.org/10.1046/j.1523-1755.2003.00217.x
Yamaguchi Y, Iwano M, Suzuki D et al (2009) Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis 54(4):653–664. https://doi.org/10.1053/j.ajkd.2009.05.009
Inoki K, Mori H, Wang J et al (2011) mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121(6):2181–2196. https://doi.org/10.1172/JCI44771
Xu Y, Gao H, Hu Y et al (2019) High glucose-induced apoptosis and necroptosis in podocytes is regulated by UCHL1 via RIPK1/RIPK3 pathway. Exp Cell Res 382(2):111463. https://doi.org/10.1016/j.yexcr.2019.06.008
Zhang Y, Chen X, Gueydan C, Han J (2018) Plasma membrane changes during programmed cell deaths. Cell Res 28(1):9–21. https://doi.org/10.1038/cr.2017.133
Khoury MK, Gupta K, Franco SR, Liu B (2020) Necroptosis in the Pathophysiology of Disease. Am J Pathol 190(2):272–285. https://doi.org/10.1016/j.ajpath.2019.10.012
Grootjans S, Vanden Berghe T, Vandenabeele P (2017) Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ 24(7):1184–1195. https://doi.org/10.1038/cdd.2017.65
He S, Huang S, Shen Z (2016) Biomarkers for the detection of necroptosis. Cell Mol Life Sci 73(11–12):2177–2181. https://doi.org/10.1007/s00018-016-2192-3
Sosna J, Voigt S, Mathieu S et al (2013) The proteases HtrA2/Omi and UCH-L1 regulate TNF-induced necroptosis. Cell Commun Signal 11:76. https://doi.org/10.1186/1478-811x-11-76
Cheng Q, Pan J, Zhou ZL et al (2021) Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin 42(6):954–963. https://doi.org/10.1038/s41401-020-00525-z
Shi J, Gao W, Shao F (2017) Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42(4):245–254. https://doi.org/10.1016/j.tibs.2016.10.004
Li X, Zeng L, Cao C et al (2017) Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res 350(2):327–335. https://doi.org/10.1016/j.yexcr.2016.12.006
Ding X, Jing N, Shen A et al (2021) MiR-21-5p in macrophage-derived extracellular vesicles affects podocyte pyroptosis in diabetic nephropathy by regulating A20. J Endocrinol Invest 44(6):1175–1184. https://doi.org/10.1007/s40618-020-01401-7
Li F, Mao X, Zhuang Q et al (2019) Inhibiting 4E-BP1 re-activation represses podocyte cell cycle re-entry and apoptosis induced by adriamycin. Cell Death Dis 10(3):241. https://doi.org/10.1038/s41419-019-1480-x
Qi YY, Zhou XJ, Cheng FJ et al (2018) Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-alpha in lupus nephritis. Ann Rheum Dis 77(12):1799–1809. https://doi.org/10.1136/annrheumdis-2018-213028
Ogawa-Akiyama A, Sugiyama H, Kitagawa M et al (2020) Podocyte autophagy is associated with foot process effacement and proteinuria in patients with minimal change nephrotic syndrome. PLoS ONE 15(1):e0228337. https://doi.org/10.1371/journal.pone.0228337
Guo C, Fu R, Zhou M et al (2019) Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J Autoimmun 103:102286. https://doi.org/10.1016/j.jaut.2019.05.014
Kim SY, Park S, Lee SW et al (2021) RIPK3 Contributes to Lyso-Gb3-Induced Podocyte Death. Cells 10:2. https://doi.org/10.3390/cells10020245
Ding F, Wickman L, Wang SQ et al (2017) Accelerated podocyte detachment and progressive podocyte loss from glomeruli with age in Alport Syndrome. Kidney Int 92(6):1515–1525. https://doi.org/10.1016/j.kint.2017.05.017
Acknowledgements
None.
Funding
This study was financially supported by the National Natural Science Foundation of China grants (81770711, 81873602, 81800610, 81974096, 81961138007, 81974097, 81900629, 82000664, 82170773, 82100794, 82100729).
Author information
Authors and Affiliations
Contributions
CZ conceptualized the review. AJ wrote a draft of the review. CZ and AS revised the paper. The figures were drawn by AJ. CZ performed the final edits.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, A., Song, A. & Zhang, C. Modes of podocyte death in diabetic kidney disease: an update. J Nephrol 35, 1571–1584 (2022). https://doi.org/10.1007/s40620-022-01269-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01269-1