Abstract
Tacrolimus has long been the cornerstone of the immunosuppressive standard-of-care in kidney transplantation. Until recently, only an immediate-release formulation of tacrolimus was available in the clinic for twice-daily administration, a schedule that is known to hamper prescription adherence and contributes to the already significant tacrolimus interactions with other drugs and meals. In order to improve patient compliance, two once-daily prolonged-release formulations of tacrolimus have recently been developed and approved. Here we will analyze the main characteristics of these two prolonged-release formulations with the aim to provide practical clinical information for a fully aware drug prescription. Finally, the theoretical advantages of the prolonged-release formulations in terms of prescription adherence, blood level steadiness and drug efficacy and tolerability will be critically reviewed, in order to define the profile of renal recipients who may benefit most from the switch to once-daily tacrolimus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The association of tacrolimus with mycophenolate and steroids represents the standard of care as maintenance immunosuppressive regimen for kidney transplant recipients [1]. Tacrolimus is the cornerstone of such regimen: it is a macrolide discovered in Tsukuba, Japan, in 1987 from the fermentation broth of the bacterium Streptomyces tsukubaensis; hence the name comes from the words Tsukuba, macrolide, and immunosuppressant. Tacrolimus belongs to the same class of calcineurin inhibitors as the older cyclosporine, which tacrolimus has almost completely replaced in the clinic, being more effective in protecting the kidney from rejection [2, 3], and it is now used by more than 90 % of transplant recipients in the United States [4].
As with cyclosporine, tacrolimus exerts its immunosuppressive function by early blocking the calcium-dependent intracellular signals that drive the activation of T-cells [5]. Upon antigen recognition by T cell receptors and appropriate co-stimulation, an increase in intracellular calcium occurs that activates the enzyme calcineurin, a calcium/calmodulin-dependent phosphatase protein. Calcineurin mediates dephosphorylation of the cytoplasmic sub-unit of nuclear factor of activated T-cells (NF-ATc) allowing its translocation into the nucleus where it binds to DNA and promotes the production, amongst others, of interleukin (IL)-2. IL-2 is a crucial mediator of the activation of T cells, the principal players in alloreactivity and rejection. Tacrolimus inhibits calcineurin by forming a complex with the immunophilin FK-binding protein 12 (FKBP-12), a complex capable of binding and blocking calcineurin [5, 6]. Unlike tacrolimus, cyclosporine, which is 10–100 times less potent than tacrolimus, blocks calcineurin activity upon the formation of a complex with a specific immunophilin, cyclophilin [6].
The original and most common formulation of tacrolimus is the immediate-release (IR) Prograf (Astellas Pharma) in capsules for twice-daily administration, which was approved in 1994 by the European Medicines Agency (EMA) and US Food and Drug Administration (FDA). Today, besides the originator drug, several generic formulations of tacrolimus for twice-daily administration have become available. For the purpose of the current review, we will refer to any twice-daily formulation (either originator or generic) as immediate-release tacrolimus (IR-Tac). Starting from 2007, a patented prolonged-release once-daily formulation named Advagraf (Astellas Pharma), also known as Astagraf XL in US, and Prograf XL in Australia, was introduced in the market. In more recent years, Envarsus (Veloxis, marketed in Europe by Chiesi Farmaceutici) has been released as another patented prolonged-release once-daily formulation with peculiar pharmacokinetic (PK) properties and improved bioavailability: for the purpose of this review, from now on these two formulations will be referred to as Once-daily XL-Tac and Once-daily LCP-Tac, respectively. Unfortunately, due to its recent release, relatively few data are available for Once-daily LCP-Tac, all of them coming from sponsored clinical trials [7, 8].
The use of tacrolimus is complicated by its narrow therapeutic index [9], and wide inter- and intra-patient variability [10]. Narrow therapeutic index means that small variations in drug exposure might significantly affect patient outcomes; therefore, tacrolimus dosing must be guided by strict therapeutic drug monitoring [1]. Therapeutic drug monitoring is usually carried out by assessing the whole blood trough level, as this parameter correlates significantly well with the 24-h drug exposure, i.e., area under the curve (AUC)0–24 [11]. During the first 3–6 months after transplantation, trough levels are typically targeted at 5–15 µg/l, with values closer to the lower or upper limit depending on the time elapsed from surgery, type of induction treatment, concomitant maintenance immunosuppressive drugs, recipient’s immunological risk profile, and organ quality. Beyond 3–6 months after transplantation, blood levels are usually kept in the range of 5–8 µg/l although what the optimal target trough levels in the long term are is far from established [12–14].
The high inter- and intra-patient variability of tacrolimus is mainly related to polymorphisms of the metabolizer cytochrome P-450, interactions with food and other drugs, varying clinical conditions, and drug adherence. Cytochrome P-450 polymorphism and drug interactions both affect tacrolimus bioavailability and hepatic metabolism [10]. Indeed, tacrolimus has a low bioavailability of approximately 15–20 % that is related to the presence of p-glycoprotein efflux protein and cytochrome P-450 enzymes 3A4 and 3A5 (CYP3A4, CYP3A5) in the gut epithelial cells and hepatocytes. When tacrolimus enters the gut epithelial cells, it is metabolized by CYP3A4 and pumped back into the gut lumen by p-glycoprotein efflux protein. Thereafter, tacrolimus arrives at the more distal segments of the bowel that contain lower amounts of both enzymes. The fraction of tacrolimus reaching the bloodstream (15–20 %, i.e., the bioavailability) distributes within erythrocytes (about 90 %) and in plasma where it bonds to albumin, α1-acid glycoprotein, and to lipoproteins. Only less than 2 % of tacrolimus is unbound in the plasma, available for entering into the lymphocytes, which are the target site of action. It is noteworthy that tacrolimus levels are measured as whole blood concentration, which variably reflects the active unbound fraction [15]. Circulating tacrolimus is eventually metabolized by CYP3A5 in the hepatocytes. Individuals expressing the CYP3A5 polymorphism (i.e., CYP3A5*1/*1 and CYP3A5*1/*3 genotype), which occurs in 2/3 of African-Americans but only in 1/20 of Caucasians, exhibit reduced tacrolimus bioavailability and increased metabolism, and require a 50–100 % dose escalation to achieve the same target levels (Table 1). Likewise, intake of food, herbal products and drugs that inhibit or increase the expression of glycoprotein p and/or CYP3A significantly affect tacrolimus bioavailability and metabolism [16, 17]. Grapefruit, azole antifungals, nucleoside/nucleotide reverse transcriptase inhibitors and non-dihydropyridine calcium antagonists increase the tacrolimus level by interfering with glycoprotein-p and/or CYP3A4 activity, whereas St. John’s Wort, rifampicin, carbamazepine, and efavirenz reduce tacrolimus levels by increasing CYP3A4 expression [18, 19]. Many other factors affect tacrolimus blood levels and the inter- and intra-patient variability such as serum albumin and hemoglobin levels, high-fat meals [20] and gastrointestinal motility [15]. Finally, for reasons unknown tacrolimus metabolism is greater overnight [21] (Table 1).
Once-daily XL-Tac was developed with the specific aim of being administered once daily, through the preparation of a granulate formulation that prolongs the release of the drug with a 90 % absorption between 6 and 12 h post-ingestion (Table 2) [22]. The granulate so obtained is filled into capsules available in 0.5, 1.0, 3.0 and 5.0 mg strengths [23]. On the other side, the development of Once-daily LCP-Tac was driven by the need to obtain a drug not only with prolonged release, but also with a significantly improved bioavailability. Once-daily LCP-Tac is in fact produced according to a proprietary MeltDose® drug delivery technology that increases the bioavailability of drugs with low water solubility by breaking them down into the smallest possible units, as single molecules [24, 25], and, in so doing, increasing the surface/volume ratio of each drug particle. A “melt solution” of the drug is obtained by heating it with a patented nozzle. Such a solution is then sprayed on a particulate carrier, on which it solidifies in a state of “solid solution” originating a granulate. The granulate, responsible for the prolonged release, is finally compressed into tablets. The tablets are available in 0.75, 1.0 and 4.0 mg strengths [26].
While the tacrolimus blood concentration–time (i.e., pharmacokinetic) profile differs between the IR formulation and the two once-daily formulations (extensively reviewed by Staatz and Teet) [27], irrespective of this the trough levels to be targeted are the same. As a rule of thumb, the dose required to maintain a similar trough level is often slightly higher with Once-daily XL-Tac than IR-Tac, whereas it is consistently lower with Once-daily LCP-Tac. In terms of total daily dose, a 1 to 1 mg conversion from IR-Tac to Once-daily XL-Tac is usually recommended in stable renal recipients [23]. However, such a conversion may result in a 10–30 % reduced tacrolimus exposure and an increased dosage is often required to maintain therapeutic TL [28–30]. As for Once-daily LCP-Tac, two studies in stable kidney and liver recipients have proven that a roughly 30 % lower dose is required to maintain the same drug exposure compared to IR-Tac, because of the improved bioavailability of Once-daily LCP-Tac [31, 32]. Therefore, a conversion rate of 0.7 ratio is recommended [26] (e.g., 5 mg/day with IR-Tac correspond to 3.5 mg/day with Once-daily LCP-Tac). Nevertheless, it should be noted that IR-Tac, Once-daily XL-Tac and Once-daily LCP-Tac are not bioequivalent, as the different formulations generate substantially different blood concentration time profiles. At individual level, a change from one formulation to another may potentially cause unexpected changes in drug exposure and tolerability. Therefore, in the case of intentional formulation conversion, it is recommended that the transplant physician closely monitor drug trough levels [33]. In the absence of specific indications, we suggest that the tacrolimus trough level be assessed roughly 5 days after the conversion, in the steady state, and fortnightly thereafter for at least 8 weeks.
As for the starting dose in de novo renal recipients, the recommendation for Once-daily XL-Tac is similar to that for IR-Tac, ranging from 0.1 to 0.3 mg/kg/day depending on the target blood level (0.1 and 0.2 mg/kg/day typically targeting blood levels of 5–10 and 10–20 µg/l, respectively), and the concomitant use of an inductive agent such as basiliximab and thymoglobulin [23]. In contrast, the recommended starting dose of Once-daily LCP-Tac is currently set to 0.17 mg/kg/day [26]. One might expect that the Once-daily LCP-Tac equivalent dose of IR-Tac 0.20 would be 0.14 mg/kg/day (i.e., 0.20 times 0.7). However, in a RCT in 63 de novo kidney recipients 37 % of patients had a trough level below 6 ng/ml, the lower threshold of the target range, on day 2 following the first dose of Once-daily LCP-Tac 0.14 mg/kg/day [34]. Based on this result, a higher starting dose of 0.17 mg/kg/day was set in order to avoid delay in reaching the target therapeutic range [7], an undesired phenomenon that has been reported with the use of Once-daily XL-Tac [33].
In terms of PK profile in adult kidney recipients, Once-daily XL-Tac exhibits a slightly lower Cmax, longer time to reach it (Tmax) [35–37], and possibly a lower variability compared to IR-Tac [29, 37]. These advantageous PK properties are even more evident for Once-daily LCP-Tac [see the section "Blood level steadiness"].
Theoretical advantages of once-daily vs. standard formulations
The once-daily tacrolimus formulation was originally introduced on the market with the alleged advantage of promoting recipients’ adherence to the immunosuppressive regimens, which are often extended in time and complex. By allowing a schedule based on a single morning dose, once-daily tacrolimus would reduce the burden of pills that transplant recipients are prescribed and give them the chance to skip the evening dose [38]. The importance of compliance to immunosuppressants gained interest when it was found that poor drug adherence is associated with antibody-mediated rejection and graft failure [39, 40]. Poor adherence to tacrolimus is reflected in the finding of inconsistent tacrolimus blood levels at routine exams despite a steady dosage: such intra-patient variability in tacrolimus exposure has been associated with poor transplant outcomes [41] and it has been the object of a number of studies that compared the efficacy of once-daily versus standard twice-daily tacrolimus formulations.
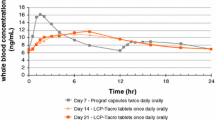
Nevertheless, difference in adherence between once-daily and standard tacrolimus formulations is not accountable alone for all intra-patient variability, as various other factors can intervene such as absorption interaction with the evening meal, metabolism circadian rhythm, interaction with other drugs and patient clinical conditions. Finally, once-daily and standard tacrolimus formulations exhibit different concentration–time profiles, such as the number of peaks in blood drug concentration (one versus two peaks), the height of peak concentration (i.e. Cmax) and the time to reach it (i.e., tmax), differences that might theoretically affect drug tolerability and efficacy.
Below, the evidence concerning each of these issues will be reviewed.
Drug adherence
There is some evidence suggesting that the use of a once-daily formulation is associated with improved drug adherence compared to IR-Tac. Indeed one of the reasons behind the development of Once-daily formulations has been to facilitate patient adherence to prescriptions by reducing the number of pills and the frequency of administration. The impact of compliance on clinical outcomes has come out over recent years, as non-adherence has turned out to be associated with antibody-mediated rejection and graft loss [39, 40]. In fact, graft recipients have a burden of pills to take [38], and as reports show that complex dose regimens are inversely associated with patient compliance, treatment simplification might improve adherence [42].
A significant and independent association between Once-daily XL-Tac and improved adherence was first reported in a cross-sectional study based on an anonymous questionnaire administered to 312 Japanese kidney recipients at Inoue Hospital (odds ratio 0.43 of non-adherence associated with Once-daily XL, p = 0.015) [43]. This finding was later confirmed by an RCT involving 219 renal recipients randomized 2:1 to Once-daily XL-Tac and IR-Tac, whose adherence was electronically monitored. The study showed a significantly superior regimen implementation with Once-daily XL-Tac: at 6 months post-randomization 81.5 % of patients on Once-daily XL-Tac were still persisting with their treatment compared to 71.9 % on IR-Tac (p = 0.0824) [44]. Among persistent patients, 88.2 % on Once-daily XL-Tac took the prescribed number of daily doses compared to 78.8 % on IR-Tac (p = 0.0009) [44]. Interestingly, for those patients on IR-Tac the evening dose was more frequently missed than the morning one (14.2 vs 11.7 %, p = 0.0035) [44]. In a prospective follow-up study in 75 renal transplant patients, the simplification of the medication regimens based on the conversion from twice-daily to once-daily drugs, including the switch from IR-Tac to Once-daily XL-Tac, increased not only self-reported adherence from 79.7 to 94.6 % (p < 0.001), but also patient satisfaction with the treatment measured with a dedicated questionnaire (on a scale from 0 to 100, treatment convenience significantly increased from 66.0 to 78.5 after the switch, p < 0.001) [45]. Similar findings on the improvement of treatment adherence following the conversion to Once-daily XL-Tac were also shown in liver and heart recipients [46]. Finally, a UK study based on a mathematical model of cost analysis, identified a substantial cost saving with Once-daily XL-Tac, despite its being a more expensive drug, mainly due to fewer graft losses and lower dialysis costs [47]. The study was based on data from the study by Kuypers et al. showing better compliance with Once-daily XL-Tac compared to IR-Tac [44] and took into consideration the impact of compliance on renal transplantation outcomes: over a 5-year period the mean cost per patient would be £ 29,328 with Once-daily XL-Tac compared to £ 33,061 with IR-Tac [48].
It must be underlined, however, that such a favorable impact of once-daily formulations on drug-adherence might depend on individual patient characteristics, with the maximum benefit expected from patients who have issues with twice daily or other complex dosing schedules. Moreover, skipping a single dose could impact blood level variability more under a once-daily regime than under twice-daily IR-Tac. Finally, randomized studies showing that once-daily formulations improve patient outcomes are still lacking. Pending the results of such trials, the authors believe that once-daily formulations do represent a valid option in many patients with poor drug adherence, and also in patients in whom for various reasons the dosing schedule needs to be simplified.
It is worth mentioning that one study examined what happens to the blood-concentration time profile by administering the standard IR-Tac formulation once-daily (i.e., the total daily dose of IR-Tac administered in the morning) [49]. Eighteen stable renal recipients were converted from twice-daily to once-daily administration of IR-Tac in the morning at 67, 85, and 100 % of the initial total daily dose. The study showed that the group receiving 85 % of the total daily dose in a unique morning administration of IR-Tac had a similar exposure to the twice-daily administration of IR-Tac. The mean AUC ratio between twice-daily and once-daily administration of 85 % of the initial dose was 1.0 [95 % confidence interval (CI) 0.9–1.1]. Finally, this small study did not show any adverse event related to the significantly higher peak concentration with once-daily administration of IR-Tac over a follow-up period of 6 to 18 months [49].
Blood level steadiness
Compared to IR-Tac, once-daily formulations are less affected by the intra-patient blood level variability [29, 50, 51] that is a risk factor for long-term poor transplantation outcomes [52]. The reasons behind the reduced variability with once-daily formulations are not fully understood. It has been hypothesized that, besides improved adherence, reduced variability might be related to the pharmacokinetic characteristics of once-daily formulations, to the lower susceptibility to cytochrome P-450 polymorphisms, and to the halved interaction with meals [15, 37].
The R2 value between AUC0–24 and trough level (i.e., the proportion of variability in AUC0–24 being accounted for by trough levels) for Once-daily XL-Tac ranges between 0.57 and 0.94 in kidney recipients, and is even wider in liver recipients, whereas it ranges between 0.56 and 1.0 with IR-Tac [27]. On the other hand, the Once-daily LCP-Tac formulation has shown some peculiarly advantageous PK properties [53]: in two studies, a similar AUC0–24 was achieved with Once-daily LCP-Tac despite a 30 % lower total daily dose than IR-Tac, and with a significantly flatter PK profile characterized by lower Cmax (11–13 vs 16–17 µg/l), longer Tmax (6 vs 1.5–1.8 h) and lower %-fluctuation (73–79 vs 127–133 %) measured as [(Cmax−Cmin)/Cmin × 100] [31, 32]. Moreover, a robust correlation between AUC0–24 and trough level was found (R2 > 0.85 in both studies) [31, 32]. Unfortunately, no peer-reviewed studies on the direct comparison between Once-daily XL-Tac and Once-daily LCP-Tac are currently available.
The tacrolimus blood level is significantly affected by the expression of different polymorphisms for genes encoding proteins involved in tacrolimus absorption and metabolism, particularly CYP3A5 [6]. An observational retrospective study in 97 Japanese renal recipients showed that tacrolimus bioavailability was linked to the CYP3A5 polymorphism and tacrolimus formulation, the impact of the first being almost double that of the latter [54]. The authors found that tacrolimus bioavailability was the lowest in the group of patients bearing the CYP3A5*1 allele and taking Once-daily XL-Tac. They speculated that the influence of the CYP3A5 polymorphism was greater on Once-daily XL-Tac than on IR-Tac, because of the longer exposure of Once-daily XL-Tac in the small intestine where the level of CYP3A5 expression varies depending on the type of polymorphism [54]. A prospective study in 40 stable renal recipients not only confirmed a reduction in intra-patient variability in 24-h tacrolimus exposure after conversion from IR-Tac to Once-daily XL-Tac (14.1 and 10.9 % respectively, p = 0.012), but identified expressers of the CYP3A5*1 allele as the recipients who gain the most in terms of variability reduction after conversion from IR-Tac to Once-daily XL-Tac (18.2 and 12.8 %, p = 0.062) [37].
Treatment efficacy
Efficacy of once-daily formulations has proven to be similar to standard IR-Tac, though a marginal trend toward an increased incidence of acute rejection has been observed with Once-daily XL-Tac compared to standard IR-Tac. Several studies have extensively investigated the efficacy of once-daily formulations, mainly as Once-daily XL-Tac compared to IR-Tac, both in de novo and conversion settings in renal recipients. The primary end-points of such studies were recipient and graft survival rates or a composite of survival rates, renal allograft rejection episodes and graft function deterioration.
Overall outcomes were generally similar between Once-daily XL-Tac and IR-Tac formulations in terms of recipient and graft survival [55]. However, the rate of biopsy-proven acute rejection (BPAR) often resulted higher, albeit not significantly, with Once-daily XL-Tac than with IR-Tac [53]. In a multicenter double-blind RCT involving 668 de novo kidney recipients, treatments with Once-daily XL-Tac or IR-Tac in combination with mycophenolate were not inferior to cyclosporine and mycophenolate in regard to treatment failure, that was a composite endpoint of death, graft failure and BPAR at 1-year post-transplantation (14.0, 15.1, and 17.0 %, respectively). However, the incidence of BPAR at 6 and 12 months was statistically lower in the IR-Tac arm, but not in the Once-daily XL-Tac one, compared to cyclosporine (respectively 3.8 vs 11.8 %, p < 0.04; 7.9 vs 11.8 %, NS) [56]. Nevertheless, 1-year estimated glomerular filtration rate (GFR) was superior in both of the tacrolimus arms compared to cyclosporine (59.7 and 58.6 vs 55.0 ml/min/1.73 m2, p < 0.05) [56], a finding confirmed also in the 4-year follow-up extension of the study [57]. The incidence of BPAR under Once-daily XL-Tac was found higher, although non significantly, than under IR-Tac also in another double-blind RCT in 667 de novo renal recipients, the BPAR rate at 6 months post-transplantation being 20.4 vs 15.8 % (p = 0.18, but with the 95 % CI of the risk difference just outside the pre-specified 10 % non-inferiority margin) [58]. Interestingly, the early trough level with Once-daily XL-Tac resulted lower than with IR-Tac (12.9 vs 15.3 ng/ml at week 1 after transplantation, p < 0.005), but this finding was not correlated to graft rejection [58]. Although Once-daily XL-Tac failed to demonstrate non-inferiority to IR-TAC due to BPAR incidence, no difference in patient and graft survival could be found at 12 months (97.5 vs 96.9 %, and 92.8 vs 91.5 %, NS) [58]. No significant difference in the incidence of acute rejection was instead found in a RCT in 124 de novo renal recipients (19.4 % with Once-daily XL-Tac vs 16.1 % with IR-Tac, p = 0.638) [59]. Finally, Once-daily XL-Tac at the starting total daily dose of 0.20 mg/kg/day with mycophenolate and steroids (arm 2) proved not inferior to IR-Tac at the same initial total daily dose (arm 1) in an open-label RCT in 1251 de novo kidney recipients that also included patients treated with Once-daily XL-Tac at the starting total daily dose of 0.30 mg/kg/day (arm 3) and patients treated with Once-daily XL-Tac at the starting total daily dose of 0.20 mg/kg/day and inductive basiliximab (arm 4): the primary end-point, a composite of graft loss, BPAR and graft dysfunction, was 42.1 % in arm 2 vs 40.5 % in arm 1 [60].
Fewer data are available for Once-daily LCP-Tac, and all are from sponsored RCTs. A multicenter RCT in 326 renal recipients randomly maintained on twice-daily IR-Tac or converted to Once-daily LCP-Tac at 0.7 ratio (0.85 for blacks) found a similar rate of treatment failures—a composite primary endpoint of death, graft failure, BPAR or loss to follow-up-between the two arms at 12 months (treatment failure was 2.5 % in both arms, p > 0.999) [61]. Similarly, Once-daily LCP-Tac proved not inferior to IR-Tac concerning treatment failure at 12 months in another multicenter double-blind RCT involving 543 de novo kidney recipients [62]. The result was then confirmed in the study extension to 24 months [63]: the proportion of patients with treatment failure was 18.3 and 23.1 % with Once-daily LCP-Tac, and 19.6 and 27.3 % with IR-Tac, respectively at 12 and 24 months post-transplantation, the statistical difference being well within the 10 % pre-specified non-inferiority margin [62, 63]. No difference in BPAR rate was found between Once-daily LCP-Tac and IR-Tac (17.1 vs 18.2 % over the first 24 months after transplantation, p = 0.7) [63]. Moreover, a post hoc subgroup analysis showed fewer treatment failures in older, black, or female recipients (−25.89 %, p = 0.067; −23.33 %, p = 0.414; and −11.70 %, p = 0.091 respectively) [63], populations known to be at higher risk of early rejection, graft loss or death. It has been supposed that this result might depend on the more rapid attainment of therapeutic TL with Once-daily LCP-Tac compared to IR-Tac: indeed 80.6 % of patients on Once-daily LCP-Tac had tacrolimus concentrations above the minimum trough level after 7 days of treatment compared to 52.8 % on IR-Tac (p < 0.001) [7].
It is worth mentioning two recent studies in the setting of liver transplantation. The first, a single-center retrospective comparison between 60 liver recipients maintained on IR-Tac and 129, either early or late, converters to Once-daily XL-Tac, showed a roughly fourfold reduction in rejection rate (p < 0.001) and a significantly smaller GFR deterioration (−35 µmol/l in IR-Tac arm vs −6 µmol/l in late Once-daily XL-Tac, p < 0.01) at 6 months after conversion to Once-daily XL-Tac [64]. The second was a retrospective analysis of the European Liver Transplant Registry on 4357 liver recipients from 21 Centers with at least 1 month of follow-up [65]. Treatment with Once-daily XL-Tac was associated with a striking improvement in patient and graft survival at 3 years post-transplant compared to IR-Tac (risk ratio for patient survival 1.72, p = 0.004; risk ratio for graft survival 1.81, p = 0.001) [65]. These results should be interpreted with caution, as it is uncertain to what extent residual confounding in the data analysis (i.e., distortion that remains after controlling for confounding factors in the design and analysis of the study) could explain the study findings.
Drug safety and tolerability
The studies conducted so far have consistently shown a similar safety profile between once-daily and standard formulations of tacrolimus [46]. Therefore, only the studies that have identified potential differences between the two formulations will be commented here.
In a prospective study involving 26 renal graft recipients, Once-daily XL-Tac was associated with improved glucose sensitivity, with a significant increase in homeostasis model assessment of pancreas β-cell function (HOMAβ) at 4 and 24 weeks after a 1 to 1 conversion from IR-Tac to Once-daily XL-Tac (p = 0.012 and 0.004, respectively) [66, 67]. However, such a difference in glucose metabolism was not confirmed in several other studies [55].
In a blind RCT in 44 stable kidney recipients, the conversion from IR-Tac to Once-daily LCP-Tac at 0.7 ratio resulted in a significant improvement in tremors measured by both an accelerometer and by blinded neurologists who applied a dedicated scale to videotapes recorded at 2 h post-dosing before and 7 days after conversion [68]. Conversion to Once-daily LCP-Tac achieved a 5.35 score improvement (p < 0.0001) and 21.58 % reduction in tremor amplitude (p = 0.03) from baseline. The authors speculated that tremor reduction might be linked to the PK profile of Once-daily LCP-Tac, which is characterized by a significantly lower Cmax compared to IR-Tac [68].
Conclusions
Once-daily prolonged-release tacrolimus formulations are now available as an effective alternative to the standard twice-daily immediate-release formulation, both for de novo and conversion immunosuppression in renal transplantation. While no study has proven so far a major benefit on renal recipient and allograft outcomes, once-daily formulations due to their inherent pharmacokinetic profile may represent a preferable option for some categories of renal allograft recipients, such as those who may need simplification in the treatment schedule to improve adherence. However, increased surveillance is recommended to ensure that prolong ed-release formulations are beneficial and not harmful in non-adherent patients. Similarly, clinicians must be aware that a close drug and clinical monitoring is also required after the switch from one tacrolimus formulation to another, as they are not bioequivalent.
References
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9(Suppl 3):S1–S155. doi:10.1111/j.1600-6143.2009.02834.x
Ekberg H, Tedesco-Silva H, Demirbas A et al (2007) Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357(25):2562–2575
Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC (2005) Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ 331(7520):810
Hart A, Smith JM, Skeans MA et al (2016) OPTN/SRTR annual data report 2014. Am J Transplant 16(S2):11–46. doi:10.1111/ajt.13666 (Special Issue)
Halloran PF (2004) Immunosuppressive drugs for kidney transplantation. N Engl J Med 351(26):2715–2729
Provenzani A, Santeusanio A, Mathis E et al (2013) Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J Gastroenterol 19(48):9156–9173. doi:10.3748/wjg.v19.i48.9156
Grinyó JM, Petruzzelli S (2014) Once-daily LCP-Tacro MeltDose tacrolimus for the prophylaxis of organ rejection in kidney and liver transplantations. Expert Rev Clin Immunol. 10(12):1567–1579. doi:10.1586/1744666X.2014.983903
Garnock-Jones KP (2015) Tacrolimus prolonged release (Envarsus®): a review of its use in kidney and liver transplant recipients. Drugs 75(3):309–320. doi:10.1007/s40265-015-0349-2
www.fda.gov/downloads/Drug/GuidanceComplianceRegulatoryInformation/Guidance/UCM181006.pdf. Accessed 16 Mar 2016
Staatz CE, Tett SE (2004) Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 43(10):623–653
Prograf (2013) Prescribing information. Astellas Pharma US, Inc; Northbrook
Ekberg H, Bernasconi C, Tedesco-Silva H et al (2009) Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant 9(8):1876–1885. doi:10.1111/j.1600-6143.2009.02726.x
Malvezzi P, Rostaing L (2015) The safety of calcineurin inhibitors for kidney-transplant patients. Expert Opin Drug Saf 14(10):1531–1546. doi:10.1517/14740338.2015.1083974
Cippà PE, Schiesser M, Ekberg H et al (2015) Risk stratification for rejection and infection after kidney transplantation. Clin J Am Soc Nephrol 10(12):2213–2220. doi:10.2215/CJN.01790215
Vanhove T, Annaert P, Kuypers DR (2016) Clinical determinants of calcineurin inhibitor disposition: a mechanistic review. Drug Metab Rev 48(1):88–112. doi:10.3109/03602532.2016.1151037
Paine MF, Khalighi M, Fisher JM et al (1997) Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 283(3):1552–1562
Masuda S, Uemoto S, Goto M, Fujimoto Y, Tanaka K, Inui K (2004) Tacrolimus therapy according to mucosal MDR1 levels in small-bowel transplant recipients. Clin Pharmacol Ther 75(4):352–361
Christians U, Jacobsen W, Benet LZ, Lampen A (2002) Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet 41(11):813–851
Nowack R (2008) Herb-drug interactions in nephrology: documented and theoretical. Clin Nephrol 69(5):319–325
Bekersky I, Dressler D, Mekki QA (2001) Effect of low- and high-fat meals on tacrolimus absorption following 5 mg single oral doses to healthy human subjects. J Clin Pharmacol 41(2):176–182
Park SI, Felipe CR, Pinheiro-Machado PG, Garcia R, Tedesco-Silva H Jr, Medina-Pestana JO (2007) Circadian and time-dependent variability in tacrolimus pharmacokinetics. Fundam Clin Pharmacol 21(2):191–197
http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000712/WC500022237.pdf. Accessed 16 Mar 2016
http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000712/WC500022234.pdf. Accessed 16 Mar 2016
MeltDose® Technology by the US Patent and Trademark Office. US7217431
Nigro V, Glicklich A, Weinberg J (2013) Improved bioavailability of MELTDOSE once-daily formulation of tacrolimus (LCP-Tacro) with controlled agglomeration allows for consistent absorption over 24 Hrs: a scintigraphic and pharmacokinetic evaluation. Am J Transplant. Poster, Abstract# B1034
http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002655/WC500170412.pdf. Accessed 16 Mar 2016
Staatz CE, Tett SE (2015) Clinical pharmacokinetics of once-daily tacrolimus in solid-organ transplant patients. Clin Pharmacokinet 54(10):993–1025. doi:10.1007/s40262-015-0282-2
de Jonge H, Kuypers DR, Verbeke K, Vanrenterghem Y (2010) Reduced C0 concentrations and increased dose requirements in renal allograft recipients converted to the novel once-daily tacrolimus formulation. Transplantation 90(5):523–529. doi:10.1097/TP.0b013e3181e9feda
Wu MJ, Cheng CY, Chen CH et al (2011) Lower variability of tacrolimus trough concentration after conversion from prograf to advagraf in stable kidney transplant recipients. Transplantation 92(6):648–652. doi:10.1097/TP.0b013e3182292426
Hougardy JM, Broeders N, Kianda M et al (2011) Conversion from Prograf to Advagraf among kidney transplant recipients results in sustained decrease in tacrolimus exposure. Transplantation 91(5):566–569. doi:10.1097/TP.0b013e3182098ff0
Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S (2013) Conversion from twice-daily tacrolimus capsules to once-daily extended-release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation 96(2):191–197. doi:10.1097/TP.0b013e3182962cc1
Alloway RR, Eckhoff DE, Washburn WK, Teperman LW (2014) Conversion from twice daily tacrolimus capsules to once daily extended-release tacrolimus (LCP-Tacro): phase 2 trial of stable liver transplant recipients. Liver Transpl 20(5):564–575. doi:10.1002/lt.23844
Barraclough KA, Isbel NM, Johnson DW, Campbell SB, Staatz CE (2011) Once- versus twice-daily tacrolimus: are the formulations truly equivalent? Drugs 71(12):1561–1577. doi:10.2165/11593890-000000000-00000
Alloway RR, Mulgaonkar S, Ueda D, et al (2011) A phase 2b, open-label, multi-center, prospective, randomized study to compare the pharmacokinetics and safety of LCP-Tacro™ tablets once-a-day to Prograf® capsules twice-a-day in de novo kidney transplant patients. Am J Transplant. Poster, Abstratct #1106
Niioka T, Satoh S, Kagaya H et al (2012) Comparison of pharmacokinetics and pharmacogenetics of once- and twice-daily tacrolimus in the early stage after renal transplantation. Transplantation 94(10):1013–1019. doi:10.1097/TP.0b013e31826bc400
Tsuchiya T, Ishida H, Tanabe T et al (2013) Comparison of pharmacokinetics and pathology for low-dose tacrolimus once-daily and twice-daily in living kidney transplantation: prospective trial in once-daily versus twice-daily tacrolimus. Transplantation 96(2):198–204. doi:10.1097/TP.0b013e318296c9d5
Stifft F, Stolk LM, Undre N, van Hooff JP, Christiaans MH (2014) Lower variability in 24-hour exposure during once-daily compared to twice-daily tacrolimus formulation in kidney transplantation. Transplantation 97(7):775–780. doi:10.1097/01.TP.0000437561.31212.0e
Hardinger KL, Hutcherson T, Preston D, Murillo D (2012) Influence of pill burden and drug cost on renal function after transplantation. Pharmacot 32(5):427–432. doi:10.1002/j.1875-9114.2012.01032.x
Sellarés J, de Freitas DG, Mengel M et al (2012) Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12(2):388–399. doi:10.1111/j.1600-6143.2011.03840.x
Tielen M, van Exel J, Laging M et al (2014) Attitudes to medication after kidney transplantation and their association with medication adherence and graft survival: a 2-year follow-up study. J Transplant. doi:10.1155/2014/675301
Shuker N, van Gelder T, Hesselink DA (2015) Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev 9(2):78–84. doi:10.1016/j.trre.2015.01.002
Claxton AJ, Cramer J, Pierce C (2001) A systematic review of the associations between dose regimens and medication compliance. J Clin Ther 23(8):1296–1310
Obi Y, Ichimaru N, Kato T et al (2013) A single daily dose enhances the adherence to immunosuppressive treatment in kidney transplant recipients: a cross-sectional study. Clin Exp Nephrol. 17(2):310–315. doi:10.1007/s10157-012-0713-4
Kuypers DR, Peeters PC, Sennesael JJ et al (2013) Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation 95(2):333–340. doi:10.1097/TP.0b013e3182725532
van Boekel GA, Kerkhofs CH, Hilbrands LB (2013) Treatment satisfaction in renal transplant patients taking tacrolimus once daily. Clin Ther 35(11):1821–1829. doi:10.1016/j.clinthera.2013.09.014
Singh N, Von Visger J, Zachariah M (2015) Extended release once a day tacrolimus. Curr Opin Organ Transplant. 20(6):657–662. doi:10.1097/MOT.0000000000000251
Butler JA, Peveler RC, Roderick P, Horne R, Mason JC (2004) Measuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoring. Transplantation 77(5):786–789
Muduma G, Shaw J, Hart WM, Odeyemi A, Odeyemi I (2014) Cost utility analysis of immunosuppressive regimens in adult renal transplant recipients in England and Wales. Pat Pre Adher 8:1537–1546. doi:10.2147/PPA.S69461
Hardinger KL, Park JM, Schnitzler MA, Koch MJ, Miller BW, Brennan DC (2004) Pharmacokinetics of tacrolimus in kidney transplant recipients: twice daily versus once daily dosing. Am J Transplant 4(4):621–625
Alloway R, Steinberg S, Khalil K et al (2005) Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen. Transplant Proc. 37(2):867–870
van Hooff J, Van der Walt I, Kallmeyer J et al (2012) Pharmacokinetics in stable kidney transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. Ther Drug Monit 34(1):46–52. doi:10.1097/FTD.0b013e318244a7fd
Borra LC, Roodnat JI, Kal JA, Mathot RA, Weimar W, van Gelder T (2010) High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 25(8):2757–2763. doi:10.1093/ndt/gfq096
Revollo J (2015) Update on the clinical utility of once-daily tacrolimus in the management of transplantation. Drug Des Dev Ther 9:2581–2583. doi:10.2147/DDDT.S84301
Niioka T, Kagaya H, Miura M et al (2013) Pharmaceutical and genetic determinants for interindividual differences of tacrolimus bioavailability in renal transplant recipients. Eur J Clin Pharmacol 69(9):1659–1665. doi:10.1007/s00228-013-1514-8
Posadas Salas MA, Srinivas TR (2014) Update on the clinical utility of once-daily tacrolimus in the management of transplantation. Drug Des Dev Ther 8:1183–1194. doi:10.2147/DDDT.S55458
Silva HT Jr, Yang HC, Abouljoud M et al (2007) One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transpl 7(3):595–608
Silva HT Jr, Yang HC, Meier-Kriesche HU et al (2014) Long-term follow-up of a phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation 97(6):636–641. doi:10.1097/01.TP.0000437669.93963.8E
Krämer BK, Charpentier B, Bäckman L et al (2010) Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant 10(12):2632–2643. doi:10.1111/j.1600-6143.2010.03256.x
Han DJ, Park JB, Kim YS et al (2012) A 39-month follow-up study to evaluate the safety and efficacy in kidney transplant recipients treated with modified-release tacrolimus (FK506E)-based immunosuppression regimen. Transpl Proc 44(1):115–117. doi:10.1016/j.transproceed.2011.12.070
Albano L, Banas B, Klempnauer JL, Glyda M, Viklicky O, Kamar N (2013) OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation 96(10):897–903. doi:10.1097/TP.0b013e3182a203bd
Bunnapradist S, Ciechanowski K, West-Thielke P et al (2013) Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transpl 13(3):760–769. doi:10.1111/ajt.12035
Budde K, Bunnapradist S, Grinyó JM et al (2014) Novel once-daily extended-release tacrolimus (LCPT) versus twice-daily tacrolimus in de novo kidney transplants: one-year results of Phase III, double-blind, randomized trial. Am J Transpl 14(12):2796–2806. doi:10.1111/ajt.12955
Rostaing L, Bunnapradist S, Grinyó JM et al (2016) Novel once-daily extended-release tacrolimus versus twice-daily tacrolimus in de novo kidney transplant recipients: two-year results of phase 3, double-blind, randomized trial. Am J Kidney Dis 67(4):648–659. doi:10.1053/j.ajkd.2015.10.024
Considine A, Tredger JM, Heneghan M et al (2015) Performance of modified-release tacrolimus after conversion in liver transplant patients indicates potentially favorable outcomes in selected cohorts. Liver Transpl 21(1):29–37. doi:10.1002/lt.24022
Adam R, Karam V, Delvart V et al (2015) Improved survival in liver transplant recipients receiving prolonged-release tacrolimus in the European Liver Transplant Registry. Am J Transplant 15(5):1267–1282. doi:10.1111/ajt.13171
Uchida J, Kuwabara N, Machida Y et al (2012) Conversion of stable kidney transplant recipients from a twice-daily prograf to a once-daily tacrolimus formulation: a short-term study on its effects on glucose metabolism. Transpl Proc 44(1):128–133. doi:10.1016/j.transproceed.2011.11.005
Uchida J, Iwai T, Kabei K et al (2014) Effects of conversion from a twice-daily tacrolimus to a once-daily tacrolimus on glucose metabolism in stable kidney transplant recipients. Transpl Proc 46(2):532–536. doi:10.1016/j.transproceed.2013.11.146
Langone A, Steinberg SM, Gedaly R et al (2015) Switching STudy of Kidney TRansplant PAtients with Tremor to LCP-TacrO (STRATO): an open-label, multicenter, prospective phase 3b study. Clin Transplant 29(9):796–805. doi:10.1111/ctr.12581
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Giovanni Piotti reports receiving consultant fees from Chiesi Farmaceutici SPA as medical research physician for the development of the medical product Envarsus. Elena Cremaschi declares that she has no conflict of interest. Umberto Maggiore is an Advisory Board or has received lecture fees from Teva, Sandoz, Chiesi, Astellas, Novartis.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Piotti, G., Cremaschi, E. & Maggiore, U. Once-daily prolonged-release tacrolimus formulations for kidney transplantation: what the nephrologist needs to know. J Nephrol 30, 53–61 (2017). https://doi.org/10.1007/s40620-016-0316-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-016-0316-3