Abstract
Purpose
This study aimed to analyze the expression of the IGF type-1 receptor gene (IGF-1r) and IGF-I, GH, testosterone, and IGFBP-3 concentrations in young people subjected to 10 weeks of muscle hypertrophy training.
Methods
IGF-1r expression, serum concentrations of IGF-I, IGFBP-3, GH, and total testosterone, as well as body composition, fat percentage, and body mass index, were determined for 22 healthy young males at three moments of resistance training (first, fifth, and tenth week of training).
Results
Throughout the 10 weeks of training, a reduction was observed in the relative expression of the IGF-1r gene (2−ΔΔCT) and an increase in IGF-I and GH concentrations. A reduction in total testosterone concentrations was detected during the recovery period in the fifth week. The IGFBP-3 concentrations did not change throughout the training.
Conclusions
The resistance training protocol prescribed for muscle hypertrophy did not suppress the GH-IGF-I axis, but it did cause alterations in IGF-1r gene expression and in IGF-I kinetics compatible with increased IGF bioactivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth is the main characteristic that differentiates children and adolescents from adult individuals [1]. The regulation of growth involves a complex and continuous interaction of genes, hormones, nutrients, and the physical environment. The integrity of the growth hormone (GH)—insulin- like growth factors (IGFs) axis, composed of the GH, IGF-I, and IGF-II hormones, IGF binding proteins (IGFBPs 1–6), and by the GHR, IGF-1r, IGF-2r, and insR receptors, plays an essential role in the human growth process, enabling the individual to achieve their full genetic potential [1]. The GH-IGF axis also participates in intermediary metabolism regulation, cellular multiplication and differentiation, as well as influencing the tissue development process [2]. Increases in anabolic hormones, such as testosterone, GH, and IGF-I, can cause muscle growth and strength gain [2, 3].
The importance of the GH/IGF-I axis in adapting to physical exercise continues to be a topic of great interest, due to its role in protecting myocardial cells, glucose homeostasis, and skeletal muscle hypertrophy [3]. However, changes in the GH/IGF-I axis induced by exercise and physical training and their consequences are far from being fully understood [4,5,6]. The safety of resistance training for adolescents in relation to the possible impact that this sporting activity can have over growth and final stature, as well as the possible modifications induced in the different components of the GH-IGF-I axis, continues to be a topic of debate. It is sometimes asked whether this type of training should be restricted to adult individuals.
Therefore, this study aimed to analyze the impact of resistance training over components of the GH/IGF axis, particularly over serum concentrations of IGF-I, IGFBP-3, GH, and testosterone and IGF-1r gene expression in young male adults.
Materials and method
Individuals
Twenty-two healthy young males aged 22 ± 2.8 years old, with a body weight of 75 ± 3.3 kg and height of 175 ± 3.3 cm, were studied in a convenience sample. As inclusion criteria the volunteers had to be aged between 18 and 25, have a minimum of 6 months experience in weight training, and they could not present any orthopedic injury or use anabolic steroids or food supplements. A minimum of 6 months experience with weight training was adopted as the efficacy measurement of a training protocol in an untrained population is hard to observe, since untrained individuals respond favorably to a myriad of training stimuli [7]. Hence, the responses of these physically active volunteers would better represent the responses of many young males who engage in this exercise modality.
Experimental design
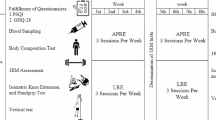
All participants were subjected to 10 weeks of resistance training and assessed in the first (M1), fifth (M2), and tenth (M3) weeks. An anthropometric assessment and blood collection for dosing the serum concentrations of IGF-I, IGFBP-3, GH, and testosterone and analyzing IGF1r expression were carried out at each one of those moments (M1, M2, and M3). The blood collections were taken before starting the training session (pre M1; pre M2; pre M3), preceded by 15 min of rest, 30 min after ending the training session (post M1; post M2; post M3), and on the following day 24 h after the initial sample (24 h M1; 24 h M2; 24 h M3), before starting the new training session (Fig. 1). In total, nine samples were obtained from each individual. The collections occurred in the afternoon, between 4 and 6 pm. The samples were stored between 0 and 4 °C until being processed and subsequently stored at − 80 °C until the analyses. The anthropometric assessments were carried out before the first training session in each phase.
The resistance training protocol was applied from Monday to Friday in the afternoon between 4 and 6 pm over 10 weeks.
Training protocol
The protocol was elaborated based on the recommendations of the American College of Sport Medicine [8]. Before the first week of training, the volunteers carried out a week of leveling and familiarization with the training protocol in the same protocol of exercises as the experiment, but executing sets with a greater number of repetitions (15–20 repetitions). The objective of the familiarization was to teach the appropriate execution technique for each exercise, familiarize the participants with all exercises, and guarantee that the participants started the study with comparable grounding. The intensity was determined per zone of maximum repetitions (10–12 maximum repetitions). The participants were directed to use a load that enabled them to execute at least 10 and at most 12 repetitions. When they managed to execute more than 12 repetitions for a particular resistance, the load was increased, so that no more than 12 repetitions could be executed [8, 9].
The training protocol was divided into three training sessions: A, B, and C. Training A was composed of straight bench press, incline bench press, cross-over, dumbbell shoulder rotation, dumbbell lateral raise, dumbbell shoulder shrug, pulley tricep, and cable tricep exercises. Training B was composed of back pulldown, seated rowing, supinated grip pulldown, inverted dumbbell cross, bar curl, Scott curl, and hammer curl exercises. Training C was composed of free squats, 45º leg press, seated leg extension, table flexion, seated flexion, seated adductor, seated calf, and abdominal exercises. The volunteers carried out 3 sets of 10–12 repetitions in each exercise with a 60–90 s rest interval. The weekly training frequency was five days. All the participants carried out the three training sessions, that is, on the first day the volunteers did training A, on the second they did training B, and on the third they did training C. On the fourth day they went back to training A and so on. In all the training sessions the volunteers were monitored by a physical education professional. The frequency of the training sessions was greater than 80% (40 sessions).
Diet and control of physical activity
All the participants were instructed to maintain their normal dietary habits and to not consume supplements during the study. The participants were instructed to abstain from other physical activities throughout the experimental protocol.
Immunoassays
The serum concentrations of IGF-I and IGFBP-3 were determined by specific immunocolorimetric assays (Immulite 2000, Siemens, Los Angeles, CA, USA). All the samples were analyzed in duplicate in a single assay. The intra-assay coefficients of variation for IGF-I and IGFBP-3 were 2.4% and 2.3%, respectively.
The serum concentrations of GH were determined by chemiluminescence (Immulite 2000, Siemens, Los Angeles, CA, USA). All the samples were analyzed in duplicate in a single assay. The intra-assay coefficient of variation was 2.3%.
The serum concentrations of total testosterone were determined by radioimmunoassay (RIA). All the samples were analyzed in duplicate in a single assay. The intra-assay coefficient of variation was 9.4%.
IGF-1r mRNA expression
Lymphocyte IGF-1r mRNA expression has been considered as a possible marker of IGF-1r expression in the whole body [10]. Therefore, the IGF-1r expression assessment was carried out through mRNA extraction from peripheral blood lymphocytes after separation using the TRISOL technique. The extracted mRNA was converted to cDNA by means of the reverse transcription technique using the Applied High Capacity kit and quantified by specific probes (TaqMan®) using the real-time PCR technique (quantitative PCR). The IGF-1r gene was analyzed in duplicate using the Real-time PCR System device (Applied Biosystems) and as an endogenous reference the β2 microglobulin and β-glucoronidase (GUSβ) genes were used. As a calibrator a sample obtained from a normal individual with age-appropriate weight and height was used [11, 12]. IGF-1r gene expression was determined using the 2−ΔΔCT method [13].
Anthropometric measures
Heights were obtained on a stadiometer fixed to the wall. Body weight was measured by a Lucastec—Ple 180 electronic balance. The skin fold measures were tricep (TR), subscapular (SB), suprailiac (SI), and abdominal (AB), using a Cescorf adipometer, following the standardization of Behnke and Wilmore [14]. The fat percentage estimates were obtained according to the equation proposed by Faulkner [15]. After the fat percentage prediction the formulas of Guedes and Guedes [16] were used to determine lean mass. For the body muscle mass estimate the equation proposed by Lee et al. [17] was used. All the anthropometric measures were taken by the same investigator.
Statistical treatment
Bayesian linear regression models, including a random effect to contemplate the dependence of the measures on the same individual over time, were adjusted after the description of the variables according to the time in weeks (pre M1 vs. pre M2; pre M1 vs. pre M3; pre M2 vs. pre M3; post M1 vs. post M2; post M1 vs. post M3; post M2 vs. post M3; 24h1 vs. 24h2; 24h1 vs. 24h3; 24h2 vs. 24h3) and within the same moment (pre vs. post; pre vs. 24 h; post vs. 24 h at M1, M2, and M3). Thus, the differences between the means and the 95% confidence intervals (95% CIs) were estimated. The interpretation of these intervals is that the probability of the true difference between means (population) being within the lower limit (LL) and the upper limit (UL) is 95%. When the zero value is outside these limits we can infer a possible difference between the times. The JAGS package from the R 3.5.1 software was used.
Results
IGF-1r expression
The relative expression of the IGF-1r gene (2−ΔΔCT) decreased throughout the 10 weeks of training. A reduction in IGF-1r expressions was observed at the pre M2 (− 0.19; 95% CI − 0.32, − 0.05) and pre M3 (− 0.15; 95% CI − 0.29, − 0.01) moments when compared to the pre M1 IGF-1r values (Table 1). No significant differences were found in any of the other comparisons made.
IGF-I
Over the 10 weeks of training it was possible to observe increases in IGF-I concentrations. The pre M3 and post M3 IGF-I concentrations increased when compared to the pre M1 (23.08; 95% CI 4.6, 41.53) and post M1 (14.83; 95% CI 0.85, 29.23) IGF-I concentrations, respectively (Table 1). No significant differences were found in any of the other comparisons made.
IGFBP-3
The variations in IGFBP-3 concentrations over the 10 weeks of training did not obtain statistical significance.
GH
Increases in GH concentrations were observed in the post-training samples when compared with the pre-training samples at M1 (2.72; 95% CI 0.82, 4.54) and at M2 (2.16; 95% CI 0.36, 4.01) and M3 (3.74; 95% CI 1.22, 6.3). Moreover, post 24 h GH serum concentrations were also lower than in the post training sample at M3 (− 3.79; 95% CI − 6.33, − 1.42) (Table 1). No significant differences were found in any of the other comparisons made.
Testosterone
The serum concentrations of total testosterone presented significant alterations only at M2. It was possible to observe a reduction in total testosterone concentrations after 24 h (24 h M2) when compared with pre M2 total testosterone concentrations (− 38.94; 95% CI − 69.25, − 9.25) and a reduction in 24 h (24 h M2) total testosterone concentrations when compared with post M2 total testosterone concentrations (− 59.47; 95% CI − 88.61, − 30.43) (Table 1). No significant differences were found in any of the other comparisons made.
Anthropometry
A reduction in fat percentage was observed between M1 and M2 (− 0.54; 95% CI − 1.04, − 0.02). Body weight, lean mass, muscle mass, and body mass index did not present alterations at any moment evaluated (Table 2).
Discussion
This study observed in an original way alterations in IGF-1r expression and in IGF-I kinetics over 10 weeks of resistance training in young males compatible with increased IGF-I bioactivity. After 10 weeks of resistance training a reduction was observed in IGF-1r expression and an increase in IGF-I concentrations. Acute increases in GH concentrations were also observed.
Greater IGF-1r mRNA expression is described in cases of reduced IGF-I bioactivity, such as hypoxia [11], partial GH/IGF-I resistance [10, 18], and in girls with decelerated growth velocity due to central precocious puberty treatment with GnRH analogue [19], suggesting the presence of an ultrashort feedback cycle dependent on IGF-I bioactivity. However, greater IGF-1r expression is often associated with greater proliferation and growth of tissues and in tall obese children [20]. The expression of an additional allele of the IGF-1r gene has been associated with tallness [21] and inactivating mutations have been described in cases of low height [22] This suggests regulation of the U-shaped IGF-1r expression curve, with greater expression in cases of reduced bioactivity or an increase in the IGF system and lower expression in normal eutrophic individuals [20]. This would enable the modular organism to express the receptor in order to offset the alteration of the action provided by the modification of the binder concentrations. In the present study the increase in IGF-I, without the corresponding increase in IGFBP-3, could be responsible for increasing the bioactivity of this hormone and inducing a compensatory reduction in the expression of its main receptor. It is worth mentioning that the metabolic stress caused by exercise creates an accumulation of metabolites, especially lactate, pyruvate, and hydrogen ions [23] which can increase anabolic hormone concentrations. This metabolic stress could thus explain the increases in GH and IGF-I concentrations [24] with it being important to highlight the possibility of the increase in IGF-I concentrations also being influenced by the increase in GH concentrations [25].
Gonzalez et al. [26] compared the acute effects of two resistance training protocols, one with a high volume (5 sets of 10–12 repetitions) and the other with a high intensity (5 sets of 2–5 repetitions). The authors observed greater IGF-1r phosphorylation in the high-volume training protocol, acute increases in IGF-I and GH concentrations after the two protocols were executed, and a reduction in testosterone concentration one and two hours after training. Under this approach, Fragala et al. [27] observed increased IGF-1r expression and IGF-I concentrations in volunteers after three days of a high- intensity training protocol (4 sets of 10 repetitions). Unlike in the cited studies, in the present study the volunteers were subjected to a protocol of high-volume exercises for 10 weeks, where a reduction in IGF- 1r expression and increases in IGF-I and GH concentrations were observed.
Researching acute and chronic endocrine responses in young people (aged 18–25) with experience with resistance training and monitored for 12 weeks, McCall et al. [28] did not observe any differences in IGF-I, GH, and testosterone concentrations after the 12 weeks, in which the volunteers trained three times a week with a high volume (3 sets of 10 repetitions). Investigating acute endocrine responses in physically active young people (± 25 years old), Walker et al. [29] monitored their volunteers for 20 weeks of resistance training. The training sessions were applied twice a week, with 2–3 sets of 12–14 repetitions being executed for 10 weeks and 3–5 sets of 8–10 repetitions and a 1–2 min interval being executed for 10 weeks. An acute increase in total testosterone and hGH concentrations was observed immediately after the exercises, remaining like that for 15 min after the end of the training. Recently, Miranda et al. [30] researched acute endocrine responses in 12 trained volunteers (± 25 years old) after executing a resistance training session. The volunteers were subjected to a traditional training protocol (3 sets of 10 repetitions) and a protocol with bi-set training exercises (agonistic and antagonistic; 3 sets of 10 repetitions). The authors verified an acute increase in total testosterone and hGH concentrations after the exercise in the traditional protocol. In the present study, it was observed that the protocol of resistance exercises prescribed with a high volume executed five days a week chronically increased IGF-I concentrations and acutely increased GH concentrations over the 10 weeks and, unlike in the cited studies, it caused a reduction in testosterone concentrations only in the fifth week of training(M2). The higher weekly load in the present experimental design may have contributed to the differences observed.
Eliakin and Nemet [31] postulated the possibility of there being a biphasic kinetic of the GH/IGF-I axis, that is, a catabolic phase that is accompanied by a reduction in the concentrations of those hormones and that would last approximately 3–5 weeks, and a second anabolic phase that would occur 5–6 weeks into training. According to these same authors, exactly how and when this phase change occurs and whether a catabolic phase is needed for the occurrence of a second, so-called anabolic phase remains unknown. As a result of this evidence, Tourinho Filho et al. [32] studied young swimmers during a season and observed a biphasic kinetic of the GH/IGF-I axis. A reduction was observed in IGF-I concentrations during the most intense phase of training and an increase in IGF-I concentrations during the polishing phase. Fornel et al. [33] also observed a biphasic kinetic of the GH/IGF-I axis when monitoring young soccer players during a season. The authors found increases in IGF-I concentrations during the initial and intermediate phases and a reduction at the end of the season. The present study only observed a physiological increase in IGF-I concentrations, coinciding with the postulated anabolic phase of the exercise. It is believed that this increase was identified as a result of an adaptation to the exercise protocol.
The bioavailability of muscle IGF-I and IGF-1r is controlled by the IGF binding proteins [34]. Thus, the adaptations of the IGFBP-3 binding protein to the resistance training were measured due to their potential action over IGF-I. Kraemer and Ratamess [9] also add that little is known about the kinetics of IGFBP-3 in chronic protocols in resistance training. In the study conducted by Borst et al. [35] a 20% reduction was observed in IGFBP-3 concentrations between the 13th and 25th week of weight training. Unlike in the present study, IGFBP-3 was not sensitive to the effects of the training. IGFBP-3 showed independent and insensitive kinetics over the 10 weeks of training.
Despite no alterations being observed in lean and muscle mass, it is not possible to ignore the action exerted by the hormonal alterations. Increases in anabolic hormones, such as testosterone, hGH, and IGF-I, can cause muscle growth and strength gain [9]. For Gomes et al. [36] a reduced quantity of muscle growth after resistance training is observed in trained individuals. Grgic et al. [37] also adds that as the “adaptation window” decreases during resistance training over the long run, more scientific recommendations are needed to adequately address the elaboration of a training program focused on trained populations that seek to increase strength and muscle hypertrophy.
The limitations of the study include the sample size. However, the number of subjects who participated is in line with the literature. Another limitation was the lack of gene expression analysis of GH receptors, Testosterone and IGFBP-3 binding protein. We believe future studies could include them.
In conclusion, the resistance training protocol prescribed for muscle hypertrophy did not suppress the GH-IGF-I axis, but it did cause alterations in IGF-1r gene expression and in IGF-I kinetics compatible with increased IGF bioactivity. The reduction in IGF-1r expression would reflect greater IGF- I action, through an increase in serum concentrations or alterations in bioavailability. These alterations could be induced both by the acute increase in GH in each training cycle and by the metabolic state triggered by the exercises.
Data availability
The entire dataset supporting the results of this study was published in the article itself.
References
Martinelli CE Jr, Custódio RJ, Oliveira MHA (2008) Physiology of the GH-IGF axis. Arq Bras Endocrinol Metab 52:717–725
Eliakim A, Nemet D, Cooper M (2005) Exercice, training and the GH-IGF-I axis. In: Kraemer WJ, Rogol AD (eds) The endocrine system in sports and exercise. Blackwell Publishing, Chennai, p 165
Grandys M, Majerczak J, Kuczek P, Sztefko K, Duda K, Zoladz JA (2017) Endurance training- induced changes in the GH-IGF-I axis influence maximal muscle strength in previously untrained men. Growth Horm IGF Res 32:41–48
Fink J, Schoenfeld BJ, Nakazato K (2008) The role of hormones in muscle hypertrophy. Phys Sportsmed 46:129–134
Frystyk J (2010) Exercise and the growth hormone-insulin-like growth factor axis. Med Sci Sports Exerc 42:58–66
Nindl BC, Pierce JR (2010) Insulin-like growth factor I as a biomarker of health, fitness, and training status. Med Sci Sports Exerc 42:39–49
Mangine GT, Hoffman JR, Gonzalez AM, Townsend JR, Wells AJ, Jajtner AR (2015) The effect of training volume and intensity on improvements in muscular strength and size in resistance-trained men. Physiol Rep 3:e12472
American College of Sports Medicine (2009) Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41:687–708. https://doi.org/10.1249/MSS.0b013e3181915670
Kraemer WJ, Ratamess NA (2005) Hormonal responses and adaptations to resistance exercise and training. Sports Med 35:339–361
Eshet R et al (1993) Up-regulation of insulin-like growth factor-I (IGF-I) receptor gene expression in patients with reduced serum IGF-I levels. J Mol Endocrinol 10:115–120. https://doi.org/10.1677/jme.0.0100115
Custodio RJ, do Carmo Custodio VI, Scrideli CA, Sader Milani SL, Cervi MC, Cupo P et al (2012) Impact of hypoxia on IGF-I, IGF-II, IGFBP-3, ALS and IGFBP-I regulation and on IGF- IR gene expression in children. Growth Horm IGF Res 22:186–191
Sambrook J, Russell DW (2001) Molecular cloning. A laboratory manual 2 vol, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Behnke AR, Wilmore JH (1974) Field methods. Prentice Hall, New Jersey
Faulkner JA (1968) Physiology of swimming and diving. In: Falls H (ed) Exercise physiology. Academy Press, Baltimore
Guedes DP, Guedes JERP (2006) Practical manual for assessment in physical education. Barueri, Manole
Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB (2000) Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr 72:796–803
Milani SS, Custódio RJ, Martinelli Jr CE (2009) IGF1r gene expression in patients with idiopathic short stature according to GH and IGF-I status. In: 37th meeting of the British Society for Paediatric Endocrinology and Diabetes, Reading, United Kingdon. Endocrine-Abstracts 23 OC1.3-OC1.3.
Sarti de Paula MTA (2015) Study of IGF1r mRNA expression in girls with central precocious puberty before and during GnRH analogue treatment. Dissertation, Ribeirão Preto Medical School, University of São Paulo
Ricco RC, Ricco RG, Queluz MC, de Paula MTS, Atique PV, Custódio RJ et al (2018) IGF-1R mRNA expression is increased in obese children. Growth Horm IGF Res 39:1–5. https://doi.org/10.1016/j.ghir.2017.11.001
Okubo Y, Siddle K, Firth H, O’Rahilly S, Wilson LC, Willatt L et al (2003) Cell proliferation activities on skin fibroblasts from a short child with absence of one copy of the type 1 insulin- like growth factor receptor (IGF1r) gene and a tall child with three copies of the IGF1r gene. J Clin Endocrinol Metab 88:5981–5988. https://doi.org/10.1210/jc.2002-021080
Labarta JI, Barrio E, Audí L, Fernández-Cancio M, Andaluz P, de Arriba A, Puga B et al (2013) Familial short stature and intrauterine growth retardation associated with a novel mutation in the IGF-I receptor (IGF1r) gene. Clin Endocrinol (Oxf) 78:255–262
Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M et al (2009) Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. J Appl Physiol 106:1119–1124
Hansen S, Kvorning T, Kjaer M, Sjøgaard G (2001) The effect of short-term strength training on human skeletal muscle: the importance of physiologically elevated hormone levels. Scand J Med Sci Sports 11:347–354
Sotiropoulos A, Ohanna M, Kedzia C, Menon RK, Kopchick JJ, Kelly PA et al (2006) Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up- regulation. Proc Nati Acad Sci USA 103:7315–7320
Gonzalez AM, Hoffman JR, Townsend JR, Jajtner AR, Boone CH, Beyer KS et al (2005) Intramuscular anabolic signaling and endocrine response following high volume and high intensity resistance exercise protocols in trained men. Physiol Rep 3:e12466
Fragala MS, Jajtner AR, Townsend JR, Gonzalez AM, Wells AJ, Oliveira LP, Hoffman JR et al (2015) Leukocyte IGF-I receptor expression during muscle recovery. Med Sci Sports 47:929. https://doi.org/10.1249/MSS.0000000000000392
McCall GE, Byrnes WC, Fleck SJ, Dickinson A, Kraemer WJ (1999) Acute and chronical hormonal responses to resistance training designed to promote muscle hypertrophy. Can J Appl Physio 24:96–107
Walker S, Santolamazza F, Kraemer W, Häkkinen K (2015) Effects of prolonged hypertrophic resistance training on acute endocrine responses in young and older men. J Aging Phys Act 23:230–236. https://doi.org/10.1123/japa.2013-0029
Miranda SS, de Souza JAAA, Scudese E, Paz GA, Salerno VP, Vigário PDS et al (2018) Acute Hormone responses subsequent to agonist-antagonist paired set vs traditional straight set resistance training. J Strength Cond Res 34:15911599. https://doi.org/10.1519/JSC.0000000000002633
Eliakim A, Nemet D (2010) Exercise training, physical fitness and the growth hormone-Insulin- Like growth factor-1 axis and cytokine balance. Med Sport Sci 55:128–140
Tourinho-Filho H, Pires M, Puggina EF, Papoti M, Barbieri R, Martinelli CE Jr (2017) Serum IGF-I, IGFBP-3 and ALS concentrations and physical performance in young swimmers during a training season. Growth Horm IGF Res 32:49–54. https://doi.org/10.1016/j.ghir.2016.12.004
Fornel RG, Bianchi KE, Martinelli Junior CE, Tourinho Filho H (2020) Metabolic biomarkers in young soccer players during a competitive season. Arch Sports Med 4:209–214. https://doi.org/10.36959/987/254
Toigo M, Boutellier U (2006) New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol 97:643–663. https://doi.org/10.1007/s00421-006-0238-1
Borst SE, De Hoyos DV, Garzarella L, Vincent K, Pollock BH, Lowenthal DT et al (2001) Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med Sci Sports Exerc 33:648–653
Gomes GK, Franco CM, Nunes PRP, Orsatti FL (2019) High-frequency resistance training is not more effective than low-frequency resistance training in increasing muscle mass and strength in well-trained men. J Strength Cond Res 33(Suppl 1):S130–S139
Grgic J, Schoenfeld BJ, Davies TB, Lazinica B, Krieger JW, Pedisic Z (2018) Effect of resistance training frequency on gains in muscular strength: a systematic review and meta- analysis. Sports Med 48:1207–1220
Acknowledgements
The authors are grateful to National Council for Scientific and Technological Development (CNPq) (CNPq—Proc. no. 142327/2018-2), Brazil, for scholarships.
Author information
Authors and Affiliations
Contributions
MCJ, HTF, and CEMJ contributed to conception and design of the study. HSCC organized the database. MCJ and CEMJ wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
This study was approved by the research ethics committee of the Clinical Hospital of the School of Medicine of Ribeirão Preto, of the University of São Paulo, in São Paulo (state).
Informed consent
The informed consent form was read and signed by all the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Junior, M.C., Cerqueira, H.S.C., Filho, H.T. et al. Muscle hypertrophy training does not suppress the GH/IGF axis in young adult males. J Endocrinol Invest 46, 2601–2607 (2023). https://doi.org/10.1007/s40618-023-02116-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02116-1