Abstract
Severe maternal morbidity (SMM) is 50 to 100 times more common than maternal death, and has increased disproportionately among ethnic/racial minority women in the United States. However, specific knowledge about how the types and timing of severe maternal morbidities deferentially affect ethnic/racial minority women is poorly understood. This study examines racial/ethnic disparities in severe maternal morbidity during antepartum (AP), intrapartum (IP), and postpartum (PP) hospital admissions in the United States (US) for 2002–2014. We identified AP, IP, and PP hospitalizations in the National Inpatient Sample. Distribution of sociodemographic, behavioral and hospital characteristics, insurance, comorbidities, and SMM occurrence was summarized using descriptive statistics. Through Joinpoint regression, temporal SMM trends of hospitalizations were examined and stratified by race. Multivariate logistic regression assessed the association between race and SMM. We found black women have the highest proportion of SMM across all pregnancy intervals with a 70% greater risk of SMM during AP after adjusting for all cofactors. In the PP period, Hispanic women’s risk of SMM is 19% less when compared to white women. Racial/ethnic disparities in SMM vary in timing and SMM type. Systematic investigation is needed to understand risks to black women and the protective factors associated with Hispanic women in the PP. Addressing racial disparities in maternal morbidity and mortality requires national policies and initiatives tailored to black women that address the specific types and timings of life-threatening obstetric complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe maternal morbidity (SMM) is 50 to 100 times more common than maternal death [1,2,3], and has increased disproportionately among ethnic/racial minority women in the United States (US) [4, 5]. SMM is defined as unintended consequences of pregnancy that may result in significant short-term or long-term adverse consequences to a woman’s health [6, 7]. Indicators of SMM cover a range of conditions, including acute renal failure, eclampsia, sepsis, adult respiratory distress syndrome, and amniotic fluid embolism [6,7,8,9]. Because SMM is associated with high rates of preventability and cost [6, 10], a more nuanced understanding may improve efforts to address poor maternal outcomes, including maternal death, among minority women and lower healthcare costs in the US [1].
SMM in the US increased 200% from 1993 to 2014 and accounts for more than 1% of all births, costing women, their families, taxpayers, and the healthcare system billions of dollars [7]. The underlying cause of the rate increase is not entirely understood, but often attributed to increasing risk factors among women of reproductive age including obesity, cesarean sections, advanced maternal age, and comorbidities [11]. However, black and other minority women face substantially higher risk of SMM and maternal death [4]. While some research suggests higher prevalence of comorbidities (obesity, hypertension, and heart disease) among black women drives the maternal health disparity [12,13,14], other research point to structural racism: unequal access to healthcare, education, housing, stress, and implicit bias [15,16,17,18].
No published national studies have examined racial/ethnic differences in types of SMM women experience or when they occur along the pregnancy continuum. Most studies on SMM use state-level hospital billing data and aggregate 21 indicators of SMM focusing mainly on intrapartum (IP), or delivery, hospitalization. Missing from these analyses are the severe complications related to pregnancy that may arise during the antepartum (AP) period (during pregnancy until the patient is admitted for labor and birth) and during the postpartum (PP) period (from hospital discharge following birth through the first 42 days after birth). Given the current crisis in maternal death, particularly among black women, more nuanced data on SMM has the potential to inform targeted interventions and policies. This paper attempts to fill these gaps by presenting racial/ethnic variations in SMM during AP, IP, and PP hospitalizations, using the most recent nationwide trend data for racial and ethnic disparities in SMM from 2002 to 2014.
Methods
Design, Data Source, and Study Population
We conducted a retrospective cross-sectional study using the National Inpatient Sample (NIS) dataset from the Healthcare Cost and Utilization Project (HCUP) for the period of 2002–2014. The NIS is the largest all-payer hospital discharge database from non-federal community hospitals from participating states. On average, the NIS dataset represents about 97% of the US inpatient population [19, 20]. Average annual hospitalizations were weighted to reflect national estimates. The NIS has been validated against databases such as National Hospital Discharge Survey and Medicare Provider Analysis and Review file [21]. Our sample consisted of AP, IP, and PP hospitalizations for women 15–49 years of age.
Identification of SMM Cases
We limited our study population to pregnancy-related hospitalizations identified by the “NEOMAT” variable provided by HCUP. Self-reported maternal race is the exposure variable. The outcome variable was hospitalization with SMM, defined as the presence of hospitalization with one more SMM indicator. We followed Callaghan et al.’s (2012) list of 25 SMM indicators. The presence of SMM was determined by scanning all diagnosis fields in the dataset for the presence of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes that indicate one or more of the 25 SMM indicators. To determine the timing (AP, IP, or PP) of hospitalization with SMM, we used a previously validated algorithm [6, 17]. Women with an indicator for SMM who did not survive to hospital discharge were excluded from analysis in order to focus specifically on SMM, a population of women underrepresented in the literature compared to maternal mortalities. IP-related discharges were identified using IP-specific ICD-9-CM diagnosis, procedure, and diagnosis-related group (DRG) codes [22]. PP-related discharges were identified using V24 code, PP DRG codes, and presence of fifth digit “4” in ICD-9-CM codes [23]. Pregnancy-related hospitalizations that did not have a code for IP or PP conditions were classified as AP hospitalization [21,22,23]. Supplemental file 1 (S1) provides the list of ICD-9-CM diagnosis, procedure, and DRG codes used in this study.
Sickle cell disease is one of the indicators of SMM identified by the CDC [9]. Considering that most people living with sickle cell disease are of African descent, we conducted a sensitivity analysis to assess the impact excluding cases with sickle cell disease might have on racial disparity in the prevalence of SMM.
Covariates
Patient age in years was classified into three categories: 15–24, 25–34, and 35–49. We used median household income in quartiles of residents in the patient’s zip code (provided in the NIS dataset) as a proxy measure for participants’ socioeconomic status. Participants insurance payer status was grouped into three categories: government (Medicare and Medicaid), private (commercial carrier, private health maintenance organization, and preferred provider organization), and other sources (includes self-pay and charity). Hospital characteristics such as region (Northeast, Midwest, South, or West), location, and teaching status (rural, urban non-teaching, urban teaching) were considered as potential confounders and included in the multivariate models. In addition to personal and hospital characteristics, we adjusted for the effect of behavioral characteristics, including tobacco, alcohol, and drug use on SMM. We employed the Elixhauser comorbidity software [24] to assess the distribution of comorbidities and adjust for the impact of these comorbidities in the association between race and risk of developing SMM.
Statistical Analyses
Descriptive and Trend Analyses
The distribution of sociodemographic and behavioral variables, hospital characteristics, insurance, and comorbidities by whether a SMM occurred was summarized using descriptive statistics across the pregnancy continuum. Total counts of SMM during the study period were divided by the duration of the study period in years (13), to calculate average annual proportions of SMM. Temporal trends of SMM during the study period by timing of hospitalization (AP, IP, PP) were examined by Joinpoint regression and stratified by race. Joinpoint regression analysis is a statistical method that captures changing trends over time, and the extent of increase or decrease within each segment [25].
Multivariate Analysis
To examine the association between race or ethnicity and SMM, a crude and two multivariable models were constructed. The first multivariable model adjusted for sociodemographic and behavioral variables, and hospital characteristics; the second included additional adjustment for Elixhauser comorbidities [24]. All analyses were conducted using SAS software (SAS 9.4; SAS Institute Inc., Cary, NC), with a 5% type I error rate and two-sided hypothesis tests.
Results
During the 13-year study period, there were over 58.7 million maternal hospitalizations among women aged 15–49 years. Among these maternal hospitalizations, 698,496 (1.2%) had at least one indicator for SMM. The majority (89.5%) occurred during IP hospitalizations followed by AP (9%) and PP (1.5%) hospitalizations. Although the PP period represents only 1.5% of the total number of maternal hospitalizations, we found that the highest proportional rate of SMM occurs during the PP period, followed by the AP period. We observed a statistically significant association between race and risk of SMM in each pregnancy interval. Irrespective of the timing, black women experience significantly higher proportions of SMM (average 2.1%) when compared to white (average 1.0%) and Hispanic (average 1.0%) women. During the study period, women with SMM incurred $6,024,878,156 additional cost of inpatient care when compared to women without SMM in the study.
Antepartum
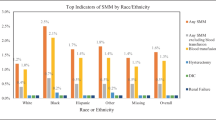
The largest racial disparity in proportions of SMM occurred during antepartum hospitalizations. During the study period, 11,120 AP hospitalizations had an indicator for SMM (Table 1). In total, 4.5% of black women hospitalized during the AP experienced SMM, compared to 2.3% (whites) and 2.2% (Hispanics) (Fig. 1). When compared to their white counterparts, black women are at 70% (aOR = 1.7, 95% CI (1.6–1.8)) increased risk of experiencing SMM during AP after adjusting for potential demographic, behavioral, hospital, insurance, and clinical confounders (Table 2). The highest proportions of AP hospitalizations with SMM were in women between the ages of 25–34 years for whites and Hispanics. On the other hand, the majority of black women with SMM were 15–24 years of age. Irrespective of race, hospitalizations for SMM during the AP period are more likely to occur in the South among women who use government insurance, have low household income, and receive care in teaching hospitals (Table 1).
Among AP hospitalizations of women with SMM, 18.7% whites and 18.4% Hispanics had two or more SMM indicators, compared to only 11.3% black hospitalizations. Sickle cell disease (44.7%), sepsis (23.9%), and sepsis (17.9%) were the primary indicators of AP SMM among black, Hispanic, and white women respectively (Table S1abc). The trend in SMM during AP hospitalization increased by 4.5% (95% CI 2.5–6.5) annually from 2002 to 2009 and remained steady afterwards (Fig. 2).
Intrapartum
The majority of hospitalizations for intrapartum management occur without SMM.
Therefore, although the largest total number of SMM occur during IP hospitalizations, the proportion of IP hospitalizations with SMM is smaller, compared to the proportions of SMM in AP or PP hospitalizations. We found relatively less racial disparity compared to AP and PP hospitalizations; however, the rate of SMM among black women (1.3%) was still nearly twice that of white (0.7%) and Hispanic (0.8%) women (Fig. 1) (Table 1). After controlling for demographic, behavioral, hospital type, insurance, and 29 Elixhauser comorbidities, black and Hispanic women endured 40% and 20% more risk of developing SMM when compared to their white counterparts (Table 2).
Intrapartum hospitalizations with SMM are more likely to occur among women in the 25–34 age group, who have low household income, use government insurance, and receive care in a teaching hospital. Among IP hospitalizations with a SMM, disseminated intravascular coagulation (DIC) was the leading indicator in white (27.6%) and Hispanic (21.5%) women and the second leading indicator among black (16.2%) women (Table S1b). The rate of SMM during IP hospitalizations is increasing (from 745 to 955 per 100,000 IP hospitalizations), a 2.1% (95% CI 1.4–2.7) increment annually (Fig. 2). Compared to women without SMM, those with SMM incurred an average extra inpatient care cost of $8007 per hospitalization translating to $3,544,410,648 during the study period.
Postpartum
The rate of SMM during PP hospitalizations represents the second largest racial disparity (next to AP). Among the 8559 hospitalizations with an indicator for SMM, 16% of black women experienced SMM compared to 12% among white, and 9.4% of Hispanic women (Table 1 and Fig. 1). After adjustment for a wide range of clinical and non-clinical confounders, Hispanic women experienced 19% less (aOR = 0.81, 95% CI 0.76–0.86) risk of SMM when compared to white hospitalizations during the PP period. On the other hand, black women experienced 18% (aOR = 1.18 (1.13–1.24)) more risk of SMM when compared to white women (Table 2).
When compared to the AP and IP periods, the proportion of SMM-related hospitalizations with two or more indicators is higher during the PP period for all racial groups, with 26.1 (black), and 25.8% (Hispanic), and 25.4% (white) (Table S1c). The rate of SMM among PP hospitalizations increased from 10,463 to 14,454 per 100,000 PP hospitalizations, translating to a 2.4% (95% CI 1.7–3.2) increase every year during the study period (Fig. 2). Women with SMM during the PP period also incurred on average an additional $12,038 per hospitalization and $1,339,408,070 overall during the study period.
Sensitivity Analysis
To examine the impact of sickle cell disease on racial disparity in SMM prevalence, we conducted a sensitivity analysis by excluding women who have had sickle cell disease as the only indicator of SMM. In the restricted sample, on average, there were yearly 9113 (AP), 33,689 (IP), and 8456 (PP) SMM-related maternal hospitalizations from 2002 to 2014. The overall rate of SMM remained higher among black women (1.8%) compared to 1% for white and Hispanic women.
After adjusting for a wide range of potential demographic, hospital, behavioral, and clinical confounders, black women overall continued to have 27% (AOR = 1.27, 95% CI = 1.21–1.32) more risk of experiencing SMM during pregnancy-related hospitalization when compared to white women. When stratified by timing of pregnancy period, black women were found to have 33% (AOR = 1.33, 95% CI = 1.25–1.41) and 13% (AOR = 1.13, 95% CI = 1.08–1.19) more risk of experiencing SMM during IP and PP periods respectively when compared to white women. However, compared to white women, without sickle cell disease, the risk of SMM was 9% (AOR = 0.91, 95% CI = 0.87–0.95) lower in black women during the AP period.
In the restricted sample, the risk of SMM among Hispanics was not significantly different from their white counterparts. In the stratified analysis, we found that Hispanics experience 7% (AOR = 0.88–0.98) and 19% (0.76–0.86) and less risk of SMM during the AP and PP periods respectively. However, the risk of SMM among Hispanics was 17% (AOR 1.17, 95% CI = 1.08–1.27) higher than that of white women during IP hospitalization.
Discussion
Racial Disparities
Similar to other studies, our data demonstrates disparities in proportions of severe maternal morbidity by race as they relate to pregnancy interval, comorbidities, and sociodemographic characteristics [26,27,28]. Black women consistently had higher proportions of SMM compared to Hispanic and white women. This disparity was especially pronounced during the AP period, and for black women residing in the South and delivering in teaching hospitals. High proportions of sickle cell disease in the black population increase the AP rate of SMM, as pregnancy exacerbates risks for sickle cell crises [29].
Intrapartum care is the leading cause of hospital admission in the United States, accounting for more than $16.1 billion in healthcare costs [30] and intense public health scrutiny of the processes and procedures related to labor and birth outcomes. However, a significantly higher proportion of SMM occur in the PP and AP periods, accounting for nearly $2.5 billion dollars in healthcare spending between 2002 and 2014. While it is more likely that a woman is admitted in AP or PP for a complication, this finding also suggests a need for increased attention to minority women during pregnancy and after IP discharge. PP hospitalizations for SMM were 66% more expensive than hospitalizations for SMM during IP or AP periods. The American College of Obstetricians and Gynecologists recently redesigned its recommendation on PP care to include earlier and more comprehensive visits. Recognition of the importance of the “fourth trimester” and timely follow-up may be crucial to reduce SMM and maternal mortality among women with chronic conditions, of which black women are disproportionately affected.
Traditional explanations for maternal health disparities focus on individual-level comorbidities more common in minority populations (e.g., obesity, hypertension, heart disease) that also increase maternal risk [31,32,33]. However, increasing attention is shifting to the impact of social and other health determinants, as well as the quality of obstetrical care [28]. One study examining racial disparities in maternal mortality found that black women did not have higher prevalence of five common high-risk obstetric complications but were 2.4–3.3 more likely to die from them than white women [27]. Our finding that black women fare significantly worse than white or Hispanic women in the South is supported by this and other research where the hospital location influences maternal morbidity and mortality [34, 35]. Black people in the South have among the country’s highest proportions of morbidity and mortality [36]. While our dataset does not allow us to directly measure structural racism, the data indicating racial variation in SMM by geographic location and hospital type support that the underlying disparities include structural factors. Instead of focus narrowing in on individual risk factors, future investigations of racial disparities in maternal morbidities should consider structural racism as an underlying etiology [15, 16]. Manifestations of structural racism in healthcare include racial biases resulting in treatment recommendation disparities for patients of color [37]. These disparities, which persist at all three (AP, IP, PP) pregnancy intervals, are not often considered by public and healthcare establishments because they are hard to measure and structural racism has taught us to normalize the outcome.
Hispanic Paradox
Hispanic women in this study consistently had a lower proportion of hospitalizations, and lower SMM prevalence during hospitalization (AP, IP, and PP) compared to black and white women. Similar to previous research, SMM during the PP hospitalizations were significantly lower for Hispanic women compared to black and white women [8, 38]. This phenomenon is referred to as the Hispanic paradox and describes the contrasting observations among Hispanics in the US. Despite having socioeconomic profiles that are comparable to blacks, Hispanics in the US have either comparable or better proportion of morbidity [39], proportion of mortality [40], and birth outcomes [8] than whites. Several explanations have been provided such as the healthy migrant hypothesis that suggests that the paradox results from selection of healthy Hispanic migrants into the US. However, no final conclusions are currently available to understand these contrasting observations.
Our study supports previous findings that Hispanic and black populations have different health profiles that necessitate attentions from both clinicians and researchers. In addition, Hispanic women seem to have protective factors during the PP period that result in significantly lower hospitalizations generally, and in SMM-related hospitalizations specifically, compared to black and white women. In-depth exploration of the PP period among Hispanic women might identify protective health indicators that can be translated to other ethnic minorities.
Limitations and Strengths
Similar to most retrospective studies that use existing datasets, our study has some inherent limitations worth mentioning. First, we were not able to assess the method used to identify each SMM condition. For example, proportions may vary depending on whether a given condition was ascertained with relevant laboratory and/or imaging test when appropriate. Our decision to exclude those patients who might have been considered cases of SMM simply because of blood transfusion in the absence of any other SMM indication might have minimized the risk of overdiagnosing SMM. Second, although we reported comorbidities, we had no information about previous pregnancy history, trimester for those hospitalized during antenatal period, and general patient condition during initial hospitalization, limiting our ability to assess the impact of these conditions on the risk of developing SMM. Third, the variable race/ethnicity is not reported by five states accounting for about 25% of the sample. However, we believe the unknown race values were random and not systematic, impacting all race groups equally. The age distribution of the group with unknown race values was not different than the groups with assigned race values. The current study reported findings for those with missing race value so that readers have an idea about SMM in these specific groups. Last, the identification of cases and clinical comorbidities using ICD-9-CM codes could be subject to errors in coding. However, we do not expect substantial differences in misclassification of SMM across racial groups during the pregnancy continuum.
Despite these limitations, the NIS, a multiyear and nationwide dataset, allowed us to calculate national prevalence and trends, and examine outcomes of SMM by race across the pregnancy continuum (AP, IP, PP). The NIS dataset provides a representative sample of pregnancy-related hospitalizations in the US, along with a range of clinical and non-clinical variables. The availability of these variables enabled us to adjust for multiple confounders in the multivariate analysis assessing the risk of SMM across racial groups during the pregnancy continuum.
Implication for Policy
Although significant attention has been paid to racial/ethnic disparities in increasing prevalence of maternal mortality in the US, the incidence of maternal mortality at any given hospital is relatively rare. By focusing solely on mortality, and not severe morbidities, previous studies may have missed the opportunity to positively impact pregnancy outcomes. The increased prevalence of maternal morbidities offers more opportunities to understand underlying mechanisms and develop targeted prevention strategies that will inherently reduce maternal mortality prevalence as well. Focusing prevention strategies on the morbidities and women at highest risk will reduce racial disparities and their associated costs.
The relationships between race and ethnicity, pregnancy interval, and severe maternal morbidities and mortalities also warrant further investigation and attention in order to reduce health disparities. With the use of more precise data, it is possible to direct interventions and policy at explicit points along the pregnancy continuum, based on known risks. For example, given the disproportionate prevalence of sickle cell disease among black women during the AP period, targeted efforts to reduce crises has strong potential to impact prevalence of SMM and their associated costs. Likewise, further research into protective factors in low-risk groups may yield valuable information that inform interventions for high-risk groups.
Some steps have been made to move healthcare policy in this direction. Our data support the “2018 Committee Opinion” issued by the American College for Obstetricians and Gynecologists to expand postpartum care and tailor services in order to improve maternal health outcomes [41]. However, more can be done. The identification of racial/ethnic disparities in health outcomes between populations of women also warrants investigation and intervention into structural and/or interpersonal racism within healthcare and society. Historically, these findings have been understood within the context of the behaviors or biology of the affected groups, without consideration for how racism may create risk factors as well as inform medical treatments that control health outcomes. When considering the clinical or policy implications of these disparities, a critical examination of healthcare structures and provision should be prioritized.
References
Lewis G. Beyond the numbers: reviewing maternal deaths and complications to make pregnancy safer. Br Med Bull. 2003;67(1):27–37. https://doi.org/10.1093/bmb/ldg009.
Cecatti CJG, Souza JP, Cecatti JG, Parpinelli MA, Helena De Sousa M, Serruya SJ. Systematic review of near miss maternal morbidity. Int J Gynaecol Obstet. 2006;22(2):255–64.
Geller SE, Rosenberg D, Cox SM, Brown ML, Simonson L, Driscoll CA, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–44. https://doi.org/10.1016/j.ajog.2004.05.099.
Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008–2010. Am J Obstet Gynecol. 2014;210(5):435.e1–8. https://doi.org/10.1016/j.ajog.2013.11.039.
Creanga AA, Berg CJ, Ko JY, Farr SL, Tong VT, Bruce FC, et al. Maternal mortality and morbidity in the United States: where are we now? J Women's Health (Larchmt). 2014;23(1):3–9. https://doi.org/10.1089/jwh.2013.4617.
Kilpatrick SK, Ecker JL, Ecker JL. Severe maternal morbidity: screening and review. Am J Obstet Gynecol. 2016;215(3):B17–22. https://doi.org/10.1016/j.ajog.2016.07.050.
CDC. Severe maternal morbidity in the United States | Pregnancy | Reproductive Health |CDC. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html#anchor_References. Accessed June 13, 2017.
Brown HL, Chireau MV, Jallah Y, Howard D. The “Hispanic paradox”: an investigation of racial disparity in pregnancy outcomes at a tertiary care medical center. Am J Obstet Gynecol. 2007;197(2):197.e1–9. https://doi.org/10.1016/j.ajog.2007.04.036.
Center for Disease Control and Prevention. Severe maternal morbidity indicators and corresponding ICD Codes during delivery hospitalizations. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm. Published 2018. Accessed January 14, 2019.
Lawton B, MacDonald E, Brown S, Wilson L. Preventability of severe acute maternal morbidity. Am J Obstet Gynecol. 2014. http://www.sciencedirect.com/science/article/pii/S0002937813022436. Accessed February 8, 2017.
Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31(5) http://care.diabetesjournals.org/content/31/5/899.long. Accessed August 24, 2017.
Hinkle SN, Sharma AJ, Kim SY, Park S, Dalenius K, Brindley PL, et al. Prepregnancy obesity trends among low-income women, United States, 1999–2008. Matern Child Health J. 2012;16(7):1339–48. https://doi.org/10.1007/s10995-011-0898-2.
Martin JA, Brady MPH, Hamilton E, et al. National vital statistics reports Vol 62, No. 1 June 28, 2013. 2011. http://www.cdc.gov/nchs/VitalStats.htm. Accessed January 9, 2019.
Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med (Baltim). 2013;56(6):372–8. https://doi.org/10.1016/j.ypmed.2013.02.015.
Gelber SE, Grünebaum A, Chervenak FA. Reducing health care disparities: a call to action. Am J Obstet Gynecol. 2016;215(2):140–2. https://doi.org/10.1016/J.AJOG.2016.06.058.
Kozhimannil KB, Henning-Smith CE, Hardeman RR. Reducing maternal health disparities: the rural context. Am J Obstet Gynecol. 2017;216(2):193–4. https://doi.org/10.1016/J.AJOG.2016.09.090.
Hardeman RR, Medina EM, Kozhimannil KB. Structural racism and supporting black lives — the role of health professionals. N Engl J Med. 2016:NEJMp1609535. https://doi.org/10.1056/NEJMp1609535.
Shavers VL, Fagan P, Jones D, Klein WMP, Boyington J, Moten C, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health. 2012;102(5):953–66. https://doi.org/10.2105/AJPH.2012.300773.
Pfuntner A, Wier LM, Elixhauser A. Overview of hospital stays in the United States, 2010. 2010. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb144.pdf. Accessed August 31, 2017.
Pfuntner A, Lauren MW, Steiner C. Costs for hospital stays in the United States, 2011 - statistical brief #168. Healthcare Cost and Utilization Project. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb168-Hospital-Costs-United-States-2011.jsp. Published 2011. Accessed August 31, 2017.
Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–85. https://doi.org/10.1161/HYPERTENSIONAHA.110.150821.
Kuklina EV, Whiteman MK, Hillis SD, Jamieson DJ, Meikle SF, Posner SF, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12(4):469–77. https://doi.org/10.1007/s10995-007-0256-6.
Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012:1. https://doi.org/10.1097/AOG.0b013e31826d60c5.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Source Med Care. 1998;36(1):8–27 http://www.jstor.org/stable/3766985. Accessed August 25, 2017.
Kim H-J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med Stat Med. 2000;19(335). https://doi.org/10.1002/(SICI)1097-0258(20000215)19:33.3.CO;2-Q.
Harper MA, Espeland MA, Dugan E, Meyer R, Lane K, Williams S. Racial disparity in pregnancy-related mortality following a live birth outcome. Ann Epidemiol. 2004;14(4):274–9. https://doi.org/10.1016/S1047-2797(03)00128-5.
Tucker MJ, Berg CJ, Callaghan WM, Hsia J. The black-white disparity in pregnancy-related mortality from 5 conditions: differences in prevalence and case-fatality rates. Am J Public Health. 2007;97(2):247–51. https://doi.org/10.2105/AJPH.2005.072975.
Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. Am J Obstet Gynecol. 2010;202(4):335–43. https://doi.org/10.1016/j.ajog.2009.10.864.
Villers MS, Jamison MG, De Castro LM, James AH. Morbidity associated with sickle cell disease in pregnancy. Am J Obstet Gynecol. 2008;199(2):125.e1–5. https://doi.org/10.1016/j.ajog.2008.04.016.
Podulka J, Stranges E, Steiner C. Hospitalizations related to childbirth, 2008. Healthcare Cost and Utilization Project (HCUP) statistical brief. 2011; p. 110. https://www.ncbi.nlm.nih.gov/books/NBK56040/. Accessed April 2017.
Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006–2010. Obstet Gynecol. 2015;125(1):5–12. https://doi.org/10.1097/AOG.0000000000000564.
Goffman D, Madden RC, Harrison EA, Merkatz IR, Chazotte C. Predictors of maternal mortality and near-miss maternal morbidity. J Perinatol. 2007;27(10):597–601. https://doi.org/10.1038/sj.jp.7211810.
Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol. 2013;25(2):124–32. https://doi.org/10.1097/GCO.0b013e32835e0ef5.
Howell EA, Zeitlin J, Hebert PL, Balbierz A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–41. https://doi.org/10.1001/jama.2014.13381.
Howell EA, Egorova N, Balbierz A, Zeitlin J, Hebert PL. Black-white differences in severe maternal morbidity and site of care. Am J Obstet Gynecol. 2016;214(1):122.e1–7. https://doi.org/10.1016/J.AJOG.2015.08.019.
Murray CJL, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3(9):e260. https://doi.org/10.1371/journal.pmed.0030260.
Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296–301. https://doi.org/10.1073/pnas.1516047113.
Abraído-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB. The Latino mortality paradox: a test of the "salmon bias" and healthy migrant hypotheses. Am J Public Health. 1999;89(10):1543–8 http://www.ncbi.nlm.nih.gov/pubmed/10511837. Accessed August 15, 2017.
Medina-Inojosa J, Jean N, Cortes-Bergoderi M, Lopez-Jimenez F. The Hispanic paradox in cardiovascular disease and total mortality. Prog Cardiovasc Dis. 2014;57(3):286–92. https://doi.org/10.1016/j.pcad.2014.09.001.
Ruiz JM, Steffen P, Smith TB. Hispanic mortality paradox: a systematic review and meta-analysis of the longitudinal literature. Am J Public Health. 2013;103(3):e52–60. https://doi.org/10.2105/AJPH.2012.301103.
ACOG. ACOG Committee opinion number 736 optimizing postpartum care. Obstet Gynecol. 2018;131(5):e140–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was granted exempt status from the University of Illinois Institutional Review Board. All authors have fulfilled all criteria for authorship. Furthermore, the authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 41.5 kb)
Rights and permissions
About this article
Cite this article
Liese, K.L., Mogos, M., Abboud, S. et al. Racial and Ethnic Disparities in Severe Maternal Morbidity in the United States. J. Racial and Ethnic Health Disparities 6, 790–798 (2019). https://doi.org/10.1007/s40615-019-00577-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-019-00577-w