Abstract
Background
Although discrimination among African Americans (AAs) has been linked to various health outcomes, few studies have examined associations of multiple measures of discrimination with prevalent subclinical disease in a large sample of AAs.
Objectives
To examine the associations of measures of discrimination and coping responses to discrimination with prevalent subclinical disease among AAs in the Jackson Heart Study (JHS); and whether this association is modified by sex.
Methods
We examined the associations of everyday, lifetime, and burden of lifetime discrimination with carotid intima-media thickness (cIMT), and left ventricular hypertrophy (LVH) among 3029 AAs in the JHS. Prevalence ratios (PR 95% confidence interval—CI) and odds ratios (OR 95% CI) were estimated for above-median cIMT and LVH, respectfully, adjusting for demographic, behavioral, and clinical risk factors.
Results
No significant associations were found between everyday and lifetime discrimination and median cIMT and LVH. Participants who reported high (vs. no) burden of lifetime discrimination had a 48% reduced odds of LVH (OR, 0.52; 95% CI, 0.29, 0.94) after full adjustment. There was evidence of effect modification by sex in the association of coping with everyday discrimination and LVH after full adjustment (p value for interaction < 0.01). Women who actively (vs. passively) coped with everyday discrimination had a greater odds of prevalent LVH (OR, 2.49; 95% CI, 1.39, 4.46).
Conclusions
This study suggests that the manner by which AA women cope with discriminatory events is associated with subclinical disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) affects about 14 million men and women in the USA [1]. African Americans (AAs) have disproportionately higher rates of CVD and related risk factors such as hypertension, obesity, and diabetes [2]. CVD disparities often stem from not only biological and behavioral factors, but from socioeconomic, environmental, and stress-related factors [3]. Studies have shown that racial discrimination (a stressor) is associated with poorer physical health [4,5,6,7], particularly hypertension among AAs [7, 8].
Research has reported associations between perceived discrimination and CVD risk factors. Sims et al. [9] found that greater lifetime and burden of lifetime discrimination were associated with higher hypertension prevalence among AAs. Findings from the Multi-Ethnic Study of Atherosclerosis (MESA) showed that everyday discrimination was associated with increased risk of CVD among men but not women [10]. Another study found no association of perceived discrimination with risk of CVD or heart failure (HF) hospitalization among AAs [11]. Few studies have examined the association of perceived discrimination with subclinical disease [12]. However, Lewis et al. found that chronic exposure to discrimination was associated with coronary artery calcification (CAC) in AA women in the Study of Women’s Health Across the Nation (SWAN) [13].
Discrimination affects CVD at the preclinical stages, which eventually develops into heart disease or stroke. An examination of the association between discrimination and subclinical disease is important because it elucidates the pathways by which discrimination influences CVD. For this reason, our study links self-reported measures of discrimination with markers of subclinical disease. No study, to our knowledge, has linked the association of multiple dimensions of and coping responses to discrimination with subclinical disease in a large sample of AAs. Examining multiple dimensions of discrimination (everyday, lifetime, burden of lifetime discrimination) could reveal the extent to which various forms of discrimination are associated with subclinical disease. For example, chronic exposures to discrimination (everyday) could affect subclinical disease differently than acute exposures to discrimination (lifetime), in that everyday discrimination may be responsible for the rapid progression of illness, while lifetime experiences of discrimination may trigger episodic events of CVD [4, 14].
Because discrimination negatively affects the health of AAs, it is also important to consider how AAs cope with or respond to discrimination and whether this mitigates the negative effects. One study found that coping styles, such as active (e.g., speaking out) and passive (e.g., keep silent) were associated with differences in blood pressure by sex [15].
In light of this gap, we examined the associations of multiple measures of discrimination (everyday, lifetime, and burden) with prevalent subclinical disease (carotid intima-media thickness [cIMT] and left ventricular hypertrophy [LVH]) among 3029 AA men and women in the Jackson Heart Study (JHS). We also examined the extent to which coping with discrimination modifies the association of discrimination with subclinical disease. We hypothesized that discrimination would be positively associated with subclinical disease, and associations would be modified by coping responses to discrimination.
Methods
Study Design and Participants
The JHS is a single-site prospective cohort study of CVD among AAs in Jackson, MS. Participants, aged 21 to 84, were recruited between 2000 and 2004 from the tri-county area of the Jackson, MS metropolitan area. All participants provided informed consent and the study was approved by the institutional review boards of the participating institutions: the University of Mississippi Medical Center, Jackson State University, and Tougaloo College. Further details of the study design have been previously reported [16].
Measures
Outcome variables included two subclinical disease markers—LVH and cIMT. JHS staff measured left ventricular (LV) mass as a continuous measure (LV mass values normalized by height2.7) using echocardiography, which followed standards of the American Society of Echocardiography. Nunez et al. [17] derived a categorical measure of LVH using an optimal threshold [LV mass index (LVMI) ≥ 51.2 g/m2.7], which was used in this study. JHS trained staff also measured carotid images of the walls from the common carotid artery, bifurcation of the carotid artery, and internal carotid artery using an electrocardiography-gated, B-mode, and spectral steered Doppler with an integrated recorder ultrasound machine [18]. We calculated the continuous measure of cIMT by estimating the maximum likelihood estimate of the averages of the right and left common carotid far wall. As there are a number of recommendations for selecting an optimal cIMT threshold [19, 20], we performed sensitivity analyses with several measures. The overall average cIMT in our sample exceeds the suggested 0.57 threshold derived by Mohan et al. [21]. In addition, and as reported in the literature, cIMT increases with age with higher medians occurring among male participants. In order to account for the association between age and cIMT and the overall increased thickness in our sample, we defined the prevalence of abnormal cIMT as any cIMT above the age-adjusted sample median (where medians were grouped in 10-year periods) and adjusted for sex in the models.
We assessed perceived discrimination using the JHS Discrimination Instrument (JHSDIS). The JHSDIS measures the occurrence, frequency, attribution, and coping responses to everyday, lifetime, and burden of lifetime discrimination [22]. We adapted the everyday discrimination measure from the Williams’ Everyday Discrimination scale [5] (9-items) (α = 0.88). Participants were asked how often certain experiences occurred on a daily basis such as, “You are called names or insulted,” “You are treated with less respect than other people,” etc. We summed their responses (0 = “never” to 7 = “several times a day”) for a total score (range 0–6), which was also transformed into z scores to form quartiles [1 = no discrimination (referent), 2 = low discrimination, 3 = moderate discrimination, 4 = high discrimination].
We derived the lifetime discrimination from the Krieger scale [7, 8]; it had good internal consistency within JHS (α = 0.78). Participants were asked if they had ever been treated unfairly (yes/no) in nine domains: receiving services, on the street/in public, receiving medical care, getting resources or money, finding housing, at work, getting a job, at school, and other. We additionally summed their responses to produce a summary score (range 0–9 points) and then transformed them into z scores to form quartiles similar to everyday discrimination.
We measured burden of lifetime discrimination using only the participants who reported lifetime discrimination in at least one domain. Participants were asked the following: “… how much has discrimination interfered with you having a full and productive life—a lot, some, a little, not at all”, “… how much harder has your life been because of discrimination—a lot, some, a little, not at all” (coded 4–1), “when you had experiences like these over your lifetime, have they been—very stressful, moderately stressful, or not stressful” (coded 4, 2.5, and 1). We summed, averaged, and converted their responses into z scores to form quartiles. Internal consistency was 0.63.
Participants reported behavioral coping responses to everyday and lifetime discrimination. For everyday discrimination, participants selected one of 12 coping responses, which were categorized into active coping (speak up, try to change, work harder, or pray), passive coping (accept it, ignore it, keep it to yourself, avoid it, or forget it), and external/other (blame yourself or get violent). We measured lifetime discrimination coping on two continuous scales ranging from 0 to 1: lifetime passive coping and lifetime active coping. Participants responded “yes” or “no” for each coping behavior. We coded each lifetime coping response as a 1 or 0 and each participant received an average score for passive and active coping. Due to a very small number of external/other responses, our analysis focused on passive and active coping for both everyday and lifetime discrimination.
We included baseline self-report sociodemographic measures, clinical risk factors, and health behaviors as covariates. Education was categorized as the highest level of completed education: less than high school, high school graduate/GED, some college/associate’s degree, or bachelor’s degree or higher. Income was based on family income, family size, and US Census poverty levels at the year of data collection and classified as poor, lower-middle, upper-middle, and affluent. Occupation was categorized into five categories: management/professional, sales, service, production/construction, and other (which includes participants who were not in the workforce or were unemployed). Clinical risk factors included fasting total cholesterol, fasting triglyceride levels, hypertension, and BMI. Total cholesterol and triglycerides were measured from fasting blood samples. Hypertension was determined by the presence of blood pressure greater than or equal to 140/90 mmHg, the use of blood pressure lowering medication, or having ever been told by a physician that the participant had hypertension. Health behaviors included a history of smoking (yes/no), excessive alcohol intake (14 drinks or more per week), and physical activity, measured using the validated JHS Physical Activity Instrument (JPAC) for sports and exercise [23, 24].
Statistical Analysis
For the following analyses, participants were excluded due to missing data for covariates [education (n = 15), occupation (n = 4), triglycerides (n = 248), smoking (n = 6), alcohol (n = 48), physical activity (n = 221)], discrimination measures (n = 86), and outcome variables (n = 2191). Differences in participant characteristics were compared by sex using χ2 test for categorical variables. ANOVA tests were used for normally distributed continuous variables, and Wilcoxon rank-sum (Mann-Whitney) tests were used for variables with non-normal distributions. Linear trends across quartiles for everyday, lifetime, and burden of lifetime discrimination were also tested.
Due to the skewed distributions of cIMT, we used generalized linear models with a gamma distribution and log link (gamma regression) to examine associations of cIMT (continuous) with discrimination. Multivariable logistic regression analysis estimated associations of discrimination with LVH (yes/no), where odds ratios [OR, 95% confidence intervals (CI)] estimated the relative odds of LVH. Multivariable Poisson regression with robust variance [24] estimated prevalence ratios (PR, 95% CI) for associations of discrimination with abnormal cIMT (yes/no). Multivariable models were adjusted with differing covariates for each outcome. Specifically, age was used as a covariate in cIMT and LVH models, but not with abnormal cIMT because our definition of abnormal cIMT accounts for participant age groups.
For each subclinical outcome, multivariable models were estimated sequentially. Model 1 was the unadjusted model. Model 2 adjusted for age, sex, socioeconomic status (SES—education, income, and occupation), clinical risk factors (hypertension status, total cholesterol, and triglycerides), and health behaviors (smoking status, physical activity, and alcohol use). To assess the effect modification of sex in the association of discrimination coping with subclinical disease, interaction terms for sex and coping were also analyzed in the fully adjusted models. Where significant interactions were found (p < 0.05), we reported sex-stratified analyses.
There was a significant amount of missing data for income (16%) which was corrected using multiple imputations (MI). Six imputed datasets were created based on variables included in the analyses using MI chained equations (MICE) in STATA 14 [25]. Sensitivity analysis was performed to compare estimates from the original data, imputed data, and data where dummy categories were created for missing responses. Results for the original versus imputed data were similar; therefore, we present the imputed results. All statistical analyses were performed using SAS version 9.4 [26] and STATA 14 [27].
Results
Characteristics for the 3029 participants (63.2% women) are presented in Table 1. Men reported greater levels of everyday, lifetime, and burden of lifetime discrimination than women (p < 0.001). Women were more likely to report active (vs. passive) coping responses to everyday discrimination (p = 0.01). The prevalence of LVH was slightly higher among women than men, but the difference was not significant (7.6 and 6.1%, respectively; p = 0.13). However, the median cIMT for men was significantly higher than women (p < 0.001).
Table 2 presents select sample characteristics by levels of discrimination. Female sex and age were linearly associated with each measure of discrimination. The percent of college educated participants was inversely patterned by everyday and lifetime discrimination (p for trend < 0.01), while affluent income was positively associated with everyday and lifetime discrimination (p for trend < 0.05). Overall, prevalent hypertension declined with increasing everyday discrimination (p for trend < 0.05). For example, for participants who reported low everyday discrimination (quartile 1), hypertension prevalence was 64%; for those who reported high everyday discrimination (quartile 4), hypertension prevalence was 43%. Prevalent hypertension also declined with increasing lifetime discrimination (p for trend < 0.01). Specifically, for participants who reported low lifetime discrimination (quartile 1), hypertension prevalence was 60%; for those who reported high lifetime discrimination (quartile 4), hypertension prevalence was 47%. A history of smoking increased with burden of lifetime discrimination (p for trend 0.001). Specifically, for participants who reported low burden of lifetime discrimination (quartile 1), the percentage of history of smoking was 27%; for those who reported high burden of lifetime discrimination (quartile 4), history of smoking was 34%. Prevalent LVH declined with increasing everyday discrimination (p for trend 0.01).
Levels of everyday, lifetime, and burden of lifetime discrimination were not associated with prevalent cIMT (Table 3). However, in the unadjusted model, participants who reported high (vs no) everyday discrimination had a 50% reduction in odds of LVH (OR, 0.50; 95% CI, 0.31, 0.81). After full adjustment, this finding attenuated and became nonsignificant. There was an inverse gradient in the association of low-to-high levels of lifetime discrimination with LVH despite statistical insignificance. In the unadjusted model, participants who reported low (vs no) burden of lifetime discrimination had a 48% reduced odds of LVH (OR, 0.52; 95% CI, 0.30, 0.89). After full adjustment this finding remained significant (OR, 0.54; 95% CI, 0.29, 1.00). Participants who reported high (vs no) burden of lifetime discrimination had a 48% reduced odds of LVH (OR, 0.52; 95% CI, 0.29, 0.94) after full adjustment.
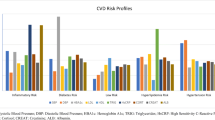
Although everyday and lifetime coping were not associated with either cIMT or LVH in the main effect models, the introduction of the coping-sex interaction term produced a significant association in the everyday discrimination models (Fig. 1). In unadjusted models, women who reported using active (vs passive) coping for everyday discrimination were more than 87% likely to have LVH (p = 0.012). This pattern continued after adjustments for SES, risk factors, and health behaviors; the association increased after full adjustment (OR, 2.49; 95% CI, 1.39, 4.46; p = 0.002). The association of coping responses to everyday discrimination LVH among men did not vary by passive or active coping status.
Overall, men had increased odds of having above-median cIMT regardless of coping style and no significant interactions occurred between sex and coping style in association with above-median cIMT. Additionally, no associations between lifetime coping and LVH were significant for men or women (data not shown).
Discussion
This study examined the association of multiple dimensions of discrimination and coping responses to discrimination with subclinical disease markers in a large sample of AAs. Continuous measures of everyday, lifetime, and burden of lifetime discrimination were not associated with cIMT and LVH in fully adjusted models. However, when measured as categories, everyday discrimination was associated with LVH in the unadjusted model but attenuated after full adjustment. In fully adjusted models, participants who reported high (vs no) burden of lifetime discrimination had a lower odds of LVH. There was a significant sex interaction with coping responses to everyday discrimination and LVH. After full adjustment, women who reported active (vs passive) coping with everyday discrimination had a greater odds of LVH. Sex did not modify the associations of coping with lifetime discrimination with cIMT or LVH.
Previous studies have found associations of discrimination with subclinical disease markers. In a sample of 109 AA and 225 white women, Troxel et al. [12] found that composite chronic stress and everyday unfair treatment were positively associated with higher IMT scores among AA women only. In a follow-up study, Lewis et al. [13] found that cumulative chronic experiences of everyday discrimination over time were positively associated with CAC scores among a sample of 181 middle-aged AA women. After full adjustment, we found greater everyday, lifetime, and burden of lifetime discrimination scores were not significantly associated with cIMT or LVH. Participants who reported high (vs no) everyday and lifetime discrimination had lower mean cIMT and reduced odds of LVH, though findings were not significant. LVH also significantly declined with increasing everyday discrimination, which may also contribute to our findings.
These null findings may also be due to either chronicity of discrimination, or high prevalence of discrimination. First, the chronicity (or the average over time) of everyday discrimination may need to be considered in order to detect an association with early development of atherosclerosis. Rather than examining the occurrence of an acute event during one’s lifetime or the occurrence of minor events in a given year, an examination of the accumulation of everyday discrimination over time may be warranted. Second, the high prevalence of discrimination may have contributed to ceiling effects in the association of discrimination with the outcomes [9, 22]. In other words, because the prevalence of everyday and lifetime discrimination was greater than 60% in this sample, the level of variance in discrimination measures no longer had an effect (a ceiling effect) on subclinical disease, thereby resulting in null findings.
Participants who reported high (vs no) burden of lifetime discrimination had a reduced odds of LVH (p < 0.05) after full adjustment. We expected that greater burden of lifetime discrimination would be associated with an increased odds of LVH. Perhaps the aggregation of items in the burden index impacted the findings, and the examination of its individual components (i.e., made life stressful and hard, or prevented productive life) may produce different results. Also, age may have played a role as younger (vs older) participants report greater levels of burden of lifetime discrimination and also have a lower prevalence of LVH, which may have contributed to the protective findings. Younger participants may also exhibit more resilience via having greater psychosocial resources (e.g., social support, optimism). These resources could mitigate the negative effects discrimination may have on biological responses (dysregulation of the HPA-axis), which in turn may reduce the odds of LVH.
We found that sex modified the association of coping responses to everyday discrimination and LVH. Specifically, women who reported active coping (speak up, try to change, work harder, and pray) with everyday discrimination had a greater odds of LVH than women who reported passive coping (accept it, ignore it, keep it to yourself, avoid it, and forget it) (p value for interaction < 0.01). This pattern was not detected in men. Previous studies have found that sex modifies relationships between stressors and cardiovascular outcomes [28,29,30,31]. In a prospective cohort study of AAs and whites, Krieger and Sydney [8] reported that AA women (n = 1143) were 1.5 times more likely than white women to respond to lifetime discrimination by keeping it to themselves. Additionally, they found that among AA working-class women, systolic blood pressure was 4 mmHg higher among those who coped with lifetime discrimination by keeping it to themselves and accepting it as a way of life than those who did something about it and talked to others. We found that active (vs passive) coping was associated with greater LVH, while Krieger found that passive (vs active) coping among women was associated with higher blood pressure, a prominent risk factor for LVH.
Perhaps active rather than passive behavioral responses to everyday discrimination (stressor) contribute to preclinical conditions that are manifested over time as LVH among AA women in the JHS. Studies have found that AA women report more stressors than men, such as obligations to manifest strength, help others, and succeed despite limited economic resources, and this adds heightened susceptibility to subsequent illness [32]. In the JHS, AA women (vs men) report more life events and global stress, but also exhibit active stress coping strategies which may place them at greater risk for subclinical disease [12, 28, 30]. Further analysis on the physiological pathway suggests that active coping produces increases in heart rate and blood pressure via β-adrenergic vasodilatory responses and increases in catecholamines, which may explain why active coping was associated with subclinical disease in this study [33]. However, research has found that both active and passive coping may result in increases in blood pressure, though the pathway by which this occurs varies. For instance, Williams et al., [34] found that passive coping increases blood pressure via α-adrenergic vasoconstrictory activity.
One limitation of this study is that it was conducted in a single southern metropolitan area, which may limit the generalizability of our results to other AA populations. Also, the predictors and outcomes were cross-sectional, which precludes us from assessing the extent to which changes in discrimination were related to changes in subclinical disease and from drawing causal inferences. A strength of this study includes utilizing JHS, the largest study of CVD in AAs, to examine discrimination and subclinical disease markers in AAs. In addition, the use of multiple dimensions of experiences with and responses to discrimination is novel, in that most work in this area of research focuses on single measures of discrimination among women.
In conclusion, we did not find a direct association of discrimination with subclinical disease. However, we did find that coping responses to everyday discrimination were associated with prevalent LVH among women. Understanding the impact perceived discrimination has on preclinical morbidities such as LVH may provide insight on how the reduction in psychosocial stressors may, in part, contribute towards the reduction in subclinical disease in AAs, particularly women.
References
Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke Statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447–54. https://doi.org/10.1161/CIR.0000000000000366.
Wyatt SB, Williams DR, Calvin R, Henderson FC, Walker ER, Winters K. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315–31.
Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10(9):e0138511. https://doi.org/10.1371/journal.pone.0138511.
Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32(1):20–47. https://doi.org/10.1007/s10865-008-9185-0.
Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335–51. https://doi.org/10.1177/135910539700200305.
Lewis TT, Williams DR, Tamene M, Clark CR. Self-reported experiences of discrimination and cardiovascular disease. Curr Cardiovasc Risk Rep. 2014;8(1):365. https://doi.org/10.1007/s12170-013-0365-2.
Krieger N. Racial and gender discrimination: risk factors for high blood pressure? Soc Sci Med. 1990;30(12):1273–81. https://doi.org/10.1016/0277-9536(90)90307-E.
Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA study of young black and white adults. Am J Public Health. 1996;86(10):1370–8. https://doi.org/10.2105/AJPH.86.10.1370.
Sims M, Diez-Roux AV, Dudley A, Gebreab S, Wyatt SB, Bruce MA, et al. Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. Am J Public Health. 2012;102(S2):S258–65. https://doi.org/10.2105/AJPH.2011.300523.
Everson-Rose SA, Lutsey PL, Roetker NS, et al. Perceived discrimination and incident cardiovascular events the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2015:kwv035.
Dunlay SM, Lippmann SJ, Greiner MA, O’Brien EC, Chamberlain AM, Mentz RJ, et al. Perceived discrimination and cardiovascular outcomes in older African Americans: insights from the Jackson Heart Study. In Mayo Clinic Proceedings. 2017;92(5):699–709 Elsevier. https://doi.org/10.1016/j.mayocp.2017.01.024.
Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol. 2003;22(3):300–9. https://doi.org/10.1037/0278-6133.22.3.300.
Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, et al. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: the SWAN Heart Study. Psychosom Med. 2006;68(3):362–8. https://doi.org/10.1097/01.psy.0000221360.94700.16.
Bhattacharyya MR, Steptoe A. Emotional triggers of acute coronary syndromes: strength of evidence, biological processes, and clinical implications. Prog Cardiovascular Dis. 2007;49(5):353–65. https://doi.org/10.1016/j.pcad.2006.11.002.
Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA Study of young black and white adults. Am J Public Health. 1996 Oct;86(10):1370–8. https://doi.org/10.2105/AJPH.86.10.1370.
Taylor HA Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6–4.
Nunez E, Arnett DK, Benjamin EJ, Liebson PR, Skelton TN, Taylor H, et al. Optimal threshold value for left ventricular hypertrophy in blacks the atherosclerosis risk in communities study. Hypertension. 2005;45(1):58–63. https://doi.org/10.1161/01.HYP.0000149951.70491.4c.
Carpenter MA, Crow R, Steffes M, Rock W, Skelton T, Heilbraun J, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131–44. https://doi.org/10.1097/00000441-200409000-00001.
Hurst RT, Ng DW, Kendall C, Khandheria B. Clinical use of carotid intima-media thickness: review of the literature. J Am Soc Echocardiogr. 2007;20(7):907–14. https://doi.org/10.1016/j.echo.2007.02.028.
Mohan A, Sada S, Kumar BS, Sarma KV, Devi BV, Rao PV, et al. Subclinical atherosclerosis in patients with rheumatoid arthritis by utilizing carotid intima-media thickness as a surrogate marker. Indian J Med Res. 2014;140(3):379–86.
Tosetto A, Prati P, Baracchini C, Manara R, Rodeghiero F. Age-adjusted reference limits for carotid intima-media thickness as better indicator of vascular risk: population-based estimates from the VITA project. J Thromb Haemost. 2005;3(6):1224–30. https://doi.org/10.1111/j.1538-7836.2005.01440.x.
Sims M, Wyatt SB, Gutierrez ML, Taylor HA, Williams DR. Development and psychometric testing of a multidimensional instrument of perceived discrimination among African Americans in the Jackson heart study. Ethn Dis. 2009;19(1):56–64.
Dubbert PM, Robinson JC, Hye Sung J, et al. Physical activity and obesity in African Americans: the Jackson Heart Study. Ethn Dis. 2010;20(4):383–9.
Smitherman TA, Dubbert PM, Grothe KB, Sung JH, Kendzor DE, Reis JP, et al. Validation of the Jackson Heart Study physical activity survey in African Americans. J Phys Act Health. 2009;6(1):S124–32. https://doi.org/10.1123/jpah.6.s1.s124.
Coutinho L, Scazufca M, Menezes PR. Methods for estimating prevalence ratios in cross-sectional studies. Rev Saúde Pública. 2008;42(6):992–8. https://doi.org/10.1590/S0034-89102008000600003.
SAS 9.4. Cary, NC; 2013.
StataCorp. Stata statistical software: release 14. College Station, TX: StataCorp LP; 2015.
Cooper DC, Thayer JF, Waldstein SR. Coping with racism: the impact of prayer on cardiovascular reactivity and post-stress recovery in African American women. Ann of Behav Med. 2014;47(2):218–30. https://doi.org/10.1007/s12160-013-9540-4.
Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26(1):469–500. https://doi.org/10.1146/annurev.publhealth.26.021304.144542.
Matthews DD, Hammond WP, Nuru-Jeter A, Cole-Lewis Y, Melvin T. Racial discrimination and depressive symptoms among African-American men: the mediating and moderating roles of masculine self-reliance and John Henryism. Psychol Men Masc. 2013;14(1):35–46. https://doi.org/10.1037/a0028436.
Pascoe EA, Richman LS. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009;135(4):531–54. https://doi.org/10.1037/a0016059.
Woods-Giscombé CL. Superwoman schema: African American women’s views on stress, strength, and health. Qual Health Res. 2010;20(5):668–83. https://doi.org/10.1177/1049732310361892.
Hamer M, Malan L. Psychophysiological risk markers of cardiovascular disease. Neurosci Biobehav Rev. 2010 Sep 30;35(1):76–83. https://doi.org/10.1016/j.neubiorev.2009.11.004.
Williams RB. Patterns of reactivity and stress. Handbook of stress, reactivity, and cardiovascular disease. 1986.
Acknowledgements
The authors thank the staff and participants of the Jackson Heart Study for their important contributions and ongoing support.
Funding Sources
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD). Dr. Sims is supported by the grants P60MD002249 and U54MD008176 from the NIMHD; 15SFDRN26140001 and P50HL120163 from American Heart Association; and 1R01HL116446 from the NHLBI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All participants provided informed consent and the study was approved by the institutional review boards of the participating institutions: the University of Mississippi Medical Center, Jackson State University, and Tougaloo College.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Okhomina, V.I., Glover, L., Taylor, H. et al. Dimensions of and Responses to Perceived Discrimination and Subclinical Disease Among African-Americans in the Jackson Heart Study. J. Racial and Ethnic Health Disparities 5, 1084–1092 (2018). https://doi.org/10.1007/s40615-017-0457-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-017-0457-7