Abstract
Objective
The purpose of this study was to predict the poor and excessive ovarian response using anti-Müllerian hormone (AMH) levels following a long agonist protocol in IVF candidates.
Research design and methods
Through a prospective cohort study, the type of relationship and appropriate scale for AMH were determined using the fractional polynomial regression. To determine the effect of AMH on the outcomes of ovarian stimulation and different ovarian responses, the multi-nominal and negative binomial regression models were fitted using backward stepwise method. The ovarian response of study subject who entered a standard long-term treatment cycle with GnRH agonist was evaluated using prediction model, separately and in combined models with (ROC) curves.
Results
The use of standard long-term treatments with GnRH agonist led to positive pregnancy test results in 30 % of treated patients. With each unit increase in the log of AMH, the odds ratio of having poor response compared to normal response decreases by 64 % (OR 0.36, 95 % CI 0.19–0.68). Also the results of negative binomial regression model indicated that for one unit increase in the log of AMH blood levels, the odds of releasing an oocyte increased 24 % (OR 1.24, 95 % CI 1.14–1.35). The optimal cut-off points of AMH for predicting excessive and poor ovarian responses were 3.4 and 1.2 ng/ml, respectively, with area under curves of 0.69 (0.60–0.77) and 0.76 (0.66–0.86), respectively.
Conclusion
By considering the age of the patient undergoing infertility treatment as a variable affecting ovulation, use of AMH levels showed to be a good test to discriminate between different ovarian responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical knowledge and technological advances in recent years have greatly contributed to the success of assisted reproductive technologies, particularly IVF. However, the number of oocytes produced by ovaries after hormonal stimulation is still one of the most important factors for success in this field [1]. In other words, one of the major limiting factors in the success of IVF is the poor ovarian response which is observed in 10–15 % of women undergoing IVF [2]. Thus, study of ovarian reserve before assisted reproductive treatments is necessary [3]. Ovarian reserve as potential ovarian function reflects the quantity and quality of oocytes in the ovary [4].
Today, with advances in reproductive medicine, a large part of the research is focused on the study of ovarian reserve. Overall goals of these researches are as follows: (a) improving the safety of ovarian stimulation techniques by identifying patients with high responsiveness (who are at higher risk of OHSS), (b) improving the effectiveness of ovarian stimulation techniques (through adjustment of stimulation dose) and (c) the use of ovarian reserve as a tool for predicting the outcome of IVF. Therefore, we can say that identification of young women with low ovarian reserve who are in similar conditions to older premenopausal women and informing them about this issue as a clinical need is of great importance [4].
Achieving satisfactory results in assisted reproductive technology requires careful evaluation of the patient and study of her ovarian reserve [5]. A proper ovarian reserve test should be able to predict the odds of pregnancy and birth of live babies in an infertile population that refer for fertility treatment and determine the optimum dose of the hormone selected for ovarian stimulation [6].
Some studies have introduced ovarian volume measurement and antral follicle count (AFC) as useful tests for assessment of ovarian reserve [7–10]. Among the other ovarian reserve tests, determining FSH (follicle-stimulating hormone) levels, inhibin-B serum levels and AFC can be mentioned [4].
AM is one of the hormones that have recently been taken into consideration as a marker for predicting ovarian response before application of assisted reproductive technology [11–13]. This hormone is produced by ovarian granulosa cells and its level slowly starts to decline after puberty and disappears at menopause [5]. Inhibition of initial follicular recruitment, inhibition of FSH-dependent growth, and selection of preantral and small antral follicles are among the functions of this hormone [14]. Since anti-Müllerian hormone (AMH) serum levels are correlated with the number of early antral follicles, it can be used to assess the fertility potential and ovarian response in IVF [5]. Based on a study, the measurement of AMH level is currently the ideal test to determine ovarian reserve which is equal to AFC, but better than FSH, estradiol, LH, and inhibin-B in terms of sensitivity and specificity [15]. FSH, inhibin-B, and estradiol have a low sensitivity in the early stages of ovarian reserve reduction. These three hormones are part of a feedback system and their serum levels are not independent of each other. In addition, changes in serum levels of these three hormones occur relatively late in reproductive aging process, when the ovarian reserve has reached the crisis point and chances of pregnancy have significantly decreased [16]. However, AMH serum level is independent of menstrual cycle and is not affected by GnRH agonists or oral contraceptives [17]. Although AMH is currently known as a reliable and promising marker in predicting ovarian response before using assisted reproductive technology, the cut-off level of this hormone to determine the minimum and maximum ovarian response is still being discussed and different values have been reported in different studies. Since determining the optimal cut-off point of the hormone for prediction of ovarian response can play an important role in making crucial clinical decisions for infertile women, this study aimed to predict poor and excessive ovarian response using AMH levels in IVF candidates.
Materials and methods
In this prospective study, all infertile patients referring to the infertility clinic of Mahdieh Hospital since the beginning of 2011 until the end of 2012 were enrolled in case of having these criteria (a) no underlying endocrine disease, (b) no use of hormonal drugs during the last 3 months and (c) no diagnosis of polycystic ovary syndrome (PCOS) based on the Rotterdam criteria and no diagnosis of azoospermia or severe oligozoospermia. For all infertile patients referring to the infertility clinic of Mahdieh Hospital who met the inclusion criteria and were candidates for IVF, levels of AMH (ELIZA, ng/ml), FSH (RIA, IU/ml) and E2 (ECL, pg/ml) were measured at day 2 or 3 of the menstrual cycle. None of the patients had received hormonal treatment for at least one month. In the next step, patients entered a standard long-term treatment cycle with short-acting GnRH agonist (Sinafact, Sinagen group) with daily dose 50 IU/sQ. It should be noted that Gonadotropin starting dose was based on patient age and dose adjustment was done based on ovarian response. Higher age is accompanied with need to higher stimulation dose.
GnRH agonist long protocol is a standard approach for ovarian stimulation and for reducing bias in this study the same protocol was used for all patients. Then, at the beginning of the menstrual cycle (days 1–3), patients who entered the study underwent basic ultrasound to ensure the absence of any underlying pathology. In this study, controlled ovarian hyperstimulation started at days 3–4 of the cycle and the required dose of human urinary-derived HMG (Merional-IBSA-75 IU/ml Amp) was determined and administered based on the patient’s age and according to the protocol adopted by infertility clinic of Mahdieh Hospital in Tehran. The control ultrasound was performed every 3–4 days; the treatment was continued based on the ovarian response; and the control ultrasound was performed again after 2–3 days. By observation of the dominant follicle (16–18 mm), the final intervention was done by injecting HCG (10,000 IU, Choriomon, IBSA) and oocytes were harvested 35–36 h later and passed to the embryologist. Embryo transfer was performed 36–48 h later if they were appropriate. Luteal phase support started on the day of oocyte retrieval using vaginal progesterone (Cyclogest, 400 mg, Actover), and continued until week 10 of pregnancy in case of pregnancy. The results of all ultrasounds, tests, ovarian response of each patient and the dose of used medication in each cycle were recorded in the patients’ files. Patients were classified into three groups of poor ovarian response (oocytes ≤3), normal ovarian response (4–12 oocytes) and excessive ovarian response (oocytes >12) based on the number of oocytes and embryos.
Anti-Müllerian hormone (AMH) assay
We used the AMH Gen II (catalogue number A79765) (Beckman Coulter, Chaska, MN, USA), which has a sensitivity of 0.57 pmol/l, and reported intra- and inter-assay coefficients of variation of less than 5.4 and 5.6 %, respectively, according to the product insert.
Statistical analysis
Continuous baseline demographic and clinical data are presented as mean ± standard deviation and grouped data as frequencies and percentages. Chi square test or Fisher’s exact test were used to determine the independence of the two categorical variables. One-way ANOVA followed by Tukey’s test were employed to investigate the mean difference between different ovarian responses. Pearson correlation coefficient was used to investigate the correlation between the studied variables and outcome and other independent variables. Given that the distribution of AMH concentration was not normal at the beginning, this was done by changing the scale to natural logarithm. In the next and previous steps of fitting a suitable model for calculating the area under curve of the predictor variables, the type of relationship (linear or nonlinear) and its appropriate scale were determined at first using Lowess smoother (locally weighted scatterplot smoothing) and Fracpoly (fractional polynomial regression) and then, the appropriate model for data fitting was used to draw the ROC curve. Comparing the results of Fracpoly with different models in all three multiple regression models of nominal, ordinal and negative binomial showed that in all these models, 0.5 power or AMH natural logarithm scale is the best case to fit them. Considering the continuous nature of AMH concentration in serum and the disadvantages listed for categorization of continuous data, these models were used. Details relating to these models have already been published [18–20]. In order to determine the effect of AMH on the outcomes of ovarian stimulation and different ovarian responses following adjustment of associated variables, the multiple regression models of nominal, ordinal and negative binomial with regarding the over-dispersion criterion were used. All the above models were fitted using backward stepwise selection. The criterion to select the best model was AIC of these models. Note that in the nominal and ordinal regression models, the response variable was different ovarian responses (no response, poor response, normal response and excessive response) but in the nbreg model, the response variable was the number of oocytes released during the menstrual cycle. Details relating to these models have already been published [21, 22]. R i386 3.0.2 software was used to determine the best cut-off point, the area under the curve, positive and negative predictive values and also the confidence levels for each of the listed values.
Results
This study used data from 188 cases of totally 193 cases of candidates for IVF referring to Mahdieh Hospital in Tehran. First, we examined the basic data from the cases studied in Table 1 and then, we discussed the univariate and multivariate analyses and determined the suitable cut-off point for predicting the AMH levels regarding different forms of ovarian response. One-way ANOVA results showed that the mean AMH blood level was different in different groups of ovarian response (no response, poor response, normal response and excessive response) (F = 8.36, p < 0.001). The results also revealed that 7.8 % (15) of patients had no ovarian response to treatment, 11.4 % (22) had poor response, 50.8 % (98) had normal response and the rest had excessive ovarian response. Subsequent Tukey’s analysis results and the other basic data from the studied cases are summarized in Table 1 based on the type of ovarian response.
According to the table, the use of AM hormone for ovarian stimulation in this study resulted in a positive β-hCG test result or in other words, 30.1 % successful pregnancies. The results of Chi square analysis demonstrated that there was no significant statistical relationship between different ovarian responses and positive pregnancy test results (p = 0.071). Pearson correlation analysis results show that there was a strong direct correlation between the concentration of AMH and the number of released oocytes (ovarian response) (Pearson correlation = 0.401 and p < 0.001). Furthermore, evaluation of the correlation between the concentration of FSH and ovarian response of the studied subjects indicated that this was an inverse relationship, i.e. the higher the concentration of FSH, the lower the ovarian response (Pearson correlation = −0.245 and p = 0.001). These findings can be observed by looking at the numbers given in Table 1.
In order to investigate the effects of different levels of AMH on ovarian response, three different regression models of multi-nominal, ordinal and negative binomial were used with regard to the over-dispersion criterion. Fractional polynomial regression was used to examine the shape of association between the independent variable (in this study, AMH) and the outcome and also the suitable scale for continuous variables. This model showed that the use of AMH hormone logarithm scale in all three models had the lowest AIC among the investigated models.
Table 2 shows the effects of different blood concentrations of AM hormone on the type of ovarian response in the multi-nominal regression models. All models were fitted based on the backward stepwise method.
Note that in the ordinal model, response variables were defined as no response, poor response, normal response and excessive ovarian response. Regarding the multi-nominal regression, these responses were considered to be nominal. For the negative binomial model, the response variable was considered as the number of oocytes released during the study period.
The results of this model were reported with inserting the normal response as the reference class and use of AIC criterion for fitting the best model. The results showed that with each unit increase in the log of AMH, the odds ratio of having poor response compared to normal response decreases by 64 %. It should also be said that in case of each unit increase in the log of AMH, this value was 71 % greater for excessive response group compared to normal group.
The results of the regression model with proportional odds showed that the odds of individuals to be in each of the classes of ovarian responses (no response, poor response, normal response and excessive response) different than the previous or next classes would be 2.29 (OR 2.29, 95 % CI 1.64–3.19, p value < 0.001).
By placing the number of oocytes released after stimulation by AMH as the response variable, the results of negative binomial regression model indicated that for one unit increase in the log of AMH blood levels, the odds of releasing an oocyte increased 24 % (OR 1.24, 95 % CI 1.14–1.35). Note that in all fitted models, the variable of maternal age was one of the variables affecting the results of the study. For example, the results in Table 2 show that with one unit increase in maternal age, the odds of having a poor response was 1.33 times more than odds of having a normal response. Moreover, with each unit increase in maternal age in nbreg model, the chance of release of each oocyte in the studied subjects decreased 4 % (OR 0.96, 95 % CI 0.93–0.99).
The results also show that the only variable affecting the number of embryos during the treatment was directly related to AMH levels and inversely related to maternal age at the time of infertility treatment. These findings suggest that with each unit increase in the concentration of AMH, the odds of formation of an embryo increased approximately 0.3 % (OR 1.025, 95 % CI 1.01–1.04), also with each year increase in maternal age, the odds of formation of an embryo decreased approximately 2 % (OR 0.98, 95 % CI 0.96–0.99).
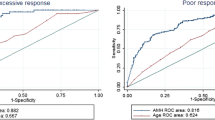
In the next step, the cut-off points for predicting poor, excessive and no ovarian responses compared to normal response will be discussed. Figure 1 shows the relationship between AMH blood levels and poor and excessive ovarian responses.
Figure 2 indicates the area under the curve and the optimal cut-off point of AMH in association with different ovarian responses.
In all the above cases, normal response was used as the reference in comparison to excessive, poor and no responses. Further details are provided in Table 3.
The results of this table show that the AMH plasma levels under 1.2 ng/ml with the area under curve of 0.87 % would very well predict the no ovarian response. This finding shows that it can be very well used to distinguish between normal and no ovarian response with 79 % sensitivity and 93 % specificity. Furthermore, given that in this study DLR+ was greater than 1 for all three ovarian responses, the test is suitable for predicting different ovarian responses. Note that, given that the excessive ovarian response level was greater than 3.4 ng/ml and poor ovarian response level was 1.2, the level of AMH associated with normal ovarian response should be between 1.2 and 3.4 ng/ml. The other results in this table can be interpreted similarly.
Discussion
The results of this study showed that, generally, the use of standard long-term treatments with GnRH agonist led to positive pregnancy test results in 30 % of treated patients. The optimal cut-off points of AMH for predicting excessive and poor ovarian responses were 3.4 and 1.2 ng/ml, respectively, with area under curves of 0.69 and 0.76 %, respectively. Furthermore, considering the estimates done for the poor and excessive ovarian responses, the normal ovarian response should be between 1.2 and 3.4 ng/ml. In mature women, AMH is only secreted by the granulosa cells of preantral and small antral follicles and helps the regulation of ovarian function and follicular steroidogenesis. Due to the exclusive production of this hormone in mature women, it can be used as a marker of ovarian activity [23]. In addition, sustained secretion of this hormone (AMH) during the menstrual cycle with no significant changes during and out of the cycle [24, 25] and its plasma levels not being affected by the use of external hormones [17] justifies the use of this indicator for research purposes and determination of secondary causes of oligo-amenorrhoea. In recent years, numerous studies have examined the role of AMH in predicting ovarian response in controlled ovarian hyperstimulation in IVF candidates. One of the recent studies conducted in this area is by Hamdine et al. [26], and the results of this study indicate that the use of AMH levels alone and as a test has a great accuracy in predicting excessive and poor ovarian responses, with the difference that the accuracy was greater for excessive ovarian response compared to poor response. In our study, the accuracy for prediction of poor ovarian response and no ovarian response was greater than excessive ovarian response. Perhaps the reason for this difference was the distribution of individuals in different ovarian response groups in the two studies. Several markers have been used in previous studies for the prediction of different ovarian responses or ovarian reserve where the antral follicle count (AFC) can be mentioned. The use of this indicator for predicting ovarian reserve prior to IVF is suggested. However, although the ability of this indicator to predict has been reported much better than basal FSH [10], the predictive value of AMH is higher and the unique characteristics of this indicator make the use of this marker for clinical use more logical [26]. In this study, the successful pregnancy rate was approximately 31 % and a negative relationship was observed between age and number of embryos. In the Ficicioglu et al. study, this rate was 39 % and they reported a negative relation between age and AMH levels. This study showed that blood levels of AMH lower than or equal to 1 ng/ml can very well predict the poor ovarian response [27]. With regard to the use of different regression models in this study, it can be stated that the only variables affecting the outcome of the study were AMH serum levels along with the maternal age (in nominal and ordinal multiple regressions of variable responses, different ovarian responses were due to controlled ovarian stimulation where once was considered nominal and once ordinal). In the multi-nominal model it was shown that with each unit increase in the log of AMH, the odds of having a poor response rather than a normal response decreased 64 %. Notable in this model is the role of maternal age, so that with each year increase in maternal age, the chance of having a poor response increased 33 %. Given that categorization of quantitative variables causes residual error in the model (this error can be modified by increasing the number of groups and decreasing the interval between them, but it does not disappear), this study used a model that considered the number of oocytes and embryos as the response variable. This finding is more tangible and understandable for many physicians who do not have much knowledge of the science of statistics. The results showed that for every one unit increase in the log of AMH blood levels, the odds ratio of releasing an oocyte increased 24 %. This model also confirmed the findings of previous models and it was shown that with each unit increase in maternal age, the odds of releasing an oocyte decreased 4 % in the studied individuals. These findings indicate the great importance of considering the maternal age and instruction for treatment in younger ages for mothers who do not have children in the early years of common life. It should also be noted that young women with minimal ovarian reserve who are in fact in the same conditions as older premenopausal women need higher clinical care [4]. Ganidou et al. [28] demonstrated that the use of maternal age, AMH and FSH variables can very well and with high accuracy predict the excessive ovarian response. The study by Vural et al. [29] also showed that maternal age is directly related with poor ovarian response and the odds of a poor response will be greater with the increase in maternal age.
Assessment of ovarian reserve before utilizing assisted reproductive technology is a very important issue, and knowing that the ovarian response would be poor or excessive, allows the doctor to choose the final method of stimulation to reduce the side effects such as OHSS and to minimize cycle cancellation [30]. The present study suggests that prediction of poor ovarian response is more accurate than excessive ovarian response (areas under curve for poor and excessive response were 0.76 (0.66–0.86) and 0.69 (0.60–0.77), respectively, with a confidence interval of 95 %). Regarding the poor ovarian response, the diagnostic ability of the test to distinguish individuals whose tests were positive and were really sick was 0.94 %, while this value was 0.56 % for excessive response. In this study, for categorization of ovarian responses using the variable of the number of oocytes released, each of these responses were made using binary mode and inserted into the next models. Importantly, the response variable of no ovarian response only included individuals who did not release any oocytes following the stimulation but the poor response variable included individuals who released 3 or less oocytes or entirely did not ovulate. Thus, it can be seen that the optimal cut-off points for predicting poor ovarian response and no response are 1.2. However, with considering a greater area under curve for no response compared to poor response and the lower number of false positives for no ovarian response, the probability of an individual with AMH level less than 1.2 being in the no response class was higher than being in poor response class. It should be noted that different categories have been presented for the ovarian reserve in various studies all of which are similar [29, 31], also the estimated areas under the ROC curves in this study are better compared to the past and recent studies and indicates better accuracy of estimates in this study [26, 32]. It should also be noted that in this study, the positive diagnostic likelihood ratios, which were related to former and latter likelihood of developing the disease, were numbers greater than 1 and along with the other reported add values in Table 3, encourage the physicians to use AMH levels for predicting ovarian response in women with infertility problems. Similarly, negative diagnostic likelihood ratios were related to the absence of disease and the more this value was less than 1, the value of the test for prediction of absence of disease was better. Further information about the add values and the use of ROC curves have been previously published [33]. In this study, precise statistical methods were used for predicting and assessing the relationship between the studied variables before determining the optimal cut-off points which resulted in more accurate estimates and better understanding of the results for use in clinics by physicians [18]. Finally, it should be noted that knowing the chances of pregnancy in each cycle allows the physicians to consult with their patients after assessment of the patients’ condition and before the assisted reproduction intervention and if necessary, use gamete donation or adoption [34].
Considering the age of the patient undergoing infertility treatment as a variable affecting ovulation and the use of AMH levels to predict poor and excessive ovarian responses as a standard test with high diagnostic value can be very helpful in determining the strategy for treatment of these patients. Larger studies with focus on all the variables affecting the infertility and its underlying causes are highly recommended.
References
Ubaldi F, Vaiarelli A, D’Anna R, Rienzi L (2014) Management of poor responders in IVF: is there anything new? Biomed Res Int 2014:Art ID 352098. doi:10.1155/2014/352098
Jirge PR, Chougule SM, Gavali VG, Bhomkar DA (2014) Impact of dehydroepiandrosterone on clinical outcome in poor responders: a pilot study in women undergoing in vitro fertilization, using bologna criteria. JHRS 7:175
Ravhon A, Lavery S, Michael S, Donaldson M, Margara R, Trew G et al (2000) Dynamic assays of inhibin B and oestradiol following buserelin acetate administration as predictors of ovarian response in IVF. Hum Reprod 15:2297–2301
Fauser B, Diedrich K, Devroey P (2008) Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update 14:1–14
Yassin MM, Sharif FA, Laqqan MM (2013) Anti-mullerian hormone as a predictor of ovarian reserve and ovarian response in IVF women from Gaza strip. Iran J Reprod Med 11:261
Maheshwari A, Fowler P, Bhattacharya S (2006) Assessment of ovarian reserve—should we perform tests of ovarian reserve routinely? Hum Reprod 21:2729–2735
Lass A, Skull J, McVeigh E, Margara R, Winston R (1997) Measurement of ovarian volume by transvaginal sonography before ovulation induction with human menopausal gonadotrophin for in vitro fertilization can predict poor response. Hum Reprod 12:294–297
Broekmans FJ, Faddy M, te Velde ER (2005) Ovarian reserve and reproductive age may be determined from measurement of ovarian volume by transvaginal sonography. Hum Reprod 20:1114–1115
Chang M-Y, Chiang C-H, Hsieh T, Soong Y, Hsu K (1998) Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril 69:505–510
Hendriks DJ, Mol BWJ, Bancsi LF, Te Velde ER, Broekmans FJ (2005) Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril 83:291–301
Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N (2010) A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril 93:855–864
Van Rooij I, Broekmans F, Te Velde E, Fauser B, Bancsi L, De Jong F et al (2002) Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod 17:3065–3071
La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC et al (2010) Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 16:113–130
Lin P-Y, Huang F-J, Kung F-T, Chiang H-J, Lin Y-J, Lin Y-C et al (2014) Evaluation of serum anti-mullerian hormone as a biomarker of early ovarian aging in young women undergoing IVF/ICSI cycle. Int J Clin Exp Patho 7:6245
Chang HJ, Han SH, Lee JR, Jee BC, Lee BI, Suh CS et al (2010) Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril 94:343–349
Singh N, Malik E, Banerjee A, Chosdol K, Sreenivas V, Mittal S (2013) “Anti-Mullerian hormone: marker for ovarian response in controlled ovarian stimulation for IVF patients”: a first pilot study in the Indian population. J Obstet Gynecol India 63:268–272
Li HWR, Wong CYG, Yeung WSB, Ho PC, Ng EHY (2011) Serum anti-müllerian hormone level is not altered in women using hormonal contraceptives. Contraception 83:582–585
Royston P, Altman DG, Sauerbrei W (2006) Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 25:127–141
Jacoby WG (2000) Loess. a nonparametric, graphical tool for depicting relationships between variables. Electoral Stud 19:577–613
Royston P, Sauerbrei W (2008) Multivariable model-building: a pragmatic approach to regression anaylsis based on fractional polynomials for modelling continuous variables. Wiley, London
Harrell FE (2001) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer, Berlin
Hilbe JM (2011) Negative binomial regression. Cambridge University Press, Cambridge
Anderson R, Nelson S, Wallace W (2012) Measuring anti-Müllerian hormone for the assessment of ovarian reserve: when and for whom is it indicated? Maturitas 71:28–33
Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, De Ziegler D (2008) Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril 90:395–400
La Marca A, Stabile G, Artenisio AC, Volpe A (2006) Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod 21:3103–3107
Hamdine O, Eijkemans M, Lentjes E, Torrance H, Macklon N, Fauser B et al (2015) Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod 30:170–178
Ficicioglu C, Cenksoy PO, Yildirim G, Kaspar C (2014) Which cut-off value of serum anti-Müllerian hormone level can predict poor ovarian reserve, poor ovarian response to stimulation and in vitro fertilization success? A prospective data analysis. Gynecol Endocrinol 30:372–376
Ganidou MA, Kolibianakis EM, Venetis CA, Gerou S, Makedos GA, Klearchou N et al (2014) Is assessment of anti-mullerian hormone and/or antral follicle count useful in the prediction of ovarian response in expected normal responders treated with a fixed dose of recombinant FSH and GnRH antagonists? A prospective observational study. Gynecol Endocrinol 30:817–821
Vural B, Cakiroglu Y, Vural F, Filiz S (2014) Hormonal and functional biomarkers in ovarian response. Arch Gynecol Obstet 289:1355–1361
Broekmans F, Kwee J, Hendriks D, Mol B, Lambalk C (2006) A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 12:685–718
Tokura Y, Yoshino O, Ogura-Nose S, Motoyama H, Harada M, Osuga Y et al (2013) The significance of serum anti-Müllerian hormone (AMH) levels in patients over age 40 in first IVF treatment. J Assist Reprod Genet 30:821–825
Verhagen T, Hendriks D, Bancsi L, Mol B, Broekmans F (2008) The accuracy of multivariate models predicting ovarian reserve and pregnancy after in vitro fertilization: a meta-analysis. Hum Reprod Update 14:95–100
Cook NR (2008) Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem 54:17–23
Elder K, Dale B (2010) In-vitro fertilization. Cambridge University Press, Cambridge
Acknowledgments
The author would like to thank Infertility and Reproductive Health Research Center of Shahid Beheshti University of Medical Sciences for their financial support of this study.
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All the individuals had been informed of the purposes of the study and gave their oral informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heidar, Z., Bakhtiyari, M., Mirzamoradi, M. et al. Prediction of different ovarian responses using anti-Müllerian hormone following a long agonist treatment protocol for IVF. J Endocrinol Invest 38, 1007–1015 (2015). https://doi.org/10.1007/s40618-015-0297-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0297-4