Abstract
Purpose
Several clinical studies testify the critical role played by estrogens in male bone metabolism. The aim of our study is to assess the effect of a single injection of testosterone enanthate in a group of hypogonadal men on 17β estradiol serum levels and some bone metabolic parameters.
Method
Twenty-one hypogonadal males were given one testosterone enanthate injection (250 mg). Blood samples were drawn before the injection and after 1, 2 and 3 weeks. The following variables were measured: Total testosterone (TT), 17β estradiol (17β E2), Sex hormone binding globulin, total alkaline phosphatase, osteocalcin, and C-telopeptide of type I collagen (CTx).
Results
After testosterone injection, both TT and 17β E2 increased, peaking 1 week after the injection. Individual observation of the response of 17β E2 to testosterone showed that a subgroup (n = 9) failed to respond with any increase in 17β E2 at any of the weekly tests (group E2−), while the remainder (n = 12) showed a significant increase in 17β E2, which reached a mean value three times higher than at baseline (group E2+). The E2− patients reached a TT peak lower than that observed in the E+ group. CTx serum levels declined progressively in the E2+ group, reaching the significance (p = 0.03) at the end of the study, while it did not change in E− group.
Conclusion
This study suggests that a single injection of testosterone might have different effects on the production of endogenous estrogens, and a significant reduction of bone resorption parameters takes place only in the patients who show a significant increase of 17ß estradiol in response to testosterone administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sex hormones (androgens and estrogens) act independently on bone, by regulating modeling during growth and reducing bone loss during aging [1–3]. Reduced levels of testosterone and estrogens correlate with decreased BMD and increased fracture risk in both sexes [4–7]. Both testosterone and estrogen have specific receptors in bone cells [1, 8, 9]. In primary cultures of human and rodent osteoblasts, androgens increase osteoblast proliferation and inhibit osteoblast apoptosis [10–12]. Furthermore, estrogens stimulate osteoclast apoptosis [13] and enhance bone formation by increasing osteoblast differentiation and activity [14–17], and inhibiting osteoblasts apoptosis. Until a few years ago it was thought that testosterone was the major regulator of bone metabolism in men, as estrogens in women.
In 1994, a 28-year-old man with an estrogen receptor gene mutation was first described [18] and, in the following year, a mutation of the gene encoding for aromatase was identified in a 24-year-old man [19]. Both were found to have osteoporosis, suggesting that estrogen could play a major role in regulating bone metabolism in males too. This hypothesis was subsequently confirmed by other AA, who reported a restoration of bone mass after estrogen treatment in two males suffering from aromatase deficiency [20, 21].
Many clinical studies have been conducted to assess the effect of testosterone replacement therapy on BMD and, in most cases, there is a general agreement that the hormone treatment determines an increase of BMD in hypogonadal men [22–24].
At present, several clinical studies testify the critical role played by estrogens in male bone metabolism [25–28]. It is common knowledge that male hypogonadism is associated with bone loss and increased risk of fractures, but to what degree this might be due to androgen deficiency or to any consequent estrogen deficiency has yet to be fully elucidated.
In most cases, data on the effect of testosterone on bone remodeling parameters are related to the long-term effect of the hormone.
The aim of our study is to assess the effect of a single injection of testosterone enanthate in a group of hypogonadal men on 17β estradiol serum levels and some bone metabolic parameters.
Materials and methods
Our study involved 21 males with hypogonadism (total testosterone: range 2.44–12.15 nmol/L; mean ± SD: 7.57 ± 3.00 nmol/L; normal range 10.5–31.4 nmol/L) referring to our outpatients clinic from April to September 2011.
Eight patients had hypergonadotropic hypogonadism (LH 12.9 ± 5.09 mIU/mL, normal range: 1.5–9.2; FSH 35.16 ± 12.46 mIU/mL, normal range: 1–12): Five patients had karyotype XXY (Klinefelter), one had received abdominal radiotherapy for lymphatic leukemia, and two had testicular atrophy. Thirteen patients had hypogonadotropic hypogonadism (LH 0.77 ± 0.34 mIU/mL; FSH 2.28 ± 2.6 mIU/mL): three of them had undergone surgery for pituitary adenomas and received hormonal replacement therapy for thyroid and adrenal insufficiency, one had suffered a cranial trauma due to an accident, four were idiopathic, two were hypothalamic, and three had Kallman syndrome.
Twelve patients had been on testosterone enanthate [Geymonat S.p.A. Anagni (FR), Italy] treatment, 250 mg im. every 3 weeks, for a period ranging from a few months to 6 years, while nine patients did not receive any prior testosterone therapy. The endocrine deficiencies other than hypogonadism were corrected with appropriate replacement therapies.
None of the patients suffered from any other diseases or took any other medication that might interfere with bone metabolism.
For each participant, plasma samples were drawn before the injection and after 1, 2 and 3 weeks (at 3 weeks, samples were only obtained from 14 individuals for BGP and 9 individuals for ALP). Fasting blood samples were collected from 0800 to 1000 hours. Each sample collected at the baseline was tested for total testosterone (TT), 17β estradiol (17β E2), sex hormone binding globulin (SHBG), calcemia, phosphoremia, plasma creatinine, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), total alkaline phosphatase (ALP), osteocalcin (OC), 25-hydroxy vitamin D (25-OHD), parathyroid hormone (PTH), and serum C-telopeptide of type I collagen (CTx).
The following variables were measured in all blood samples drawn after the testosterone injection: TT, 17β E2, SHBG, ALP, OC, CTx. At each sample, we calculated the free androgen index (FAI), an indirect indicator of the amount of free testosterone, according to the formula:
Serum calcium, phosphorus, creatinine, urinary calcium, and ALP were determined with common laboratory methods. Parathyroid hormone (PTH) was measured by IRMA (Intact PTH Bridge, Adaltis, Milano, Italy): inter- and intra-assay coefficient of variation (CV) were 4 and 3.5 %, respectively; detection limit was 10 pg/mL. 25-hydroxy vitamin D was determined by radioimmunoassay method using a commercial kit from DiaSorin, Saluggia, Italy: inter- and intra-assay CV were 9 % in both cases; detection limit was 1.5 ng/mL. Serum OC was determined by IRMA using a commercial kit from Adaltis, Milano, Italy: inter- and intra-assay CV were 5 and 3 %, respectively; detection limit was 0.3 ng/mL. Serum CTx was determined with immunoenzymatic assay using a commercial kit from Nordic Bioscience Diagnostics, Herlev, Denmark: inter- and intra-assay CV were 5.4 and 5 %, respectively; detection limit was 0.010 ng/mL. TT was determined by radioimmunoassay using a commercial kit from Adaltis, Milano, Italy: inter- and intra-assay CV were 8–4 %, respectively; detection limit was 0.09 nmol/L. 17β estradiol was determined by estradiol MAIA kit from Adaltis, Milano, Italy: inter- and intra-assay CV were 9.7 and 8.8 %, respectively; detection limit was 6.5 pg/mL. LH and FSH were determined by immunoenzymometric assay using a commercial kit from Adaltis, Milano, Italy: inter- and intra-assay CV were 8.6 and 4.6 %, respectively, for FSH, and 11.8 and 8 %, respectively, for LH; detection limit was 0.084 IU/L for FSH and 0.8 IU/L for LH. Individual values of TT and 17β estradiol have been also calculated after BMI adjustment, considering for all patients BMI = 23. The formula used is
SHBG was measured by immunoenzymometric assay using a commercial kit from Adaltis, Milano, Italy: inter- and intra-assay CV were 5.9 and 2.5 %; detection limit was 1 nmol/L.
Statistical analysis was performed using the SPSS software rel. 17.0 and PRISM 5.0 (GRAPH PAD, CA, USA). Differences between the parameters were calculated using Student’s t test for paired data when comparing values for the same subject, and with the Mann–Whitney nonparametric method for unpaired data in the other cases.
One-way analysis of variance (ANOVA) was used to test differences between means for each variable measured before and after testosterone administration.
The response of 17β estradiol to testosterone enabled us to identify two subgroups of patients responding or not responding to the treatment (see “Results”). Bone remodeling parameters (ALP, OC and CTx) were checked for normality with Shapiro–Wilk test and were compared in responders and not responders with a mixed linear model of analysis of covariance for repeated measures with compound symmetry variance–covariance matrix considering the baseline values as covariate. Statistical significance was set at the 5 % level.
All the individuals had been informed of the purposes of the study and gave their oral informed consent. The local ethical committee approved this study.
Results
Table 1 provides personal and anthropometric details, as well as the baseline values of the various biochemical parameters for the patients.
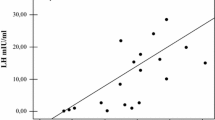
After testosterone enanthate injection, there was a significant increase in both TT and 17β E2 (absolute values as well as values adjusted for BMI), showing a trend that peaked 1 week after the injection (Fig. 1; Table 2). The increase in testosterone levels coincided with a significant drop in serum levels of LH and FSH (Fig. 2). Individual variations of TT and 17β E2 were similar in pre-treated and in naïve patients (Fig. 3).
Sex hormones behavior after testosterone injection. Total serum testosterone (on the left side, a) and 17β estradiol (on the right side, b) after testosterone enanthate injection in hypogonadal patients. In both cases peak is reached 1 week after the injection, and returned to baseline at the end of the study, 3 weeks after the injection
Percent individual variations of total testosterone and estradiol (1 week vs baseline) in patients previously treated (closed figures) or untreated (open figures) with testosterone enanthate. The circles indicate total testosterone, the triangles indicate 17β estradiol. For both parameters the difference between patients previously treated or untreated is not significant
Among the other parameters, only the FAI increased significantly 1 week after testosterone injection (Table 2).
When the hypogonadal patients were observed individually, it became evident that a subgroup (n = 9) failed to respond with any increase in 17β E2 at any of the weekly tests (group E2−), while the remainder (n = 12) showed a significant increase in 17β E2, which reached a mean value three times higher than that found at baseline at week 1 and then progressively decreased to levels comparable to baseline measurements (group E2+) (Fig. 4a). The two subgroups did not differ in age, BMI and any of the other laboratory variables (Table 3). The peak of testosterone levels was significantly lower in E2− subgroup than in E2+ one (Fig. 4b).
17β estradiol and total testosterone behavior among hypogonadal patients after testosterone enanthate injection. On the left side (a), patients are divided into two groups according to the response of 17β estradiol to testosterone. The dotted line indicates patients who failed to increase 17β estradiol (group E−), while the continuous line indicates those who showed a three-fold average increase of 17β estradiol (group E+). On the right side (b) is shown the response of testosterone after testosterone enanthate injection in the two groups of patients (E+ and E−). The E− group shows a weaker response than the E+ group
Although the baseline bone formation parameters (ALP and OC) were higher in the E2− subgroup, the difference was not significant and the trends after injection were similar in the two subgroups.
In contrast, CTx serum levels showed a significant decrease at the end of the study in E2+ group (p = 0.035) while it did not significantly change in the E2− group. In the E2+ group the percent reduction at the end of the study was −15.4 ± 6.2 compared to baseline (p = 0.03), while in the group E2− the values found at any time did not significantly differ from baseline. The estradiol response effect on CTx resulted statistically significant (p = 0.0105), showing that the behavior of CTx is significantly different between E2+ and E2− group (Table 4; Fig. 5).
a CTX values before and after injection of testosterone in responders and not responders patients. Points indicate the mean values calculated with a linear mixed model of analysis of covariance for repeated measures with compound symmetry variance–covariance matrix, considering the baseline values as a covariate. b, c individual changes of CTX at baseline and at the end of the study. Patients who showed a significant increase of estrogens after testosterone injection (E2+ group) showed a significant decrease in CTx values, while those that showed no changes in estrogen serum levels (E2− group) did not show any significant changes in CTX values. d Percent individual changes in CTx values measured at baseline and at the end of the study in both groups of patients (E2+ and E2−)
There is not any difference in the data observed at baseline and after testosterone injection between patients who had been already treated with testosterone and those who just started therapy. Similarly, there was not any difference in the etiology of hypogonadism.
Discussion
Our data show that, when the whole population is considered, a single testosterone injection was unable to significantly affect the behavior of the bone metabolism markers in our hypogonadal patients as a whole. This leads us to believe that testosterone is unable to appreciably modify bone turnover when administered acutely, and that such an effect emerges only after prolonged treatment, as amply demonstrated by various studies [29, 30]. Since more than half of our patients had already been on testosterone treatment for few months to several years, we might believe that any testosterone-induced changes in skeletal turnover had already taken place as a consequence of the previous treatment, and that the bone was therefore no longer responsive to a single androgen dose. However, the lack of response of markers of bone remodeling to a single injection of testosterone also occurs in patients who were given the hormone for the first time.
Looking at individual cases, there is a wide range of response of 17β E2 to testosterone, some patients being unresponsive, and others showing a rise in estrogens three times or more compared to baseline levels.
A limited conversion into estrogens might be influenced by the patient’s body composition because the enzyme activity is prevalent in the adipose tissue [31, 32]. Lakshman et al. [33], after weekly administration of testosterone enanthate in man with gonadotropin-releasing hormone (GnRh) agonist-induced hypogonadism, demonstrated that the estrogen increase was higher in the older subjects, partly related to their higher percentage of fat mass.
The long-term therapy with testosterone is known to have significant effects on body composition, increasing lean body mass and decreasing fat tissue. Because fat tissue is the main site of conversion of androgens to estrogens [34], we also evaluated possible differences in estradiol values after adjustment for BMI, but we did not detect any difference between pre-treated and untreated patients.
Although in our case the two subgroups of patients did not differ in terms of age and BMI, we can not exclude a relationship between our data and adipose tissue because we did not measure the total amount of fat or its distribution or waist circumference, which more accurately reflects the central adiposity.
Another hypothesis is that the lack of estrogen response might correlate with a potential aromatase down-regulation mechanism induced by repeated testosterone injections; however, we found the same situation in patients who had just started testosterone replacement therapy.
Nakazawa et al. [35] measured the hormone profiles after intramuscular injection of testosterone enanthate in nine hypogonadal patients and found that 17β E2 was increased 1.7-fold 1 day after injection, returning to baseline after 14 days. Curves for the individual behavior of estrogen are not shown in the paper, but the large standard deviation suggests that in some patients the increase was very modest or absent.
In patients in which 17β E2 did not change, all markers of bone metabolism remained stable, while patients who had a significant increase in estrogen, showed a significant decrease in CTx, beside the substantial stability of ALP and OC.
This finding is consistent with two studies, in which healthy males were first treated with GnRh analogues to make them become hypogonadal, then given different supplementation, i.e. one group took testosterone plus an aromatase inhibitor, the other took testosterone alone. This enabled an assessment of the independent effect of androgens and estrogens, showing that the latter is more effective in reducing bone resorption [36, 37].
This finding is also consistent with a reported reduction of CTx and NTx after treatment with raloxifene and micronized estradiol in osteopenic males [38–41].
Other studies have shown that age-related bone loss and fracture risk are lower in patients who have a particular type of polymorphism of the gene encoding for aromatase, increasing its activity [42–44].
Despite the presence of androgen receptors on osteoblasts, no significant changes were observed in the bone formation parameters after a single injection of testosterone enanthate. The lack of response of bone formation indices might be explained by the fact that estrogens mainly exert an anti-resorption activity and the bone formation phase following the resorption blockade takes much longer to become apparent.
The reason why in some patients estrogen levels increase after the administration of testosterone while in others do not, remains to be elucidated. It may be that patients whose estrogen levels fail to increase have a significantly lower testosterone peak than the others.
The evidence that only the patients who reached a higher testosterone peak had an increase in 17β E2 might lead us to believe that a sufficient amount of substrate (testosterone) must be available to reach such an effect, although there are no experimental grounds supporting this hypothesis.
In conclusion, this study suggests that a single injection of testosterone might have different effects on the production of endogenous estrogens, and a significant reduction of bone resorption parameters takes place only in the patients who show a significant increase of 17β estradiol in response to testosterone administration. This finding could have a clinical implication, when testosterone treatment is given to prevent bone damage: in these cases it might be useful to assess the estrogens’ response to testosterone administration to verify the efficacy of the androgen therapy on bone metabolism.
References
Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C (2004) Androgens and bone. Endocr Rev 25:389–425
Bouillon R, Bex M, Vanderschueren D, Boonen S (2004) Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab 89:6025–6029
Taes Y, Lapauw B, Vandewalle S, Zmierczak H, Goemaere S, Vanderschueren D, Kaufman JM, T’Sjoen G (2009) Estrogen-specific action on bone geometry and volumetric bone density, longitudinal observations in an adult with complete androgen insensitivity. Bone 45:392–397
Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL, Meikle AW, Center JR, Eisman JA, Seibel MJ (2008) Endogenous sex hormones and incident fracture risk in older men, the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med 14:47–54
Bjørnerem A, Ahmed LA, Joakimsen RM, Berntsen GK, Fønnebø V, Jørgensen L, Øian P, Seeman E, Straume B (2007) A prospective study of sex steroids, sex hormone-binding globulin, and non-vertebral fractures in women and men, the Tromso Study. Eur J Endocrinol 157:119–125
Goderie-Plomp HW, van der Klift M, de Ronde W, Hofman A, de Jong FH, Pols HA (2004) Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women, the Rotterdam Study. J Clin Endocrinol Metab 89:3261–3269
Amin S, Zhang Y, Felson DT, Sawin CT, Hannan MT, Wilson PW, Kiel DP (2006) Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. Am J Med 119:426–433
Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S (2007) Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130:811–823
Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC (2010) The estrogen receptor α in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 24:323–334
Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C (2004) Androgens and bone. Endocr Rev 25:389–425
Lin IC, Slemp AE, Hwang C, Sena-Esteves M, Nah HD, Kirschner RE (2007) Dihydrotestosterone stimulates proliferation and differentiation of fetal calvarial osteoblasts and dural cells and induces cranial suture fusion. Plast Reconstr Surg 120:1137–1147
Wiren KM, Toombs AR, Semirale AA, Zhang X (2006) Osteoblast and osteocyte apoptosis associated with androgen action in bone, requirement of increased Bax/Bcl-2 ratio. Bone 38:637–651
Garcia AJ, Tom C, Guemes M, Polanco G, Mayorga ME, Wend K, Miranda-Carboni GA, Krum SA (2013) ERα signaling regulates MMP3 expression to induce FasL cleavage and osteoclast apoptosis. J Bone Miner Res 28:283–290
Chen X, Deng Y, Zhou Z, Tao Q, Zhu J, Li X, Chen J, Hou J (2010) 17beta-estradiol combined with testosterone promotes chicken osteoblast proliferation and differentiation by accelerating the cell cycle and inhibiting apoptosis in vitro. Vet Res Commun 34:143–152
Kondoh S, Inoue K, Igarashi K, Sugizaki H, Shirode-Fukuda Y, Inoue E, Yu T, Takeuchi JK, Kanno J, Bonewald LF, Imai Y (2014) Estrogen receptor α in osteocytes regulates trabecular bone formation in female mice. Bone 60:68–77
Gao Y, Huang E, Zhang H, Wang J, Wu N, Chen X, Wang N, Wen S, Nan G, Deng F, Liao Z, Wu D, Zhang B, Zhang J, Haydon RC, Luu HH, Shi LL, He TC (2013) Crosstalk between Wnt/β-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS One 12:e82436. doi:10.1371/journal.pone.0082436
Börjesson AE, Lagerquist MK, Windahl SH, Ohlsson C (2013) The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell Mol Life Sci 70:4023–4037
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB e Korach KS (1994) Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061
Morishima A, Grubach MM, Simpson ER, Fischer C, Qin K (1995), Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:1841–1845
Carani C, Qin K, Simoni M, faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpsom ER (1997) Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–95
Bilezikian JP, Morishima A, Bell J, Grumbach MM (1998) Increase bone mass as a result of estrogentherapy in a man with aromatase deficiency. N Engl J Med 339:599–603
Tenover JS (1992) Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab 75:1092–1098
Vermuelen A (1991) Androgens in the aging male. J Clin Endocrinol Metab 73:221–224
Wang C, Swerloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N (2001) Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clin Endocrinol 54:739–750
Leder B (2007) Gonadal steroids and bone metabolism in men. Curr Opin Endocrinol Diabetes Obes 14:241–246
Nuti R, Martini G, Merlotti D, De Paola V, Valleggi F, Gennari L (2007) Bone metabolism in men, role of aromatase activity. J Endocrinol Invest 30(6 Suppl):18–23
Vandenput L, Ohlsson C (2009) Estrogens as regulators of bone health in men. Nat Rev Endocrinol 5:437–443
Vandenput L, Ohlsson C (2010) Sex steroid metabolism in the regulation of bone health in men. J Steroid Biochem Mol Biol 121:582–588
Katznelson L (1998) Therapeutic role of androgens in the treatment of osteoporosis in men. Clin Endocrinol Metab 12:453–470
De Rosa M, Paesano L, Nuzzo V, Zarrilli S, Del Puente A, Oriente P, Lupoli G (2001) Bone mineral density and bone markers in hypogonadotropic and hypergonadotropic hypogonadal men after prolonged testosterone treatment. J Endocrinol Invest 24:246–252
Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ (2011) Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res 4:329–346
Ghosh S, Hu Y, Li R (2010) Cell density is a critical determinant of aromatase expression in adipose stromal cells. J Steroid Biochem Mol Biol 118:231–236
Lakshman KM, Kaplan B, Travison TG, Basaria S, Knapp PE, Singh AB, LaValley MP, Mazer NA, Bhasin S (2010) The effect of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab 95:3955–3964
Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A (2005) Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol 3:280–293
Nakazawa R, Baba K, Nakano M, Katabami T, Saito N, Takahashi T, Iwamoto T (2006) Hormone profiles after intramuscular injection of testosterone enanthate in patients with hypogonadism. Endocr J 53:305–310
Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S (2000) Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106:1553–1560
Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS (2003) Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab 88:204–210
Duschek EJ, Gooren LJ, Netelenbos C (2004) Effects of raloxifene on gonadotrophins, sex hormones, bone turnover and lipids in healthy elderly men. Eur J Endocrinol 150:539–546
Doran PM, Riggs BL, Atkinson EJ, Khosla S (2001) Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. J Bone Miner Res 16:2118–2125
Taxel P, Kennedy D, Fall P, Willard A, Shoukri K, Clive J, Raisz LG (2000) The effect of short-term treatment with micronized estradiol on bone turnover and gonadotrophins in older men. Endocr Res 26:381–398
Taxel P, Fall PM, Albertsen PC, Dowsett RD, Trahiotis M, Zimmerman J, Ohannessian C, Raisz LG (2002) The effect of micronized estradiol on bone turnover and calciotropic hormones in older men receiving hormonal suppression therapy for prostate cancer. J Clin Endocrinol Metab 87:4907–4913
Masi L, Becherini L, Gennari L, Amedei A, Colli E, Falchetti A, Farci M, Silvestri S, Gonnelli S, Brandi ML (2001) Polymorphism of the aromatase gene in postmenopausal Italian women, distribution and correlation with bone mass and fracture risk. J Clin Endocrinol Metab 86:2263–2269
Salmen T, Heikkinen AM, Mahonen A, Kröger H, Komulainen M, Pallonen H, Saarikoski S, Honkanen R, Mäenpää PH (2003) Relation of aromatase gene polymorphism and hormone replacement therapy to serum estradiol levels, bone mineral density, and fracture risk in early postmenopausal women. Ann Med 35:282–288
Zarrabeitia MT, Hernández JL, Valero C, Zarrabeitia AL, García-Unzueta M, Amado JA, González-Macías J, Riancho JA (2004) A common polymorphism in the 5′-untranslated region of the aromatase gene influences bone mass and fracture risk. Eur J Endocrinol 150:699–704
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camozzi, V., Bonanni, G., Frigo, A. et al. Effect of a single injection of testosterone enanthate on 17β estradiol and bone turnover markers in hypogonadal male patients. J Endocrinol Invest 38, 389–397 (2015). https://doi.org/10.1007/s40618-014-0183-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-014-0183-5