Abstract
Purpose
Success rates of any artificial reproduction techniques depend on a correct protocol for ovarian stimulation. This can be decided only by proper assessment of ovarian reserve before commencing ovarian stimulation. This study has been conducted to investigate the role of hormonal and functional biomarkers in the prediction of ovarian response.
Methods
A total of 689 women between July 2012 and July 2013 undergoing IVF at Kocaeli University have been enrolled in the study. Patients have been categorized into three groups according to the number of oocytes retrieved: 0–3 oocytes (poor responders), 4–15 oocytes (normoresponders), and >16 oocytes (hyperresponders). Groups have been compared according to follicle-stimulating hormone (FSH), E2, luteinizing hormone (LH) levels, antral follicle counts, and E2 levels on hCG days. Furthermore, regression analysis has been performed with parameters such as age, FSH, LH, E2, anti-mullerian hormone (AMH) and antral follicle counts (AFC) that can affect the total number of oocytes retrieved and pregnancy rates and their interactions with each other have been investigated.
Results
FSH, age, hCG day LH level, cycle cancellation rate, total gonadotropin dose were significantly higher in the poor responder group, but in this group, AFC, AMH, hCG day E2 level, and the number of MII oocytes were significantly lower. Cut-off values of normal responders for FSH, AMH, and AFC were 8.43 area under curve [AUC: 0.541 (0.491–0.590)], 0.62 [AUC: 0.704 (0.638–0.764)], and 6 [AUC: 0.715 (0.667–0.760)], respectively. Cut-off values for the absolute poor response group (cycle cancellation) were 12.75 for FSH [AUC: 0.533 (0.49–0.57)], 0.23 for AMH [AUC: 0.678 (0.618–0.733)], and 6 for AFC [AUC: 0.576 (0.531–0.613)]. AMH and AFC were the best markers for the prediction of total oocyte count, independent of age, FSH, and LH levels.
Conclusions
AMH and AFC were found to be the best ovarian reserve tests that can determine the total oocyte count retrieved, without any significant effects on pregnancy rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian response can be defined as the reaction of the ovaries to exogenous stimulus. It varies considerably among individuals and in the same individual as well. In spite of many advances in assisted reproductive technology (ART), the prediction of ovarian response following controlled ovarian hyperstimulation (COH) is still a considerable problem in clinical practice. For several years, age and day-3 levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) have been used as indicators of ovarian response to ART [1, 2]. In fact, the basal FSH concentration is the most common test used for ovarian screening; however, it has been reported that the increase in FSH levels occurs late in the sequence of events associated with ovarian ageing [3]. Therefore, if fertility is considered the end point, this increase may be of limited clinical use as a marker.
Recent studies have shown that anti-mullerian hormone (AMH) and antral follicle count (AFC) may be predictors of ovarian response to COH in ART and superior to basal FSH and chronological age [4–6]. However, the role of AMH and AFC in success rates of IVF is contradictory. AMH is produced by granulosa cells of pre-antral and small antral follicles, correlating with the primordial follicle pool. AMH serum levels reflect the ovarian reserve potential with high accuracy [7]. Compared to antral follicle count, AMH concentrations could reliably and equally predict the poor response to ovarian stimulation in IVF cycles [8]. This could be attributed to the greater number of oocytes retrieved in women with high AMH levels, given that high basal AMH concentrations indicate a great number of selectable follicles. On the other hand, the results of a large meta-analysis showed that AMH levels are very poor predictors of pregnancy outcome [9].
This study has been conducted to investigate the role of ovarian reserve markers in predicting ovarian response.
Materials and methods
This study was designed retrospectively among women who have undergone IVF/ICSI in Kocaeli University Faculty of Medicine Assisted Reproductive Techniques Unit. Clinical details of all treatment cycles were prospectively entered in a computer, which were retrieved for analysis, retrospectively.
Patient selection
A total of 689 women who fulfill the inclusion criteria between July 2012 and June 2013 undergoing IVF at Kocaeli University have been enrolled in the study. Patients with previous ovarian surgery, diagnosis of endometriosis or adnexal masses, hyperprolactinemia, thyroid dysfunction, systemic diseases and patients more than 45 years of age were not included in the study. All patients underwent detailed infertility evaluation such as spermiogram, hysterosalphingography or laparoscopy for tubal patency-pelvic pathology, insulin level, serum day-3 hormone levels of prolactin, estradiol, testosterone, and FSH, LH, TSH, AMH levels. The homeostasis model assessment of insulin resistance (HOMA-IR) was derived from calculations according to the following formula: fasting insulin (mU/ml) × fasting glucose (mmol/l)/22.5.
Ovarian reserve assessment
The total number of antral follicles measuring 2–5 mm in diameter was calculated. All patients’ measurements of AMH were determined in duplicate using the “Gen II microELISA” method (Beckman Coulter, Inc. 250 S. Kraemer Blvd. Brea, CA 92821, USA). The sensitivity of the assay was 0.017 ng/ml. The intra- and inter-assay variations were 5 and 8 %, respectively. Serum LH, FSH, and estradiol levels have been examined on the third day of cycle and on hCG day by Electrochemiluminescence Immunoassay “ECLIA” method (Roche Diagnostics GmbH, D-68298, Mannheim, Germany). According to this, the intra-assay repeatability values for FSH, LH, and estradiol were 1.4–2.0 %, 0.8–1.8 %, and 1.6–5.7 % respectively; and the inter-assay repeatability values for FSH, LH, and estradiol were 2.9–5.3 %, 1.9–5.2 %, and 2.3–6.2 %, respectively.
Transvaginal ultrasonography was performed in all patients at the third day of menstrual cycle to exclude any pelvic pathology and antral follicle count. All ultrasound examinations were performed by one of the authors (BV, YC) using an Ultrasound Machine [Medison Sonoace 8× Ultrasound Machine (manufacturer), 4–8 MHz]. To determine the diameter of the follicle, the mean of measurements in two perpendicular directions was taken. The numbers of follicles in both ovaries were added for the total antral follicle count (AFC). The follicles visualised and counted by TVS in the early follicular phase are 2–10 mm in size.
Stimulation protocol
All patients received gonadotropin-releasing hormone agonist long protocol or antagonist protocol. Patients were monitored for serum E2, LH, and progesterone levels with serial transvaginal ultrasonographic examinations. When at least two follicles reached a mean diameter of 18 mm or 3 or more follicles reached 17 mm, subcutaneous hCG (recombinant–choriogonadotropin alpha; Ovitrelle® 250 μg; Serono, Switzerland) was administered. When only one follicle was more than 17 mm or serum progesterone level was more than 1.5 ng/ml on rHCG day, the cycle was cancelled.
Oocytes retrieval was carried out under transvaginal ultrasound under sedation–analgesia. Patients with 0–3 oocytes retrieved were evaluated as poor responders, 4–15 oocytes as normoresponders, and >16 oocytes as hyperresponders. Intracytoplasmic sperm injection (ICSI) was performed in all patients. Embryo transfer was initiated after ICSI procedure. Luteal phase support was maintained by vaginal progesterone gel (Crinone® 8 % gel, Serono, Switzerland).
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences (SPPS) 14 software (SPSS Inc., IL, Chicago, USA) and MedCalc software version 12.3.0 (MedCalc Software, Broekstraat 52, 9030 Mariakerke, Belgium). Data are expressed as mean, standard deviation, and percentage. T test was used for the comparison of different variables. The relationships between the data were evaluated using Pearson’s correlation coefficient. The effect of multiple independent and parametric variables on dependent variables was investigated by multivariate linear regression analysis. The effect of multiple independent and nonparametric variables on dependent variables was investigated by logistic regression analysis. Receiver operating curve (ROC) analysis with area under curve (AUC) (ROC AUC) was used to determine the predictive value of day-3 AMH, FSH, hCG day E2 levels, and AFC for poor response to COH. All the data were evaluated within 95 % confidence interval in both directions. A p value of <0.05 was considered statistically significant.
Results
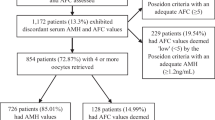
A total of 689 fresh cycles have been investigated according to their response to conventional IVF stimulation protocol. Sociodemographic, laboratory, ultrasonographic parameters and response to stimulation between the groups are demonstrated in Table 1. Table 1 also shows the total gonadotropin doses used. Accordingly, high gonadotropin doses were used in poor responders with no affect of age. High gonadotropin doses were used in normoresponders >40 years compared to normoresponders <40 years. There were no patients >40 years in hyperresponders.
Table 2 shows the correlation analysis among sociodemographic, laboratory, ultrasonographic parameters, and the total number of oocytes. The total number of oocytes was negatively correlated with chronologic age and day-3 FSH level, and positively correlated with AFC, day-3 E2 level, E2 level on hCG day, AMH level, testosterone, and DHEAS level. There was no statistically significant correlation between the total number of oocytes and BMI, insulin level, HOMA-IR, day-3 LH, day-3 progesterone.
A poor ovarian response was defined as fewer than three oocytes during the OPU procedure. ROC curve analysis was performed to determine cut-off values for AMH, FSH, and AFC for the prediction of poor response. The optimal cut-off values of AMH, FSH, and AFC for the prediction of poor response are <0.62 ng/ml, >10.6 IU/ml, and <6, respectively [AUC: 0.704 (0.638–0.764), AUC: 0.541 (0.491–0.590), AUC: 0.715 (0.667–0.760), respectively]. In our study, we calculated the optimal cut-off values of AMH, FSH, and AFC for the prediction of cycle cancellation and reported to be <0.23 ng/ml, >12.75 IU/ml, and <6, respectively [AUC: 0.678 (0.618–0.733); AUC: 0.576 (0.531–0.619); AUC: 0.533 (0.494–0.576)]. The analysis is demonstrated in Fig. 1.
Patients with 4–15 oocytes retrieved were defined as normoresponders. ROC curve analysis was performed. The optimal cut-off values of AMH, FSH, and AFC for the prediction of normo-response are >0.62 ng/ml, <8.43 IU/ml, and >6, respectively. [AUC: 0.541 (0.491–0.590), AUC: 0.704 (0.638–0.764), AUC: 0.715 (0.667–0.760), respectively]. In our study, we calculated the optimal cut-off values of AMH, FSH, and AFC for the prediction of cycle cancellation in the normoresponder patients and reported to be <0.41 ng/ml, >6.8 IU/ml, and <9, respectively [AUC: 0.710 (0.618–0.733), AUC: 0.652 (0.593–0.708), AUC: 0.735 (0.676–0.787)]. The analysis is demonstrated in Fig. 2.
A hyperovarian response was defined as more than 15 oocytes during the OPU procedure and patients were defined as hyperresponders. ROC curve analysis was performed to determine cut-off values for AMH, FSH, and AFC for the prediction of hyper-response. The optimal cut-off values of AMH, FSH, and AFC for the prediction of hyper-response are >5.1 ng/ml, <6.76 IU/ml, and >14, respectively [AUC: 0.520 (0.432–0.607), AUC: 0.571 (0.517–0.624), AUC: 0.653 (0.597–0.706), respectively].
Linear regression analysis model was performed for factors effecting total oocyte count (AMH, day-3 FSH, LH, BMI, age, and AFC were independent variables). According to this model, AMH and AFC were the only independent predictors of total oocyte count (dependent variable) (R 2: 181; p: 0.000). The model is given in Table 3. Table 4 shows MII oocyte predictors by regression analysis model (R 2: 78.2; p: 0.000). AFC and age were the only independent variables that significantly affect MII oocyte count.
Tables 5, 6, and 7 demonstrate logistic regression analysis results for pregnancy rates of poor responders, hyperresponders, and normoresponders. The cut-off values for poor, hyper- and normo-response in our study were taken as independent variables. None of the parameters had significant and independent effect on pregnancy rates.
Discussion
This study describes the predictive value of ovarian reserve tests especially FSH, AMH, and AFC. According to our results, AMH and AFC are the most valuable parameters that can be used for the prediction of ovarian reserve as poor, normo- or hyper-response.
In the literature, ovarian tests including FSH, estradiol, inhibin B, AFC, and ovarian volume have been described as predictors of ovarian reserve [1, 2, 10, 11]. Among these tests, AMH and AFC are the most common tests that have been investigated and have been researched [12, 13]. Before start of an IVF cycle for an individual patient, both the physician and the patient want to have ideas about the outcome of that cycle. The dose of the medication can be modified according to the reserve tests to avoid the risk of cycle cancellation due to non-responsiveness or the risk of OHSS due to higher doses.
AMH has been investigated in the literature briefly and in much detail [14]. Since the test is not cycle day dependent, it has the advantage of performing at any day of the cycle and in that way the patient does not need to wait for the next cycle. We have determined 0.62 ng/ml as a cut-off value for the prediction of poor response. Values in between 0.1 and 1.66 ng/ml have been reported as a cut-off value [15–20]. Our cut-off value stays almost in the middle of the previously reported values. Our results indicated a cut-off value of 5.1 ng/ml for the prediction of hyper-response. In the literature, values in between 0.63 and 5.98 have been reported as cut-off values for the prediction of hyper-response [19, 20].
AFC has also been investigated in the literature recently [21, 22]. Determination of AFC is operator dependent and has to be analysed at the initial days of menstruation, so a time lapse may be needed for sonographic examination. Frattarelli et al. [23] have investigated AFC before initiating IVF and have concluded that patients <4 antral follicles had higher chance (41 %) of cycle cancellation. In our study, we have reported <6 antral follicles as a cut-off value for both prediction of cycle cancellation and poor response. Also patients with >14 follicles at initial evaluation has the risk of hyper-response according to our study.
AMH and AFC have been compared to each other in the literature [24, 25]. Even though AFC has been reported as the best single predictor of response in most of the articles reported, our study indicated that both of the tests have equal power on the prediction of response [26]. Majumder et al. have evaluated AMH and AFC and reported significant correlations with the number of oocytes retrieved and AMH and AFC. Kunt et al. [27] have compared the predictive values of AMH and AFC prediction of poor response and stated AMH as a promising marker for oocyte response.
In our study, we have compared FSH, AMH, and AFC. Different from previous studies, we have determined the cut-off values for the prediction of ovarian response according to the number of oocytes retrieved and cycle cancellation rates even though it was a retrospective study. According to our results, if patients have AFC <6 and AMH <0.23 ng/ml, specialist should share the possibility of cycle cancellation. If patients have AFC >14 with AMH >5 ng/ml, specialist should take precaution for the risk of OHSS.
In conclusion, in this study, AMH and AFC are found to be the best ovarian reserve tests that can determine the total oocyte count retrieved. For correct decision making, both AMH and AFC should be measured before stimulation program to decrease the risk of OHSS and cycle cancellation. However, these markers independently do not predict pregnancy rates.
References
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB (2006) A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 12(6):685–718
Sills ES, Alper MM, Walsh AP (2009) Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol 146(1):30–36. doi:10.1016/j.ejogrb.2009.05.008
Yang YS, Hur MH, Kim SY, Young K (2011) Correlation between sonographic and endocrine markers of ovarian aging as predictors for late menopausal transition. Menopause 18(2):138–145
Majumder K, Gelbaya TA, Laing I, Nardo LG (2010) The use of anti-Müllerian hormone and antral follicle count to predict the potential of oocytes and embryos. Eur J Obstet Gynecol Reprod Biol 150(2):166–170. doi:10.1016/j.ejogrb.2010.02.029
Kalaiselvi VS, P S, K P, Krishna GP (2012) The anti mullerian hormone—a novel marker for assessing the ovarian reserve in women with regular menstrual cycles. J Clin Diagn Res 6(10):1636–1639
Panchal S, Nagori C (2012) Comparison of anti-mullerian hormone and antral follicle count for assessment of ovarian reserve. J Hum Reprod Sci 5(3):274–278
Sahmay S, Cetin M, Ocal P, Kaleli S, Senol H, Birol F, Irez T (2011) Serum anti-Müllerian hormone level as a predictor of poor ovarian response in in vitro fertilization patients. Reprod Med Biol 10:9–14
Himabindu Y, Sriharibabu M, Gopinathan K, Satish U, Louis TF, Gopinath P (2013) Anti-mullerian hormone and antral follicle count as predictors of ovarian response in assisted reproduction. J Hum Reprod Sci 6(1):27–31. doi:10.4103/0974-1208.112377
Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, Mitchell P, Ambrose P, Fleming R (2009) Anti-Müllerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod 24(4):867–875. doi:10.1093/humrep/den480
Fiçicioglu C, Kutlu T, Baglam E, Bakacak Z (2006) Early follicular antimüllerian hormone as an indicator of ovarian reserve. Fertil Steril 85(3):592–596
Alviggi C, Humaidan P, Ezcurra D (2012) Hormonal, functional and genetic biomarkers in controlled ovarian stimulation: tools for matching patients and protocols. Reprod Biol Endocrinol 10(9):1–9
Broer SL, Mol BW, Hendriks D, Broekmans FJ (2009) The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril 91:705–714
Celik H, Bıldırcın D, Güven D, Cetinkaya MB, Alper T, Batuoğlu AS (2012) Random anti-Müllerian hormone predicts ovarian response in women with high baseline follicle-stimulating hormone levels: anti-Müllerian hormone in poor responders in assisted reproductive treatment. J Assist Reprod Genet 29(8):797–802. doi:10.1007/s10815-012-9794-y (Epub 2012 May 10)
La Marca A (2013) Whatever its variability, antimüllerian hormone remains the most stable hormonal predictor. Fertil Steril 100(1):e1. doi:10.1016/j.fertnstert.2013.04.010
Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E (2008) Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod 23:1359–1365
Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G (2006) Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod 21(8):2022–2026
Muttukrishna S, Suharjono H, McGarrigle H, Sathanandan M (2004) Inhibin B and anti-Mullerian hormone: markers of ovarian response in IVF/ICSI patients? BJOG 111(11):1248–1253
Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, Laing I (2009) Circulating basal anti- Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril 92(5):1586–1593. doi:10.1016/j.fertnstert.2008.08.127
Nakhuda GS, Sauer MV, Wang JG, Ferin M, Lobo RA (2007) Müllerian inhibiting substance is an accurate marker of ovarian response in women of advanced reproductive age undergoing IVF. Reprod Biomed Online 14(4):450–454
La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, Volpe A (2007) Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod 22(3):766–771
Ben-Haroush A, Farhi J, Zahalka Y, Sapir O, Meizner I, Fisch B (2012) Correlations between antral follicle count and ultrasonographic ovarian parameters and clinical variables and outcomes in IVF cycles. Gynecol Endocrinol 28(6):432–435
Melo MA, Garrido N, Alvarez C, Bellver J, Meseguer M, Pellicer A, Remohí J (2009) Antral follicle count (AFC) can be used in the prediction of ovarian response but cannot predict the oocyte/embryo quality or the in vitro fertilization outcome in an egg donation program. Fertil Steril 91(1):148–156. doi:10.1016/j.fertnstert.2007.11.042
Frattarelli JL, Levi AJ, Miller BT, Segars JH (2003) A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil Steril 80(2):350–355
Van Disseldorp J, Lambalk CB, Kwee J, Looman CW, Eijkemans MJ, Fauser BC, Broekmans FJ (2010) Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod 25(1):221–227. doi:10.1093/humrep/dep366
Panchal S, Nagori C (2012) Comparison of anti-mullerian hormone and antral follicle count for assessment of ovarian reserve. J Hum Reprod Sci 5(3):274–278. doi:10.4103/0974-1208.106340
Nahum R, Shifren JL, Chang Y, Leykin L, Isaacson K, Toth TL (2001) Antral follicle assessment as a tool for predicting outcome in IVF—is it a better predictor than age and FSH? J Assist Reprod Genet 18(3):151–155
Kunt C, Ozaksit G, Keskin Kurt R, Cakir Gungor AN, Kanat-Pektas M, Kilic S, Dede A (2011) Anti-Mullerian hormone is a better marker than inhibin B, follicle stimulating hormone, estradiol or antral follicle count in predicting the outcome of in vitro fertilization. Arch Gynecol Obstet 283(6):1415–1421. doi:10.1007/s00404-011-1889-7
Conflict of interest
There are no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vural, B., Cakiroglu, Y., Vural, F. et al. Hormonal and functional biomarkers in ovarian response. Arch Gynecol Obstet 289, 1355–1361 (2014). https://doi.org/10.1007/s00404-013-3132-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-3132-1