Abstract
Estrogens are important endocrine regulators of skeletal growth and maintenance in both females and males. Studies have demonstrated that the estrogen receptor (ER)-α is the main mediator of these estrogenic effects in bone. Therefore, estrogen signaling via ERα is a target both for affecting longitudinal bone growth and bone remodeling. However, treatment with estradiol (E2) leads to an increased risk of side effects such as venous thromboembolism and breast cancer. Thus, an improved understanding of the signaling pathways of ERα will be essential in order to find better bone specific treatments with minimal adverse effects for different estrogen-related bone disorders. This review summarizes the recent data regarding the intracellular signaling mechanisms, in vivo, mediated by the ERα activation functions (AFs), AF-1 and AF-2, and the effect on bone, growth plate and other estrogen responsive tissues. In addition, we review the recent cell-specific ERα-deleted mouse models lacking ERα specifically in neuronal cells or growth plate cartilage. The newly characterized signaling pathways of estrogen, described in this review, provide a better understanding of the ERα signaling pathways, which may facilitate the design of new, bone-specific treatment strategies with minimal adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
General introduction
Estrogens are important endocrine regulators of skeletal growth and maintenance in both females and males. Experimental and human studies have demonstrated that the estrogen receptor (ER)-α is the main mediator of these estrogenic effects in bone [1–8]. Therefore, estrogen signaling via ERα is a target both for affecting longitudinal bone growth and for bone remodeling. 17β-estradiol (E2) is the most potent estrogen and it is important for preventing bone loss. E2 also affects longitudinal bone growth and low levels of E2 during sexual maturation is essential for the longitudinal growth spurt, whereas high E2 levels in late puberty result in growth plate closure and thereby cessation of longitudinal bone growth in humans [9]. Since E2 is important for the different stages of longitudinal bone growth, it is possible to affect the longitudinal bone growth by manipulating the estrogen signaling in young patients with idiopathic short stature or constitutionally tall stature. Estrogen deficiency after menopause reduces bone mineral density (BMD), which leads to an increased risk of fractures. Therefore, it is possible to prevent bone loss in postmenopausal women by E2 administration [10]. However, there are several estrogen responsive tissues expressing ERs, e.g., the reproductive system, central nervous system (CNS), and blood vessels [11–13]. Therefore, estrogen treatment would not only have positive effects on the bone but could also lead to an increased risk of adverse effects, such as venous thromboembolism and breast cancer [14, 15]. Thus, it would be beneficial to develop bone-specific estrogen treatments. To achieve this, it is important to further characterize the estrogenic signaling pathways in bone versus other tissues in vivo.
Growth plate physiology
The growth plates consist of three principal zones of chondrocytes: the resting zone, the proliferative zone, and the hypertrophic zone, where the hypertrophic chondrocytes are the most differentiated [16, 17]. Longitudinal bone growth of the long bones occurs at the growth plates through a process called endochondral ossification. In this process, cartilage is formed and then replaced by bone tissue at the metaphyseal border of the growth plate [18].
Low E2 levels enhance skeletal growth during early sexual maturation (i.e. the pubertal growth spurt), whereas high E2 levels during late puberty result in growth plate fusion and thereby cessation of longitudinal bone growth in humans [9]. The mechanisms of action for these two seemingly opposite effects of estrogens on longitudinal bone growth are not fully understood but clearly depend on maturational stage and serum levels of E2 [9, 19]. Estrogens are crucial regulators of the growth hormone/insulin-like growth factor 1 (GH/IGF-I) axis, and, therefore, some of the effects of estrogens on skeletal growth may be indirect via modulation of the GH/IGF-I axis, while other effects may be direct [9, 20–22]. Low-dose E2 treatment has been shown to increase serum GH and IGF-I, which may contribute to the pubertal growth spurt. An effect via the GH/IGF-I axis is supported by the fact that ER blockade down-regulates the GH/IGF-I axis [3, 9]. A key role for E2 in the regulation of longitudinal bone growth and growth plate closure in humans was demonstrated by the findings that both males and females with estrogen deficiency, caused by a mutation in the aromatase gene, do not show a pubertal growth spurt and continue to grow after sexual maturation due to unfused growth plates [3, 23–26]. ERα was demonstrated to be the main mediator of this effect when a man with a similar phenotype was shown to have a non-functional ERα due to a point mutation in exon 2 (estrogen-resistant man). This man had elevated serum E2 levels, to which he was non-responsive [6, 27]. In contrast, the growth phenotype of the aromatase deficient patients could be rescued by E2 treatment [3, 23–25]. The mechanism for the estrogenic effects on growth plate fusion is not well understood. However, it has been demonstrated that estrogen accelerates the fusion of the growth plate in rabbits by advancing the senescence of the growth plate via proliferative exhaustion of the chondrocytes [28].
Estrogen and bone remodeling
The process in which osteoclasts resorb bone and osteoblasts form new bone is called bone remodeling. This process is important for enabling the bone to respond and adapt to load induced strain, replace old or damaged bone tissue, and to maintain calcium homeostasis [29]. Estrogen is important for maintaining the balance between bone resorption and bone formation. At menopause, when the E2 levels drop, there is an imbalance in bone resorption and bone formation leading to an accelerated bone loss [1]. It has been suggested that, although the trabecular bone loss is accelerated at menopause, the trabecular bone is not as sensitive as the cortical bone to the estrogen deprivation at menopause, since the trabecular bone loss starts already in young adult life when the levels of estrogens are normal [1].
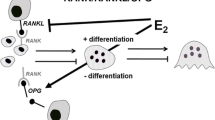
Estrogen has fundamental effects on bone metabolism by several different mechanisms, acting both directly and indirectly on the skeleton. Estrogen affects the expression of certain factors in osteoblastic cells, e.g., it increases osteoprotegerin (OPG) and decreases receptor activator of nuclear κB ligand (RANKL) and tumor necrosis factor (TNF)-α, resulting in suppressed bone resorption [30–32]. It has been suggested by Khosla et al. [1]that the most consistent effect of estrogen on bone is to (1) induce commitment of precursor cells to the osteoblast lineage at the expense of the adipocyte lineage and (2) prevent apoptosis of osteoblastic cells [1, 33–37]. In addition, estrogen also reduces bone resorption by inhibiting differentiation and promoting apoptosis of osteoclasts [36, 38–42].
Structure of estrogen receptors
The first estrogen receptor discovered, ERα, was cloned in 1986, although its existence had already been suggested in 1962 [43–45]. Ten years later, a second estrogen receptor, ERβ, was cloned [46]. In 2005, there were reports suggesting that a seven-transmembrane G protein-coupled receptor (GPCR) named G protein-coupled ER-1 (GPER-1 or GPR30) was a membrane-associated ER [47–49]. However, there are contradictory studies suggesting that GPER-1 is not an estrogen receptor [49–53].
Both ERα and ERβ belong to the nuclear receptor superfamily of ligand-activated transcription factors, and they can dimerize to form both homo- and heterodimers. The primary structure of these receptors is divided into six functional domains, A–F, which display, overall, high sequence homology (Fig. 1) [46, 54, 55]. The A/B domains in the N-terminus contain the ligand-independent activation function-1 (AF-1) [56]. The C domain comprises the DNA binding domain (DBD) and the area important for dimerization. The D domain contains the nuclear localization signal and is also a hinge, which increases the flexibility between the C- and E/F domains. The ligand binding domain is found in the E/F domains in the C-terminus together with the ligand-dependent AF-2 [46, 55–57]. The most abundant ERα isoform is the 66-kDa protein, but there is also a truncated, less expressed 46-kDa ERα isoform that lacks most of the A/B domains (Fig. 1) [58].
Schematic presentation of the ERα proteins expressed in the different mouse models. There are two wild-type (WT) ERα isoforms; the full length 66-kDa protein and the less abundant, 46-kDa protein. The ERα−/− mice do not express any ERα protein. The ERαAF-10 mice have a normal expression of a 49-kDa protein, lacking amino acids 2–148 and also the 46-kDa isoform. The ERαAF-20 mice have a normal expression of two ERα proteins, which are slightly smaller than the WT proteins due to the deletion of amino acids 543–549. The first described ERKO mice from the group of Korach have a low expression of a 61-kDa isoform, lacking amino acids 92–155 which are replaced by 7 amino acids from the Neomycin sequence (NEO), and also an expression of the normal 46-kDa isoform. Adapted and reproduced with permission from Am J Physiol Endocrinol Metab (2012) 302:E1381–1389
Both AFs are important for recruiting cofactors essential for gene regulation. The cofactors that bind to the AFs are suggested to be dependent on the ER, the cell type and promoter context [59, 60]. When a ligand binds to an ER, the ER undergoes a conformational change so that helix 12 in the ligand binding domain folds in the agonistic orientation [61]. Helix 12 has a key role in forming the ERα AF-2, together with helices 3, 4, and 5, which can attract cofactors important for gene regulation, hence the AF-2 is ligand-dependent [61–63]. Although the ERβ AF-1 is suggested to be weaker than the ERα AF-1 [64], they both interact with cofactors independently of ligand being bound to the receptor or not, and therefore the AF-1 is ligand-independent [56, 65]. In vitro studies of ERα have shown that the E2-induced transactivation is dependent on either one of the AFs but that most promoter contexts require synergism between them for full transcriptional activity [56, 59, 66–68].
ERα is the main ER in bone
The bone-sparing effects of estrogen are primarily mediated via ERα. This has been demonstrated using different mouse models lacking ERα, ERβ, both ERα and ERβ, or GPER-1. ERα has been shown to be crucial for mediating the estrogenic effects in both trabecular and cortical bone, while ERβ plays a less important role and GPER-1 has been demonstrated to be dispensable for the estrogenic preservation of bone mass [2, 4, 5, 49, 69, 70]. Female and male mice that are estrogen deficient due to ovariectomy (ovx) or orchidectomy (orx) lose bone. However, both trabecular and cortical bone mass can be restored by E2 treatment in both genders. In contrast, the bone mass cannot be restored in E2-treated ovx or orx mice lacking ERα (ERα−/− mice), demonstrating a crucial role for ERα in both the female and male skeleton [2, 4, 5, 69]. ERβ has only been shown to slightly modulate the effects of ERα in female mice but not in male mice [2, 8, 71–73]. This modulating effect of ERβ was shown by studying female adolescent ERβ−/− mice. These mice displayed an increased cortical bone mineral content but no trabecular phenotype [73]. One-year-old female ERβ−/− mice were also studied and shown to maintain the cortical phenotype seen in the adolescent mice, but also to have a higher trabecular bone mass compared to age-matched controls [72]. This suggests that ERβ normally has a slightly inhibiting effect on ERα in the female bone [72, 73]. In addition, estrogen-regulated transcriptional activity in bone was evaluated in female ERα−/−, ERβ−/−, and ERαβ−/− mice, demonstrating that ERβ reduces ERα-regulated gene transcription in bone in the presence of ERα but partially replaces ERα in the absence of ERα [74]. Although both ERα and ERβ have an AF-1, this region is not well conserved. The ERβ AF-1 is weaker than the ERα AF-1, and it has been shown that the estrogenic effect mediated by ERα is slightly repressed when an ERα/ERβ heterodimer is formed [64].
Signaling pathways of estrogen
Primary target cells involved in bone regulation
Both ERs have been shown to be expressed throughout the skeleton in osteoblasts, osteoclasts, osteocytes, and chondrocytes [75–79]. The expression of ERs in these different bone cells demonstrates that the estrogenic effects can be exerted locally in the skeleton. The relative importance of the different estrogenic target cells for bone regulation is not yet completely understood. It would be valuable to fully characterize the primary target cells for estrogen, and how these cells exert effects on bone regulation when stimulated with E2, in order to develop cell-specific treatments. To investigate the importance of ERs in the different bone cells for the estrogenic skeletal effects, mouse models have been developed that specifically lack ERα in one cell type. Specific deletion of ERα in osteoclasts in female, but not male, mice led to trabecular bone loss, and this effect was due to a decreased apoptosis of the osteoclasts [38, 39]. Further experiments are required in order to find the target cells for the estrogenic effects in trabecular bone in male mice and in cortical bone in both male and female mice.
Bone is traditionally considered to be regulated by peripheral signaling, which is controlled by hormones (e.g., estrogen), autocrine/paracrine signals, and mechanical loading. However, bone is an innervated tissue and, lately, central signaling, via the CNS, has also been recognized to have a major role in bone regulation [80]. The first clear evidence showing this was when mice deficient of leptin were studied. These mice had, despite being hypogonadal, a high bone mass phenotype, which was reduced by intracerebroventricular injections of leptin [81]. In addition, mice lacking the β2-adrenergic receptor, which binds the main sympathetic neurotransmitter noradrenaline, were resistant to the central bone-reducing effects of leptin, demonstrating that central leptin signaling may exert its negative effects on bone via the sympathetic nervous system [82, 83].
Intracellular signaling
At the cellular level, the ERs are generally considered to have four signaling pathways, three of which are classified as ligand-dependent and one which is classified as ligand-independent [84]. The ERs are transcription factors that either directly or indirectly bind to the DNA. The AF-1 and AF-2 in the ERs are important for recruiting certain coactivators, which in turn can interact with the transcriptional preinitiation complex and thereby initiate transcription of specific estrogen-regulated genes [85, 86]. There are two genomic ligand-dependent signaling pathways involving dimerization and translocation of the ERs from the cytosol to the nucleus when ligand binds; (1) in the classical (direct) genomic pathway the ER-ligand complex binds directly to estrogen response elements (ERE) on the DNA and regulates gene transcription, and (2) in the non-classical (indirect) genomic pathway, other transcription factors are bound to the DNA and the ER-ligand complex binds to these transcription factors and thereby regulates gene transcription [54, 84, 87]. Fos/Jun and SP-1 are examples of transcription factors involved in the non-classical genomic pathway and these transcription factors can interact with both the ERs and specific sites on the DNA, not harboring EREs. Fos/Jun proteins form the transcriptional complex AP-1 (activator protein 1), which interacts with AP-1 responsive elements in the DNA [88, 89]. The SP-1 (specificity protein 1) complex interacts with GC-rich SP-1 motifs in the DNA [90]. Interaction between the ER-ligand complex and the described transcription factors results in activation of gene transcription. The ligand-dependent non-genomic pathway is not very well understood. The rapid responses of this pathway imply that no gene regulation occurs. Instead, it involves signaling cascades, which are activated when ligand binds to an ER that could be located either in the membrane or in the cytoplasm [84, 91]. Some of these rapid responses to E2 are mediated by second messenger systems, e.g., cAMP and protein kinase A (PKA), while other responses are mediated by membrane-based ion fluxes, involving, e.g., Ca2+ and Ca2+-dependent K+ channels, which are capable of responding to estrogens [91, 92]. GPER-1 has been suggested to be involved in this non-genomic pathway and there is also some evidence for a membrane-localized ERα [47, 49, 93]. The ligand-independent pathway does not involve any ligand binding to the ER. Instead, other factors are involved (e.g., growth factors (GFs) like epidermal growth factor (EGF) and IGF) that can activate extracellular-signal-regulated kinases (ERKs) which in turn can activate the ER by phosphorylation [84, 94–97]. It has also been shown that phosphorylation of the ER increases after E2 binding [98]. The phosphorylated ER translocates to the nucleus where it will recruit coactivators and mediate gene transcription [84, 98]. ERα has several sites for phosphorylation and most of them are found in the AF-1 [99].
Estrogen and modulation of longitudinal bone growth
Estrogens are important both for the longitudinal growth spurt and for cessation of growth by inducing growth plate closure [9]. It is possible to manipulate the longitudinal bone growth by affecting the estrogenic signaling pathways. High-dose E2 treatment will result in growth plate closure and thereby cessation of growth, leading to a shorter adult stature than expected. In contrast, treatment with aromatase inhibitors (attenuating the conversion of androgens into estrogens) will delay the closure of the growth plates, thereby increasing the final height [100–102]. The long-term effects of high-dose estrogen treatment or treatment with aromatase inhibitors are only recently being evaluated. It has been suggested that high-dose estrogen treatment in adolescent girls may lead to reduced fertility later in life [103–105]. There are also concerns for increased risks of breast or gynecological cancers [101, 106]. In addition, boys treated with aromatase inhibitors were shown to have a high rate of vertebral body deformities after treatment [107]. Further characterization of the estrogenic signaling pathways in bone and growth plate will therefore be important in order to find better treatments for manipulating longitudinal bone growth, without increasing the risk of adverse effects later in life.
Selective ER modulators (SERMs)
SERMs have the ability to bind to an ER and act as an ER agonist or antagonist in a tissue-specific manner. Tamoxifene was the first developed SERM and is used for breast cancer treatment since it has an antagonizing effect in breast tissue. In addition, Tamoxifene was shown to be an agonist in bone but unfortunately also an agonist in uterus, increasing the risk of endometrial cancer [108, 109]. Another SERM, Raloxifene, shown to be an ER agonist in bone and an ER antagonist in breast, was the first SERM approved for the prevention and treatment of postmenopausal osteoporosis [110]. More SERMs have been developed, but all available SERM treatments for osteoporosis today, including Raloxifene, will not only lead to a reduced fracture risk but also to adverse effects such as increased risk of thromboembolism [110–112]. Therefore, further studies of the signaling pathways of estrogen are required in order to find more specific SERMs.
In vitro studies have shown that the SERMs have a bulky side chain, which upon binding to ERα protrudes from the ligand binding pocket. This hinders the optimal conformational change of the ligand binding domain of the ERα by preventing the folding of helix 12 in the agonistic orientation. Instead, helix 12 is able to bind to the static region of ERα AF-2; formed by residues from helices 3, 4, and 5. Helix 12 then prevents/limits the interaction between the static region of ERα AF-2 and certain coactivators and corepressors. Because of the lack of cofactors binding to the ERα AF-2 after SERM binding, ERα AF-1 is suggested to be the main mediator of the SERM effects [59, 61–63, 113–115]. Variation in the expression of cofactors and the recruitment of cofactors to the ER in different cell types appear to have an important role for the tissue-specific effects of the SERMs [61, 114]. Regarding treatment strategies for osteoporosis, development of new SERMs that have the positive effects of estrogen in bone but lack the negative effects in other tissues would be optimal. In addition, a SERM treatment directed specifically towards the growth plate in constitutionally tall adolescents could reduce their final height without leading to systemic adverse effects. To achieve this, the characterization of the ER signaling pathways in bone versus other tissues is of importance. Since the phenotypes of the different global ER-deleted mouse models have been described in detail in previous reviews [116–118], we do not focus on these mouse models in this review.
Roles of transactivating functions 1 and 2 of ERα in bone
The two transactivation functions; ERα AF-1 and AF-2, have been shown, in vitro, to interact with cofactors (coactivators and corepressors) and are therefore important for mediating the gene-regulating effects of ERα. Further characterization of these AFs in vivo is therefore of importance in understanding whether they can act independently of each other to mediate gene regulation and whether this is dependent on the tissue. Evaluation of these AFs in vivo was possible after the development of two new mouse models lacking either the ERα AF-1 (ERαAF-10; [119]) or the ERα AF-2 (ERαAF-20; [69]). The ERαAF-10 mice have a deletion of 441 bp of exon 1, corresponding to amino acid (aa) 2–148, with a preserved translational initiation codon in exon 1 (ATG1) (Fig. 1). The ERαAF-10 mice do not express any full-length 66-kDa protein [69, 119]. Instead, they express a truncated 49-kDa ERα protein that lacks AF-1 and also the physiologically occurring but less abundantly expressed 46-kDa ERα isoform, initiated by a second translational initiation codon in exon 2 (ATG2). ERαAF-20 mice have a deletion of the AF-2 core, which resides within exon 9 and corresponds to aa 543–549 (Fig. 1) [69]. The sizes of the ERαAF-20 proteins are slightly smaller than the WT ERα proteins of 66 and 46-kDa, respectively (corresponding to the 7 aa truncation located in the AF-2 region; [69]). In order to evaluate the involvement of the AFs for the bone protective effects of estrogen the ERαAF-10 and ERαAF-20 mice were studied together with mice completely devoid of ERα protein (ERα−/−; [120]) and WT control mice.
The ERαAF-10, ERαAF-20, and ERα−/− mice were shown to have elevated serum levels of E2, luteinizing hormone (LH) and testosterone compared to WT controls, demonstrating that these mouse models all have a disturbed negative feedback regulation of serum sex steroid levels [69]. Thus, both intact ERα AF-1 and AF-2 are important for a normal negative feedback regulation of serum sex steroids. Because of the elevated sex steroid levels in these mouse models, gonadal-intact mice were not ideal for studying the estrogenic response. Therefore, only ovx mice were studied when evaluating the roles of ERα AF-1 and AF-2 for the estrogenic effects in bone and other estrogen responsive tissues. The ovx ERαAF-10, ERαAF-20, ERα−/−, and WT control mice were treated with either E2 or placebo, and thereafter the estrogenic effects on the skeleton and other estrogenic target tissues were evaluated. E2 treatment increased the amount of trabecular and cortical bone in ovx WT mice. Neither the trabecular nor the cortical bone responded to E2 treatment in ovx ERα−/− or ovx ERαAF-20 mice. Interestingly, ovx ERαAF-10 mice displayed a normal E2 response in cortical bone but no E2 response in trabecular bone (Fig. 2) [69]. This was shown by performing both pQCT and μCT analyses on femurs and μCT analyses on the L5 vertebrae, demonstrating that the ERαAF-10 mice had a normal E2 response on cortical thickness and vBMD, while there was no response on trabecular bone volume/tissue volume (BV/TV) or trabecular number. Also, immune parameters and the major estrogen responsive tissues, uterus and liver, were evaluated. It was demonstrated that none of the tissues evaluated showed any estrogenic response in the ovx ERα−/− or ovx ERαAF-20 mice. However, the estrogenic response in ERαAF-10 mice was tissue-dependent with no significant estrogenic response on thymus, a clearly reduced estrogenic response on uterus weight and bone marrow cellularity, an intermediate response on the frequency of B-lymphocytes in the bone marrow, and a normal response on liver weight (Fig. 2) [69]. Together, these results suggest that ERα AF-2 is required for all the estrogenic effects in the evaluated tissues while the role of ERα AF-1 is tissue-dependent. This implies that, while some tissues are completely dependent or independent of ERα AF-1, other tissues require both ERα AF-1 and AF-2 for a full estrogenic effect.
The role of ERα AF-1 is tissue-dependent. Ovariectomized (ovx) ERα−/−, ERαAF-20 and ERαAF-10 and their corresponding wild-type (WT) mice were treated with either vehicle or estradiol (E2) for 4 weeks. As expected, E2 treatment resulted in a significant effect on several estrogen responsive bone parameters (increased total body aBMD, cortical thickness, cortical volumetric BMD, trabecular BV/TV, and trabecular number), bone marrow parameters (reduced bone marrow cellularity and frequency of B-lymphocytes), and non-bone parameters (increased uterine weight and liver weight but reduced thymus weight) in ovx WT mice. To illustrate the role of ERα AF-1 and ERα AF-2 for the effect of E2 on these parameters, the estrogenic response in E2 treated ovx WT mice is for each parameter set to 100 %. The bars represent the estrogenic response in % for the E2-treated ovx ERα−/−, ERαAF-20, and ERαAF-10 mice compared to the E2 response in their ovx WT mice, respectively. Thus, 0 % means no E2 response while 100 % is a normal WT E2 response. Values are mean ± SEM (n = 8–12). BM bone marrow, BV/TV bone volume/total volume, BW body weight, vBMD volumetric bone mineral density, aBMD areal bone mineral density. Reproduced with permission from Proc Natl Acad Sci USA (2011) 108:6288–6293
Lately, it has been suggested that the trabecular and cortical bone compartments are different and that they could have somewhat different estrogenic signaling mechanisms [1, 39, 121]. Data supporting that the cellular environment as well as the specific cell type decide the expression levels of different cofactors have also been reported [122–124]. The mechanism(s) behind the crucial role of ERα AF-1 in the trabecular but not the cortical bone is still unknown. A possible explanation for this difference may be that the trabecular and cortical bone compartments have different cofactor expression patterns and this would then explain why the estrogen signaling pathways in these bone compartments differ. One possible pathway involves the steroid receptor coactivator (SRC)-1, which has been shown, in vitro, to be cooperatively recruited by AF-1 and AF-2 in ERα and thereby mediate synergism between the two AFs [125]. Another study has shown that different cell types express different amounts of SRC-1, which in turn lead to different responses to SERM treatment. Since SERMs are suggested to act mainly via ERα AF-1, these results suggestthat SRC-1 is important for mediating the effect of SERMs by interacting with ERα AF-1 [126, 127]. In addition, when mice with a specific inactivation of SRC-1 (SRC-1 KO) were ovx and treated with E2, the normal estrogenic response was absent in the trabecular bone, while it was normal in cortical bone [121]. Thus, the estrogenic response in the SRC-1 KO and ERαAF-10 mice showed a similar pattern. This suggests that SRC-1 is involved in the AF-1-dependent E2 effects in trabecular bone but not in the AF-1-independent E2 effects in cortical bone in female mice [69].
It has also been suggested that the estradiol-mediated phosphorylation of residues in ERα AF-1 is different, dependent on the cell type [98]. This suggests that phosphorylation of residues of ERα AF-1 could be involved in the AF-1-dependent estrogenic effects in trabecular bone but not in cortical bone. This may be due to different expression patterns of kinases and/or phosphatases in trabecular and cortical bone.
Finding the cell types responsible for the estrogenic effects in bone have been the focus of several research groups during recent years. So far, ERα has been specifically inactivated in osteoclasts [38, 39] using the Cre-loxP system in mice. These studies have shown that ERα in osteoclasts is of importance for the estrogenic effect in the trabecular but not the cortical bone compartment in female mice [38, 39]. These findings, together with the results from ERαAF-10 mice, suggest that ERα AF-1 is crucial for the estrogenic effect in trabecular but not cortical bone, and that estrogen preserves the trabecular bone via osteoclast ERα involving AF-1 [69].
The mice lacking ERα AF-1 have also been reported to have retained vasculoprotective effects of E2 treatment [119]. The results from the mice lacking ERα AF-1 or AF-2 could be useful for the development of new SERMs, and suggest that SERMs activating ERα AF-1 minimally could exert beneficial actions in cortical bone and the vascular system, while minimizing the AF-1-dependent effects on reproductive organs [69].
The role of ERα and its AF-1 for growth plate closure in female mice
Although the rodent growth plates do not fuse during their lifetime, elderly mice do have a reduced longitudinal bone growth, and growth plate closure can be induced in adult mice by high-dose E2 treatment [128]. The estrogen-resistant man, lacking a functional ERα, had a continued linear growth long into adulthood, due to unfused growth plates [6, 27]. A mouse model, believed to lack the entire ERα (K-ERα−/−; [129]) had an opposite growth plate phenotype compared to the estrogen-resistant man, in that these mice completely fused their growth plates [130, 131]. However, the K-ERα−/− mouse model, developed in the Korach and Smithies laboratories, was later shown to have low expression of truncated ERα isoforms, lacking AF-1 (Fig. 1) [129, 132–134]. The question then arose whether these truncated ERα isoforms could explain the opposite effects in the growth plates in mouse and man, and also what role ERα AF-1 has in regulating growth plate fusion. To answer these questions, both longitudinal bone growth and growth plate closure were studied in female ERα−/− [120] and ERαAF-10 mice [119, 132].
Young adult (4 months old) female ERα−/− mice had normal longitudinal bone growth. Interestingly, old (16–19 months) female ERα−/− mice showed continued longitudinal bone growth, resulting in longer bones, associated with increased growth plate height compared with WT mice (Fig. 3). The increased growth was not associated with any changes in serum IGF-I levels [132]. These results showed that the ERα−/− mice and the estrogen-resistant man, with a dysfunctional ERα, have both increased longitudinal bone growth and wide, unfused growth plates [6], confirming the ERα to be the main mediator of the estrogenic effects on growth plate reduction in mouse and growth plate closure in man [132].
Altered growth plate heights in old ERα−/− and ERαAF-10 mice. Representative pictures of growth plates from proximal tibiae of old female ERα−/− and ERαAF-10 mice and their wild-type (WT) controls. KO knock-out. Adapted and reproduced with permission from Am J Physiol Endocrinol Metab (2012) 302:E1381–1389
In contrast, the longitudinal bone growth in ERαAF-10 female mice had already ceased in the young adult mice, and this was even more pronounced in the old ERαAF-10 female mice, due to growth plate closure (Fig. 3). The changes in longitudinal bone growth were not related to changes in the serum IGF-I levels [132]. These results are similar to what was earlier shown in the K-ERα−/− mice [130], but more specifically show that it is the lack of the ERα AF-1 that is responsible for this phenotype. In a study of growth plate fusion in rabbits, it was demonstrated that estrogen accelerates growth plate fusion by advancing the senescence of the growth plate via proliferative exhaustion of the chondrocytes [28]. Consistent with these findings, the ERαAF-10 mice, prior to growth plate closure, displayed reduced height of the proliferative zone in the growth plate [132]. In addition, immunostaining showed that there was a tendency towards a reduction in chondrocyte proliferation and an increase in chondrocyte apoptosis. This led to a significantly altered balance between chondrocyte apoptosis and proliferation, which is suggested to be involved in the mechanism for growth plate closure in the ERαAF-10 mice [132].
The signaling of estrogen via the ERα involves the interaction of cofactors with the ERα AFs. It is likely that there are cofactors specific for ERα AF-1 that have repressive effects on the ERα-mediated growth plate senescence pathway. This could explain the growth plate phenotype in the ERαAF-10 mice, where the ERα AF-1 interaction with corepressors would not be present and, thus, the repressive effect of ERα AF-1 on the growth plate senescence would be lost. The premature growth plate fusion, and thereby cessation of longitudinal bone growth, in the ERα AF-1-deficient mice could, therefore, be the result of a hyperactive ERα that is activated by the ERα AF-2 with no interference from the ERα AF-1 [132]. However, ERαAF-10 mice have been shown to have elevated serum levels of E2 which could lead to the threshold level for estrogen-induced growth plate closure being reached. This would mean that the AF-1-deleted ERα still has the capacity to mediate growth plate closure when exposed to supra-physiological E2 levels. Regardless of the process occurring in the growth plate, these results suggest that growth plate closure is induced by functions of the ERα that do not require AF-1 [132].
Although the growth plate physiology in mouse and human is different, and one should be careful to extrapolate data from mice to humans, the recent results from the ERα−/− and ERαAF-10 mice indicate that mice are more similar to humans than was previously believed [132]. The prolonged substantial longitudinal bone growth observed in elderly ERα−/− mice resembles the continued post-pubertal growth seen in humans with ERα inactivation or aromatase deficiency. In contrast, the spontaneous growth plate closure in the ERαAF-10 mice implies that it may be beneficial to use ERαAF-10 mice as a model for studying E2-mediated signaling pathways in growth plate closure. Also, it would be interesting to study whether some patients with short stature have an inactivating mutation in the ERα AF-1 region [132].
The role of ERα in growth plate cartilage for longitudinal bone growth
The effects of estrogens on longitudinal bone growth and growth plate closure are generally believed to be mediated either indirectly via regulation of the GH/IGF-I axis or via direct effects on locally expressed ERα in the growth plate chondrocytes. Until the development of a mouse model lacking ERα specifically in cartilage (Col2α1-ERα−/−; [135]), it was not possible to evaluate the relative roles of these two pathways for the effects of estrogens on longitudinal bone growth in vivo.
The Col2α1-ERα−/− mice were developed by crossing mice expressing Cre recombinase under the control of the collagen type II, α 1 (Col2α1) promoter [136] with mice in which exon 3 of the ERα gene was flanked by the loxP sequence (ERαflox/flox; [120]). The offspring of this breeding were then bred in a specific scheme in order to generate littermate conditional mutant Col2α1-ERα−/− and control (ERαflox/flox) mice [135].
The role of ERα for longitudinal bone growth during sexual maturation was investigated by studying ERα−/− and Col2α1-ERα−/− mice until 4 months of age. The male ERα−/− mice had a reduced longitudinal bone growth during sexual maturation, resulting in shorter bone length, while the male Col2α1-ERα−/− mice had a normal growth [135]. The reduced growth in male ERα−/− mice was associated with a significant reduction of serum IGF-I levels and disturbed GH secretion as indicated by reduced major urinary protein (MUP) expression in the liver [21]. This demonstrates that in male mice, indirect, probably GH/IGF-I-mediatedeffects not requiring ERα in growth plate cartilage are responsible for the role of ERα to modulate skeletal growth during early sexual maturation. Furthermore, this indicates that ERα via altered activity in the GH/IGF-I axis is of importance for the pubertal growth spurt in male mice [135]. One-year-old female Col2α1-ERα−/− mice were also evaluated, and it was found that local ERα in the growth plate is important for the normal reduction in longitudinal bone growth seen in elderly mice [135]. This resembles the phenotype seen in old ERα−/− mice, which also displayed a continued growth after sexual maturation [132]. Thus, ERα in the growth plate is important for mediating normal age-related reduction in longitudinal bone growth [135].
High-dose E2 treatment induces growth plate closure in rodents [128]. To examine the importance of local ERα for this effect, sexually mature female and male Col2α1-ERα−/− and WT mice were gonadectomized (gnx) and treated with supraphysiological levels of E2 or placebo. The E2 treatment resulted in normal estrogenic responses on bone mass, uterus, and thymus in both WT and Col2α1-ERα−/− mice [135]. As expected, both female and male WT mice displayed reduced growth plate height after E2 treatment compared to placebo-treated mice. Interestingly, this effect was not seen in either female or male Col2α1-ERα−/− mice, demonstrating an essential role of cartilage-located ERα for the effect of high-dose E2 to reduce growth plate height in both males and females [135]. Quantitative histological measurements of the growth plates in female mice showed that the reduced growth plate height in the WT mice was due to a reduction of the proliferative zone, while the height of the hypertrophic zone was unchanged. The Col2α1-ERα−/− mice did not display any changes in the proliferative or hypertrophic zones [135]. This is consistent with previous experiments showing that E2 accelerates the proliferative exhaustion, and thereby senescence, of growth plate chondrocytes [28, 132]. A reduced height in the proliferative zone was also seen in the ERαAF-10 mice before growth plate closure occurred [132]. This suggests that the process of growth plate senescence and closure is dependent on growth plate-located ERα via a signaling pathway that does not require ERα AF-1.
Although there are clear species differences between man and mouse, the findings of the importance of ERα in growth plate cartilage, both for high-dose E2-induced reduction of the growth plate height in adult mice and for age-dependent reduction of longitudinal bone growth in mice, suggest that growth plate-located ERα might also be essential for growth plate fusion and cessation of longitudinal bone growth in humans [135].
The results from the Col2α1-ERα−/− mice suggest that the high E2 levels during late puberty (humans) or after E2 treatment (humans and mice) reduce bone growth via a direct effect on ERα in growth plate cartilage (Fig. 4) [135]. Also, growth plate-located ERα is of importance for the reduced longitudinal bone growth in elderly mice. Therefore, studies in both mice [132] and humans [6] strongly suggest that ERα is the dominant ER for the normal age-dependent reduction and cessation of longitudinal bone growth observed in mouse and man, respectively, and that this effect is mediated via growth plate-located ERα (Fig. 4) [135].
Proposed role of ERα for longitudinal bone growth. Our findings demonstrate that a indirect, probably GH/IGF-I-mediated effects, not requiring ERα in growth plate cartilage, are responsible for the role of ERα to modulate skeletal growth during early sexual maturation associated with low serum E2 levels, while b direct effects of ERα in growth plate cartilage are required for the effect of a high E2 dose to reduce the growth plate height in adult mice and for reduction of longitudinal bone growth in elderly mice. We propose that low E2 levels in early puberty enhance longitudinal bone growth via actions on the GH/IGF-I axis, while the higher E2 levels during late puberty (humans) or after high-dose E2 treatment (humans and mice) reduce bone growth via a direct effect on ERα in the growth plate cartilage. + Stimulatory action, − inhibitory action. Reproduced from J Bone Miner Res (2010) 25:2690–2700 with permission of the American Society for Bone and Mineral Research
ERα expression in neuronal cells affects bone mass
Peripheral estrogen signaling via ERα is important for the regulation of bone, which has been shown by evaluating mouse models that lack ERα in either chondrocytes [135] or osteoclasts [38, 39]. The CNS is a target for estrogen, and ERs are expressed in many different parts of the brain. In order to evaluate the importance of central ERα, a mouse model with a specific inactivation of ERα in nervous tissue (nestin-ERα−/−) was developed [137].
The nestin-ERα−/− mice were generated by crossing mice which express the Cre recombinase specifically in neuronal and glial precursor cells (nestin-Cre; [138]) with ERαflox/flox mice [120]. The offspring were then bred according to a specific scheme in order to generate littermate conditional mutant nestin-ERα−/− and control (ERαflox/flox) mice [137].
Female nestin-ERα−/− mice had an increased trabecular and cortical vBMD together with increased femoral bone strength. The high bone mass seen in nestin-ERα−/− mice was mainly caused by increased bone formation. Thus, prevention of ERα signaling in the nervous system leads to increased bone density and increased bone strength, which suggests that central estrogen signaling has a negative impact on bone [137]. The female nestin-ERα−/− mice did not have a disturbed negative feedback regulation of sex steroids, indicating that increased levels of sex steroids are not the primary cause of the increased bone mass. The main mechanism for the increased bone mass in nestin-ERα−/− mice was suggested to be mediated via decreased central leptin sensitivity in the hypothalamus, and thereby increased secretion of leptin from white adipose tissue, which in turn results in increased peripheral leptin-induced bone formation [137]. It was also shown that the increased bone mass seen in nestin-ERα−/− mice was not due to a higher bone accrual during early development, but is a dynamic event. In addition, there was no evidence that altered serotonin regulation or sympathetic tone was responsible for the bone phenotype seen in nestin-ERα−/− mice. These data are in accordance with previous results, suggesting a more prominent role for ERβ in the regulation of the central serotonergic system [137, 139].
In conclusion, the balance between peripheral and central ERα actions is important for the regulation of bone mass, since central ERα signaling was shown to have inhibitory effects, and peripheral ERα signaling is known to have stimulatory effects, on bone mass [137]. This suggests that it would, probably, be favorable to develop an ERα-specific agonist with low penetrance to the CNS for having positive effects on bone. Such an agonist would lead to stimulatory peripheral bone effects without having inhibitory central bone effects. However, further studies are required to characterize the relative importance of centralversus peripheral ERα-signaling for other nonbone-related estrogen effects [137].
Concluding remarks
The ongoing research about the relative importance of local versus central effects of ERα and the understanding of how estrogen signals in different tissues and cell types, together with the characterization of the roles of the different domains in ERα, is important in order to map the signaling pathways of estrogen. A better knowledge of the estrogenic signaling pathways may in turn facilitate the design of new, improved SERMs with minimal adverse effects.
The results described in this review recognize that an ERα-specific SERM that would not activate the ERα AF-1 could have beneficial effects on cortical bone, while the effects on reproductive tissues would be minimal. Such a SERM could perhaps also be considered for inducing growth plate closure in constitutionally tall adolescents with reduced risks of adverse effects. The importance of local ERα for reducing growth plate height suggests that, if it was possible to modulate the ERα signaling pathways locally in the growth plate chondrocytes, this could potentially reduce or increase the final height of the patient without the risk of systemic adverse effects. In addition, the results from the mice lacking ERα in neuronal tissue suggest that a SERM with low penetrance to the CNS or with antagonistic effects in the CNS would have a more pronounced effect on bone mass.
It would be optimal to attain local estrogenic effects without having systemic effects. One appealing approach for this emerged when Finan et al. [140] recently reported a method for targeted estrogen delivery. They showed that estrogen conjugated to a glucagon-like peptide-1 (GLP-1) only affected tissues expressing the GLP-1 receptor. This GLP-1-estrogen conjugate did not lead to adverse estrogenic effects in the reproductive system or oncogenicity [140]. If estrogen could be conjugated to a peptide which specifically targets skeletal tissue or the growth plate, this could potentially lead to beneficial estrogenic effects locally, without resulting in systemic adverse effects.
References
Khosla S, Melton LJ 3rd, Riggs BL (2011) The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res 26:441–451
Lindberg MK, Weihua Z, Andersson N, Moverare S, Gao H, Vidal O, Erlandsson M, Windahl S, Andersson G, Lubahn DB, Carlsten H, Dahlman-Wright K, Gustafsson JA, Ohlsson C (2002) Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol 174:167–178
MacGillivray MH, Morishima A, Conte F, Grumbach M, Smith EP (1998) Pediatric endocrinology update: an overview. The essential roles of estrogens in pubertal growth, epiphyseal fusion and bone turnover: lessons from mutations in the genes for aromatase and the estrogen receptor. Horm Res 49(Suppl 1):2–8
Moverare S, Venken K, Eriksson AL, Andersson N, Skrtic S, Wergedal J, Mohan S, Salmon P, Bouillon R, Gustafsson JA, Vanderschueren D, Ohlsson C (2003) Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci USA 100:13573–13578
Sims NA, Clement-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M, Baron R (2003) A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest 111:1319–1327
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS (1994) Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061
Vandenput L, Ohlsson C (2009) Estrogens as regulators of bone health in men. Nat Rev Endocrinol 5:437–443
Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C (2000) Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci USA 97:5474–5479
Juul A (2001) The effects of oestrogens on linear bone growth. Hum Reprod Update 7:303–313
Ettinger B (1988) Prevention of osteoporosis: treatment of estradiol deficiency. Obstet Gynecol 72:12S–17S
Farhat MY, Lavigne MC, Ramwell PW (1996) The vascular protective effects of estrogen. FASEB J 10:615–624
Gillies GE, McArthur S (2010) Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev 62:155–198
Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863–870
Collaborative Group on Hormonal Factors in Breast Cancer (1997) Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 350:1047–1059
Daly E, Vessey MP, Hawkins MM, Carson JL, Gough P, Marsh S (1996) Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 348:977–980
Chagin AS, Chrysis D, Takigawa M, Ritzen EM, Savendahl L (2006) Locally produced estrogen promotes fetal rat metatarsal bone growth; an effect mediated through increased chondrocyte proliferation and decreased apoptosis. J Endocrinol 188:193–203
Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423:332–336
Stevens DA, Williams GR (1999) Hormone regulation of chondrocyte differentiation and endochondral bone formation. Mol Cell Endocrinol 151:195–204
van der Eerden BC, Karperien M, Wit JM (2003) Systemic and local regulation of the growth plate. Endocr Rev 24:782–801
Jansson JO, Eden S, Isaksson O (1985) Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6:128–150
Ohlsson C, Mohan S, Sjogren K, Tivesten A, Isgaard J, Isaksson O, Jansson JO, Svensson J (2009) The role of liver-derived insulin-like growth factor-I. Endocr Rev 30:494–535
Veldhuis JD, Metzger DL, Martha PM Jr, Mauras N, Kerrigan JR, Keenan B, Rogol AD, Pincus SM (1997) Estrogen and testosterone, but not a nonaromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth hormone)-insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement. J Clin Endocrinol Metab 82:3414–3420
Bilezikian JP, Morishima A, Bell J, Grumbach MM (1998) Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med 339:599–603
Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER (1997) Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–95
Conte FA, Grumbach MM, Ito Y, Fisher CR, Simpson ER (1994) A syndrome of female pseudohermaphrodism, hypergonadotropic hypogonadism, and multicystic ovaries associated with missense mutations in the gene encoding aromatase (P450arom). J Clin Endocrinol Metab 78:1287–1292
Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K (1995) Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698
Smith EP, Specker B, Bachrach BE, Kimbro KS, Li XJ, Young MF, Fedarko NS, Abuzzahab MJ, Frank GR, Cohen RM, Lubahn DB, Korach KS (2008) Impact on bone of an estrogen receptor-alpha gene loss of function mutation. J Clin Endocrinol Metab 93:3088–3096
Weise M, De-Levi S, Barnes KM, Gafni RI, Abad V, Baron J (2001) Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci USA 98:6871–6876
Raisz LG (1999) Physiology and pathophysiology of bone remodeling. Clin Chem 45:1353–1358
Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL (2003) Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest 111:1221–1230
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL (1999) Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 140:4367–4370
Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R (2001) Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 98:13960–13965
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297
Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Lowik CW (2002) Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res 17:394–405
Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, Tomiyama H, Sakamoto Y, Matsumoto T (2002) Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology 143:2349–2356
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23:576–581
Qu Q, Perala-Heape M, Kapanen A, Dahllund J, Salo J, Vaananen HK, Harkonen P (1998) Estrogen enhances differentiation of osteoblasts in mouse bone marrow culture. Bone 22:201–209
Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC (2010) The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 24:323–334
Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S (2007) Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130:811–823
Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF (1996) Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med 2:1132–1136
Oursler MJ, Osdoby P, Pyfferoen J, Riggs BL, Spelsberg TC (1991) Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci USA 88:6613–6617
Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A, Yang NN, Hakeda Y, Kumegawa M (1997) Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med 186:489–495
Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P (1986) Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320:134–139
Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J (1986) Sequence and expression of human estrogen receptor complementary DNA. Science 231:1150–1154
Jensen EV (1962) On the mechanism of estrogen action. Perspect Biol Med 6:47–59
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930
Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630
Thomas P, Pang Y, Filardo EJ, Dong J (2005) Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632
Filardo EJ, Thomas P (2012) Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153:2953–2962
Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C (2010) A critical review of fundamental controversies in the field of GPR30 research. Steroids 75:603–610
Levin ER (2009) G protein-coupled receptor 30: estrogen receptor or collaborator? Endocrinology 150:1563–1565
Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH (2009) GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80:34–41
Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH (2008) G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 149:4846–4856
Pettersson K, Grandien K, Kuiper GG, Gustafsson JA (1997) Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol Endocrinol 11:1486–1496
Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S (1997) Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J Biol Chem 272:25832–25838
Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P (1989) The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 59:477–487
Krust A, Green S, Argos P, Kumar V, Walter P, Bornert JM, Chambon P (1986) The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J 5:891–897
Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F (2000) Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J 19:4688–4700
Berry M, Metzger D, Chambon P (1990) Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J 9:2811–2818
Green S, Chambon P (1988) Nuclear receptors enhance our understanding of transcription regulation. Trends Genet 4:309–314
Pike AC (2006) Lessons learnt from structural studies of the oestrogen receptor. Best Pract Res Clin Endocrinol Metab 20:1–14
Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M (1997) Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758
Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL (1998) The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95:927–937
Hall JM, McDonnell DP (1999) The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578
Tremblay A, Tremblay GB, Labrie F, Giguere V (1999) Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell 3:513–519
Gronemeyer H, Laudet V (1995) Transcription factors 3: nuclear receptors. Protein Profile 2:1173–1308
Kraus WL, McInerney EM, Katzenellenbogen BS (1995) Ligand-dependent, transcriptionally productive association of the amino- and carboxyl-terminal regions of a steroid hormone nuclear receptor. Proc Natl Acad Sci USA 92:12314–12318
Metzger D, Losson R, Bornert JM, Lemoine Y, Chambon P (1992) Promoter specificity of the two transcriptional activation functions of the human oestrogen receptor in yeast. Nucleic Acids Res 20:2813–2817
Borjesson AE, Windahl SH, Lagerquist MK, Engdahl C, Frenkel B, Moverare-Skrtic S, Sjogren K, Kindblom JM, Stubelius A, Islander U, Antal MC, Krust A, Chambon P, Ohlsson C (2011) Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc Natl Acad Sci USA 108:6288–6293
Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B, Swanson C, Moverare-Skrtic S, Savendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C (2009) The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab 296:E490–E496
Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R (2002) Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 30:18–25
Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G (2001) Female estrogen receptor beta−/− mice are partially protected against age-related trabecular bone loss. J Bone Miner Res 16:1388–1398
Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C (1999) Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(−/−) mice. J Clin Invest 104:895–901
Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C (2003) Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol 17:203–208
van der Eerden BC, Gevers EF, Lowik CW, Karperien M, Wit JM (2002) Expression of estrogen receptor alpha and beta in the epiphyseal plate of the rat. Bone 30:478–485
van der Eerden BC, Lowik CW, Wit JM, Karperien M (2004) Expression of estrogen receptors and enzymes involved in sex steroid metabolism in the rat tibia during sexual maturation. J Endocrinol 180:457–467
Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS, Lanyon LL (2006) Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res 21:1297–1306
Ushiyama T, Ueyama H, Inoue K, Ohkubo I, Hukuda S (1999) Expression of genes for estrogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis Cartilage 7:560–566
Vidal O, Kindblom LG, Ohlsson C (1999) Expression and localization of estrogen receptor-beta in murine and human bone. J Bone Miner Res 14:923–929
Gajda M, Litwin JA, Cichocki T, Timmermans JP, Adriaensen D (2005) Development of sensory innervation in rat tibia: co-localization of CGRP and substance P with growth-associated protein 43 (GAP-43). J Anat 207:135–144
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520
Takeda S, Karsenty G (2008) Molecular bases of the sympathetic regulation of bone mass. Bone 42:837–840
Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931
Meyer ME, Gronemeyer H, Turcotte B, Bocquel MT, Tasset D, Chambon P (1989) Steroid hormone receptors compete for factors that mediate their enhancer function. Cell 57:433–442
Onate SA, Tsai SY, Tsai MJ, O’Malley BW (1995) Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357
Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC (2004) Anatomy of the estrogen response element. Trends Endocrinol Metab 15:73–78
Chinenov Y, Kerppola TK (2001) Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20:2438–2452
Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P (2000) Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74:311–317
Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S (2000) Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem 275:5379–5387
Rickard DJ, Subramaniam M, Spelsberg TC (1999) Molecular and cellular mechanisms of estrogen action on the skeleton. J Cell Biochem Suppl 32–33:123–132
Segars JH, Driggers PH (2002) Estrogen action and cytoplasmic signaling cascades. Part I: membrane-associated signaling complexes. Trends Endocrinol Metab 13:349–354
Pedram A, Razandi M, Levin ER (2006) Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009
Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P (1995) Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491–1494
Driggers PH, Segars JH (2002) Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab 13:422–427
Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, Korach KS (1996) Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA 93:12626–12630
Newton CJ, Buric R, Trapp T, Brockmeier S, Pagotto U, Stalla GK (1994) The unliganded estrogen receptor (ER) transduces growth factor signals. J Steroid Biochem Mol Biol 48:481–486
Lannigan DA (2003) Estrogen receptor phosphorylation. Steroids 68:1–9
Murphy LC, Seekallu SV, Watson PH (2011) Clinical significance of estrogen receptor phosphorylation. Endocr Relat Cancer 18:R1–R14
Drop SL, De Waal WJ, De Muinck Keizer-Schrama SM (1998) Sex steroid treatment of constitutionally tall stature. Endocr Rev 19:540–558
Karimian E, Savendahl L (2011) Estrogen signaling in growth plate cartilage. Endocr Dev 21:42–51
Hero M, Norjavaara E, Dunkel L (2005) Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. J Clin Endocrinol Metab 90:6396–6402
Hendriks AE, Drop SL, Laven JS, Boot AM (2012) Fertility of tall girls treated with high-dose estrogen, a dose-response relationship. J Clin Endocrinol Metab 97:3107–3114
Hendriks AE, Laven JS, Valkenburg O, Fong SL, Fauser BC, de Ridder MA, de Jong FH, Visser JA, van Ginneken AM, Boot AM, Drop SL (2011) Fertility and ovarian function in high-dose estrogen-treated tall women. J Clin Endocrinol Metab 96:1098–1105
Venn A, Bruinsma F, Werther G, Pyett P, Baird D, Jones P, Rayner J, Lumley J (2004) Oestrogen treatment to reduce the adult height of tall girls: long-term effects on fertility. Lancet 364:1513–1518
Rookus MA, van Leeuwen FE (1994) Oral contraceptives and risk of breast cancer in women aged 20–54 years. Netherlands oral contraceptives and breast cancer study group. Lancet 344:844–851
Hero M, Makitie O, Kroger H, Nousiainen E, Toiviainen-Salo S, Dunkel L (2009) Impact of aromatase inhibitor therapy on bone turnover, cortical bone growth and vertebral morphology in pre- and peripubertal boys with idiopathic short stature. Horm Res 71:290–297
Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Silfversward C, Skoog L, Somell A, Theve T, Wilking N et al (1989) Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet 1:117–120
Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL (1992) Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 326:852–856
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple outcomes of raloxifene evaluation (MORE) investigators. JAMA 282:637–645
Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J, Armstrong RA, Thompson DD, Powles T, Zanchetta J, Kendler D, Neven P, Eastell R (2010) Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med 362:686–696
Silverman SL, Chines AA, Kendler DL, Kung AW, Teglbjaerg CS, Felsenberg D, Mairon N, Constantine GD, Adachi JD (2012) Sustained efficacy and safety of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled study. Osteoporos Int 23:351–363
Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M (1994) Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science 264:1455–1458
Smith CL, Nawaz Z, O’Malley BW (1997) Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol 11:657–666
Nettles KW, Greene GL (2005) Ligand control of coregulator recruitment to nuclear receptors. Annu Rev Physiol 67:309–333
Windahl SH, Andersson G, Gustafsson JA (2002) Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab 13:195–200
Syed F, Khosla S (2005) Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 328:688–696
Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C (2004) Androgens and bone. Endocr Rev 25:389–425
Billon-Gales A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, Chambon P, Arnal JF (2009) The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci USA 106:2053–2058
Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M (2000) Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127:4277–4291
Modder UI, Sanyal A, Kearns AE, Sibonga JD, Nishihara E, Xu J, O’Malley BW, Ritman EL, Riggs BL, Spelsberg TC, Khosla S (2004) Effects of loss of steroid receptor coactivator-1 on the skeletal response to estrogen in mice. Endocrinology 145:913–921
Bebermeier JH, Brooks JD, DePrimo SE, Werner R, Deppe U, Demeter J, Hiort O, Holterhus PM (2006) Cell-line and tissue-specific signatures of androgen receptor-coregulator transcription. J Mol Med (Berl) 84:919–931
Ghosh S, Thakur MK (2008) Tissue-specific expression of receptor-interacting protein in aging mouse. Age (Dordr) 30:237–243
Smith CL, O’Malley BW (2004) Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71
Metivier R, Penot G, Flouriot G, Pakdel F (2001) Synergism between ERalpha transactivation function 1 (AF-1) and AF-2 mediated by steroid receptor coactivator protein-1: requirement for the AF-1 alpha-helical core and for a direct interaction between the N- and C-terminal domains. Mol Endocrinol 15:1953–1970
Shang Y, Brown M (2002) Molecular determinants for the tissue specificity of SERMs. Science 295:2465–2468
Borjesson AE, Farman HH, Engdahl C, Koskela A, Sjogren K, Kindblom JM, Stubelius A, Islander U, Carlsten H, Antal MC, Krust A, Chambon P, Tuukkanen J, Lagerquist MK, Windahl SH, Ohlsson C (2012) The role of activation functions 1 and 2 of estrogen receptor-a for the effects of estradiol and selective estrogen receptor modulators (SERMs) in male mice. J Bone Miner Res [Epub ahead of print]
Vanderschueren D, Boonen S, Ederveen AG, de Coster R, Van Herck E, Moermans K, Vandenput L, Verstuyf A, Bouillon R (2000) Skeletal effects of estrogen deficiency as induced by an aromatase inhibitor in an aged male rat model. Bone 27:611–617
Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS (1995) Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol 9:1441–1454
Chagin AS, Lindberg MK, Andersson N, Moverare S, Gustafsson JA, Savendahl L, Ohlsson C (2004) Estrogen receptor-beta inhibits skeletal growth and has the capacity to mediate growth plate fusion in female mice. J Bone Miner Res 19:72–77
Parikka V, Peng Z, Hentunen T, Risteli J, Elo T, Vaananen HK, Harkonen P (2005) Estrogen responsiveness of bone formation in vitro and altered bone phenotype in aged estrogen receptor-alpha-deficient male and female mice. Eur J Endocrinol 152:301–314
Borjesson AE, Windahl SH, Karimian E, Eriksson EE, Lagerquist MK, Engdahl C, Antal MC, Krust A, Chambon P, Savendahl L, Ohlsson C (2012) The role of estrogen receptor-alpha and its activation function-1 for growth plate closure in female mice. Am J Physiol Endocrinol Metab 302:E1381–E1389
Denger S, Reid G, Kos M, Flouriot G, Parsch D, Brand H, Korach KS, Sonntag-Buck V, Gannon F (2001) ERalpha gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol Endocrinol 15:2064–2077
Kos M, Denger S, Reid G, Korach KS, Gannon F (2002) Down but not out? A novel protein isoform of the estrogen receptor alpha is expressed in the estrogen receptor alpha knockout mouse. J Mol Endocrinol 29:281–286
Borjesson AE, Lagerquist MK, Liu C, Shao R, Windahl SH, Karlsson C, Sjogren K, Moverare-Skrtic S, Antal MC, Krust A, Mohan S, Chambon P, Savendahl L, Ohlsson C (2010) The role of estrogen receptor alpha in growth plate cartilage for longitudinal bone growth. J Bone Miner Res 25:2690–2700
Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR (2000) Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis 26:145–146
Ohlsson C, Engdahl C, Borjesson AE, Windahl SH, Studer E, Westberg L, Eriksson E, Koskela A, Tuukkanen J, Krust A, Chambon P, Carlsten H, Lagerquist MK (2012) Estrogen receptor-alpha expression in neuronal cells affects bone mass. Proc Natl Acad Sci USA 109:983–988
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23:99–103
Nomura M, Akama KT, Alves SE, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S (2005) Differential distribution of estrogen receptor (ER)-alpha and ER-beta in the midbrain raphe nuclei and periaqueductal gray in male mouse: predominant role of ER-beta in midbrain serotonergic systems. Neuroscience 130:445–456
Finan B, Yang B, Ottaway N, Stemmer K, Muller TD, Yi CX, Habegger K, Schriever SC, Garcia-Caceres C, Kabra DG, Hembree J, Holland J, Raver C, Seeley RJ, Hans W, Irmler M, Beckers J, de Angelis MH, Tiano JP, Mauvais-Jarvis F, Perez-Tilve D, Pfluger P, Zhang L, Gelfanov V, Dimarchi RD, Tschop MH (2012) Targeted estrogen delivery reverses the metabolic syndrome. Nat Med 18:1847–1856
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Börjesson, A.E., Lagerquist, M.K., Windahl, S.H. et al. The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell. Mol. Life Sci. 70, 4023–4037 (2013). https://doi.org/10.1007/s00018-013-1317-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1317-1