Abstract
Objective
The imbalance of gut microbiota has been linked to manifold endocrine diseases, but the association with Graves’ disease (GD) is still unclear. The purpose of this study was to investigate the correlation between human gut microbiota and clinical characteristics and thyroidal functional status of GD.
Methods
14 healthy volunteers (CG) and 15 patients with primary GD (HG) were recruited as subjects. 16SrDNA high-throughput sequencing was performed on IlluminaMiSeq platform to analyze the characteristics of gut microbiota in patients with GD. Among them, the thyroid function of 13 patients basically recovered after treatment with anti-thyroid drugs (oral administration of Methimazole for 3–5 months). The fecal samples of patients after treatment (TG) were sequenced again, to further explore and investigate the potential relationship between dysbacteriosis and GD.
Results
In terms of alpha diversity index, the observed OTUs, Simpson and Shannon indices of gut microbiota in patients with GD were significantly lower than those in healthy volunteers (P < 0.05).The difference of bacteria species was mainly reflected in the genus level, in which the relative abundance of Lactobacillus, Veillonella and Streptococcus increased significantly in GD. After the improvement of thyroid function, a significant reduction at the genus level were Blautia, Corynebacter, Ruminococcus and Streptococcus, while Phascolarctobacterium increased significantly (P < 0.05). According to Spearman correlation analysis, the correlation between the level of thyrotropin receptor antibody (TRAb) and the relative abundance of Lactobacillus and Ruminococcus was positive, while Synergistetes and Phascolarctobacterium showed a negative correlation with TRAb. Besides, there were highly significant negative correlation between Synergistetes and clinical variables of TRAb, TPOAb and TGAb (P < 0.05, R < − 0.6).
Conclusions
This study revealed that functional status and TRAb level in GD were associated with composition and biological function in the gut microbiota, with Synergistetes and Phascolarctobacterium protecting the thyroid probably, while Ruminococcus and Lactobacillus may be novel biomarkers of GD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A complex microbial ecosystem in the intestines of human hosts is constituted by gut microflora, which is of great significance to human health [1, 2]. The gut microbiota plays vital roles in immunomodulation, metabolism, barrier protection and nutrition absorption in humans [3, 4]. Numerous studies have shown a close correlation between the alteration of gut microbiota and metabolic diseases such as obesity or diabetes, and the two may interact as both cause and effect. The dysbiosis of intestinal microflora is considered to chiefly produce an effect on the glycolipid metabolism in the host recently. There are significant quantitative and qualitative differences between the gut microbiota of the lean and fat people, as well as between the diabetic and the healthy subjects [5,6,7,8]. There is a consensus that obesity is usually associated with a decrease in Bacteroides and an increase in Firmicutes [9,10,11,12,13], but this conclusion is currently controversial [14,15,16]. Undeniably, gut microbiota and its metabolites can participate in host nutritional absorption and metabolism by regulating appetite, fat production, gluconeogenesis and inflammation [17]. Therefore, gut microbiota is probably one of the contributing factors of metabolic diseases such as obesity and diabetes. Further analysis of gut microbiota and its metabolites is crucial.
At present, related studies on gut microbiota and thyroid disease have been widely reported at home and abroad. Among them, there are still inconsistent conclusions about GD and gut microbiota. Hyperthyroidism refers to the hyperactivity of thyroid and the release of redundant-free thyroid hormone, which leads to hypermetabolism throughout the body [18]. GD is the most common form of hyperthyroidism. A considerable portion of hyperthyroidism is caused by GD, an organ-specific autoimmune disease characterized by thyrotoxicosis, thyroncus and the production of TRAb. TRAb stimulates the proliferation and hypersecretion of thyroid cells, leading to autoimmune hyperthyroidism [19]. Zhou and his team found that the number of Bifidobacterium and Lactobacillus decreased significantly while Enterococcus increased in patients with hyperthyroidism [20]. The study of Ishaq showed the obvious increase of Haemophilus parainfluenza in patients with GD, which may be one of the candidate pathogens [21]. Shi reported an evident raise in the abundance of Bacteroidetes in patients with Graves’ ophthalmopathy [22]. Although these studies have found differences in the gut microbiota between hyperthyroid patients and normal persons in terms of composition and diversity, however, there may be insufficient understanding of the role of gut microbiota in the occurrence and development of GD due to the individual differences of subjects, the inadequateness of sample size and the depth of sequencing. Besides, there are few studies on the relevance between gut microbiota and TRAb levels.
In this study, the 16S rDNA sequencing of fecal samples comes from 15 patients with primary GD and 14 healthy volunteers were performed and the thyroid-related antibodies of their blood were detected. Moreover, the treatment group was set up, aiming to find out the remarkable feature of gut microbiota of patients with GD that had never located. The association between the detected characteristic flora and the serum thyroid antibodies of GD patients was analyzed, so as to put forward the new insights for the pathogenesis of GD.
Methods
Sample selection
15 patients with primary GD were recruited as the hyperthyroidism group (HG) from February 2018 to April 2019 in the Department of Endocrinology, Jinling Hospital of Southeast University, School of Medical. In the meantime, 14 healthy volunteers were recruited as the control group (CG). All the patients with GD were only treated with anti-thyroid drugs (Methimazole, Merck Company). And then, specimens were taken again after 3–5 months of treatment when subjects’ thyroid function is basically restored to normal, a total of 13 cases were taken as the treatment group (TG). All patients with GD met the diagnostic criteria of the “Guideline for the Diagnosis and Treatment of Chinese Thyroid Diseases”, and the enrolled cases without treatment were excluded from transient hyperthyroidism such as subacute thyroiditis, painless thyroiditis and drug-induced hyperthyroidism. All the subjects were defined between 18 and 65 years old. None of the subjects had history of autoimmune disease or long-term hormone treatment, diabetes and other metabolic diseases, constipation or chronic diarrhea such as inflammatory bowel disease (IBD), cancer and familial genetic disease, severe dysfunction of multiple organ such as heart, liver and kidney. No one had taken antibiotics or microbiological preparations within one month prior to participation. We also excluded vegetarians and pregnant or lactating women. And the remained subjects were without history of gastrointestinal surgery and addiction of alcohol or drug. Besides, the patients and the controls were all from the local area of Jiangsu Province, located in the east coast of China, and were on a similar diet. Prior to the collection of clinical information and samples, all subjects signed an informed consent, meeting the requirements of ethical review.
Sample collection
The stool specimens were collected by the subjects themselves, and the procedure of collection was as follows: discharge the stool on a piece of dry and clean paper, pick up the center part with the pick rod of the sterile stool sample box and put into the stool sample box immediately. After that, the researcher placed the specimen on ice instantly to make sure that the temperature was lower than 4 ℃ and transported to the endocrinology laboratory within one hour. Then the central part was picked out in the ventilation cupboard after disinfection and sterilization within 30 min. All of the samples were divided into two parts, about the size of soybean grains, and stored in 1.5-mL EP tubes. The samples were frozen at − 80 ℃ refrigerator promptly after collection. The collection and storage of samples were conducted in strict accordance with the rules and regulations of the Department of Endocrinology, Jinling Hospital of Southeast University, School of Medical, and all samples had no history of repeated freeze–thaw and contamination.
DNA extraction and PCR amplification
DNA extraction and concentration determination are carried out according to the instructions of Magen Hipure Soil DNA kit and Qubit ®dsDNA HS Assay kit. Then the 16 s rDNA V3-V4 variable region was amplified by PCR with upstream primer 5′-CCTACGGRRBGCASCAGKVRVGAAT-3′ and downstream primer 5′-GGACTACNVGGGTWTCTAATCC-3′. The amplification procedure is as follows: pre-denaturation at 94 °C for 3 min, denaturation at 94 °C for 5 s, annealing at 57 °C for 90 s, extension at 72 °C for 10 s, additional extension at 72 °C for 5 min, and there are altogether 24 cycles. The 25 μL amplification system is composed of2.5 μL TransStart buffer, 2 μL dNTPs, two of 1 μL primer, 0.5 μL TransStart polymerase, 20 ng DNA template and the rest supplemented with ddH2O. The specificity and size of PCR products were detected by 1.5% agarose gel electrophoresis. The amplified PCR products were purified and eluted, then the library was constructed which was detected and quantified by Qubit3.0 Fluorometer fluorescence quantitative instrument (Invitrogen, Carlsbad, CA), and finally analyzed on the computer.
16S rDNA gene sequencing and data processing
Perform the PE250/PE300 double-terminal sequencing according to the instructions of the Illumina MiSeq sequencer, read the sequencing data by the MiSeq Control Software (MCS) of MiSeq, perform quality control on the original sequencing data, and obtain valid data after assembly and filtering. Amplicon sequences with 99% similarity were classified as an operational taxonomy unit (OTU), then QIIME software was used for OUT clustering and species annotation, and species abundance and diversity index analysis were performed based on the results of OTU cluster analysis. The statistical analysis of the composition structure of gut microbiota was carried out at various taxonomic levels such as phylum level and genus level.
Statistical analysis
Histogram of colony composition and heat map of species distribution among groups were drawn by R-language. The abundance of a species in a group was the average of all sample abundances of the species under the group. Obtain significant differences between groups with Kruskal–Wallis non-parametric test (q < 0.05), linear discriminant analysis (LDA) and LDA effect size (LEfSe) (LDAscore > 2.0), draw the heatmap of the distribution of different species based on R-language. In addition, Spearman rank test was used to analyze the correlation between gut microbiota and thyroid-related antibodies.
Results
Baseline characteristics
There was no obvious difference in age, blood pressure and body mass index between 15 patients with GD (7 males and 8 females) and 14 healthy volunteers (6 males and 8 females) (Table 1). The relevant clinical variables between group HG and CG were compared through the independent samples t test in SPSS (Table 1). The related indexes between group HG and TG (6 males and 7 females) were analyzed by paired-samples t test. As shown in Table 2, the thyroid function indexes such as FT3 and FT4 in group TG have basically recovered and TRAb decreased significantly. The data obtained were expressed as mean ± standard deviation (x ± s), with P < 0.05 representing statistically significant differences.
Alpha diversity
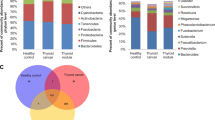
Alpha diversity index was used to evaluate the species abundance and diversity of microflora [23], in which Goods Coverage could reflect the depth of sample detection, Simpson index and Shannon index were a reflection of microflora diversity, Chao1 index and observation-OTUs index were a reflex of microflora richness. The Goods coverage of each group was more than 99.9%, indicating that the sequencing results of gut microbiota represent the true situation of the sample. The sample dilution curve of this study was drawn according to observed-OTUs. As shown in Fig. 1a and b, the curve has been smooth when the amount of sequencing data reached more than 5000, showing that the amount of sequencing data was sufficient to cover all species in the samples. Therefore, the sequencing data of this study was extremely reasonable. Compared with the CG group, the abundance and diversity of gut microbiota in group HG decreased significantly, among which the Shannon, Simpson and observation-OTUs indexes were visibly declined (Table 1, P < 0.05). After improvement of thyroid function, the Shannon index and Chao1 index in the TG group increased significantly (Table 2, P < 0.05). This was also confirmed by the Venn diagram of OTU distribution (Fig. 1c, d), which intuitively showed the common and unique number of OTU in each group. In addition to the common parts, the controls had the most unique OTUs, while primary GD patients had a significant reduction, and the OTUs was significantly improved after treatment.
Species composition of gut microbiota
The composition and structure of gut microbiota between GD patients and healthy volunteers were significantly different at the phylum and genus levels. From the phylum level, the histogram (Fig. 2a, b) showed that the dominant bacteria of both patients and controls were Firmicutes and Bacteroidetes, and the ratio of the two (F/B) was 3.79 in CG and 3.01 in HG (Table 1, P = 0.444). The results were not statistically significant probably because of individual differences or small sample size in the research. As can be seen from the heatmap (Fig. 3a, c), the relative abundances of Proteobacteria and Synergistetes in HG were significantly reduced compared with the CG group, while the Proteobacteria in TG was significantly increased compared with the HG group. From the genus level (Figs. 2b, d, 3c, d), there was a high relative abundance of Phascolarctobacterium in the CG group. Compared with CG, the relative abundances of Lactobacillus, Veillonella and Streptococcus increased in HG. Statistical analysis showed that Veillonella (Table 1, P = 0.039) had significant difference. Compared with HG, the abundance of Phascolarctobacterium increased, while Blautia, Lactobacillus, Veillonella and Streptococcus decreased in the TG group. Statistical analysis showed that the differences were statistically significant except for Veillonella (Table 2, P < 0.05).
Differential species analysis
Kruskal–Wallis test is used to get the P value based on the species abundance table. And the Q value was obtained after the P value was modified by FDR (Benjamini and Hochberg False Discovery Rate). With Q < 0.05 as the threshold value, the species with significant difference between groups were obtained. Among them, no significant differences in species among groups were obtained at the phylum level of each group. The analysis of different species at genus level (Tables 1, 2) and LDA analysis (Fig. 4) showed that, compared with the CG group, Phascolarctobacterium decreased significantly in HG, while the relative abundances of microorganisms were significantly increased, including Streptococcus, Blautia, Lactobacillus, Veillonella, Bacilli and so on. Compared with HG, the relative abundances of Ruminococcus, Streptococcus and Blautia decreased significantly, while the abundance of Phascolarctobacterium increased significantly in TG.
Potential correlation between gut microbiota and thyroid-related antibodies
Spearman correlation analysis was used to explore the potential correlation between different bacterial abundances and clinical thyroid-related antibodies in GD patients (Fig. 4c). We calculated the Spearman correlation coefficient R between gut microbiota and immune indexes including TRAb. As shown in Table 3, among all, Synergistetes was negatively correlated with TRAb (P = 0.000, R = − 0.702), TGAb (P = 0.003, R = − 0.624) and TPOAb (P = 0.001, R = − 0.711), while Lactobacillus was positively correlated with TRAb (P = 0.024, R = 0.489) and TPOAb (P = 0.006, R = 0.607). TRAb was also negatively correlated with Phascolarctobacterium (P = 0.011, R = − 0.544). Through the correlation analysis of the indexes and gut microbiota of GD patients before and after treatment (Table 4), it was found that there was a positive correlation between TRAb and Ruminococcus (P = 0.021, R = 0.553). We also analyzed the correlation between the changes of index and bacterial abundance in GD patients before and after treatment (Fig. 4d, e). The results indicated that the abundance difference value of Ruminococcus (P = 0.035, R = 0.638) and Phascolarctobacterium (P = 0.046, R = 0.610) had a highly positive correlation with the difference value of TRAb level. Therefore, it is speculated that the increase of TRAb level in GD patients is related to the increase of Ruminococcus and Lactobacillus, as well as the decrease of Phascolarctobacterium and Synergistetes. These microbiota may be involved in the immune process of GD.
Discussion
The gut microbiota plays an important role in gastrointestinal homeostasis and may be one of the contributing factors of thyroid disease [24, 25]. Patients with GD are prone to suffer from digestive system symptoms such as hunger, anorexia, vomiting and increased defecation [26, 27]. The increased intestinal motility in patients may lead to changes in intestinal physiology, and thereby further remodeling gut microbiota composition [28]. Through the detection of gut microbiota and thyroid-related antibodies in 14 normal subjects and 15 GD patients, as well as the analysis of the changes of microflora and serum antibodies in GD patients before and after treatment, this study explored the correlation between gut microbiota and immune status represented by TRAb in GD, to provide new biological indicators for GD, new insights for the pathogenesis of GD and a new perspective for the treatment of GD. In this study, we found that the gut microbiota of GD patients had significant changes compared with the controls. The abundance and diversity of gut microbiota were significantly reduced in GD patients, while increased apparently after treatment along with the improvement of microflora.
The alpha diversity and richness of gut microbiota in patients with GD were significantly lower than those in healthy volunteers, which was in agreement with some previous findings [29]. In addition, we observed that when the thyroid function of these GD patients basically recovered after treatment, the microflora diversity index was also improved. Therefore, we believe that the decrease of bacterial diversity may be closely related to the progress of the disease.
At the phylum level, Synergistetes of CG subjects were significantly increased compared to that of HG patients. Synergistetes is more common in oral diseases such as periodontitis [29]. Spearman correlation analysis showed that the relative abundance of Synergistetes was negatively correlated with TRAb, TGAb and TPOAb levels, suggesting a possible association between Synergistetes and thyroid autoantibodies. A study found that, in SLE patients [30], the abundance of intestinal Synergetics was negatively correlated with serum anti-dsDNA antibody titer, and positively correlated with protective natural IgM antibodies against phospholcholine, which indicated that the deficiency of synergetics in SLE patients resulted in compatible synthesis or secret of anti-phospholcholine IgM in B1 cells. Patricia et al. [31] confirmed through in vitro culture that the flora isolated from the feces of SLE patients can promote the activation of immature CD4 + lymphocytes and the differentiation of Th17 cells, and prevent them from differentiating into Tregs, while Bifidobacterium supplementation can inhibit the over activation of CD4 + lymphocytes. In recent years, other studies [32] have confirmed that some bacteria can affect the differentiation balance of Th17/Treg by secreting inflammatory cytokines or small molecules such as polysaccharide a, short chain fatty acids, etc. These studies suggest that synergistetes may be involved in regulating the synthesis and secretion of autoantibodies in patients with autoimmune diseases by affecting the differentiation balance of Th17/Treg. Our study shows that the number of intestinal Synergistes in patients with Gd is reduced, which is consistent with the previous studies. Therefore, we speculate that the decrease of Synergistetes in GD patients may lead to the balance of Th17/Treg differentiation, which further mediates the synthesis and secretion of serum antibodies such as TRAb, TGAb and TPOAb.
Ruminococcus and Lactobacillus were abundant in the intestines of patients with GD. And we found the two genus were positively associated with the level of TRAb. Compared with HG patients, the relative abundance of Phascolarctobacterium in both of the CG and TG subjects was significantly increased. And Phascolarctobacterium was negatively correlated with TRAb. Phascolarctobacterium colonizes and exerts beneficial effects in the human gastrointestinal tract, producing short-chain fatty acids (SCFAs) including acetate and propionate. A previous study showed that SCFAs and their receptors play an important role in gastrointestinal physiology and pathology, and participate in metabolism and emotion regulation of the host [33]. Moreover, The involvement of SCFAs in the regulation of intestinal motility, hormone secretion, maintain epithelial barrier and immune function, could be of great significance for inflammation and immune response under pathological conditions [34]. The decrease of Phascolarctobacterium may lead to the imbalance of immune homeostasis of human host, making it more susceptible to digestive system diseases and metabolic diseases. To some extent, the reduction of the levels of Phascolarctobacterium in GD patients may promote the progress and aggravation of the disease.
Ruminococcus can produce propionate [35], a kind of short chain fatty acid, which can induce the differentiation of regulatory T cells to produce cytokines such as IL-10 [35]. It has negative effects on intestinal health in inflammatory and allergic diseases [37]. Lactobacillus is rarely pathogenic in the human body. Contrarily, it plays an important role in gut microbiota improving and defecation, and has a certain preventive and therapeutic role in inflammatory bowel disease, immune disease and even cancer. The diarrhea symptoms of GD patients may be associated with the high level of Lactobacillus. It was found that Lactobacillus can induce macrophages to secrete various effector cytokines such as IL-10, IL-8, IL-6 and TNF-α [38]. And several studies have discovered that Ruminococcus and Lactobacillus participate in the pathological process of autoimmune diseases through cytokines such as TNF-α, IL-6 and IL-10. Previous studies have found that TNF-α and IL-6 are significantly correlated with Graves’ disease [39,40,41,42]. TNF-α has been discovered to play an important role in the initiation of humoral immunity, participating in the upregulation of HLA class I, the activation of phagocytes and the induction of IL-1, IL-6 and TNF-α itself, as well as enhancing the expression of HLA II class in cooperation with IFN-γ [43,44,45], thus inducing cell apoptosis and participating in the regulation of thyroid autoimmunity[46]. However, some studies have found that Ruminococcus decrease in certain autoimmune diseases, such as autoimmune hepatitis [47], and induces metabolic diseases. Therefore, the homeostasis of Ruminococcus is crucial in autoimmune diseases. Speculate that the increase or decrease of Ruminococcus abundance will induce different immune responses. Short chain fatty acids produced by Ruminococcus can induce the differentiation of regulatory T cells, increase the number of Th1 and Th17 cells, and upregulate IL-10 which plays key parts in T cell proliferation and B cell differentiation, stimulating B lymphocyte responses and promoting TRAb production by enhancing B cell HLA class II expression and stimulating B cell proliferation [48]. And when Ruminococcus abundance is substantially decreased, the activation of effector T cell cause redundant local and systemic inflammatory responses [49], leading to the occurrence of certain autoimmune diseases. Therefore, Ruminococcus and Lactobacillus were speculated to regulate the secretion of cytokines which leads to the promotion of TRAb production and eventually participate in the immune mechanism of GD.
Veillonella is composed of a variety of Gram-negative bacteria and appears in the development of many common oral diseases, such as dental caries and periodontitis [50, 51]. Studies have reported that Veillonella acts as a pathogen in a variety of inflammation such as periodontitis, bacteremia and pneumonia [52,53,54]. It can recognize microorganisms and metabolites through Toll-like receptors as well as trigger inflammatory and immune responses to pathogens [55]. Streptoccus is also a pathogenic bacterium of many diseases. It has been found that Veillonella and Streptoccus coexist in the ecosystem. There is a metabolic interaction between the two [56], which can induce dendritic cells to secrete cytokines, and significantly enhance the response of cytokines such as IL-8, IL-6, IL-10 and TNF-α [57]. Excessive immune response may break the tolerance of thyroid autoimmunity. Thus, we hypothesized that Veillonella and Streptoccus may play an auxiliary role in the pathogenesis of autoimmune hyperthyroidism under certain circumstances.
Blautia had a significant increase in patients with GD. It can produce butyrate and acetate, and reduce body weight by regulating G-protein-coupled receptors 41(GPR41) and GPR43 [58, 59]. In addition, it has been found that Blautia is negatively correlated with visceral fat accumulation [60]. The latest research also shows that Blautia is consumed in large amounts in the intestinal tract of obese subjects [61]. Therefore, we believe that the emaciation of GD patients may not only be caused by the high metabolism of thyroid hormone. Blautia is likely to play a promoting role in the emaciation of GD patients.
In summary, GD has a significant correlation with the composition and structure of gut microbiota, but the microbiota composition found in GD patients might be an effect of hyperthyroidism and might not necessarily be involved in the pathogenesis. The direct role of gut microbiota in the occurrence and development of GD remains to be elucidated in the future research. The main advantages of this study are the collection of samples before the treatment and the setting of the treatment group for the matching analysis of fecal samples before and after the treatment. But there are still some limitations in this study. For one hand, individual differences among samples are inevitable, including incomplete control of drug, diet, environment and other confounding factors. For another, the insufficient sample size and the limitations of 16SrDNA sequencing method itself are also the defects of the research. The results of the study can only provide evidence of relevance rather than causality. Further research needs to expand the sample size and establish animal models to explore the mechanism of specific microflora in depth.
Conclusion
In brief, the analysis of bacterial community diversity showed that the gut microbiota diversity of GD patients was lower than that in healthy controls and subjects with thyroid function recovered after treatment. Blautia, Veillonella, Streptococcus, Ruminococcus and Lactobacillus were enriched in GD, while Phascolarctobacterium and Synergistetes were consumed in large quantities. The current research reveals that immune responses against gut microflora could be of great relevance to the regulation of TRAb observed in GD patients, with the decreased Synergistetes and Phascolarctobacterium probably have a protective role in the pathogenesis of GD, while the increased Ruminococcus and Lactobacillus possibly play a role in the immune mechanism of Graves’ disease. Our findings provide a few references for elucidating the mechanism of gut microbiota disorder in Graves’ disease. Nevertheless, there are still many problems needed to be solved and more further studies needed to be conducted.
References
Burcelin R, Nicolas S, Blasco-Baque V (2016) Microbiotes and metabolic diseases: the bases for therapeutic strategies. Med Sci (Paris) 32:952–960. https://doi.org/10.1051/medsci/20163211010
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W et al (2012) Host-gut microbiota metabolic interactions. Science 336:1262–1267. https://doi.org/10.1126/science.1223813
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK (2014) Specialized metabolites from the microbiome in health and disease. Cell Metab 20:719–730. https://doi.org/10.1016/j.cmet.2014.10.016
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F et al (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60. https://doi.org/10.1038/nature11450
Diamant M, Blaak EE, de Vos WM (2011) Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev 12:272–281. https://doi.org/10.1111/j.1467-789X.2010.00797.x
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. https://doi.org/10.1038/nature05414
Fei N, Zhao L (2013) An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 7:880–884. https://doi.org/10.1038/ismej.2012.153
Bervoets L, Van Hoorenbeeck K, Kortleven I, Van Noten C, Hens N, Vael C et al (2013) Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog 5:10. https://doi.org/10.1186/1757-4749-5-10
Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW et al (2013) Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring) 21:E607–E615. https://doi.org/10.1002/oby.20466
Xu P, Li M, Zhang J, Zhang T (2012) Correlation of intestinal microbiota with overweight and obesity in Kazakh school children. BMC Microbiol 12:283. https://doi.org/10.1186/1471-2180-12-283
Ferrer M, Ruiz A, Lanza F, Haange SB, Oberbach A, Till H et al (2013) Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environ Microbiol 15:211–226. https://doi.org/10.1111/j.1462-2920.2012.02845.x
Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL et al (2010) Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59:3049–3057. https://doi.org/10.2337/db10-0253
Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI et al (2011) Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 94:58–65. https://doi.org/10.3945/ajcn.110.010132
Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C et al (2010) Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18:190–195. https://doi.org/10.1038/oby.2009.167
Yun Y, Kim HN, Kim SE, Heo SG, Chang Y, Ryu S et al (2017) Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol 17:151. https://doi.org/10.1186/s12866-017-1052-0
Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Martinez JA (2019) Diet, Gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr 10:S17–S30. https://doi.org/10.1093/advances/nmy078
Bahn CR, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I et al (2011) Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid 21:593–646. https://doi.org/10.1089/thy.2010.0417
Rees SB, McLachlan SM, Furmaniak J (1988) Autoantibodies to the thyrotropin receptor. Endocr Rev 9:106–121. https://doi.org/10.1210/edrv-9-1-106
Zhou L, Li X, Ahmed A, Wu D, Liu L, Qiu J et al (2014) Gut microbe analysis between hyperthyroid and healthy individuals. Curr Microbiol 69:675–680. https://doi.org/10.1007/s00284-014-0640-6
Ishaq HM, Mohammad IS, Shahzad M, Ma C, Raza MA, Wu X et al (2018) Molecular alteration analysis of human gut microbial composition in Graves’ disease patients. Int J Biol Sci 14:1558–1570. https://doi.org/10.7150/ijbs.24151
Shi TT, Xin Z, Hua L, Zhao RX, Yang YL, Wang H et al (2019) Alterations in the intestinal microbiota of patients with severe and active Graves’ orbitopathy: a cross-sectional study. J Endocrinol Invest 42:967–978. https://doi.org/10.1007/s40618-019-1010-9
Hagerty SL, Hutchison KE, Lowry CA, Bryan AD (2020) An empirically derived method for measuring human gut microbiome alpha diversity: demonstrated utility in predicting health-related outcomes among a human clinical sample. PLoS ONE 15:e229204. https://doi.org/10.1371/journal.pone.0229204
Covelli D, Ludgate M (2017) The thyroid, the eyes and the gut: a possible connection. J Endocrinol Invest 40:567–576. https://doi.org/10.1007/s40618-016-0594-6
Young VB (2012) The intestinal microbiota in health and disease. Curr Opin Gastroenterol 28:63–69. https://doi.org/10.1097/MOG.0b013e32834d61e9
De Leo S, Lee SY, Braverman LE (2016) Hyperthyroidism. Lancet 388:906–918. https://doi.org/10.1016/S0140-6736(16)00278-6
Chen LY, Zhou B, Chen ZW, Fang LZ (2010) Case report: recurrent severe vomiting due to hyperthyroidism. J Zhejiang Univ Sci B 11:218–220. https://doi.org/10.1631/jzus.B0900371
Ebert EC (2010) The thyroid and the gut. J Clin Gastroenterol 44:402–406. https://doi.org/10.1097/MCG.0b013e3181d6bc3e
You M, Mo S, Watt RM, Leung WK (2013) Prevalence and diversity of Synergistetes taxa in periodontal health and disease. J Periodontal Res 48:159–168. https://doi.org/10.1111/j.1600-0765.2012.01516.x
Chen B, Sun L, Zhang X (2017) Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. J Autoimmun 83:31–42. https://doi.org/10.1016/j.jaut.2017.03.009
Lopez P, de Paz B, Rodriguez-Carrio J, Hevia A, Sanchez B, Margolles A et al (2016) Th17 responses and natural IgM antibodies are related to gut microbiota composition in systemic lupus erythematosus patients. Sci Rep 6:24072. https://doi.org/10.1038/srep24072
Omenetti S, Pizarro TT (2015) The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Front Immunol 6:639. https://doi.org/10.3389/fimmu.2015.00639
Wu F, Guo X, Zhang J, Zhang M, Ou Z, Peng Y (2017) Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp Ther Med 14:3122–3126. https://doi.org/10.3892/etm.2017.4878
Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT (2018) Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr Physiol 8:1091–1115. https://doi.org/10.1002/cphy.c170050
Park J, Lee J, Yeom Z, Heo D, Lim YH (2017) Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals. Sci Rep 7:14520. https://doi.org/10.1038/s41598-017-15163-5
Shimizu J, Kubota T, Takada E, Takai K, Fujiwara N, Arimitsu N et al (2018) Propionate-producing bacteria in the intestine may associate with skewed responses of IL10-producing regulatory T cells in patients with relapsing polychondritis. PLoS ONE 13:e203657. https://doi.org/10.1371/journal.pone.0203657
Chua HH, Chou HC, Tung YL, Chiang BL, Liao CC, Liu HH et al (2018) Intestinal dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology 154:154–167. https://doi.org/10.1053/j.gastro.2017.09.006
Rocha-Ramirez LM, Perez-Solano RA, Castanon-Alonso SL, Moreno GS, Ramirez PA, Garcia GM et al (2017) Probiotic lactobacillus strains stimulate the inflammatory response and activate human macrophages. J Immunol Res 2017:4607491. https://doi.org/10.1155/2017/4607491
Ferrari SM, Ruffilli I, Elia G, Ragusa F, Paparo SR, Patrizio A et al (2019) Chemokines in hyperthyroidism. J Clin Transl Endocrinol 16:100196. https://doi.org/10.1016/j.jcte.2019.100196
Duraes C, Moreira CS, Alvelos I, Mendes A, Santos LR, Machado JC et al (2014) Polymorphisms in the TNFA and IL6 genes represent risk factors for autoimmune thyroid disease. PLoS ONE 9:e105492. https://doi.org/10.1371/journal.pone.0105492
Inoue N, Watanabe M, Morita M, Tatusmi K, Hidaka Y, Akamizu T et al (2011) Association of functional polymorphisms in promoter regions of IL5, IL6 and IL13 genes with development and prognosis of autoimmune thyroid diseases. Clin Exp Immunol 163:318–323. https://doi.org/10.1111/j.1365-2249.2010.04306.x
Anvari M, Khalilzadeh O, Esteghamati A, Momen-Heravi F, Mahmoudi M, Esfahani SA et al (2010) Graves’ disease and gene polymorphism of TNF-alpha, IL-2, IL-6, IL-12, and IFN-γ. Endocrine 37:344–348. https://doi.org/10.1007/s12020-010-9311-y
Giusti C (2019) The Th1 chemokine MIG in Graves’ disease: a narrative review of the literature. Clin Ter 170:e285–e290. https://doi.org/10.7417/CT.2019.2149
Shiau MY, Huang CN, Yang TP, Hwang YC, Tsai KJ, Chi CJ et al (2007) Cytokine promoter polymorphisms in Taiwanese patients with Graves’ disease. Clin Biochem 40:213–217. https://doi.org/10.1016/j.clinbiochem.2006.11.009
Matsuno H, Yudoh K, Katayama R, Nakazawa F, Uzuki M, Sawai T et al (2002) The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): a study using a human RA/SCID mouse chimera. Rheumatology (Oxford) 41:329–337. https://doi.org/10.1093/rheumatology/41.3.329
Gonzalez-Diaz SN, Sanchez-Borges M, Rangel-Gonzalez DM, Guzman-Avilan RI, Canseco-Villarreal JI, Arias-Cruz A (2020) Chronic urticaria and thyroid pathology. World Allergy Organ J 13:100101. https://doi.org/10.1016/j.waojou.2020.100101
Lou J, Jiang Y, Rao B, Li A, Ding S, Yan H et al (2020) Fecal microbiomes distinguish patients with autoimmune hepatitis from healthy individuals. Front Cell Infect Microbiol 10:342. https://doi.org/10.3389/fcimb.2020.00342
Ganesh BB, Bhattacharya P, Gopisetty A, Prabhakar BS (2011) Role of cytokines in the pathogenesis and suppression of thyroid autoimmunity. J Interferon Cytokine Res 31:721–731. https://doi.org/10.1089/jir.2011.0049
Makki K, Deehan EC, Walter J, Backhed F (2018) The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23:705–715. https://doi.org/10.1016/j.chom.2018.05.012
Arif N, Sheehy EC, Do T, Beighton D (2008) Diversity of Veillonella spp. from sound and carious sites in children. J Dent Res 87:278–282. https://doi.org/10.1177/154405910808700308
Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL et al (2002) Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40:1001–1009. https://doi.org/10.1128/jcm.40.3.1001-1009.2002
Mashima I, Theodorea CF, Thaweboon B, Thaweboon S, Nakazawa F (2016) Identification of veillonella species in the tongue biofilm by using a novel one-step polymerase chain reaction method. PLoS ONE 11:e157516. https://doi.org/10.1371/journal.pone.0157516
Marriott D, Stark D, Harkness J (2007) Veillonella parvula discitis and secondary bacteremia: a rare infection complicating endoscopy and colonoscopy? J Clin Microbiol 45:672–674. https://doi.org/10.1128/JCM.01633-06
Shah A, Panjabi C, Nair V, Chaudhry R, Thukral SS (2008) Veillonella as a cause of chronic anaerobic pneumonitis. Int J Infect Dis 12:e115–e117. https://doi.org/10.1016/j.ijid.2008.03.018
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14. https://doi.org/10.1093/intimm/dxh186
Egland PG, Palmer RJ, Kolenbrander PE (2004) Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci USA 101:16917–16922. https://doi.org/10.1073/pnas.0407457101
van den Bogert B, Meijerink M, Zoetendal EG, Wells JM, Kleerebezem M (2014) Immunomodulatory properties of Streptococcus and Veillonella isolates from the human small intestine microbiota. PLoS ONE 9:e114277. https://doi.org/10.1371/journal.pone.0114277
Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T et al (2013) The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 4:1829. https://doi.org/10.1038/ncomms2852
Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S et al (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108:8030–8035. https://doi.org/10.1073/pnas.1016088108
Ozato N, Saito S, Yamaguchi T, Katashima M, Tokuda I, Sawada K et al (2019) Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 5:28. https://doi.org/10.1038/s41522-019-0101-x
Benitez-Paez A, Gomez DPE, Lopez-Almela I, Moya-Perez A, Codoner-Franch P, Sanz Y (2020) Depletion of blautia species in the microbiota of obese children relates to intestinal inflammation and metabolic phenotype worsening. mSystems. https://doi.org/10.1128/mSystems.00857-19
Funding
This study was funded by National Natural Science Foundation of China (No. 81774134 and No. 81873174); Natural Science Foundation of Jiangsu Province of China (No. BK20150558 and No. BK20171331); Postdoctoral Foundation of Jiangsu Province of China (No. 1501120C); Jiangsu Province 333 Talent Funding Project (No. BRA2017595); Young Medical Key Talents Project of Jiangsu Province (No. QNRC2016902); Key Research and Development Plan Project of Jiangsu Province—Social Development Projects (No. BE2020701).
Author information
Authors and Affiliations
Contributions
CJ, SJQ and GP contributed to the conception and design of this study. Data preparation, sample collection, data collection and analysis were conducted by CJ, WW, GZH, HSS, LHY, ZP and LB The first draft of the paper was written by CJ, and all authors participated in revising the previous version of the manuscript. All the authors have read and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Wang, W., Guo, Z. et al. Associations between gut microbiota and thyroidal function status in Chinese patients with Graves’ disease. J Endocrinol Invest 44, 1913–1926 (2021). https://doi.org/10.1007/s40618-021-01507-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01507-6