Abstract
Purpose of Review
Candida species are an important cause of both superficial and life-threatening systemic fungal infections. Historically, their study has been centered around their ability to cause human disease. However, this narrow lens limits our understanding of the overall factors that shape their evolution.
Recent Findings
We argue that from the perspective of evolutionary dynamics, pathogenic traits of Candida species contribute to only one aspect of selection, and their roles as commensal members of the healthy human mycobiome or in the environment may play a larger role in adaptation. We stress that our understanding of these species is lacking due to a limited geographical sampling and minimal study of commensal fungal populations.
Summary
By looking outside of the box of medical mycology, we can identify what we do and do not know about the factors that shape the genetic and phenotypic diversity of Candida spp. within the variety of environments they inhabit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida species account for the most prevalent fungal infections in the world and are a significant cause of human mortality and morbidity [1]. The majority of these infections are associated with Candida albicans, yet non-albicans Candida species (“NACS”) are also important opportunistic pathogens. Much of the history of Candida spp. is steeped in taxonomic confusion. Early studies of Candida species relied upon identifying differences in morphology or metabolism, an ambiguous approach for species identification and assessment of relatedness, given that many Candida spp. are capable of extensive morphological and metabolic flexibility. This lack of clarity has continued into the twentieth century, as the genus Candida has been used as an umbrella term to include yeast capable of causing human infection and lacking a conventional sexual cycle. It was not until 1991 when Susan Barns and colleagues compared ribosomal DNA (rDNA) sequences and demonstrated the phylogenetic divergence among Candida spp. By example, Candida glabrata, the second most common human-associated Candida spp. is more closely related to Saccharomyces cerevisiae than to Candida albicans [2]. Advances in sequencing technology and broader sampling of isolates have enabled higher phylogenetic resolution, and the ~ 200 identified Candida spp. belong to over 13 phylogenetically distinct clades [3]. A recent effort formally renamed species based on their phylogenetic relationships, and we include the revised names in brackets for the first use of each affected species [4] (Fig. 1). As the incidence of NACS as human pathogens as well as the incidence of multiple Candida spp. co-infections is increasing [6, 7], it will become ever more critical to identify how these species are both similar and differ.
Phylogenetic relationship among Candida spp. and close relatives. The ploidy level of the majority of sampled isolates is provided in brackets. Adapted with permission from [5]
In this review, we attempt to look outside the box of medical mycology [8] to discuss what is known and what remains unknown about the factors shaping the evolution of Candida spp. and consider the breadth of the environmental and human-associated conditions these species experience. We discuss understudied topics in genome evolution in Candida spp., from the different organizational levels variation is observed: between species, between isolates of the same species, and between cells within individual populations.
Broadening the Lens We Use to Look at Candida species Evolution
Much of the study of Candida spp. has been through the lens of their capacity to cause infections in humans [9••, 10, 11••, 12] and our understanding of the factors that drive Candida spp. evolution is likely limited by this narrow viewpoint.
Consistent with their broad phylogenetic relationships, virulence traits appear to have independently evolved in Candida spp. Despite triggering highly similar host responses in a whole-blood infection model, C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis displayed unique transcriptional responses [13]. C. dubliniensis, a close relative to C. albicans that is less virulent [14] and seldom isolated from infections [1] has been used to compare with C. albicans to identify genetic determinants of virulence. From these comparisons, it became apparent that several gene families that contribute to virulence, stress responses, and filamentation (e.g., adhesins, secreted aspartyl proteinases, subtelomeric genes) are expanded in the C. albicans genome [15, 16]. A critical virulence factor for C. albicans is its ability to transition between yeast and filamentous growth [17], which is differentially regulated in C. dubliniensis [18]. Intriguingly, in C. tropicalis and C. parapsilosis, two emerging pathogens, filamentation is not essential for virulence and may even be detrimental to survival [17, 19]. In C. albicans, genetic screens and experimental evolution revealed that commensal phenotypes are associated with reduced filamentation [20, 21••, 22], yet reduced filamentation evolves only in hosts with reduced microbial diversity [22, 23]. In most clinical cases, the commensal population gives rise to organisms that become pathogenic [24]. The selective drivers of adaptive evolution may thus emerge more from the roles as commensal members of the healthy human mycobiome (and other environmental niches, in some species) than from their roles in human infection; population-level genetic variation and the traits under selection in the context of commensalism in Candida spp. have been understudied.
In host environments, Candida spp. face complex abiotic and biotic selection pressures. Alongside the many stresses imposed by the host, including but not limited to nutrient availability, oxygen levels, and immune defenses (Fig. 2), fungal cell populations exist alongside other microbial species, and both permissive and antagonistic interactions have been documented [25, 26]. As part of the infection process and during transitions between host environments (e.g., translocation from gut to bloodstream or from bloodstream to systemic organs; Fig. 2), significant population bottlenecks occur and limit the efficiency of selection on niche-specific traits. Candida spp. have also been isolated from several other mammalian hosts, such as hedgehogs, opossums, dogs, and sheep [27, 28] and have been collected from moist soils, fallen leaves, oak trees, and coastal wetlands [27, 29, 30, 31••, 32•• 33]. The recent discovery of C. auris isolates on the coastal wetlands of India, including those that were multi-drug resistant, points to a potential environmental source for this species and signals that additional environmental surveillance of Candida species is needed [27, 29, 30, 31••, 32••, 33]. A complete understanding of how different niches and environments shape the evolution of these species will require comprehensive surveys of different environments paired with fitness measurements of Candida isolates in the diversity of conditions they encounter.
Candida cells are subjected to large population bottlenecks as they move through different host anatomical niches, each harboring diverse selective pressures. Within commensal sites such as the oral cavity, GI tract, or skin, Candida cells face additional stresses imposed by the microbiome and competition for nutrients with other species
Adaptation requires genetic variation for selection to act on. Genetic variation in Candida spp. is primarily the result of asexual reproduction, with many members having restricted or absent meiotic recombination programs [34, 35] and sparse evidence for mating [5, 36••, 37]. The prevailing view has been that limited sexual recombination could preserve the integrity of well-adapted genomes [34, 35, 38]. This also provides an opportunity for karyotypic variation (single chromosome changes or whole ploidy shifts) to play a role in adaptation. Accordingly, in addition to point mutations (single nucleotide variants, small insertions and deletions), variation in chromosome copy number is frequently observed [5, 9••, 36••, 39, 40••, 41], and whole ploidy shifts are observed in nearly all human-associated Candida spp. [42]. The outlier is the haploid C. glabrata; whether the absence of ploidy-variant isolates of C. glabrata is due to undersampling or something specific about the genetics of this species remains unknown [40••].
Different types of mutations occur at different rates and impact a different number of genes (Fig. 3). The types of mutations that arise and are selected during evolution are also influenced by baseline ploidy, which varies among Candida spp. (Fig. 1 [43]). For example, in diploid C. albicans, loss of heterozygosity (LOH) events are common in clinical isolates [10, 12, 36••] and have been identified in the C. albicans laboratory reference strain evolved under a multitude of in vitro and in vivo conditions [44, 45••, 46••]. LOH is also likely to be common in other diploid NACS [47, 48]. LOH mutations can reveal recessive alleles, though whether these events on average are neutral or beneficial is not well resolved (discussed below). Copy number variants of small regions or whole chromosomes are both generated and lost at high frequencies [11••], which might allow cells to bet-hedge the potential benefit of increased gene copy number under certain conditions while also being able to revert to the wild type when the selective pressure is removed. Chromosomal aneuploidy has been associated with replicative, metabolic, and proteotoxic stress [49, 50], and aneuploidies are likely to be temporary rather than long-term solutions. Recent genomic analyses have shown that ancient hybridization events occurred between distantly related isolates before the divergence of C. albicans, C. africana, and C. stellatoidea, a process hypothesized to mediate the emergence of new phenotypic traits and to enable adaptation to new environments [51, 52••, 53]. Hence, there are many different mechanisms on which selection can act to result in rapid genetic variation within fungal populations.
Size of observed genetic variations relative to the time needed to generate them in haploid or diploid fungal cells. Colored arrows indicate the ratio between the genetic size and the time needed to generate these events. For example, aneuploidy can arise within single cell divisions and affects entire chromosomes. At the opposite spectrum, SNPs/indels arising within single divisions affect a small number of single nucleotide positions. Reversible genetic changes are indicated by double-end arrows
Population Structure Reflects Primarily Neutral Rather Than Adaptive Processes
Population structure captures genetic variation at the species level and reflects evolutionary processes and demographic history. The population structure of Candida spp. and other fungal taxa has been assessed using short tandem repeat markers (e.g., microsatellite typing, variable number tandem repeat), sequence variation in the ITS locus, multilocus sequence typing (MLST), and whole-genome sequencing. Whole-genome sequencing offers the highest ability to differentiate among isolates and to capture genome-wide diversity; the high costs and time required for analysis have restricted its broad use for answering phylogenetic questions, though this is likely to change in the future. In C. albicans, MLST analysis is preferred over ITS or microsatellite typing since the data is unambiguous, is reproducible, and can be directly compared among research groups and isolate collections [54]. For C. glabrata [55, 56] and the C. parapsilosis species complex [57], microsatellite typing provides higher discriminatory power and has been more widely used. In C. tropicalis, microsatellite typing and MLST analysis provide similar discriminatory power [58].
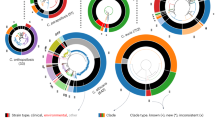
Currently, only four Candida spp. are represented in the PubMLST database, the public database for molecular typing and microbial genome diversity (https://pubmlst.org/). The distribution of isolates in all four species is skewed by the source of isolation, with bloodstream and oropharynx isolates being the most common (Fig. 4 [59]). We currently lack a complete picture of how population structure maps to environment or geography at the global level since the geographical distribution of deposited isolates is also skewed. The majority of isolates have been deposited from Asia (mainly China, Taiwan, and Iran), Europe (mainly the UK, France, and Spain), and North America (mainly the USA). Nevertheless, MLST studies in all species repeatedly indicate local clustering with frequent gene flow (e.g., C. tropicalis [60,61,62,63,64,65], C. glabrata [66,67,68,69], C. albicans [70,71,72,73,74,75,76,77,78,79]). A small number of existing phylogenetic analyses using whole-genome sequences of a global set of isolates were overall consistent with the MLST studies (C. albicans [36••], C. glabrata [80], C. tropicalis [81]). Phylogenies thus appear to primarily reflect neutral processes such as geography and gene flow rather than selection. Despite a small number of interesting associations between phenotypes of interest and sequence types (STs), the majority of studies have failed to detect STs associated with anatomical source, patient health status, or phenotypes of interest such as drug susceptibility (C. albicans [36••, 82,83,84], C. glabrata [55, 56, 66, 68, 80, 85], C. tropicalis, [60, 62, 63, 65], C. parapsilosis [86,87,88]). Furthermore, environmental isolates typically cluster with clinical isolates (C. tropicalis [81, 89], C. krusei (referred to as Pichia kudriavzevii when isolated from the environment) [90], C. albicans [31••, 36••], C. glabrata [91], C. parapsilosis [87]).
Proportion of Candida isolates deposited in the PubMLST database based on geographic location (A) and source of isolation (B). The total number of isolates for each species as of January 2021 is indicated in brackets below the species name. The number of researchers that have deposited sequences also differs by species: C. albicans, 76; C. glabrata, 14; C. tropicalis, 25; C. krusei: 7
Selection can act on isolates within specific niches to alter virulence or infection-related traits. However, it remains challenging to disentangle how biased sampling influences potentially spurious associations when looking for correlations between phylogenetic clusters and isolate characteristics. For example, in C. albicans isolates from animals, the most common ST is ST172 (6.5% of animal isolates, versus 0.12% of all other isolates), yet all come from Hungary. In contrast, the five ST172 isolates from the UK, India, Germany, France, and China were collected from oral, vaginal, and systemic infections. Distinguishing whether clustered isolates are genuinely different for specific traits of interest requires significant additional global sampling, such as the recent tour de force to acquire environmental yeast isolates [32••].
The Potential Influence of Heterozygosity
Considerable heterozygosity exists in the diploid species (Fig. 1), but it remains unclear whether this is meaningful in the context of adaptation. Allelic heterozygosity has been quantified as the average frequency of polymorphisms across the genome (i.e., number of SNPs per base [92]) and as nucleotide diversity (i.e., the average number of nucleotide differences per site between two DNA sequences in all possible pairs [36••]). Early C. albicans whole-genome studies noted a high level of heterozygosity compared to sexually reproducing species and speculated that allelic differences might have clinical consequences [84, 92]. Since then, Candida spp. allelic variation has often been interpreted through an adaptive lens. However, heterozygosity levels among diploid, asexual Candida spp. vary widely. C. albicans and C. tropicalis have similar levels of heterozygosity, with ~ 0.5% positions in the genome being heterozygous [45••, 81], ~ 70-fold higher than that observed in C. parapsilosis [93] (with the caveat that fewer C. parapsilosis strains have been examined).
Heterozygosity is theoretically advantageous from the perspective of evolvability. The effect size of many beneficial mutations is at least partially masked by a wild-type allele (i.e., the effect size of a beneficial allele as a homozygote is larger than as a heterozygote). If a particular SNP is beneficial in a specific environment, loss of heterozygosity (LOH) can provide a rapid route to homozygosity. LOH has been observed in both C. albicans and C. lusitaniae following microevolution in clinical and laboratory studies as an efficient mechanism by which cells become drug-resistant [9••, 10, 94] or increase their fitness in mammalian hosts [22, 23]. Similarly, in serial isolates recovered from patients with recalcitrant C. albicans infections, LOH events between early and late isolates were associated with increased drug resistance [10]. Although some specific LOH events have been linked directly to adaptive phenotypes, differences in LOH rate in different environments may also reflect different genome-wide mutation rates under different conditions [44, 45••]. For example, de novo LOH events reached higher frequencies in the host and under stressful conditions relative to growth in rich media in C. albicans [44, 45••, 46••], consistent with stress-induced mutagenesis observed in higher eukaryotes [95]. However, in the majority of patient-derived isolates, LOH tracts appear to be neutral [96••]. It remains challenging to evaluate the fitness consequences of specific tracts as LOH can span single polymorphisms to whole chromosomes, and studies often lack matched isogenic strains with these particular configurations.
There is some evidence for selection to maintain heterozygosity to avoid exposing recessive deleterious alleles that accumulate neutrally in the genome [97,98,99]. Heterozygosity at specific genomic regions may have direct consequences on biological processes. For example, heterozygosity at the mating-type locus (MTL) reduces the capacity for mating in C. albicans cells. [100, 101]. The majority of C. albicans clinical isolates are MTL heterozygous, and they are more virulent in mice compared to closely related MTL homozygous isolates [102, 103]. However, selection for polymorphism and selection against LOH should not be conflated. Determining whether there is selection to maintain polymorphisms per se requires finding significant correlations between genome-wide polymorphism levels and phenotypes of interest. In a set of 21 C. albicans isolates, a significant correlation was found between genome-wide heterozygosity and growth in nutrient-rich media at 30 °C, but not at 37 °C, nor growth in other examined conditions [39]. Furthermore, there was no association between heterozygosity and C. albicans virulence in a murine or insect infection model [39], nor with C. orthopsilosis virulence in an insect model [104]. While higher levels of heterozygosity have been observed in clinical isolates compared to environmental isolates of Saccharomyces cerevisiae, high levels of heterozygosity in Candida spp. do not seem to be tied to selection in the context of human infections; C. albicans strains isolated from oak trees displayed higher heterozygosity levels than clinical isolates from the same clades [31••]. While heterozygosity may be theoretically advantageous, there remains little to no empirical data supporting whether (or not) selection for broad polymorphism contributes to differences among isolates, especially in the context of clinically relevant phenotypes.
The Black Box of Within-Population Heterogeneity
Relatively little is known about the extent of genetic diversity in fungal populations within an individual host. Sequencing-based methods have identified Candida spp. as frequent members of the mycobiome of several human niches, including the gastrointestinal and vaginal tracts, oral cavity, and lungs [105]. Work from the early 1990s found that strains from the same individual isolated from different body sites are typically highly similar but not genetically identical [106]. This led to the common assumption that hosts are colonized by clonal isolates. However, this work has major limitations, including the small number of individuals and isolates examined, and sequencing methods that only reflected a small fraction of the genome.
Later work showed that healthy hosts could simultaneously harbor multiple diverse genotypes [84, 107], indicating that colonization is a dynamic process and that microevolution may shape the population structure of the colonizing isolates. C. albicans isolates were found to differ at a single or limited number of MLST loci during commensal growth in the gastrointestinal or genital tracts, with variation often associated with LOH [84, 108]. Host infection microevolution studies demonstrate that colonizing isolates differ through multiple LOH following infection [109, 110]. Examining C. albicans from 40 secondary infections identified 50 diploid STs, with LOH as the source of variation between isolates [70]. The tract size of LOH events can be used to distinguish between mitotic recombination, resulting in gene conversion, and break-induced replication, resulting in the homozygosis of partial or whole chromosomes. Both were detected within colonizing populations or following murine passage [111, 112] in experimental evolution studies that utilized different mouse models of commensalism or infection. LOH and chromosomal copy number variation frequently arose and rapidly resulted in extensive heterogeneity within cell populations impacting C. albicans fitness. For example, chromosome 7 trisomy was associated with increased fitness during colonization of the mouse gastrointestinal tract [45••], and chromosome 6 trisomy was repeatedly selected during infection of the mouse oral cavity [21••].
Recent whole-genome sequencing of C. albicans isolates from healthy individuals revealed a high degree of variation [96••]. An oral sample from a single individual contained several isolates differing from each other by multiple, short LOH tracts [96••]. Similarly, widespread genetic variation was detected in C. glabrata isolates from single individuals, with hundreds of nonsynonymous single nucleotide polymorphisms (SNPs) identified between isolate pairs and occasional aneuploidy events [40••]. Interestingly, C. glabrata SNPs were enriched in genes encoding cell wall proteins [40••], and these gene families are also a hotspot for C. albicans genetic variation during in vitro evolution [45••]. Temporal heterogeneity in Candida lusitaniae (Clavispora lusitaniae) isolates from bronchoalveolar lavage samples from a patient with cystic fibrosis has also been documented [9••]. Here, isolates derived from a common ancestor differed by hundreds of SNPs and indels, and a hotspot for mutations was observed in MRR1, a transcription factor regulating the expression of antifungal drug transporters. Mutations in MRR1 were shown to protect fungal cells from particular host and bacterial factors and indirectly selected for subpopulations resistant to antifungal drugs [9••].
The development of azole resistance in serial clinical isolates provides an excellent example of how microevolution can follow diverse routes to the acquisition of drug resistance. A comparison of C. albicans isolates before and after the development of azole resistance found that isolates differed by thousands of SNPs, yet azole resistance was primarily attributed to persistent and recurrent LOH [10]. Aneuploidy, particularly of chromosome 5, was also frequently, yet transiently, observed [10]. Since drug resistance in these clinical isolates often coincides with fitness costs in the absence of antifungals [10], the hypothesis is that aneuploidy extends the window for other beneficial mutations with smaller fitness costs to arise, at which point selection to maintain the aneuploidy would be lost.
Conclusion
In commensal or pathogenic contexts, Candida spp. have many mutational pathways available and exhibit extensive genetic variation, which can promote survival under fluctuating conditions of nutrient availability, drug exposure, microbiome competition, and immune surveillance. Outstanding questions remain regarding the nature of the relationships established between coexisting isolates and how genetic heterogeneity impacts commensalism and pathogenicity. Future studies are necessary to determine to what extent this genetic variation reflects selection or genetic drift in the respective environments.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53.
Barns SM, Lane DJ, Sogin ML, Bibeau C, Weisburg WG. Evolutionary relationships among pathogenic Candida species and relatives. J Bacteriol. 1991;173:2250–5.
Borman AMJEM. Candida, Cryptococcus, and other yeasts of medical importance. In: Carroll KCPMA, editor. Manual of clinical microbiology. 12th ed. Washington, DC: ASM Press; 2019.
Borman AM, Johnson EM. Name changes for fungi of medical importance, 2018 to 2019. J Clin Microbiol [Internet]. 2021;59. Available from:. https://doi.org/10.1128/JCM.01811-20.
Muñoz JF, Gade L, Chow NA, Loparev VN, Juieng P, Berkow EL, et al. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun. 2018;9:5346.
Miceli MH, Díaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011;11:142–51.
Medina N, Soto-Debrán JC, Seidel D, Akyar I, Badali H, Barac A, et al. MixInYeast: a multicenter study on mixed yeast infections. J Fungi (Basel) [Internet]. 2020;7:13. Available from:. https://doi.org/10.3390/jof7010013.
Morio F. Dear medical mycologists, it is time to look outside the box. FEMS Yeast Res. 2020;20:foz080. Available from:. https://doi.org/10.1093/femsyr/foz080.
•• Demers EG, Biermann AR, Masonjones S, Crocker AW, Ashare A, Stajich JE, et al (2018) Evolution of drug resistance in an antifungal-naive chronic Candida lusitaniae infection. Proc Natl Acad Sci U S A. National Academy of Sciences, 201807698. This study reports the existence of drug-resistant subpopulations of Candida lusitaniae during human infection.
Ford CB, Funt JM, Abbey D, Issi L, Guiducci C, Martinez DA, et al. The evolution of drug resistance in clinical isolates of Candida albicans. eLife. 2015;4:e00662. Available from:. https://doi.org/10.7554/elife.00662.
•• Todd RT, Selmecki A (2020) Expandable and reversible copy number amplification drives rapid adaptation to antifungal drugs. eLife 9:e58349. Available from: 10.7554/eLife.58349. This study establishes rapid acquisition of copy number variants during azole exposure of C. albicans.
Hirakawa MP, Chyou DE, Huang D, Slan AR, Bennett RJ. Parasex generates phenotypic diversity de novo and impacts drug resistance and virulence in Candida albicans. Genetics. 2017;207:1195–211.
Kämmer P, McNamara S, Wolf T, Conrad T, Allert S, Gerwien F, et al. Survival strategies of pathogenic Candida species in human blood show independent and specific adaptations. MBio. 2020;11:e02435–20. Available from:. https://doi.org/10.1128/mBio.02435-20.
Stokes C, Moran GP, Spiering MJ, Cole GT, Coleman DC, Sullivan DJ. Lower filamentation rates of Candida dubliniensis contribute to its lower virulence in comparison with Candida albicans. Fungal Genet Biol. 2007;44:920–31.
Flanagan PR, Fletcher J, Boyle H, Sulea R, Moran GP, Sullivan DJ. Expansion of the TLO gene family enhances the virulence of Candida species. PLoS One. 2018;13:e0200852.
Jackson AP, Gamble JA, Yeomans T, Moran GP, Saunders D, Harris D, et al. Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans. Genome Res. 2009;19:2231–44.
Kadosh D, Mundodi V. A Re-evaluation of the relationship between morphology and pathogenicity in Candida species. J Fungi (Basel). 2020;6:13. Available from:. https://doi.org/10.3390/jof6010013.
O’Connor L, Caplice N, Coleman DC, Sullivan DJ, Moran GP. Differential filamentation of Candida albicans and Candida dubliniensis is governed by nutrient regulation of UME6 expression. Eukaryot Cell. 2010;9:1383–97.
Banerjee M, Lazzell AL, Romo JA, Lopez-Ribot JL, Kadosh D. Filamentation is associated with reduced pathogenicity of multiple non-albicans Candida species. mSphere. 2019;4:e00656–19. Available from:. https://doi.org/10.1128/mSphere.00656-19.
Witchley JN, Penumetcha P, Abon NV, Woolford CA, Mitchell AP, Noble SM. Candida albicans morphogenesis programs control the balance between gut commensalism and invasive infection. Cell Host Microbe. 2019;25:432–43.e6.
•• Forche A, Solis NV, Swidergall M, Thomas R, Guyer A, Beach A, et al. Selection of Candida albicans trisomy during oropharyngeal infection results in a commensal-like phenotype. PLoS Genet. 2019;15:e1008137 This study links specific changes in chromosomal copy numbers to increased pathogen fitness during oropharyngeal candidiasis.
Liang S-H, Anderson MZ, Hirakawa MP, Wang JM, Frazer C, Alaalm LM, et al. Hemizygosity enables a mutational transition governing fungal virulence and commensalism. Cell Host Microbe. 2019;25:418–31.e6.
Tso GHW, Reales-Calderon JA, Tan ASM, Sem X, Le GTT, Tan TG, et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science. 2018;362:589–95.
Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001;33:1959–67.
Sam QH, Chang MW, Chai LYA. The fungal mycobiome and its interaction with gut bacteria in the host. Int J Mol Sci [Internet]. 2017;18:230. Available from:. https://doi.org/10.3390/ijms18020330.
d’Enfert C, Kaune A-K, Alaban L-R, Chakraborty S, Cole N, Delavy M, et al. The impact of the fungus-host-microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol Rev. 2020;45:e00656–19. Available from:. https://doi.org/10.1093/femsre/fuaa060.
Marples MJ. Some observations on the ecology of Candida albicans, a potential mammalian pathogen. Proc New Zealand Ecological Society. 1966:29–34.
Barnett JA. A history of research on yeasts 12: medical yeasts part 1, Candida albicans. Yeast. 2008;25:385–417. Available from:. https://doi.org/10.1002/yea.1595.
Di Menna ME. A search for pathogenic species of yeasts in New Zealand soils. J Gen Microbiol. 1955;12:54–62.
Menna MEDI, Di Menna ME. Candida albicans from grass leaves. Nature. 1958;181:1287–8. Available from. https://doi.org/10.1038/1811287b0.
•• Bensasson D, Dicks J, Ludwig JM, Bond CJ, Elliston A, Roberts IN, et al. Diverse lineages of Candida albicans live on old Oaks. Genetics. 2019;211:277–88 This study is the first to perform an in-depth genomic analysis of environmental C. albicans isolates and to compare these to clinical isolates.
•• Opulente DA, Langdon QK, Buh KV, Haase MAB, Sylvester K, Moriarty RV, et al. Pathogenic budding yeasts isolated outside of clinical settings. FEMS Yeast Res 2019;19:foz032. Available from: https://doi.org/10.1093/femsyr/foz032. This study provides an analysis of pathogenic yeast species using non-clinical isolates and raises the possibility that particular environments could represent a point of contact for human infections.
Arora P, Singh P, Wang Y, Yadav A, Pawar K, Singh A, et al. Environmental isolation of Candida auris from the coastal wetlands of Andaman Islands, India. MBio. 2021;12:e03181–20. Available from:. https://doi.org/10.1128/mBio.03181-20.
Taylor JW, Hann-Soden C, Branco S, Sylvain I, Ellison CE. Clonal reproduction in fungi. Proc Natl Acad Sci U S A. 2015;112:8901–8.
Ene IV, Bennett RJ. The cryptic sexual strategies of human fungal pathogens. Nat Rev Microbiol. 2014;12:239–51.
•• Ropars J, Maufrais C, Diogo D, Marcet-Houben M, Perin A, Sertour N, et al. Gene flow contributes to diversification of the major fungal pathogen Candida albicans. Nat Commun. 2018;9:2253 This study is the first to perform a comparative genomic analysis on a large number of Candida albicans isolates.
Wang JM, Bennett RJ, Anderson MZ. The genome of the human pathogen Candida albicans is shaped by mutation and cryptic sexual recombination. MBio. 2018;9:e01205–18.
Usher J. The mechanisms of mating in pathogenic fungi—a plastic trait. Genes. 2019;10:831.
Hirakawa MP, Martinez DA, Sakthikumar S, Anderson MZ, Berlin A, Gujja S, et al. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 2015;25:413–25.
•• Carreté L, Ksiezopolska E, Gómez-Molero E, Angoulvant A, Bader O, Fairhead C, et al. Genome comparisons of Candida glabrata serial clinical isolates reveal patterns of genetic variation in infecting clonal populations. Front Microbiol. 2019;10:112 This study uses whole-genome sequencing of Candida glabarata to catalogue genetic diversity within closely related isolates from the same patient.
Bennett RJ, Forche A, Berman J. Rapid mechanisms for generating genome diversity: whole ploidy shifts, aneuploidy, and loss of heterozygosity. Cold Spring Harb Perspect Med, Available from. 2014;4. https://doi.org/10.1101/cshperspect.a019604.
Gerstein AC, Sharp NP. The population genetics of ploidy change in unicellular fungi. FEMS Microbiol Rev. 2021; https://doi.org/10.1093/femsre/fuab006.
Turner SA, Butler G. The Candida pathogenic species complex. Cold Spring Harb Perspect Med. 2014;4:a019778.
Forche A, Abbey D, Pisithkul T, Weinzierl MA, Ringstrom T, Bruck D, et al. Stress alters rates and types of loss of heterozygosity in Candida albicans. MBio. 2011;2:e00129–11. Available from:. https://doi.org/10.1128/mBio.00129-11.
•• Ene IV, Farrer RA, Hirakawa MP, Agwamba K, Cuomo CA, Bennett RJ. Global analysis of mutations driving microevolution of a heterozygous diploid fungal pathogen. Proc Natl Acad Sci U S A. 2018;115:E8688–97 This study is the first to characterize the full spectrum of mutations that emerge during C. albicans microevolution and to measure mutation frequencies in this species.
•• Avramovska O, Hickman MA. The magnitude of Candida albicans stress-induced genome instability results from an interaction between ploidy and antifungal drugs. G3. 2019;9:4019–27 This study shows the impact of organismal ploidy on mutagenesis during exposure to antifungal drugs.
Mixão V, Saus E, Perez Hansen A, Lass-Florl C, Gabaldón T. Genome assemblies of two rare opportunistic yeast pathogens: Diutina rugosa (syn. Candida rugosa) and Trichomonascus ciferrii (syn. Candida ciferrii). G3. 2019;9:3921–7. Available from:. https://doi.org/10.1534/g3.119.400762.
Cuomo CA, Shea T, Yang B, Rao R, Forche A. Whole genome sequence of the heterozygous clinical isolate Candida krusei 81-B-5. G3. 2017;7:2883–9.
Tsai H-J, Nelliat A. A double-edged sword: aneuploidy is a prevalent strategy in fungal adaptation. Genes. 2019;10:787. Available from:. https://doi.org/10.3390/genes10100787.
Tsai H-J, Nelliat AR, Choudhury MI, Kucharavy A, Bradford WD, Cook ME, et al. Hypo-osmotic-like stress underlies general cellular defects of aneuploidy. Nature. 2019;570:117–21.
Gabaldón T. Hybridization and the origin of new yeast lineages. FEMS Yeast Res. 2020;20:foaa040. Available from:. https://doi.org/10.1093/femsyr/foaa040.
•• Mixão V, Gabaldón T. Hybridization and emergence of virulence in opportunistic human yeast pathogens. Yeast. 2018;35:5–20 This study reviews the roles hybridization could play in the evolution and emergence of pathogenic yeast species.
Mixão V, Saus E, Boekhout T, Gabaldón T. Extreme diversification driven by parallel events of massive loss of heterozygosity in the hybrid lineage of Candida albicans. Genetics. 2020;217:iyaa004 Available from: https://academic.oup.com/genetics/article-abstract/217/2/iyaa004/5995314.
McManus BA, Coleman DC. Molecular epidemiology, phylogeny and evolution of Candida albicans. Infect Genet Evol. 2014;21:166–78.
Hou X, Xiao M, Chen SC-A, Kong F, Wang H, Chu Y-Z, et al. Molecular epidemiology and antifungal susceptibility of Candida glabrata in China (August 2009 to July 2014): a multi-center study. Front Microbiol. 2017;8:880.
Abbes S, Sellami H, Sellami A, Hadrich I, Amouri I, Mahfoudh N, et al. Candida glabrata strain relatedness by new microsatellite markers. Eur J Clin Microbiol Infect Dis. 2012;31:83–91.
Tavanti A, Davidson AD, Gow NAR, Maiden MCJ, Odds FC. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J Clin Microbiol. 2005;43:284–92.
Wu Y, Zhou H-J, Che J, Li W-G, Bian F-N, Yu S-B, et al. Multilocus microsatellite markers for molecular typing of Candida tropicalis isolates. BMC Microbiol. 2014;14:245.
Muñoz M, Wintaco LM, Muñoz SA, Ramírez JD. Dissecting the heterogeneous population genetic structure of Candida albicans: limitations and constraints of the multilocus sequence typing scheme. Front Microbiol. 2019;10:1052.
Wu J-Y, Zhou D-Y, Zhang Y, Mi F, Xu J. Analyses of the global multilocus genotypes of the human pathogenic yeast Candida tropicalis. Front Microbiol. 2019;10:900.
Scordino F, Giuffrè L, Barberi G, Marino Merlo F, Orlando MG, Giosa D, et al. Multilocus sequence typing reveals a new cluster of closely related Candida tropicalis genotypes in Italian patients with neurological disorders. Front Microbiol. 2018;9:679.
Al-Obaid K, Asadzadeh M, Ahmad S, Khan Z. Population structure and molecular genetic characterization of clinical Candida tropicalis isolates from a tertiary-care hospital in Kuwait reveal infections with unique strains. PLoS One. 2017;12:e0182292.
Wu J-Y, Guo H, Wang H-M, Yi G-H, Zhou L-M, He X-W, et al. Multilocus sequence analyses reveal extensive diversity and multiple origins of fluconazole resistance in Candida tropicalis from tropical China. Sci Rep. 2017;7:42537.
Wu Y, Zhou H, Wang J, Li L, Li W, Cui Z, et al. Analysis of the clonality of Candida tropicalis strains from a general hospital in Beijing using multilocus sequence typing. PLoS One. 2012;7:e47767.
Magri MMC, Gomes-Gouvêa MS, de Freitas VLT, Motta AL, Moretti ML, Shikanai-Yasuda MA. Multilocus sequence typing of Candida tropicalis shows the presence of different clonal clusters and fluconazole susceptibility profiles in sequential isolates from candidemia patients in Sao Paulo, Brazil. J Clin Microbiol. 2013;51:268–77.
Dodgson AR, Pujol C, Denning DW, Soll DR, Fox AJ. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J Clin Microbiol. 2003;41:5709–17.
Dodgson AR, Pujol C, Pfaller MA, Denning DW, Soll DR. Evidence for recombination in Candida glabrata. Fungal Genet Biol. 2005;42:233–43.
Amanloo S, Shams-Ghahfarokhi M, Ghahri M, Razzaghi-Abyaneh M. Genotyping of clinical isolates of Candida glabrata from Iran by multilocus sequence typing and determination of population structure and drug resistance profile. Med Mycol. 2018;56:207–15.
Klotz U, Schmidt D, Willinger B, Steinmann E, Buer J, Rath P-M, et al. Echinocandin resistance and population structure of invasive Candida glabrata isolates from two university hospitals in Germany and Austria. Mycoses. 2016;59:312–8.
Wu K, Luo T, Li L, Zhang Q, Zhu J, Gao Q, et al. Multilocus sequence typing of pathogenic Candida albicans isolates collected from a teaching hospital in Shanghai, China: a molecular epidemiology study. PLoS One. 2015;10:e0125245.
Gong Y-B, Zheng J-L, Jin B, Zhuo D-X, Huang Z-Q, Qi H, et al. Particular Candida albicans strains in the digestive tract of dyspeptic patients, identified by multilocus sequence typing. PLoS One. 2012;7:e35311.
Hu L, Du X, Li T, Song Y, Zai S, Hu X, et al. Genetic and phenotypic characterization of Candida albicans strains isolated from infectious disease patients in Shanghai. J Med Microbiol. 2015;64:74–83.
Afsarian SMH, Badali H, Shokohi T, Najafipour S. Molecular diversity of Candida albicans isolated from immunocompromised patients, based on MLST method. Iran J Public Health. 2015;44:1262–9.
Chen K-W, Chen Y-C, Lin Y-H, Chou H-H, Li S-Y. The molecular epidemiology of serial Candida tropicalis isolates from ICU patients as revealed by multilocus sequence typing and pulsed-field gel electrophoresis. Infect Genet Evol. 2009;9:912–20.
Shin JH, Bougnoux M-E, d’Enfert C, Kim SH, Moon C-J, Joo MY, et al. Genetic diversity among Korean Candida albicans bloodstream isolates: assessment by multilocus sequence typing and restriction endonuclease analysis of genomic DNA by use of BssHII. J Clin Microbiol. 2011;49:2572–7.
Scordino F, Giuffrè L, Felice MR, Orlando MG, Medici MA, Marino Merlo F, et al. Genetic diversity of Candida albicans isolates recovered from hospital environments and patients with severe acquired brain injuries. Infect Genet Evol. 2019;76:104068.
Alastruey-Izquierdo A, Mandelblat M, Ben Ami R, Perlin DS, Segal E. Multilocus sequence typing of Candida albicans isolates from candidemia and superficial candidiasis in Israel. Med Mycol. 2013;51:755–8.
Mushi MF, Okamo B, Majinge DC, Gross U, Bader O, Mshana SE. Diversity of the diploid sequence type of Candida albicans clinical isolates from a tertiary-care hospital in Mwanza. Tanzania New Microbes New Infect. 2020;37:100731.
Huyke J, Martin R, Walther G, Weber M, Kaerger K, Bougnoux M-E, et al. Candida albicans bloodstream isolates in a German university hospital are genetically heterogenous and susceptible to commonly used antifungals. Int J Med Microbiol. 2015;305:742–7.
Biswas C, Marcelino VR, Van Hal S, Halliday C, Martinez E, Wang Q, et al. Whole genome sequencing of Australian Candida glabrata isolates reveals genetic diversity and novel sequence types. Front Microbiol. 2018;9:2946.
O’Brien CE, Oliveira-Pacheco J, Cinnéide EÓ, Hasse MAB, Hittinger CT, Rogers TR, et al. Population genomics of the pathogenic yeast Candida tropicalis identifies hybrid isolates in environmental samples. PLoS Pathog. 17:e1009138.
L’Ollivier C, Labruère C, Jebrane A, Bougnoux M-E, d’Enfert C, Bonnin A, et al. Using a multi-locus microsatellite typing method improved phylogenetic distribution of Candida albicans isolates but failed to demonstrate association of some genotype with the commensal or clinical origin of the isolates. Infect Genet Evol. 2012;12:1949–57.
MacCallum DM, Castillo L, Nather K, Munro CA, Brown AJP, Gow NAR, et al. Property differences among the four major Candida albicans strain clades. Eukaryot Cell. 2009;8:373–87.
Bougnoux ME, Diogo D, François N, Sendid B, Veirmeire S, Colombel JF, et al. Multilocus sequence typing reveals intrafamilial transmission and microevolutions of Candida albicans isolates from the human digestive tract. J Clin Microbiol Am Soc Microbiol. 2006;44:1810–20.
Byun SA, Won EJ, Kim M-N, Lee WG, Lee K, Lee HS, et al. Multilocus sequence typing (MLST) genotypes of Candida glabrata bloodstream isolates In Korea: Association with antifungal resistance, mutations in mismatch repair gene (Msh2), and clinical outcomes. Front Microbiol. 2018;9:1523.
Neji S, Hadrich I, Ilahi A, Trabelsi H, Chelly H, Mahfoudh N, et al. Molecular genotyping of Candida parapsilosis species complex. Mycopathologia. 2018;183:765–75.
Sabino R, Sampaio P, Rosado L, Videira Z, Grenouillet F, Pais C. Analysis of clinical and environmental Candida parapsilosis isolates by microsatellite genotyping—a tool for hospital infection surveillance. Clin Microbiol Infect. 2015;21:954.e1–8.
Desnos-Ollivier M, Bórmida V, Poirier P, Nourrisson C, Pan D, Bretagne S, et al. Population structure of Candida parapsilosis: no genetic difference between French and Uruguayan isolates using microsatellite length polymorphism. Mycopathologia. 2018;183:381–90.
Yang Y-L, Lin C-C, Chang T-P, Lauderdale T-L, Chen H-T, Lee C-F, et al. Comparison of human and soil Candida tropicalis isolates with reduced susceptibility to fluconazole. PLoS One. 2012;7:e34609.
Douglass AP, Offei B, Braun-Galleani S, Coughlan AY, Martos AAR, Ortiz-Merino RA, et al. Population genomics shows no distinction between pathogenic Candida krusei and environmental Pichia kudriavzevii: one species, four names. PLoS Pathog. 2018;14:e1007138.
Chillemi V, Lo Passo C, van Diepeningen AD, Rharmitt S, Delfino D, Cascio A, et al. Multilocus microsatellite analysis of European and African Candida glabrata isolates. Eur J Clin Microbiol Infect Dis. 2016;35:885–92.
Jones T, Federspiel NA, Chibana H, Dungan J, Kalman S, Magee BB, et al. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci U S A. 2004;101:7329–34.
Butler G, Rasmussen MD, Lin MF, Santos MAS, Sakthikumar S, Munro CA, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–62.
Coste A, Turner V, Ischer F, Morschhäuser J, Forche A, Selmecki A, et al. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics. 2006;172:2139–56.
Galhardo RS, Hastings PJ, Rosenberg SM. Mutation as a stress response and the regulation of evolvability. Crit Rev Biochem Mol Biol Informa UK Ltd UK. 2007;42:399–435.
•• Sitterlé E, Maufrais C, Sertour N, Palayret M, d’Enfert C, Bougnoux M-E. Within-host genomic diversity of Candida albicans in healthy carriers. Sci Rep. 2019;9:2563 This study uses MLST and whole-genome sequencing analyses to establish the existence of diverse C. albicans lineages in single individuals.
Ciudad T, Hickman M, Bellido A, Berman J, Larriba G. Phenotypic consequences of a spontaneous loss of heterozygosity in a common laboratory strain of Candida albicans. Genetics. 2016;203:1161–76. Available from:. https://doi.org/10.1534/genetics.116.189274.
Hickman MA, Zeng G, Forche A, Hirakawa MP, Abbey D, Harrison BD, et al. The “obligate diploid” Candida albicans forms mating-competent haploids. Nature. 2013;494:55–9.
Feri A, Loll-Krippleber R, Commere P-H, Maufrais C, Sertour N, Schwartz K, et al. Analysis of repair mechanisms following an induced double-strand break uncovers recessive deleterious alleles in the Candida albicans diploid genome. MBio. 2016;7:ee01109–16. Available from:. https://doi.org/10.1128/mBio.01109-16.
Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–10.
Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289:310–3.
Wu W, Lockhart SR, Pujol C, Srikantha T, Soll DR. Heterozygosity of genes on the sex chromosome regulates Candida albicans virulence. Mol Microbiol. 2007;64:1587–604.
Lockhart SR, Wu W, Radke JB, Zhao R, Soll DR. Increased virulence and competitive advantage of a/α over a/a or α/α offspring conserves the mating system of Candida albicans. Genetics. 2005;169:1883–90.
Schröder MS, Martinez de San Vicente K, THR P, Hammel S, Higgins DG, Bagagli E, et al. Multiple origins of the pathogenic yeast Candida orthopsilosis by separate hybridizations between two parental species. PLoS Genet. 2016;12:e1006404.
Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14:405–16.
Soll DR, Galask R, Schmid J, Hanna C, Mac K, Morrow B. Genetic dissimilarity of commensal strains of Candida spp. carried in different anatomical locations of the same healthy women. J Clin Microbiol. 1991;29:1702–10.
Xu J, Boyd CM, Livingston E, Meyer W, Madden JF, Mitchell TG. Species and genotypic diversities and similarities of pathogenic yeasts colonizing women. J Clin Microbiol. 1999;37:3835–43.
Jacobsen MD, Duncan AD, Bain J, Johnson EM, Naglik JR, Shaw DJ, et al. Mixed Candida albicans strain populations in colonized and infected mucosal tissues. FEMS Yeast Res. 2008;8:1334–8.
Forche A, May G, Magee PT. Demonstration of loss of heterozygosity by single-nucleotide polymorphism microarray analysis and alterations in strain morphology in Candida albicans strains during infection. Eukaryot Cell. 2005:156–65. Available from:. https://doi.org/10.1128/ec.4.1.156-165.2005.
Sampaio P, Gusmão L, Correia A, Alves C, Rodrigues AG, Pina-Vaz C, et al. New microsatellite multiplex PCR for Candida albicans strain typing reveals microevolutionary changes. J Clin Microbiol. 2005;43:3869–76.
Diogo D, Bouchier C, d’Enfert C, Bougnoux M-E. Loss of heterozygosity in commensal isolates of the asexual diploid yeast Candida albicans. Fungal Genet Biol. 2009;46:159–68.
Forche A, Magee PT, Selmecki A, Berman J, May G. Evolution in Candida albicans populations during a single passage through a mouse host. Genetics. 2009;182:799–811.
Acknowledgements
We thank R. Bennett, N. Sharp, and A-R Adamu Bukari for their thoughtful comments and suggestions.
Code Availability
Custom R code is available at https://github.com/acgerstein/candida-mlst/.
Funding
IVE is supported by the Pasteur Institute and NIH NIAID R21AI139592. MAH is supported by the National Science Foundation (DEB-1943415). ACG’s research program is supported by an NSERC Discovery Grant. ACG and IVE are also supported by the CIFAR Azrieli Global Scholars Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Mycology
Rights and permissions
About this article
Cite this article
Ene, I.V., Hickman, M.A. & Gerstein, A.C. The Interplay Between Neutral and Adaptive Processes Shapes Genetic Variation During Candida Species Evolution. Curr Clin Micro Rpt 8, 129–138 (2021). https://doi.org/10.1007/s40588-021-00171-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-021-00171-x