Abstract
Objectives
Glypican4 (GPC4) is a novel adipokine associated with obesity and insulin resistance. GPC4 was cleaved by the glycosylphosphatidylinositol-specific phospholipase D (GPLD1) in an anchored site of the glycosylphosphatidylinositol, and then was released into the extracellular environment. Herein, we investigated the changes of serum GPC4 and GPLD1 levels in obese subjects with different glucose metabolism status and their relationship with adipose tissue insulin resistance index (Adipo-IR) in Chinese north populations.
Methods
A total of 221 obese subjects and 37 normal controls (NC) were recruited in this study. Obese subjects were divided into normal insulin (NI) group, hyperinsulinemia (HI) group, impaired glucose tolerance (IGT) group, and type 2 diabetes mellitus (DM) group. Serum GPC4, GPLD1, and adiponectin were determined by commercially available ELISA kits.
Results
Serum GPC4 levels in the HI, IGT, and DM groups were significantly higher than those in the NC and NI groups (2.27 ± 0.58 ng/mL, 2.21 ± 0.60 ng/mL, 2.49 ± 0.67 ng/mL vs. 1.70 ± 0.33 ng/mL, 1.93 ± 0.34 ng/mL, P < 0.05). GPC4 was positively correlated with GPLD1, which was the most important influencing factor of GPC4. Adipo-IR was independently and positively associated with serum GPC4 and GPLD1. For GPC4, after adjustment for confounders, the risk of adipose tissue insulin resistance in subjects with the highest tertile was 2.974-fold that of those with the lowest tertile (OR = 2.974, P = 0.013). For GPLD1, before adjustment for lipids, the increased probability still existed (Model 2, OR = 3.568, P = 0.003).
Conclusion
GPC4 is an adipokine associated with adipose tissue insulin resistance, and its activity may be regulated by GPLD1. GPC4 may be a marker for adipose tissue insulin resistance in Chinese north obese populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipose tissue is an endocrine organ that is sensitive to insulin. In normal individuals, insulin can efficiently suppress the hydrolysis of triglyceride (TG) and the release of free fatty acids (FFA) by inactivating hormone-sensitive lipase [1], but in obese and type 2 diabetes (T2DM) individuals, this suppression is weakened [2]. The inhibitory effect of insulin on lipolysis is weakened, known as adipose tissue insulin resistance [3], which can cause excessive release of FFA, lead to ectopic fat deposition, induce liver and skeletal muscle insulin resistance, and eventually result in metabolic disorders [4]. Therefore, adipose tissue insulin resistance occurs earlier than liver and skeletal muscle insulin resistance, and is a prelude to systemic insulin resistance [4]. Studying adipose tissue insulin resistance is of great significance to glucose and lipid metabolism. Clinical research requires a reliable and simple quantitative method to measure adipose tissue insulin resistance.
The multistep pancreatic clamp technique is the gold standard for evaluating adipose tissue insulin resistance [5]. It inhibits endogenous insulin secretion using somatostatin, resulting in near-zero insulin concentrations in the body, thereby calculating the insulin concentration needed to inhibit 50% lipolysis (IC50). However, its complexity and cost limit applications in large-scale research. The adipose tissue insulin resistance index (Adipo-IR) is a simple method of measuring adipose tissue insulin sensitivity, which is calculated by multiplying the fasting insulin (FINS) concentration by the fasting FFA concentration [6]. Hydrolysis of TG in lipid droplets is enhanced by growth hormone, catecholamines, and glucagon and inhibited by insulin [1, 5]. The somatostatin used in the multistep pancreatic clamp technique can inhibit the secretion of these hormones, thus circumventing their effects on lipolysis. Although only insulin is included in the Adipo-IR index, Adipo-IR is closely related to the IC50 measured by the multistep pancreatic clamp technique (r = 0.86, P < 0.001) [5]. Therefore, Adipo-IR is a good predictor of adipose tissue insulin sensitivity and is suitable for larger population studies.
Adipose tissue plays a key role in the metabolism of glucose and lipid by secreting adipokines [7]. Adipokines have been known as important factors linking obesity and insulin resistance [8]. In recent years, many researchers have devoted themselves to studying the relationships between adipokines and insulin resistance, and have made great progress. However, there are a few studies on the association between adipokines and adipose tissue insulin resistance, and only some studies have shown that adiponectin (ADP) correlated negatively with Adipo-IR [3, 9].
Glypican4 (GPC4) is an adipokine that belongs to the heparan sulfate proteoglycan family [10]. It is anchored to the external surface of the cell membrane by the glycosylphosphatidylinositol (GPI) and can be cleaved by the lipase of GPI to be released from the cell membrane surface into the extracellular environment. Therefore, it can play a role not only on the cell membrane but also in the extracellular environment. GPC4 can enhance insulin signaling by directly binding to insulin receptors [11]. The previous studies have shown that serum GPC4 levels are significantly positively correlated with body mass index (BMI), FINS, and HOMA-IR in humans [11,12,13]. However, whether GPC4 is associated with Adipo-IR is unclear.
Glycosylphosphatidylinositol-specific phospholipase D (GPLD1) is an enzyme that can specifically cleave GPI anchors [14]. The expression of GPLD1 mRNA is highest in the liver [15]. GPLD1 is a secreted protein that is abundant in the bloodstream [16] and regulated by insulin [17]. Some previous clinical studies have shown that serum GPLD1 levels are associated with type 1 diabetes [18], insulin resistance [19], T2DM [20], and nonalcoholic fatty liver disease (NAFLD) [21]. Since GPC4 is anchored on the surface of the cell membrane through GPI, Brunner et al. speculated that GPLD1 may cleave GPC4 [22]. In this study, we explored the relationship between GPC4 and GPLD1, which can better understand the action mechanism and biological function of GPC4.
The purpose of this study was to investigate the changes of serum GPC4 and GPLD1 levels in obese subjects with different glucose metabolism status and their relationship with Adipo-IR.
Materials and methods
Study subjects and clinical and biochemical characterization
The study cohort consisted of 258 subjects (221 obese and 37 normal-weight) from our hospital outpatient. Bodyweight was stable (± 2 kg) in the past 3 months. All subjects did not take any medication that affected glucose tolerance. All obese subjects were primary obese. Obesity was defined as BMI ≥ 28Kg/m2. HOMA-IR ≥ 2.6 was regarded as insulin resistance [23]. Normal glucose tolerance (NGT) was defined as FBG < 6.1 mmol/L and 2hPBG < 7.8 mmol/L. Impaired glucose tolerance (IGT) was defined as FBG < 6.1 mmol/L and 2hPBG ≥ 7.8 mmol/L but < 11.1 mmol/L. T2DM was defined as FBG ≥ 7 mmol/L and or 2hPBG ≥ 11.1 mmol/L [24]. All subjects were divided into five groups according to BMI and glucose metabolism status: normal controls (NC), subjects with 18.5 < BMI < 24 kg/m2, NGT and normal insulin levels; normal insulin (NI), obese subjects with NGT and normal insulin levels; hyperinsulinemia (HI), obese subjects with NGT and hyperinsulinemia; IGT, obese subjects with IGT; diabetes mellitus (DM), obese subjects with T2DM.
At 7–8 AM following a 10-h overnight fast, all subjects underwent physical examinations, biochemical measurements, and oral glucose tolerance test (75 g anhydrous glucose). Height, weight, and waist circumference (WC) were measured three times and took the average. After resting quietly for 5 min, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice using an electronic sphygmomanometer (Omron HEM-907) and took the average. Liver and kidney function, lipid profiles, fasting blood glucose (FBG), and 2 h postprandial blood glucose (2hPBG) were measured by an automated biochemical analyzer. The FINS was determined by the Siemens Centaur XP system. This study was approved by the Ethics Committee of Peking Union Medica College Hospital. Each subject gave written informed consent before participating.
Mathematical formulas of simple indices for measuring insulin resistance were based on the fasting blood samples [3, 23]: HOMA-IR was calculated as [FBG (mmol/L) × FINS (mU/L)]/22.5, Adipo-IR was calculated as \({\text{[FINS (mU/L)}} \times {\text{ FFA (mmol/L)]}}\).
Serum adipokine assays
Serum GPC4, GPLD1, and ADP levels (USCNK Life Science Inc. Wuhan, China) were assayed by commercially available ELISA kits. The catalog numbers were SEA998Hu for GPC4, SEH975Hu for GPLD1, and SEA605Hu for ADP, respectively. All samples were determined in random order and duplicate. The intra-assay and inter-assay coefficients of variations were 2.94% and 6.95% for GPC4, 5.83% and 10.41% for GPLD1, 4.40% and 8.26% for ADP, respectively.
Data analysis
All statistical analyses were performed by SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Normally distributed data are expressed as mean ± standard deviation (SD), and non-normally distributed data are expressed as median (interquartile range) and were transformed into normal distribution by logarithmic transformation. One-way ANOVA analysis and Bonferroni post hoc analysis were used for comparison between multiple groups. Pearson correlation analysis was used to evaluate the bivariate relationships between GPC4, GPLD1, ADP, and clinical parameters. Multiple linear regression analysis was performed to find variables independently associated with serum GPC4, GPLD1, and ADP. Logistic regression analysis was used to evaluate the relationships between serum GPC4, GPLD1, ADP levels, and Adipo-IR. P < 0.05 was considered statistically significant.
Results
General clinical characteristics of the study subjects
As shown in Table 1, subjects in each group were matched for age and gender, and all obese subjects had similar BMI. The FBG was within the normal range in non-diabetic subjects (NC, NI, HI, and IGT) and increased in T2DM subjects, while the 2hPBG increased linearly from NGT to IGT to T2DM. The FINS progressively increased from NC to NI to HI, IGT, and DM. The fasting FFA increased markedly and linearly from NGT to IGT to T2DM. The changes in FINS and FFA reflected the progressive decrease in adipose tissue insulin sensitivity. Adipo-IR increased 4.2-fold in HI group and 3.1-fold in IGT group and 5.1-fold in DM group versus NC group (9.27 [6.22, 13.71], 6.77 [3.91, 10.64], 11.09 [7.52, 18.02] vs 2.19 [1.26, 3.28], P < 0.05). Adipo-IR was significantly higher in insulin-resistant subjects than that in insulin-sensitive.
Serum adipokine levels
As shown in Fig. 1, compared with the NC group, serum GPC4 levels in the NI, HI, IGT, and DM groups increased significantly by 13.53%, 33.53%, 30.00%, and 46.47%, respectively (1.93 ± 0.34 ng/mL, 2.27 ± 0.58 ng/mL, 2.21 ± 0.60 ng/mL, 2.49 ± 0.67 ng/mL vs. 1.70 ± 0.33 ng/mL, P < 0.05). In addition, serum GPC4 levels in the HI, IGT, and DM groups were also higher than those in the NI group (P < 0.05) (Fig. 1a). The changes in GPLD1 and GPC4 are consistent. Serum GPLD1 levels in the NI, HI, IGT, and DM groups were significantly increased by 40.70%, 70.69%, 53.66%, and 74.41%, respectively, in comparison with the NC group (12.48 ± 4.67 μg/mL, 15.14 ± 4.47 μg/mL, 13.63 ± 5.40 μg/mL, 15.47 ± 4.92 μg/mL vs. 8.87 ± 2.37 μg/mL, P < 0.05). Serum GPLD1 levels were higher in the HI and DM groups than those in the NI group (P < 0.05) (Fig. 1b). Conversely, serum ADP levels were lower in the NI, HI, and DM groups when compared with the NC group (17.77 ± 6.68 μg/mL, 15.53 ± 6.41 μg/mL, 14.31 ± 6.09 μg/mL vs. 20.49 ± 6.74 μg/mL, P < 0.05) (Fig. 1c).
There was no gender difference between serum GPC4 and GPLD1 (Fig. 2a, b). However, males versus females with DM had higher GPC4 (P < 0.05) (Fig. 2d). In males, serum GPC4 levels in insulin-resistant subjects were significantly higher than that in insulin-sensitive subjects, and that in the DM group was the highest. In females, serum GPC4 levels in obese subjects were higher than those in normal controls. Conversely, females had higher levels of ADP when compared with males (Fig. 2c), especially in the HI and DM groups (P < 0.05) (Fig. 2f).
Associations between serum adipokines and clinical parameters
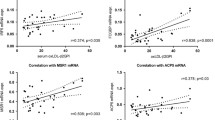
As shown in Table 2, serum GPC4 levels were positively correlated with BMI, WC, DBP, alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid (UA), TG, FFA, FINS, HOMA-IR, Adipo-IR, and GPLD1 (P < 0.05). Serum GPLD1 levels were positively correlated with age, BMI, WC, SBP, DBP, ALT, AST, total cholesterol (TC), TG, FFA, FINS, HOMA-IR, and Adipo-IR, and negatively correlated with high-density lipoprotein cholesterol (HDL-C) (P < 0.05). By contrast, serum ADP levels were negatively correlated with BMI, WC, SBP, DBP, ALT, AST, UA, TG, FFA, FINS, FBG, 2hPBG, HOMA-IR, Adipo-IR, GPC4, and GPLD1, and positively correlated with HDL-C, age, and gender (P < 0.05).
In a multivariable regression analysis, as shown in Table 3, after adjusting age, gender, BMI, SBP, AST, creatinine (Cr), TG, HDL-C, low-density lipoprotein cholesterol (LDL-C), FBG, and ADP, we found that GPLD1 (β = 0.559), Adipo-IR (β = 0.190), and UA (β = 0.128) were independent contributors to serum GPC4 levels in all subjects. GPLD1 was the most important independent influence factor for serum GPC4. Serum GPLD1 levels were independently correlated with TG (β = 0.467), GPC4 (β = 0.208), Adipo-IR (β = 0.197), and age (β = 0.107) after adjusting for gender, BMI, SBP, AST, Cr, UA, HDL-C, LDL-C, FBG, and ADP in all subjects (P < 0.05), and TG was the most important independent influence factor for serum GPLD1. Serum ADP levels were independently correlated with Adipo-IR (β = − 0.183), HDL-C (β = 0.173), gender (β = 0.145), TG (β = − 0.190), and age (β = 0.151) after adjusting for potential confounding factors (P < 0.05). Adipo-IR was the common independent contributor to serum GPC4, GPLD1, and ADP, and it was independently positively correlated with serum GPC4 and GPLD1 levels, while negatively correlated with serum ADP levels.
Relationship of serum adipokines with adipose tissue insulin resistance
To further investigate the relationships of serum GPC4, GPLD1, and ADP levels and Adipo-IR, Logistic regression analysis was performed. All subjects were stratified into trisections according to GPC4, GPLD1, and ADP tertiles, respectively. Because Adipo-IR was significantly higher in HI, IGT and DM groups than that in NC and NI groups, all subjects were divided into with and without Adipo-IR.
As shown in Table 4, the probability of adipose tissue insulin resistance in subjects with the medium and highest GPC4 levels was 1.340-fold and 6.602-fold higher than that in subjects with the lowest GPC4 levels [univariate, OR = 2.340 (1.053–3.543), 7.602 (3.751–15.408), P < 0.05]. This increased probability of adipose tissue insulin resistance in subjects with the highest GPC4 levels remained after adjusting for age, gender, and BMI [Model 1, OR = 4.928 (2.295–10.579), P < 0.001] and SBP, FBG, and AST based on Model 1 [Model 2, OR = 4.006 (1.755–9.130), P = 0.001], and TC, TG, and HDL-C based on Model 2 [Model 3, OR = 2.974 (1.262–7.001), P = 0.013].
The probability of adipose tissue insulin resistance in subjects with the highest GPLD1 levels was 5.306-fold higher than that in subjects with the lowest GPLD1 levels [univariate, OR = 6.306 (3.128–12.714), P < 0.001]. This increased probability remained after adjusting for age, gender, and BMI [Model 1, OR = 4.126 (1.920–8.868), P < 0.001] and SBP, FBG, and AST based on Model 1 [Model 2, OR = 3.568 (1.545–8.239), P = 0.003]. However, when further adjustment TC, TG, and HDL-C based on Model 2, the increased probability no longer existed.
By contrast, the risk of adipose tissue insulin resistance in subjects with the lowest serum ADP levels was 1.583-fold higher than that in those with the highest ADP levels [univariate, OR = 2.583 (1.372–4.865), P = 0.003]. This increased risk remained after adjusting for age, gender, and BMI [Model 1, OR = 2.266 (1.080–4.755), P = 0.040]. However, when further adjustment SBP, FBG, and AST in Model 2, and even further adjustment for TC, TG, and HDL-C in Model 3, the increased risk of Adipo-IR no longer existed.
Discussion
In the present study, we investigated the changes of serum GPC4 and GPLD1 levels in obese subjects with different glucose metabolism status. Serum GPC4 levels were significantly increased in obese subjects, especially in obese subjects with insulin resistance. The changing trend of GPLD1 was similar to GPC4. Serum GPC4 levels were positively correlated with BMI, WC, DBP, ALT, AST, UA, TG, FFA, FINS, HOMA-IR, Adipo-IR, and GPLD1 in all subjects. GPLD1 and Adipo-IR were the two most important independent contributors to serum GPC4 levels. Subjects with high GPC4 and GPLD1 levels were more likely to develop adipose tissue insulin resistance. All these findings suggested that GPC4 is an adipokine associated with obesity and adipose tissue insulin resistance, and its activity may be tightly regulated by GPLD1.
Insulin can effectively inhibit lipolysis and has a close inverse relationship with FFA. The research group of Gastaldelli had confirmed that in humans, the inhibition of lipolysis and FFA release has a curvilinear relationship with plasma insulin concentration, and the logarithmic conversion has a linear relationship [3]. In subjects with obesity and T2DM, this relationship moves upward, with higher FFA concentration at any given insulin concentration [25]. Therefore, the product of plasma insulin and FFA concentration provides an index of adipose tissue insulin resistance, and this index has been used to assess adipose tissue insulin sensitivity under various metabolic conditions [26]. In a large sample population, during the transition from NGT to IGT to T2DM, the adipose tissue insulin sensitivity progressively decreases [3, 27, 28]. In our study, we found that Adipo-IR was increased 4.2-fold in hyperinsulinemia and 3.1-fold in IGT and 5.1-fold in T2DM compared with normal controls. These results indicate that Adipo-IR is closely related to glucose metabolism and is one of the important pathophysiological changes of T2DM.
GPC4 is the first adipokine associated with insulin resistance found in proteoglycan family [13]. Gesta et al. found for the first time that there is a differential expression of GPC4 in human visceral and subcutaneous adipose tissues [12]. The previous studies have shown that serum GPC4 levels are significantly correlated with BMI, body fat content, waist-to-hip ratio (WHR) [11, 12], and the ratio of visceral to the subcutaneous fat area [29]. In line with these results, we showed that serum GPC4 levels were significantly increased in obese subjects, which was positively correlated with BMI and WC. All these results suggest that serum GPC4 may be a marker for BMI and visceral fat accumulation.
The previous studies had shown that serum GPC4 levels were positively correlated with FINS and HOMA-IR in humans [11,12,13]. Since adipose tissue insulin resistance may precede liver and skeletal muscle insulin resistance, we tried to investigate whether serum GPC4 levels are associated with adipose tissue insulin resistance. As expected, our results showed that serum GPC4 levels were closely related to Adipo-IR. Serum GPC4 levels may be a useful marker for adipose tissue insulin resistance. Ussar et al. showed that GPC4 can enhance insulin signaling by directly binding to insulin receptors and play an insulin-like role [11]. Obese subjects had significantly increased serum GPC4 levels, which may be a compensation mechanism for the body to reduce insulin demand and alleviate insulin resistance [8]. Maintaining higher serum GPC4 levels in obese subjects with insulin resistance or diabetes could still reduce insulin demand [11]. In the present study, serum GPC4 levels in the NI group were significantly increased compared with the NC group, which could be the compensatory mechanism before adipose tissue insulin resistance in obese subjects. When serum GPC4 levels are insufficient to compensate, adipose tissue insulin resistance will occur. Increased serum GPC4 levels may indicate the occurrence of adipose tissue insulin resistance, which is an important pathophysiological component of T2DM. Therefore, actively intervening in obese patients with elevated GPC4 levels is of great significance in preventing the occurrence of T2DM.
GPLD1 is an enzyme that cleaves GPI [17], so it is speculated that GPLD1 may cleave GPC4 [22]. However, population-based studies have not proven the relationship between GPC4 and GPLD1. In this study, we demonstrated for the first time that there was a strong correlation between serum GPC4 and GPLD1 levels, and GPLD1 was the most important influence factor for GPC4. This is of great significance for further understanding of the action mechanism of GPC4. Therefore, we speculate the possible mechanism of linking GPC4 and Adipo-IR: insulin levels increase with weight gain and increased insulin levels can cause increased GPLD1 activity, which will increase GPC4 cleavage, and ultimately lead to increased serum GPC4 levels. Elevated GPC4 binds directly to insulin receptors and plays an insulin-like role, reducing insulin demand and delaying adipose tissue insulin resistance. As the disease progresses, when adipose tissue insulin resistance is present, maintaining high serum GPC4 levels can delay adipose tissue insulin resistance progression. Thus, increased serum GPC4 levels observed in obesity may represent a new compensatory mechanism. GPC4 may become a new target for the treatment of insulin resistance and T2DM. In addition, the previous studies have found that serum GPLD1 levels are positively correlated with TG, ALT, and HOMA-IR [19, 21]. In this study, TG was found to be the most important independent factor of GPLD1. This may be the reason why the increased risk of adipose tissue insulin resistance in subjects with the highest GPLD1 tertile no longer exists after adjustment for lipids. Serum GPC4 and GPLD1 levels were significantly elevated in the HI group compared to the NI group, whereas ADP was not significantly different between the HI and NI groups. This similar changes in serum GPC4 and adiponectin levels were also found in our previous study [13]. All these findings suggest that serum GPC4 and GPLD1 levels may be altered earlier than adiponectin levels as the biochemical marker of adipose tissue insulin resistance.
Studies performed by Yoo et al. showed that Korean males had higher serum GPC4 levels than Korean females [29], which may be related to more visceral fat in males. In this study, the WC of males in each subgroup was larger than that of females, supporting males have more visceral fat. However, we found that males had higher GPC4 levels than females only in the DM group. It is unclear why the GPC4 levels in males and females differed only in DM. One possible explanation is that the sample size is small.
There are some limitations to the present study. First, as a cross-sectional study, our study is failed to establish a causal relationship between serum GPC4 levels and adipose tissue insulin resistance. Second, we do not further group T2DM subjects based on blood glucose levels, and it is unclear whether Adipo-IR is suitable for patients with severe hyperglycemia. Third, our research scale is still small. Fourth, we only studied obese subjects with insulin resistance and diabetes, and did not include lean subjects with insulin resistance and diabetes. Therefore, the association of GPC4 with Adipo-IR cannot be generalized to all insulin resistance or diabetic subjects. Further study of GPC4 changes in lean insulin-resistant or diabetic subjects and its association with Adipo-IR may be a better understanding of the physiological role of GPC4 in energy metabolism and systemic glucose.
In summary, serum GPC4 levels are significantly elevated in obese subjects, especially obese subjects with insulin resistance. Serum GPC4 is closely related to GPLD1 and supports GPLD1 to cleave GPC4. Serum GPC4 and GPLD1 levels are closely associated with Adipo-IR in different glucose tolerance status subjects. Subjects with high GPC4 levels are more likely to have adipose tissue insulin resistance after adjusting for confounders. All these findings suggested that GPC4 is an adipokine associated with obesity and adipose tissue insulin resistance, and its activity may be tightly regulated by GPLD1. Serum GPC4 levels may be a marker for BMI and adipose tissue insulin resistance in Chinese north obese populations.
References
Saponaro C, Gaggini M, Carli F, Gastaldelli A (2015) The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients 7(11):9453–9474. https://doi.org/10.3390/nu7115475
Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA (1989) Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Investig 84(1):205–213. https://doi.org/10.1172/jci114142
Gastaldelli A, Gaggini M, DeFronzo RA (2017) Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio Metabolism Study. Diabetes 66(4):815–822. https://doi.org/10.2337/db16-1167
Shulman GI (2014) Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 371(12):1131–1141. https://doi.org/10.1056/NEJMra1011035
Sondergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD (2017) How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab 102(4):1193–1199. https://doi.org/10.1210/jc.2017-00047
Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K (2009) Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, MD) 50(4):1087–1093. https://doi.org/10.1002/hep.23116
Scherer PE (2006) Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55(6):1537–1545. https://doi.org/10.2337/db06-0263
Tamori Y, Kasuga M (2013) Glypican-4 is a new comer of adipokines working as insulin sensitizer. J Diabetes Invest 4(3):250–251. https://doi.org/10.1111/jdi.12071
Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, Weiss R (2016) Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab 101(6):2423–2431. https://doi.org/10.1210/jc.2016-1376
Fico A, Maina F, Dono R (2011) Fine-tuning of cell signaling by glypicans. Cell Mol Life Sci CMLS 68(6):923–929. https://doi.org/10.1007/s00018-007-7471-6
Ussar S, Bezy O, Bluher M, Kahn CR (2012) Glypican-4 enhances insulin signaling via interaction with the insulin receptor and serves as a novel adipokine. Diabetes 61(9):2289–2298. https://doi.org/10.2337/db11-1395
Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR (2006) Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA 103(17):6676–6681. https://doi.org/10.1073/pnas.0601752103
Zhu HJ, Pan H, Cui Y, Wang XQ, Wang LJ, Li NS, Yang HB, Gong FY (2014) The changes of serum glypican4 in obese patients with different glucose metabolism status. J Clin Endocrinol Metab 99(12):E2697–2701. https://doi.org/10.1210/jc.2014-2018
Metz CN, Brunner G, Choi-Muira NH, Nguyen H, Gabrilove J, Caras IW, Altszuler N, Rifkin DB, Wilson EL, Davitz MA (1994) Release of GPI-anchored membrane proteins by a cell-associated GPI-specific phospholipase D. EMBO J 13(7):1741–1751
Masuda S, Fujishima Y, Maeda N, Tsugawa-Shimizu Y, Nakamura Y, Tanaka Y, Obata Y, Fukuda S, Nagao H, Kita S, Nishizawa H, Shimomura I (2019) Impact of glycosylphosphatidylinositol-specific phospholipase D on hepatic diacylglycerol accumulation, steatosis, and insulin resistance in diet-induced obesity. Am J Physiol Endocrinol Metab 316(2):E239–E250. https://doi.org/10.1152/ajpendo.00319.2018
Davitz MA, Hereld D, Shak S, Krakow J, Englund PT, Nussenzweig V (1987) A glycan-phosphatidylinositol-specific phospholipase D in human serum. Science (New York, NY) 238(4823):81–84. https://doi.org/10.1126/science.2443973
Raikwar NS, Bowen-Deeg RF, Du XS, Low MG, Deeg MA (2010) Glycosylphosphatidylinositol-specific phospholipase D improves glucose tolerance. Metabolism 59(10):1413–1420. https://doi.org/10.1016/j.metabol.2008.11.021
Schofield JN, Stephens JW, Hurel SJ, Bell KM, deSouza JB, Rademacher TW (2002) Insulin reduces serum glycosylphosphatidylinositol phospholipase D levels in human type I diabetic patients and streptozotocin diabetic rats. Mol Genet Metab 75(2):154–161. https://doi.org/10.1006/mgme.2001.3287
Kurtz TA, Fineberg NS, Considine RV, Deeg MA (2004) Insulin resistance is associated with increased serum levels of glycosylphosphatidylinositol-specific phospholipase D. Metabolism 53(2):138–139. https://doi.org/10.1016/j.metabol.2003.09.004
von Toerne C, Huth C, de Las Heras Gala T, Kronenberg F, Herder C, Koenig W, Meisinger C, Rathmann W, Waldenberger M, Roden M, Peters A, Thorand B, Hauck SM (2016) MASP1, THBS1, GPLD1 and ApoA-IV are novel biomarkers associated with prediabetes: the KORA F4 study. Diabetologia 59(9):1882–1892. https://doi.org/10.1007/s00125-016-4024-2
Chalasani N, Vuppalanchi R, Raikwar NS, Deeg MA (2006) Glycosylphosphatidylinositol-specific phospholipase d in nonalcoholic fatty liver disease: a preliminary study. J Clin Endocrinol Metab 91(6):2279–2285. https://doi.org/10.1210/jc.2006-0075
Brunner G, Metz CN, Nguyen H, Gabrilove J, Patel SR, Davitz MA, Rifkin DB, Wilson EL (1994) An endogenous glycosylphosphatidylinositol-specific phospholipase D releases basic fibroblast growth factor-heparan sulfate proteoglycan complexes from human bone marrow cultures. Blood 83(8):2115–2125
Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R (2003) Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 26(12):3320–3325. https://doi.org/10.2337/diacare.26.12.3320
(2018) Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 41(Suppl 1):S13–S27. 10.2337/dc18-S002
Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA (1991) The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 72(1):96–107. https://doi.org/10.1210/jcem-72-1-96
Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E, Ferrannini E, Defronzo RA (2007) Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 133(2):496–506. https://doi.org/10.1053/j.gastro.2007.04.068
Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Kitabchi AE, Mudaliar S, Ratner RE, Stentz FB, Musi N, Reaven PD, Gastaldelli A (2013) Prediction of diabetes based on baseline metabolic characteristics in individuals at high risk. Diabetes Care 36(11):3607–3612. https://doi.org/10.2337/dc13-0520
Mahendran Y, Cederberg H, Vangipurapu J, Kangas AJ, Soininen P, Kuusisto J, Uusitupa M, Ala-Korpela M, Laakso M (2013) Glycerol and fatty acids in serum predict the development of hyperglycemia and type 2 diabetes in Finnish men. Diabetes Care 36(11):3732–3738. https://doi.org/10.2337/dc13-0800
Yoo HJ, Hwang SY, Cho GJ, Hong HC, Choi HY, Hwang TG, Kim SM, Bluher M, Youn BS, Baik SH, Choi KM (2013) Association of glypican-4 with body fat distribution, insulin resistance, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab 98(7):2897–2901. https://doi.org/10.1210/jc.2012-4297
Funding
The study was supported by grants from the Beijing Natural Science Foundation [No. 7182130], the National Natural Science Foundation of China [No. 81673184, 81370898], the Chinese Academy of Medical Science Innovation Fund for Medical Science [CAMS-2016-I2M-1-008]; the National Key Program of Clinical Science [WBYZ2011-873].
Author information
Authors and Affiliations
Contributions
KZ measured serum GPC4, GPLD1, and ADP levels, analyzed the data, and wrote the original manuscript. HZ designed experiments and recruited patients. LW and HY collected clinical data and serum samples and determined biochemical parameters. HP and FG designed the experiment, directed the whole study, and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declared no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Each subject gave written informed consent before participating.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, K., Zhu, H., Wang, L. et al. Serum glypican4 and glycosylphosphatidylinositol-specific phospholipase D levels are associated with adipose tissue insulin resistance in obese subjects with different glucose metabolism status. J Endocrinol Invest 44, 781–790 (2021). https://doi.org/10.1007/s40618-020-01372-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01372-9