Abstract
Background
Emerging evidence suggests that chronic metabolic acidosis (CMA) may have significant implications in terms of worsening renal disease in CKD patients, but the effect of CMA on renal function and structure has not been fully elucidated.
Method
We studied the acute and chronic consequences of an acid load (AL) on glomerular filtration rate (GFR) and renal histology in C57BL/6 mice. FITC-inulin clearance was performed at several time points; markers of renal fibrosis were studied at mRNA and protein levels; finally, kidney expression of candidate molecules triggering changes in renal function was studied.
Results
Glomerular hyperfiltration occurred within 1–3 days from AL; after 1 week, the GFR returned to baseline and then declined progressively within 15–21 days. The GFR decline was accompanied by the onset of renal fibrosis, as shown by Masson trichrome staining. Markers of renal fibrosis, namely α-smooth muscle actin and collagen-1, increased after 1 day of acid loading in both mRNA and protein levels and remained higher than baseline for up to 21 days. Well-known mediators of renal fibrosis, including transforming growth factor (TGF)-β and the intrarenal renin–angiotensin system (RAS) axis, were increased even before the decline of the GFR.
Conclusion
Acid load caused hyperfiltration acutely and a progressive decline of the GFR chronically; the evidence of renal fibrosis indicates that structural and not only functional renal changes occurred. The concomitant upregulation of TGF-β and intrarenal RAS axis indicates that those factors may be potentially involved in the progression of kidney disease in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maintaining blood pH in the physiological range is critical for health. The kidney plays a pivotal role in acid–base balance, a function that is progressively compromised as the glomerular filtration rate (GFR) falls. Chronic metabolic acidosis (CMA) is in fact a well known complication of chronic kidney disease (CKD), and is responsible for a number of adverse effects including muscle wasting, bone disease, and cardiovascular and metabolic complications [1]. Recent clinical trials have demonstrated that alkali administration in CKD patients is associated with a slower progression of renal disease [2, 3], suggesting that metabolic acidosis may in turn have a detrimental effect on the progression of renal disease. However, the exact effect of metabolic acidosis on renal structure and function is only partially known.
The present study aimed to investigate whether an acid load (AL) is associated with changes in renal function and structure. Our results demonstrated that an acid load causes glomerular hyperfiltration acutely and a progressive decline of the GFR chronically. The latter is coupled with the onset of interstitial fibrosis that may be triggered by the increased renal expression of transforming growth factor (TGF)-β and the intrarenal renin–angiotensin system (RAS) axis.
Materials and methods
Animal models
All experiments were conducted in 4-month-old male C57BL/6 mice. All animals received standard rodent chow. Two experimental models of metabolic acidosis were used: (1) mice were given 0.3 M of ammonium chloride (NH4Cl) in drinking water (tap water) versus tap water; (2) mice received an acidic diet by adding 0.4 M hydrogen chloride (HCl) to powdered rodent chow as described elsewhere [4] versus standard diet mixed with distilled water. All animals were adapted to metabolic cages for 3 days. Daily chow, water intake and body weights were measured and urine was collected under mineral oil. One group of mice was acid loaded following protocol (1) At 1, 2, 3, 7, 14, and 21 days of treatment, animals were anesthetized with inactin hydrate (Sigma-Aldrich, Merck, Darmstadt, Germany), arterial blood was collected from the carotid and analyzed immediately for pH, blood gases and electrolytes with an IDEXX VetStat Electrolyte and Blood Gas Analyzer (Westbrook, ME, USA). Inulin clearance was measured as described below. Another set of mice was acid loaded following protocol (2) All procedures were in accordance with the ethical standards of the institution at which the studies were conducted.
Inulin clearance
FITC-inulin clearance was performed as previously described [5]. Briefly, mice were weighed and anesthetized with inactin (Sigma-Aldrich) 100 mg/kg BW, tracheostomized, and placed on a surgical table. The left jugular vein was cannulated with a PE-10 catheter for infusion via a syringe pump (KDS Legato 200 high tech infusion pump, KD Scientific, Holliston, MA, USA). The right carotid artery was catheterized to monitor blood pressure through a blood pressure recorder (BP1; WPI, Sarasota, FL, USA). The bladder was catheterized with a PE-50 tube for urine collection. Then, mice received a constant infusion of FITC-inulin (Sigma-Aldrich) at a rate of 0.15 µL/min/g BW. After 60 min of equilibration, urine samples were collected every 30 min. A total of four urine and blood collections were made. GFR was calculated using a standard clearance formula. FITC-inulin concentrations in plasma and urine were measured by a colorimetric method (EnVision).
Immunohistochemistry
Immunohistochemistry was performed as previously described [5]. Briefly, mice were anesthetized by isoflurane and perfused with 4% paraformaldehyde (PFA) through the abdominal aorta. Deparaffinized and rehydrated tissue sections (1–4 µm) were stained with Masson trichrome (Bio-optica, Milan, Italy), according to the manufacturer’s instructions. Masson trichrome sections were viewed with bright field illumination.
c-DNA synthesis and real-time PCR
For quantitative polymerase chain reaction (qPCR), mRNA was isolated from the kidneys of control and NH4Cl-treated mice using the Trizol method (Ambion; Life Technologies, ThermoFisher Scientific, Waltham, MA, USA). RNA concentration was evaluated by photometric measurement at 260/280 nm. 1 µg RNA was used for cDNA synthesis using the EasyScript cDNA Synthesis Kit (Applied Biological Materials, Richmond, BC, Canada). PCR reactions were performed using the Maxima SYBR Green/Fluorescein qPCR Master mix (2×) (Thermo Scientific). Real-time PCR reactions were set up in 96-well reaction plates. All reactions were performed in a final volume of 25 µL containing 12.5 µL of Maxima SYBR Green/Fluorescein qPCR Master mix (2×), 500 ng of cDNA, 1 µL Forward Primer (2 µM), 1 µL Reverse Primer (2 µM) and nuclease free water. Thermocycling was performed on an iCycler Real-time PCR detection system (BioRad, Hercules, CA, USA) as follows: 10 min at 95 °C, 40 cycles of 15 s at 95 °C, 1 min 60 °C, 30 s at 72 °C. Expression levels were normalized to the actin expression and calculated using the ΔΔC method.
Immunoblotting
Kidneys were sectioned in the renal cortex and medulla. Tissues were homogenized with a TissueLyser (Qiagen, Hilden Germany) in lysis buffer (Sucrose 0.3M, Imidazole 25 mM, EDTA 1 mM, PMSF 1 mM) with protease and phosphatase inhibitor cocktails (Complete Protease Inhibitor Cocktail, Santa Cruz biotechnology, Santa Cruz, CA, USA; PhosSTOP, Roche, Branchburg, NJ, USA). Total protein concentration was measured by Bradford assay (Biorad Protein Assay). Proteins were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS PAGE) on a 4–12% Bis–Tris Gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Invitrolon PVDF, Invitrogen) [6]. The membranes were incubated overnight with primary antibodies against α-smooth muscle actin (α-SMA) (Invitrogen, ThermoFisher) and collagen-1 (Abcam, Cambridge, UK); after washing, membranes were incubated for 1 h with the secondary antibody and the blots were visualized with the ECL chemiluminescent substrate reagent kit (Pierce, Thermo Scientific) and quantized by Image-j.
Statistical analysis
All data were tested for significance using Student’s t test and ANOVA as appropriate. Only data with p < 0.05 were considered statistically significant. Data are reported as means ± standard deviation (SD).
Results
Mice fed NH4Cl developed metabolic acidosis
NH4Cl administration resulted in metabolic acidosis, as indicated by the reduction of both plasma pH and bicarbonate levels compared to controls (Table 1). As expected, the degree of metabolic acidosis was more severe after 1 day of acid loading than at longer time points. The AL was associated with a trend to increased urine volume during the first day of loading. Even though the mice had reduced water intake at the beginning, later there was no difference with controls until the latest phases of the experimental conditions.
NH4Cl loading was associated with acute glomerular hyperfiltration (GH) and a chronic progressive decline of GFR
After 1 day of NH4Cl feeding there was a 24% increase in inulin clearance compared to baseline (from 0.97 ± 0.07 to 1.28 ± 0.02 mL/min, p < 0.001). GH persisted at day 2 and 3 (inulin clearance: 1.17 ± 0.02 and 1.07 ± 0.10 mL/min respectively, with a 16 and 9% increase compared to baseline), and then went back to the baseline value after 7 days of acid feeding (inulin clearance: 1.00 ± 0.01 mL/min, p = 0.51). Later the GFR declined, reaching a decrease of 26 and 261% at 15 and 21 days of acid treatment (0.77 ± 0.01 and 0.27 ± 0.14 mL/min, respectively) (Fig. 1).
Acute and chronic effect of NH4Cl loading on the GFR. The GFR was measured as inulin clearance under control conditions and after NH4Cl loading. Acid load was associated with a significant increase in the GFR after 1–3 days of NH4Cl loading, then went back to normal and finally declined within 15–21 days. p < 0.05; **p < 0.01; ***p < 0.001. #not significant. GFR glomerular filtration rate
HCl diet was associated with acute GH
In a second group of animals, mice received HCl added to food to induce metabolic acidosis. This model of AL was performed in order to evaluate whether the acute changes in GFR were the consequence of a toxic effect of NH4Cl rather than the effect of changes in systemic pH. Interestingly, in this second group we saw a similar result. Acutely, HCl administration determined a severe metabolic acidosis associated with GH (1.03 ± 0.02 in controls vs. 1.19 ± 0.07 in acid-fed animals, p < 0.04) (Supplementary Fig. 1).
Chronic NH4Cl administration resulted in renal fibrosis
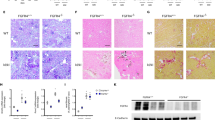
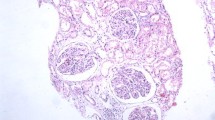
To examine whether the GFR decline was the result of hemodynamic factors or structural changes of renal parenchyma, Masson trichrome staining was performed. Interstitial fibrosis increased progressively after NH4Cl loading, as depicted in Fig. 2. This finding suggested that acid feeding led to a progressive damage of renal parenchyma, causing a decline of kidney function.
α-SMA was increased in both mRNA and protein levels after NH4Cl loading
α-SMA is the hallmark of myofibroblasts. It is barely detectable in the renal tissue of several models of renal failure [7]. Interestingly, α-SMA mRNA abundance from acid fed mice was increased soon after the acid loading. After 1 day from the acid loading it resulted fivefold higher than in controls, and remained significantly more abundant up to 21 days (Fig. 3a). In line with these data, α-SMA protein abundance was increased in both the renal cortex and medulla (Fig. 3b, c) at any time point from acid treatment.
α-SMA mRNA (a) and protein (b and c) abundance in renal tissue. Normalized α-SMA mRNA is shown in controls and after 1, 3 and 21 days of NH4Cl load (a). N = 4–6 per group. *p < 0.05, **p < 0.01 versus control. Immunoblotting showing α-SMA protein abundance in controls and in acidotic mice after 1, 3 and 21 days of loading in renal cortex (b) and medulla (c). N = 4–6 per group. *p < 0.05, **p < 0.01 versus control, ***p < 0.001 versus control. α-SMA, α-smooth muscle actin
Collagen-1 mRNA and protein abundance were both increased after NH4Cl loading
Similarly, collagen-1 mRNA abundance was more than fivefold higher than in controls after 1 day of acid loading, and remained significantly higher than baseline up to 21 days, as depicted in Fig. 4a. As Fig. 4b, c show, also at protein levels in both the cortex and medulla collagen-1 abundance increased during experimentation at both early and late time points.
Collagen-1 mRNA (a) and protein (b and c) abundance, TGF-β (d), renin (e) and angiotensinogen (f) mRNA levels in renal tissue. Normalized collagen-1 mRNA is shown in controls and in experimental mice at 1, 3 and 21 days of NH4Cl loading. N = 4–6 per group. *p < 0.05, **p < 0.01 versus control. Immunoblotting showing collagen-1 protein abundance in renal cortex (b) and in renal medulla (c). N = 4–6 per group. *p < 0.05, **p < 0.01 versus control. Normalized TGF-β (d), renin (e) and angiotensinogen (f) mRNA is shown in controls and after 1, 3 and 21 days of NH4Cl load. N = 4–6 per group. *p < 0.05, **p < 0.01, ***p < 0.001 versus control. TGF-β, transforming growth factor β
TGF-β, renin and angiotensinogen mRNA levels were augmented after NH4Cl loading
A growing number of different renal pro-fibrotic factors have been documented. TGF-β is virtually implicated in every type of CKD model, and it is believed to stimulate the epithelial-mesenchymal transition.
We found a significant increase of TGF-β mRNA abundance in total kidney at 1 day (1.5-fold vs. control, p < 0.0002), 3 days (5.2-fold vs. control, p < 0.019) and 21 days (6.6-fold vs. control, p < 0.020) after NH4Cl loading (Fig. 4d). Given the pivotal role of the RAS in regulating renal hemodynamics and mediating changes in renal structure, we measured the intrarenal expression of renin and angiotensinogen (Fig. 4e, f). Renin mRNA levels were 10-fold higher than baseline after 1 day of AL, and 24.99- and 11.76-fold higher than controls after 3 and 21 days of acid feeding. In accordance with this finding, angiotensinogen mRNA levels were significantly augmented compared to baseline (8.0-, 37.23- and 20.62-fold higher after 1, 3 and 21 days, respectively).
Discussion
The present study investigated the time-dependent changes in kidney structure and function associated with acid feeding, demonstrating that the latter caused glomerular hyperfiltration acutely and a progressive decline of the GFR chronically in mice, accompanied by the onset of renal fibrosis and the upregulation of putative pro-fibrotic agents, namely TGF-β and the intrarenal RAS axis. It is well established that the kidney plays a central role in maintaining acid base homeostasis. This is accomplished through several processes: the absorption of almost all filtered bicarbonate, the generation of new molecules of bicarbonate and NH4+ through glutamine metabolism, and the secretion of acid and protons [8].
In the face of an acid load these functions are exalted. The molecular basis underlying renal adaptive responses to metabolic acidosis has been extensively studied [9,10,11], but whether acidosis itself or compensatory adaptations to CMA may progressively determine deterioration of renal function is only partially understood. CMA is known to induce protein catabolism [12]. It has been shown that it is associated with: (1) growth retardation in children that is normalized by alkali feeding [13]; (2) the decline of lean body mass [14]; (3) increased bone resorption [15]; and (4) reduced albumin synthesis [16] in uremic patients. Paradoxically, in the rat, kidney CMA is accompanied by increased protein synthesis, and a reduction of protein degradation with consequent enlargement of kidney size [17]. But a systematic study showing the time-dependent changes in renal function and structure during CMA has never been performed.
This study shows that an acid load causes GH in the short term. The addition of ammonium chloride to drinking water resulted in a significant reduction of blood pH and plasma bicarbonate levels, increased inulin clearance and a trend to increased urine output after 1–3 days of acid loading. To investigate whether GH resulted from acidosis per se or was the effect of ammonium loading, we used a second model of acid feeding, by adding HCl to the food. Interestingly, also in the second model of acid loading we observed the occurrence of GH, after 1 day of acid administration. These results argue against the notion that NH4Cl directly causes GH; however, whether the reduced plasmatic pH or corollary effects associated with metabolic acidosis are responsible for glomerular hyperfiltration is unknown. Multiple causes may contribute to the change in glomerular filtration rate, but this study was not able to discern among the putative causative factors determining hyperfiltration.
Either reduced afferent arteriolar tone or increased efferent arteriolar tone may result in hyperfiltration [18]. Arteriolar tone is under the control of neuro-hormones and paracrine agents, and it is finely regulated also by the tubuloglomerular feedback [19,20,21]. It is well known that any factor reducing Na+ and Cl− loading to the macula densa attenuates the tubuloglomerular feedback, resulting in the reduction of afferent arteriolar vasoconstriction with consequent glomerular hyperfiltration [22]. An acid load is known to promote Na+ absorption along the proximal tubule [23], which may in turn induce GH as the result of reduced luminal Na+ concentration at the macula densa. Besides this putative mechanism, Ng et al. showed that chronic NH4Cl loading caused the activation of intrarenal RAS axis in rats [24], suggesting that it may play a role in the changes in GFR under chronic conditions [25, 26]. Our study demonstrated that an acid load increased renin and angiotensinogen mRNA levels in both the short and long term after acid feeding. This association suggests that intrarenal RAS activation may have a role in both glomerular hyperfiltration (acutely) and the progressive decline in the GFR (chronically). However, further studies are needed to confirm whether intrarenal RAS activation is implicated in the worsening renal structure and function in acidotic conditions. In addition, RAS axis activation may have systemic effects, such as increased blood pressure, but these remain to be demonstrated.
In our study, after 1 week of NH4Cl loading, the GFR went back to normal and later tended to decline. Several conditions leading to chronic renal disease feature this peculiar change in the GFR over the years, before ending up in end-stage renal disease, including diabetic nephropathy and hypertension [27,28,29]. The GFR decline was accompanied by the increased mRNA and protein abundance of early markers of renal fibrosis, such as α-SMA. The latter was higher than in controls even before the decline in GFR, and was followed by the appearance of renal fibrosis, as seen in histological sections. This demonstrates that the GFR decline is not only the result of hemodynamic changes, since it is paralleled by an alteration of the renal parenchyma, with signs of fibrosis. Multiple pathways triggering renal fibrosis have been described in CKD. TGF-β is considered an important mediator of CKD progression [30, 31]. Several studies have demonstrated that it is a key molecule mediating renal parenchyma degeneration independently of the initial kidney injury, by acting on all renal compartments [32]. In this study, we showed that NH4Cl loading caused a significant increase in renal TGF-β expression, suggesting a possible pathogenic role in this model of chronic acidosis.
Limitations of our study are: (1) the lack of interventional procedures to address whether changes of renal function and structure could be prevented or attenuated by inhibiting these pathways; and (2) the difficulties to discriminate between systemic acidosis and NH4Cl loading in the determination of renal damage under chronic conditions. However, the key strength of the study is the evidence that two models of metabolic acidosis strongly affect renal function acutely, and that chronic NH4Cl loading results in the impairment of renal structure and function, in the absence of baseline renal insufficiency.
Conclusion
In conclusion, our study shows that two experimental models of acid loading caused glomerular hyperfiltration acutely; chronic NH4Cl feeding was associated with a progressive decline of the GFR, paralleled by the onset of renal fibrosis, suggesting that the dysfunction was caused by tissue damage and not by hemodynamic fluctuations. The onset of renal fibrosis was preceded by the activation of the RAS axis and increased TGF-β expression, known mediators of renal fibrosis. Further studies are needed to explore the relationship between activation of intrarenal renin and angiotensinogen and increased TGF-β with renal dysfunction.
References
Dobre M, Rahman M, Hostetter TH (2005) Current status of bicarbonate in CKD. J Am Soc Nephrol 26(3):515–523. https://doi.org/10.1681/ASN.2014020205
Gaggi M, Sliber C, Sunder-Plassmann G (2014) Effect of oral alkali supplementation on progression of chronic kidney disease. Curr Hypertens Rev 10(2):112–120
Jeong J, Kwon SK, Kim HY (2014) Effect of bicarbonate supplementation on renal function and nutritional indices in predialysis advanced chronic kidney disease. Electrolyte Blood Press 12(2):80–87. https://doi.org/10.5049/EBP.2014.12.2.80
Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID (2010) Role of the Rhesus glycoprotein, Rh B glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299(5):F1065–F1077. https://doi.org/10.1152/ajprenal.00277.2010
Iervolino A, Trepiccione F, Petrillo F, Spagnuolo M, Scarfò M, Frezzetti D, De Vita G, De Felice M, Capasso G (2015) Selective dicer suppression in the kidney alters GSK3β/β-catenin pathways promoting a glomerulocystic disease. PLoS ONE 10(3):e0119142. https://doi.org/10.1371/journal.pone.0119142
Capobianco V, Caterino M, Iaffaldano L, Nardelli C, Sirico A, Del Vecchio L, Martinelli P, Pastore L, Pucci P, Sacchetti L (2016) Proteome analysis of humanamniotic mesenchymal stem cells (hA-MSCs) reveals impaired antioxidantability, cytoskeleton and metabolic functionality in maternal obesity. Sci Rep 29(6):25270. https://doi.org/10.1038/srep25270
Strutz F, Zeisberg M (2006) Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 17(11):2992–2998
Curthoys NP, Moe OW (2014) Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9(9):1627–1638. https://doi.org/10.2215/CJN.10391012
Bounoure L, Ruffoni D, Müller R, Kuhn GA, Bourgeois S, Devuyst O, Wagner CA (2014) The role of the renal ammonia transporter Rhcg in metabolic responses to dietary protein. Am Soc Nephrol 25(9):2040–2052. https://doi.org/10.1681/ASN.2013050466
Capasso G, Rizzo M, Pica A, Di Maio FS, Moe OW, Alpern RJ, De Santo NG (2002) Bicarbonate reabsorption and NHE-3 expression: abundance and activity are increased in Henle’s loop of remnant rats. Kidney Int 62(6):2126–2135
Laghmani K, Preisig PA, Alpern RJ (2002) The role of endothelin in proximal tubule proton secretion and the adaptation to a chronicmetabolic acidosis. J Nephrol 15(Suppl 5):S75–S87
Caso G, Garlick PJ (2005) Control of muscle protein kinetics by acid-base balance. Curr Opin Clin Nutr Metab Care 8(1):73–76
Kraut JA, Madias NE (2011) Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatr Nephrol 26(1):19–28. https://doi.org/10.1007/s00467-010-1564-4
Fahal IH (2014) Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant 29(9):1655–1665. https://doi.org/10.1093/ndt/gft070
Krieger NS, Frick KK, Bushinsky DA (2004) Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens 13(4):423–436
Grossman SB, Yap SH, Shafritz DA (1977) Influence of chronic renal failure on protein synthesis and albumin metabolism in rat liver. J Clin Invest 59(5):869–878
Lotspeich WD (1965) Renal hypertrophy in metabolic acidosis and its relation to ammonia excretion. Am J Physiol 208:1135–1142
Zacchia M, Capasso G (2015) The importance of uromodulin as regulator of salt reabsorption along the thick ascending limb. Nephrol Dial Transplant 30(2):158–160
Carlström M, Wilcox CS, Arendshorst WJ (2015) Renal autoregulation in health and disease. Physiol Rev 95(2):405–511. https://doi.org/10.1152/physrev.00042.2012
Anastasio P, Viggiano D, Zacchia M, Altobelli C, Capasso G, Gaspare De Santo N (2017) Delay in renal hemodynamic response to a meat meal in severe obesity. Nephron 136(2):151–157. https://doi.org/10.1159/000453283
Zacchia M, Capasso G (2011) Dehydration: a new modulator of klotho expression. Am J Physiol Renal Physiol 301(4):F743–F744
Mollica F, Saviano C, De Santo NG (1995) Tubule effects of glomerular hyperfiltration: an integrated view. Semin Nephrol 15(5):419–425
Twombley K, Gattineni J, Bobulescu IA, Dwarakanath V, Baum M (2010) Effect of metabolic acidosis on neonatal proximal tubule acidification. Am J Physiol Regul Integr Comp Physiol 299(5): R1360–R1368. https://doi.org/10.1152/ajpregu.00007.2010
Ng HY, Chen HC, Tsai YC, Yang YK, Lee CT (2011) Activation of intrarenal renin-angiotensin system during metabolic acidosis. Am J Nephrol 34(1):55–63. https://doi.org/10.1159/000328742
Ponticelli C, Cucchiari D (2017) Renin-angiotensin system inhibitors in kidney transplantation: a benefit-risk assessment. J Nephrol 30(2):155–157
Dattolo PC, Gallo P, Michelassi S, Paudice N, Cannavò R, Romoli E, Fani F, Tsalouchos A, Mehmetaj A, Ferro G, Sisca S, Pizzarelli F (2016) Conservative management of chronic kidney disease stage 5: role of angiotensin converting enzyme inhibitors. J Nephrol 29(6):809–815
Khairallah P, Scialla JJ (2017) Role of acid-base homeostasis in diabetic kidney disease. Curr Diab Rep 17(4):28. https://doi.org/10.1007/s11892-017-0855-6
Stel VS, Brück K, Fraser S, Zoccali C, Massy ZA, Jager K (2017) International differences in chronic kidney disease prevalence: a key public health and epidemiologic research issue. Nephrol Dial Transplant 32(suppl_2):ii129–ii135. https://doi.org/10.1093/ndt/gfw420
Perna AF, Di Nunzio A, Amoresano A, Pane F, Fontanarosa C, Pucci P, Vigorito C, Cirillo G, Zacchia M, Trepiccione F, Ingrosso D (2017) Divergent behavior of hydrogen sulfide pools and of the sulfur metabolite lanthionine, a novel uremic toxin, in dialysis patients. Biochimie 126:97–107. https://doi.org/10.1016/j.biochi.2016.04.018
Petrazzuolo O, Trepiccione F, Zacchia M, Capasso G (2010) Hypertension and renal calcium transport. J Nephrol 23(Suppl 16):S112-S117
López-Hernández FJ, López-Novoa JM (2012) Role of TGF-β in chronic kidney disease: an integration of tubular, glomerular and vascular effects. Cell Tissue Res 347(1):141–154. https://doi.org/10.1007/s00441-011-1275-6
Patschan D, Schwarze K, Henze E, Patschan S, Müller GA (2016) Endothelial autophagy and endothelial-to-mesenchymal transition (EndoMT) in eEPC treatment of ischemic AKI. J Nephrol 29(5):637–644
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with humans participants.
Informed consent
For this type of study formal consent form is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40620_2018_493_MOESM1_ESM.pptx
Supplementary material 1 (PPTX 83 KB). Supplementary figure 1. Acute effect of HCl loading on the GFR. HCl addition to the food caused metabolic acidosis (left panel) and glomerular hyperfiltration (right panel). N = 6. *p < 0.05 versus control.
Rights and permissions
About this article
Cite this article
Tammaro, G., Zacchia, M., Zona, E. et al. Acute and chronic effects of metabolic acidosis on renal function and structure. J Nephrol 31, 551–559 (2018). https://doi.org/10.1007/s40620-018-0493-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-018-0493-3