Abstract
Background
Ambulatory-BP-monitoring (ABPM) is recommended for hypertension diagnosis and management in hemodialysis patients due to its strong association with outcomes. Intradialytic and scheduled interdialytic BP recordings show agreement with ambulatory BP. This study assesses in parallel the association of pre-dialysis, intradialytic, scheduled interdialytic and ambulatory BP recordings with cardiovascular events.
Methods
We prospectively followed 242 hemodialysis patients with valid 48-h ABPMs for a median of 45.7 months to examine the association of pre-dialysis, intradialytic, intradialytic plus pre/post-dialysis readings, scheduled interdialytic BP, and 44-h ambulatory BP with outcomes. The primary end-point was a composite one, composed of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, resuscitation after cardiac arrest, hospitalization for heart failure, coronary revascularization procedure or peripheral revascularization procedure.
Results
Cumulative freedom from the primary end-point was significantly lower with increasing 44-h SBP (group 1, < 120 mmHg, 64.2%; group 2, ≥ 120 to < 130 mmHg 60.4%, group 3, ≥ 130 to < 140 mmHg 45.3%; group 4, ≥ 140 mmHg 45.5%; logrank-p = 0.016). Similar were the results for intradialytic (logrank-p = 0.039), intradialytic plus pre/post-dialysis (logrank-p = 0.044), and scheduled interdialytic SBP (logrank-p = 0.030), but not for pre-dialysis SBP (logrank-p = 0.570). Considering group 1 as the reference group, the hazard ratios of the primary end-point showed a gradual increase with higher BP levels with all BP metrics, except pre-dialysis SBP. This pattern was confirmed in adjusted analyses. An inverse association of DBP levels with outcomes was shown with all BP metrics, which was no longer evident in adjusted analyses.
Conclusions
Averaged intradialytic and scheduled home BP measurements (but not pre-dialysis readings) display similar prognostic associations with 44-h ambulatory BP in hemodialysis patients and represent valid metrics for hypertension management in these individuals.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is the most common modifiable risk factor in patients with chronic kidney disease (CKD) and is associated with an increased risk for cardiovascular disease, progression of CKD and all-cause mortality [1, 2]. Hypertension is highly prevalent among patients with end-stage renal disease (ESRD) undergoing hemodialysis (HD); an estimated 85% is considered to be hypertensive, but only 30% of patients have appropriate control of their blood pressure (BP) [3]. Both diagnosis and management of hypertension in hemodialysis patients have traditionally been based on peridialytic BP measurements [4], but pre- and post- dialysis BP recordings are poorly reproducible and have either no association or non-linear associations (“J” or “U” shaped) with cardiovascular events and mortality [5,6,7]. Additionally, pre-dialysis BP tends to overestimate, while post-dialysis BP to underestimate the interdialytic BP load [3, 8, 9], a phenomenon relevant to the progressive increase of BP during the interdialytic interval and the rapid decrease of BP during the dialysis session in most patients [10].
Over the past few years, several lines of evidence have suggested that ambulatory BP monitoring (ABPM) or home BP recordings are far more appropriate in diagnosing and managing hypertension in dialysis patients [1, 11]. In contrast to the peridialytic BP levels, the BP measured with ABPM over the intradialytic interval is strongly associated with target-organ damage and hard outcomes, as well as cardiovascular and all-cause mortality [12,13,14]. Furthermore, ABPM is the only way of assessing short-term BP variability, which has been shown to increase with advancing CKD stages and to also be independently associated with major cardiovascular outcomes and mortality in hemodialysis patients [15,16,17]. Home BP measurements display better association with interdialytic ambulatory BP [18], as well as higher prognostic ability for target-organ damage and adverse outcomes compared to peridialytic BP [12, 13], representing another reliable option for hypertension management in hemodialysis [1, 14, 19].
Although ABPM clearly exceeds among all BP metrics in predicting adverse events and mortality, its clinical application in everyday dialysis practice is extremely limited due to several reasons including the presence of non-functional old arteriovenous fistulae, patient discomfort, lack of reimbursement, physician inertia and others [1, 11]. Such restrictions created the need for investigation for alternative valid BP metrics in hemodialysis [1, 11]. In a recent cross-sectional study, we showed that, in contrast to pre- and post- dialysis BP, the average of intradialytic, intradialytic plus pre- and post- dialysis, or scheduled interdialytic BP measurements have strong correlations with 44-h ambulatory BP and could represent alternative methods for assessing the true BP load in hemodialysis patients [20]. The aim of the present study was to assess in parallel the associations of each of the above BP metrics for cardiovascular events and mortality in a cohort of hemodialysis patients.
Methods
Study population
This is a prospective cohort study including hemodialysis patients from five affiliated Hemodialysis centers in Northern Greece. Ethical approval of this study was obtained from the Ethics Committee of the School of Medicine, Aristotle University of Thessaloniki. All evaluations were performed according to the Declaration of Helsinki (2013 Amendment). Inclusion criteria were: (i) age > 18 years; (ii) ESRD treated with a standard thrice-weekly hemodialysis schedule for > 3 months; (iii) informed written consent. Exclusion criteria included: (i) chronic atrial fibrillation or other arrhythmia; (ii) non-functional arteriovenous fistula in the contralateral brachial arm area of the one used for vascular access that could interfere with proper ABP recording; (iii) modification of dry weight or antihypertensive treatment during the month before study initiation; (iv) myocardial infarction, angina pectoris and ischemic stroke during the month prior to enrollment; (v) history of malignancy or any other condition with poor prognosis.
Supplemental Fig. 1 presents the flowchart of study participants for this analysis. From a total of 312 patients assessed for eligibility, 286 patients fulfilled the inclusion/exclusion criteria and consented to participate. Of these, 37 patients had invalid 48-h ABPM and seven invalid recordings of at least one of the other BP metrics. Thus, a total of 242 patients were included in the final analysis.
Study procedures and data collection
A total 242 hemodialysis patients that met the inclusion/exclusion criteria and had valid 48-h BP recordings between February 2013 and February 2018 were included in this analysis. The initial evaluation started before a mid-week dialysis session. Patients’ demographic and anthropometric characteristics, medical history, medication, co-morbidities, as well as other dialysis-related parameters were recorded. Blood samples were obtained for routine laboratory testing.
ABPM was performed with the Mobil-O-Graph device (IEM, Stolberg, Germany), an automated oscillometric device that has been validated for brachial BP measurement according to standard protocols [21], and that was shown to provide identical values with a widely used ABPM monitor [22]. ABPM was performed using appropriate-size cuffs on the non-fistula arm of participants. The recording started before a mid-week dialysis session and was programmed to last for a complete 48-h standard intra- and inter-dialytic period. After fitting, participants underwent their regular dialysis session, during which ultrafiltration volume was programmed based on their pre-specified dry weight. Patients were instructed to follow their usual daily activities and continue their regular medication until the next session. The monitor was set to measure BP every 20 min during day-time (07:00–22:59) and every 30 min during night-time (23:00–06:59). Measurements were included in the analysis if > 80% of the total recordings were valid and a maximum of 2 non-consecutive day-hours had fewer than two valid measurements and a maximum of 1 night-hour had no valid recording. In case of invalid measurements the ABPM was repeated one week later. In order to minimize the possible effect of manual BP measurements, only measurements recorded at the pre-specified time intervals at which the device was set to take measurements were used in this analysis.
BP metrics examined
We examined the association with outcomes of the following systolic blood pressure (SBP) and diastolic blood pressure (DBP) metrics:
-
i.
Pre-dialysis SBP/DBP, recorded before initiation of the dialysis session at the level of the brachial artery in the non-fistula arm with a validated oscillometric device.
-
ii.
Intradialytic SBP/DBP, defined as the mean of 12 BP measurements that were recorded every 20 min during a 4-h dialysis session with the Mobil-O-Graph device.
-
iii.
Intradialytic plus pre/post-dialysis SBP/DBP, defined as the mean of 14 BP measurements including pre-dialysis BP, intradialytic BP measurements and post-dialysis BP, of the same dialysis session.
-
iv.
Mean home SBP/DBP, calculated by taking the average of two scheduled Mobil-O-Graph BP measurements that were recorded at 08:00 am and 08:00 pm of the out-of-dialysis day.
-
v.
Ambulatory SBP/DBP, recorded during the standard interdialytic interval (i.e. 44-h SBP and 44-h DBP).
Study endpoints
In this prospective cohort study, patients were censored on the date of the first occurrence of the endpoints under study or on May 31st, 2020. The primary end point was a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, resuscitation after cardiac arrest, hospitalization for heart failure, coronary revascularization procedure or peripheral revascularization procedure. The secondary end-point was a combination of all-cause death, non-fatal myocardial infarction and non-fatal stroke.
Statistical analysis
Statistical analysis was performed with Statistical Package for Social Sciences version 22.0 (SPSS Inc, Chicago, IL, USA). Continuous data are presented as mean and standard deviation (SD) or median and interquartile range (IQR) depending on the normality of distribution which was examined with the Shapiro–Wilk test, while categorical data are presented as frequencies and percentages (n, %). To compare differences in the occurrence of study end-points among the different levels of each studied parameter, patients were categorized into four groups, based on their BP measurements. The systolic BP groups that were formed were the following: group 1, < 120 mmHg; group 2, ≥ 120 to < 130 mmHg; group 3, ≥ 130 to < 140 mmHg; group 4, ≥ 140 mmHg. Accordingly, the diastolic BP groups were: group 1, < 70 mmHg; group 2, ≥ 70 to < 80 mmHg; group 3, ≥ 80 to < 90 mmHg; group 4, ≥ 90 mmHg. Kaplan–Meier curves were created, and the log-rank test was applied in order to compare the differences between SBP or DBP groups in freedom from the studied end-points during follow-up. Furthermore, we performed univariate Cox regression analysis and calculated hazard ratios (HRs) with relevant 95% confidence intervals (CI) on the association of SBP or DBP groups of the various BP metrics with the primary and secondary outcome. We also performed multivariate Cox regression analysis of the associations of each BP metric with the primary outcome, including age, sex, dialysis vintage, diabetes mellitus, coronary heart disease and smoking in the model. Values of p < 0.05 (two-tailed) were considered statistically significant in all comparisons.
Results
Baseline characteristics and endpoints
Demographic, clinical, laboratory and dialysis-related characteristics of the study population are presented in Table 1. In total, 242 hemodialysis patients (151 men and 91 women) with mean age 62.71 ± 14.23 years and median hemodialysis vintage 27.8 (12–56.75) months were prospectively followed for 46.7 (28.7–75.7) months. At initial evaluation, the prevalence of major cardiovascular risk factors and associated co-morbidities was 29.3% for diabetes mellitus, 90.9% for hypertension (defined as ambulatory BP ≥ 130/80 mmHg or current antihypertensive treatment), 23.8% for dyslipidemia, 25.6% for coronary heart disease, 40.5% for heart failure, 7.9% for previous stroke and 7.0% for peripheral vascular disease.
Supplemental Table 1 presents the distribution of the patient population in each of the four BP groups with each one of the BP metrics studied. With the exception of pre-dialysis BP, the SBP and DBP of the remaining BP metrics that we examined display rather similar distributions of patients (i.e. for SBP around 16–25% of patients for groups 1, 2, and 3 and 35–40% for group 4).
Table 2 summarizes the frequencies of primary and secondary endpoints and their individual components. During follow-up, a total of 122 (50.4% of total) patients died, 9 (3.7%) due to myocardial infarction, 7 (2.9%) due to stroke, 53 (21.9%) patients experienced sudden death and 53 (21.9%) due to non-cardiac causes, such as infection, cachexia or cancer.
Associations of SBP and DBP captured with the various BP metrics with the primary outcome
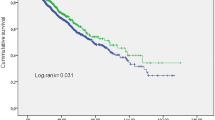
The Kaplan–Meier curves and the life tables of freedom from the primary endpoint for groups of pre-dialysis SBP, intradialytic SBP, intradialytic plus pre/post-dialysis SBP, mean home SBP and 44-h SBP are presented in Fig. 1. As shown in figure, cumulative freedom from the primary endpoint was significantly lower for groups of higher 44-h SBP (64.2%, 60.4%, 45.3% and 45.5% for group 1, group 2, group 3 and group 4, respectively; logrank-p = 0.016, Fig. 1E). Similarly, cumulative freedom from the primary endpoint was lower for higher groups of intradialytic SBP (64.7%, 57.6%, 38.5% and 49.4%, for groups 1–4, respectively; logrank-p = 0.039, Fig. 1B), intradialytic plus pre/post-dialysis SBP (63.0%, 58.3%, 44.4% and 47.6%, respectively; logrank-p = 0.044, Fig. 1C), and mean home SBP (64.9%, 50.0%, 55.1% and 44.9%, respectively; logrank-p = 0.030, Fig. 1D). In contrast to all the other SBP metrics, cumulative freedom from the primary endpoint was not significantly different between the groups of pre-dialysis SBP (73.1%, 40.6%, 55.3%, 50.7%, respectively; logrank-p = 0.570, Fig. 1A).
Kaplan–Meier survival curves and life tables for the occurrence of the primary end point (cardiovascular mortality or non-fatal myocardial infarction or non-fatal stroke or resuscitation after cardiac arrest or hospitalization for heart failure or coronary revascularization procedure or peripheral revascularization procedure) and A groups of pre-dialysis systolic blood pressure (pre-dialysis SBP), B groups of intradialytic systolic blood pressure (intradialytic SBP), C groups of intradialytic plus pre-dialysis plus post-dialysis systolic blood pressure (intradialytic plus pre/post-dialysis SBP), D groups of mean home systolic blood pressure (mean home SBP), and E groups of 44-h ambulatory systolic blood pressure (44-h SBP)
Figure 2 depicts the HRs of the primary end-point for patients categorized in the relevant groups of pre-dialysis SBP, intradialytic SBP, intradialytic plus pre/post-dialysis SBP, mean home SBP and 44-h SBP. In all cases, group 1 (i.e. SBP < 120 mmHg with each metric), served as the reference group. Mean SBP values of each group are displayed below the relevant HRs. Pre-dialysis SBP displayed an irregular pattern with regard to the association with the primary outcome, with group 2 showing an increased risk for the primary outcome (HR 3.05, 95% CI 1.28–7.25). In contrast, 44-h SBP and all other SBP metrics we examined were associated with a rather gradual increase of future risk of the primary outcome with higher BP values. Figure 3 depicts the HRs of the associations of each SBP metric with the primary outcome after adjustment for age, sex, dialysis vintage, diabetes mellitus, coronary heart disease and smoking in the model; as noted in figure, the patterns of associations of each BP metric with the primary outcome remained similar with the unadjusted analysis.
The Kaplan–Meier curves and the life tables of freedom from the primary end-point for groups of pre-dialysis DBP, intradialytic DBP, intradialytic plus pre/post-dialysis DBP, mean home DBP and 44-h DBP are presented in Supplemental Fig. 2. The HRs of the primary endpoint for patients categorized in the relevant groups of DBP with the various metrics are depicted in Supplemental Fig. 3. For all BP metrics cumulative freedom from the primary endpoint was higher for groups of higher DBP, showing an inverse association of DBP levels with outcomes. This association was significant for pre-dialysis DBP (logrank-p = 0.014), intradialytic DBP (logrank-p = 0.045), and intradialytic plus pre/post-dialysis DBP (logrank-p = 0.041), and displayed a borderline significance for mean home DBP (logrank-p = 0.071) and 44-h DBP (logrank-p = 0.111). The adjusted HRs of the associations of each DBP metric with the primary outcome are depicted in Supplemental Fig. 4. Notably, the inverse associations of 44-h BP or the surrogate BP metrics with outcomes were no longer evident in the adjusted analyses.
Associations of SBP and DBP captured with the various BP metrics with the secondary outcome
The HRs of the secondary endpoint for patients categorized in the relevant groups of pre-dialysis SBP, intradialytic SBP, intradialytic plus pre/post-dialysis SBP, mean home SBP and 44-h SBP are presented in Supplemental Fig. 5. Pre-dialysis SBP and mean home SBP show an irregular pattern, with an increased risk of group 2 for the secondary outcome, while intradialytic SBP, intradialytic plus pre/post-dialysis SBP and 44-h SBP display a gradual increase of the future risk of the secondary outcome from group 2 onwards. However, none of these associations between the various SBP metrics and the secondary outcome was statistically significant.
The HRs of the secondary endpoint for patients categorized in the relevant groups of DBP with the various metrics are depicted in Supplemental Fig. 6. All DBP metrics examined displayed a significant association with the secondary outcome, showing a decrease of the future risk with higher BP values.
Discussion
This is the first study examining the associations of pre-dialysis, intradialytic, intradialytic plus pre/post-dialysis, scheduled interdialytic and 44-h BP recordings with cardiovascular events and mortality in a cohort of hemodialysis patients. Our main finding was that with the exception of pre-dialysis BP, cumulative freedom of the primary endpoint was significantly lower and the HRs of the primary outcome were generally higher, with increasing BP levels evaluated with intradialytic, intradialytic plus pre/post-dialysis, scheduled interdialytic and 44-h SBP. An inverse association of DBP with cardiovascular events was present with all BP metrics examined. The above strongly suggest that either averaged intradialytic measurements or scheduled home measurements in the dialysis-off day display similar prognostic significance for cardiovascular events with 44-h ambulatory BP and could be valid alternatives for hypertension management in hemodialysis patients.
Several lines of evidence suggest that pre- and post-dialytic BP readings are imprecise estimates of interdialytic BP recorded with ABPM. A meta-analysis showed that pre-dialysis BP overestimated and post-dialysis BP underestimated the 44-h interdialytic BP load [23]. In a recent study in 396 hemodialysis patients, predialysis BP had 76% sensitivity and 54% specificity to diagnose hypertension evident by ABPM, while the prevalence of white-coat and masked hypertension were 18.2% and 14.1%, respectively [24]. This inability of pre- and post-dialysis BP to reflect the interdialytic BP is obviously related to the overall BP pattern in hemodialysis, with most patients having a gradual BP fall during dialysis session and a progressive increase during the interdialytic interval, and approximately 5–15% exhibiting intradialytic hypertension, i.e. a paradoxical BP increase during or immediately after the session [1, 10, 25]. In addition, hemodialysis patients represent perhaps the human patient population with the highest short-term BP variability [15, 16, 19, 25], and this adds to poor accuracy of peridialytic BP recordings.
A previous study in 135 hemodialysis patients tested the accuracy of various metrics (peridialytic, intradialytic, intradialytic including pre- and post-dialysis and the average of pre- and post-dialysis) using ambulatory BP as the reference standard. Intradialytic including pre- and post-dialysis BP was the most accurate and reproducible metric; an SBP cut-point of 135 mmHg had sensitivity and specificity of 90.4% and 75.9%, respectively, of diagnosing increased ambulatory SBP [8]. In a recent study, we tested the accuracy of pre- and post-dialysis, intradialytic, intradialytic without first/last dialysis reading, intradialytic plus pre/post-dialysis readings and scheduled interdialytic BP against 44-h BP [20]. We also observed that pre-dialysis SBP and DBP were significantly higher whereas post-dialysis SBP was significantly lower than relevant 44-h levels; pre- and post-dialysis BP readings had the worst agreement and the lowest sensitivity and specificity for ambulatory BP, among the metrics examined. In contrast, intradialytic, intradialytic plus pre/post-dialysis and scheduled interdialytic SBP, showed areas-under-the-curve of 0.850, 0.850 and 0.917 and maximal sensitivity/specificity of 73.0%/81.2%, 68.1%/88.1% and 82.3%/89.1%, respectively, for diagnosing 44-h SBP ≥ 130 mmHg.
As of this writing, no study has evaluated in parallel the prognostic significance of all the above BP metrics in hemodialysis patients. A previous study in 150 hemodialysis patients [26] examined the association of pre- and post-dialysis BP, home BP readings performed over 1 week using a validated oscillometric device and 44-h BP with cardiovascular and all-cause mortality over a median of 24 months. In contrast to peri-dialytic measurements that showed no associations with outcomes, both home BP and ambulatory BP showed a J-curve pattern of association; with the lowest BP quartile used as the reference, the second quartile displayed non-significantly lower and the fourth quartile higher HRs for the outcomes. For example, 44-h SBP > 145 mmHg were associated with HRs of 2.11 (95% CI 0.71–6.30) and 2.52 (95% CI 1.03–6.19), and home SBP > 157.9 mmHg with HRs of 2.10 (95% CI 0.70–6.25) and 1.80 (95% CI 0.78–4.15) for cardiovascular and all-cause mortality. Subsequent studies with larger populations also showed J-shaped or U-shaped associations of ambulatory SBP with outcomes [27, 28]. With regard to ambulatory DBP, an inverse association with outcomes in hemodialysis patients was also previously shown [17, 27]. Evidence from the general population suggests that the increased risk observed with low DBP values is actually explained by the association of increased pulse pressure (due to underlying arterial stiffness) with cardiovascular events [29]. In previous studies of our group in hemodialysis patients, we observed that increased pulse wave velocity has the strongest associations with cardiovascular events and is also the main determinant of the associations of BP parameters with adverse outcomes [17, 27, 30]. The J-curve is also considered by many to be an epiphenomenon related to increased mortality of severely diseased patients, including those with cachexia or advanced heart failure, which are not uncommon in the hemodialysis population. This was also confirmed by a large prospective study showing that hemodialysis patients with heart failure and/or atrial fibrillation had an inverse association of BP with mortality [28].
Our study expands the above observations from 44-h BP to other BP metrics. First, it confirms the absence of association of pre-dialysis BP and the significant association of 44-h SBP with cardiovascular events, showing that the first (44-h SBP < 120 mmHg) and second (44-h SBP ≥ 120 to < 130 mmHg) groups of ambulatory SBP signify the lowest risk. Second, it displays a rather similar pattern for scheduled home BP. Most importantly, it shows that when compared to 44-h SBP, intradialytic SBP displays a similar association and intradialytic plus pre/post-dialysis SBP shows an identical association with the primary endpoint. Finally, intradialytic, intradialytic plus pre/post-dialysis, scheduled interdialytic and 44-h DBP displayed similar inverse associations with the cardiovascular outcome in unadjusted analyses. In adjusted analyses these inverse associations were no longer evident, a finding also pointing towards that this was rather an epiphenomenon related to aging and co-morbid conditions. Again, the pattern of the associations was not morphologically different between 44-h BP and the other metrics, with the exception of pre-dialysis BP.
Consensus documents clearly suggest that ABPM is the most appropriate tool for hypertension management in hemodialysis patients but at the same time they recognize its limited application in everyday practice and call for exploration of BP metrics alternative to pre- and post-dialysis BP [1, 11]. The recent Kidney-Disease-Improving-Global-Outcomes (KDIGO) BP Guidelines advocate for the first time the use of an initial ABPM and, thereafter, home BP measurements to supplement standardized office BP in pre-dialysis patients with CKD [31]. In an ideal world ABPM would be used in routine intervals (i.e. once a year) or when deemed necessary in all hemodialysis patients to effectively obtain information on the complex BP phenotypes of these individuals; however, the reality is that in the majority of clinical settings worldwide hypertension management is still based on pre- and post-dialysis BP readings, which are largely inaccurate [3, 8, 9]. Our findings on the similarity of prognostic associations of the aforementioned BP metrics with those of 44-h BP are added to previous cross-sectional data on the validity of these metrics to reflect the 44-h BP load [8, 20]. As such, in situations where ABPM can be used, our data suggest that averaged intradialytic or averaged intradialytic plus pre/post-dialysis or scheduled home readings on the dialysis-off day could complement ABPM performance for hypertension management. In clinical settings where ABPM is not available or feasible, averaged intradialytic or home BP metrics should at least substitute the use of pre- and post-dialysis BP, as there are far more accurate approaches to identify the true BP load.
This study has strengths and limitations. It is the first to evaluate the prognostic significance of BP indices alternative to ambulatory BP in hemodialysis patients. We included valid 48-h recordings, which is a difficult task in diseased populations such as hemodialysis patients, and tested several candidate BP metrics with careful analyses. Our study population consisted of 242 patients and the mean follow-up lasted more than 4 years, which corresponds to the largest sample and follow-up period available among hemodialysis studies with 48-h ABPM. The main limitation is that we did not perform typical home BP recordings with validated oscillometric devices [32], but used the average of readings obtained at 8 am and 8 pm on a single non-dialysis day with the ABPM monitor. Thus, our findings refer to readings obtained outside the dialysis unit that are not typical home BP readings. However, most experts agree that home BP readings and awake ambulatory BP readings offer to a large extent similar information regarding the actual BP levels, while home BP and awake ambulatory BP share the same threshold for identifying hypertension [14, 32, 33]. Thus, although these scheduled interdialytic readings may be essentially similar to appropriate home BP readings at the relevant time points, our findings on this matter should be further confirmed with studies employing typical home BP. Furthermore this study, like most longitudinal studies in the field, assessed the association of BP recorded at a single time-point and not the association of BP trajectories over time with cardiovascular outcomes; thus, it is not known whether changes in BP control over time existed and whether they influenced the observed results.
Recent consensus documents recognize the limitations in expanded everyday use of ABPM and call for studies assessing the utility of different types of readings, such as simple BP readings, averaged intradialytic BP readings, and scheduled home BP readings compared to ABPM [11]. This study answers this call, showing that, in contrast to pre-dialysis readings, averaged intradialytic BP, averaged intradialytic plus pre- and post-dialysis BP, as well as scheduled interdialytic BP recordings at the out-of-dialysis day display significant associations with cardiovascular outcomes, that are similar to those of 44-h interdialytic ambulatory BP. These findings of similar prognostic significance are major additions to previous diagnostic accuracy studies, demonstrating that intradialytic, intradialytic plus pre/post-dialysis and scheduled interdialytic BP recordings show good agreement with ambulatory BP. Overall, our study suggests these three metrics are better tools for hypertension diagnosis and management in hemodialysis patients than pre- or post-dialysis BP; future recommendations should incorporate these findings.
Data availability
Not applicable.
Code availability
Not applicable.
References
Sarafidis PA, Persu A, Agarwal R et al (2017) Hypertension in dialysis patients: a consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) and the Hypertension and the Kidney working group of the European Society of Hypertension (ESH). Nephrol Dial Transplant 32:620–640. https://doi.org/10.1093/ndt/gfw433
United States Renal Data System (2020) USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA
Sarafidis PA, Mallamaci F, Loutradis C et al (2018) Prevalence and control of hypertension by 48-h ambulatory blood pressure monitoring in haemodialysis patients: a study by the European Cardiovascular and Renal Medicine (EURECA-m) working group of the ERA-EDTA. Nephrol Dial Transplant 33:1872. https://doi.org/10.1093/ndt/gfy263
(2005) K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45:S1–153
Foley RN, Herzog CA, Collins AJ, United States Renal Data System (2002) Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int 62:1784–1790. https://doi.org/10.1046/j.1523-1755.2002.00636.x
Robinson BM, Tong L, Zhang J et al (2012) Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 82:570–580. https://doi.org/10.1038/ki.2012.136
Kovesdy CP, Bleyer AJ, Molnar MZ et al (2013) Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med 159:233–242. https://doi.org/10.7326/0003-4819-159-4-201308200-00004
Agarwal R, Metiku T, Tegegne GG et al (2008) Diagnosing hypertension by intradialytic blood pressure recordings. Clin J Am Soc Nephrol 3:1364–1372. https://doi.org/10.2215/CJN.01510308
Zoccali C, Tripepi R, Torino C et al (2015) Moderator’s view: ambulatory blood pressure monitoring and home blood pressure for the prognosis, diagnosis and treatment of hypertension in dialysis patients. Nephrol Dial Transplant 30:1443–1448. https://doi.org/10.1093/ndt/gfv241
Karpetas A, Sarafidis PA, Georgianos PI et al (2015) Ambulatory recording of wave reflections and arterial stiffness during intra- and interdialytic periods in patients treated with dialysis. Clin J Am Soc Nephrol 10:630–638. https://doi.org/10.2215/CJN.08180814
Flythe JE, Chang TI, Gallagher MP et al (2020) Blood pressure and volume management in dialysis: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 97:861–876. https://doi.org/10.1016/j.kint.2020.01.046
Agarwal R, Brim NJ, Mahenthiran J et al (2006) Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension 47:62–68. https://doi.org/10.1161/01.HYP.0000196279.29758.f4
Agarwal R (2010) Blood pressure and mortality among hemodialysis patients. Hypertension 55:762–768. https://doi.org/10.1161/HYPERTENSIONAHA.109.144899
Parati G, Ochoa JE, Bilo G et al (2016) Hypertension in chronic kidney disease part 1: out-of-office blood pressure monitoring: methods, thresholds, and patterns. Hypertension 67:1093–1101. https://doi.org/10.1161/HYPERTENSIONAHA.115.06895
Karpetas A, Loutradis C, Bikos A et al (2017) Blood pressure variability is increasing from the first to the second day of the interdialytic interval in hemodialysis patients. J Hypertens 35:2517–2526. https://doi.org/10.1097/HJH.0000000000001478
Alexandrou ME, Loutradis C, Schoina M et al (2020) Ambulatory blood pressure profile and blood pressure variability in peritoneal dialysis compared with hemodialysis and chronic kidney disease patients. Hypertens Res 43:903–913. https://doi.org/10.1038/s41440-020-0442-0
Sarafidis PA, Loutradis C, Karpetas A et al (2019) The association of interdialytic blood pressure variability with cardiovascular events and all-cause mortality in haemodialysis patients. Nephrol Dial Transplant 34:515–523. https://doi.org/10.1093/ndt/gfy247
Agarwal R, Andersen MJ, Bishu K, Saha C (2006) Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int 69:900–906. https://doi.org/10.1038/sj.ki.5000145
Parati G, Ochoa JE, Bilo G et al (2016) Hypertension in chronic kidney disease part 2: role of ambulatory and home blood pressure monitoring for assessing alterations in blood pressure variability and blood pressure profiles. Hypertension 67:1102–1110. https://doi.org/10.1161/HYPERTENSIONAHA.115.06896
Sarafidis P, Theodorakopoulou MP, Loutradis C, Iatridi F, Alexandrou M-E, Karpetas A, Koutroumpas G, Raptis V, Ferro CJ, Papagianni A (2021) Accuracy of peridialytic, intradialytic, and scheduled interdialytic recordings in detecting elevated ambulatory blood pressure in hemodialysis patients. Am J Kidney Dis 78(5):630–639.e1. https://doi.org/10.1053/j.ajkd.2021.01.022
Wei W, Tölle M, Zidek W, van der Giet M (2010) Validation of the mobil-O-Graph: 24 h-blood pressure measurement device. Blood Press Monit 15:225–228. https://doi.org/10.1097/MBP.0b013e328338892f
Sarafidis PA, Lazaridis AA, Imprialos KP et al (2016) A comparison study of brachial blood pressure recorded with Spacelabs 90217A and Mobil-O-Graph NG devices under static and ambulatory conditions. J Hum Hypertens 30:742–749. https://doi.org/10.1038/jhh.2016.11
Agarwal R, Peixoto AJ, Santos SFF, Zoccali C (2006) Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol 1:389–398. https://doi.org/10.2215/CJN.01891105
Sarafidis PA, Mallamaci F, Loutradis C et al (2019) Prevalence and control of hypertension by 48-h ambulatory blood pressure monitoring in haemodialysis patients: a study by the European Cardiovascular and Renal Medicine (EURECA-m) working group of the ERA-EDTA. Nephrol Dial Transplant 34:1542–1548. https://doi.org/10.1093/ndt/gfy147
Bikos A, Angeloudi E, Memmos E et al (2018) A comparative study of short-term blood pressure variability in hemodialysis patients with and without intradialytic hypertension. Am J Nephrol 48:295–305. https://doi.org/10.1159/000493989
Alborzi P, Patel N, Agarwal R (2007) Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol 2:1228–1234. https://doi.org/10.2215/CJN.02250507
Sarafidis PA, Loutradis C, Karpetas A et al (2017) Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all-cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension 70:148–157. https://doi.org/10.1161/HYPERTENSIONAHA.117.09023
Mayer CC, Matschkal J, Sarafidis PA et al (2018) Association of ambulatory blood pressure with all-cause and cardiovascular mortality in hemodialysis patients: effects of heart failure and atrial fibrillation. J Am Soc Nephrol 29:2409–2417. https://doi.org/10.1681/ASN.2018010086
Kannel WB, Wilson PWF, Nam B-H et al (2004) A likely explanation for the J-curve of blood pressure cardiovascular risk. Am J Cardiol 94:380–384. https://doi.org/10.1016/j.amjcard.2004.04.043
Sarafidis PA, Loutradis C, Mayer CC et al (2019) Weak within-individual association of blood pressure and pulse wave velocity in hemodialysis is related to adverse outcomes. J Hypertens 37:2200–2208. https://doi.org/10.1097/HJH.0000000000002153
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group (2021) KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 99:S1–S87. https://doi.org/10.1016/j.kint.2020.11.003
Parati G, Stergiou GS, Asmar R et al (2010) European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens 24:779–785. https://doi.org/10.1038/jhh.2010.54
Parati G, Stergiou G, O’Brien E et al (2014) European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 32:1359–1366. https://doi.org/10.1097/HJH.0000000000000221
Acknowledgements
None.
Funding
This paper was not supported by any source and represents an original effort of the authors.
Author information
Authors and Affiliations
Contributions
Research idea and study design: AP, PAS; data acquisition: FI, MPT, AK, AB, M-EA, IT; statistical analysis: FI, MPT, AK; data analysis and interpretation: PAS, A-BH, CCM, supervision or mentorship: AP, GP.
Corresponding author
Ethics declarations
Conflict of interest
All authors disclose that they have no financial or other relationships which might lead to a conflict of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Ethical approval
Ethical approval of this study was obtained from the Ethics Committee of the School of Medicine, Aristotle University of Thessaloniki. All evaluations were performed according to the Declaration of Helsinki (2013 Amendment).
Informed consent
All participants provided informed written consent prior to study entry.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iatridi, F., Theodorakopoulou, M.P., Karpetas, A. et al. Association of peridialytic, intradialytic, scheduled interdialytic and ambulatory BP recordings with cardiovascular events in hemodialysis patients. J Nephrol 35, 943–954 (2022). https://doi.org/10.1007/s40620-021-01205-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-021-01205-9