Abstract
Purpose of Review
Immunotherapy has emerged as a promising cancer treatment; however, success in only select clinical indications underscores the need for novel approaches. Recently Listeria monocytogenes–based vaccines have been developed to drive tumor-specific T cell responses. Here, we discuss recent preclinical studies using L. monocytogenes vaccines, innate immune pathways that influence T cell priming, and new vaccine strategies in clinical trials.
Recent Findings
Recent studies indicate that in addition to inducing antigen-specific T cell responses, L. monocytogenes vaccines remodel the TME. In addition, several innate immune pathways influence adaptive immune responses to L. monocytogenes and modulating these pathways holds promise to enhance antitumor T cell responses.
Summary
The interplay between innate and adaptive immune responses to L. monocytogenes is poorly understood. Understanding these interactions will facilitate the design of better anti-cancer vaccines and improved use of combination therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To achieve a robust and durable antitumor response, an immunotherapy approach must achieve two goals: (1) the generation of antigen-specific T cell responses [1, 2] and (2) modulation of the immunosuppressive tumor microenvironment (TME) [3]. Checkpoint inhibitors have revolutionized the treatment landscape for many tumor types by reinvigorating a preexisting pool of T cells. Yet, only a small fraction of patients respond [4], with a large proportion eventually becoming treatment resistant [5]. Likewise, many tumors are poorly immunogenic, and methods to elicit antigen-specific T cell responses have failed for most tumor types [6,7,8], demanding novel approaches.
Bacteria, a long-forgotten treatment for cancer, have the potential to overcome the immunosuppressive TME and drive antigen-specific T cell responses and as such are poised to make a resurgence as part of a therapeutic regimen. Indeed, the ability of bacteria to stimulate antitumor immune responses was first appreciated in the 1890s when William Coley observed tumor regressions in sarcoma patients purposefully infected with Streptococci [9]. Despite remarkable responses, the use of bacteria to treat cancer fell out of practice in favor of more consistent treatments such as radiotherapy and cytotoxic agents. However, evidence accumulated over the twentieth century indicating that the immune system has an active role against cancer. In the 1950s, Paul Ehrlich proposed the cancer immunosurveillance hypothesis and the 1970s saw a revival of the use of bacteria to treat cancer when Alvaro Morales demonstrated that intravesical administration of attenuated Mycobacterium tuberculosis (the Bacillus Calmette-Guerin [BCG] vaccine) could prevent recurrence of non-muscle-invasive bladder cancer [10].
There are currently eighteen active or recruiting clinical trials utilizing bacteria to treat cancer with countless others completed in the last two decades. Of the active/recruiting trials, three will employ Salmonella, one will use Clostridium novyi, two will use Enterococcus gallinarum, one will use Bifidobacterium longum, and eleven will see Listeria monocytogenes used as a therapeutic platform [11]. L. monocytogenes has unique and advantageous properties compared with other bacteria or oncolytic viruses used to treat cancer. In this review, we will highlight aspects of L. monocytogenes biology that make it a particularly attractive immunotherapy agent as well as describe our current understanding of the mechanisms of L. monocytogenes–induced antitumor activity while highlighting recent and current clinical trials.
L. monocytogenes Life Cycle and Induction of Antigen-Specific T Cell Responses
L. monocytogenes is a gram-positive bacterium best known as a food-borne pathogen and the causative agent of listeriosis [12]. L. monocytogenes infection occurs when the bacterium is ingested and disseminates to the liver, spleen, and central nervous system. Cellular access is gained through phagocytosis or by receptor-mediated endocytosis. Two L. monocytogenes proteins, internalin A and internalin B, facilitate receptor-mediated endocytosis through interactions with host E-cadherin or C-met, respectively [13, 14]. Once internalized, L. monocytogenes is encapsulated in a phagosome and secretes phospholipases and a pore-forming toxin, listeriolysin O (LLO), to escape the maturing phagolysosome [15, 16]. In the host cytosol, L. monocytogenes replicates and expresses ActA, a secreted protein that polymerizes host actin to propel itself into neighboring cells facilitating an almost exclusively intracellular lifecycle [17].
Internalized L. monocytogenes undergo one of two fates inside the host cell. Inside antigen-presenting cells (APCs), some bacteria are degraded in the phagolysosome [18], facilitating antigen processing and presentation on major histocompatibility class II (MHC-II) complexes and induction of CD4+ T cell responses [19], while some bacteria escape the phagosome and enter the cytosol. Here, secreted antigens are degraded by the proteasome and loaded onto major histocompatibility class I (MHC-I) complexes facilitating CD8+ T cell responses [20,21,22]. Access to the host cytosol is required for a strong CD8+ T cell response and L. monocytogenes lacking LLO do not induce protective CD8+ T cell responses [22]. In fact, CD8+ T cell responses are suppressed by infection with L. monocytogenes that fail to escape host phagosomes [23]. However, LLO-deficient L. monocytogenes elicit CD4+ T cell responses indicating that cytosolic access is not necessary for MHC-II antigen presentation [22]. Similarly, antigen secretion is required to generate robust antigen-specific CD8+ T cell populations. L. monocytogenes expressing non-secreted antigens induce poor memory T cell responses compared with L. monocytogenes expressing secreted versions of the same antigens [24].
Thus, in comparison with other intracellular bacteria which have been proposed as therapeutic platforms (like Salmonella which tend to reside within host phagosomes [25]), L. monocytogenes is uniquely adept at generating strong antigen-specific T cell responses due to its ability to escape the phagosome and thrive in the host cytosol. Survival in the APC cytosol supports CD8+ T cell priming and the generation of a pool of memory T cells capable of providing protective immunity upon secondary challenge. Discussed in more detail below, aspects of L. monocytogenes biology such as cell-to-cell spread and host cell tropism can be manipulated to engineer safe and effective cancer vaccines.
Therapeutic L. monocytogenes Platforms
L. monocytogenes vaccines are primarily administered intravenously (IV) and are phagocytosed by host APCs [26]. To utilize a pathogen as a cancer vaccine, two essential steps must be taken: (1) the pathogen’s ability to cause disease must be attenuated while retaining immunogenicity and (2) the bacterium must secrete a target antigen(s) to drive antitumor CD8+ T cell responses. Two main companies, Advaxis and Aduro Biotech, as well as a variety of academic labs, have pioneered this technology. A comprehensive review of possible attenuation strategies can be found elsewhere [27]; therefore, we will focus on strategies that are currently in clinical trials. Importantly, as discussed below, the Aduro Biotech and Advaxis platforms differ in both mechanisms of attenuation and mechanisms of tumor-associated antigen (TAA) expression; however, these platforms have not been directly compared.
Aduro Biotech’s therapeutic platform, termed Live Attenuated Double Deleted (LADD), utilizes a strain of L. monocytogenes lacking actA and inlB. Loss of ActA prevents cell-to-cell spread and dissemination of bacteria, while deletion of InlB curbs liver toxicity by preventing receptor-mediated endocytosis into hepatocytes [28]. ActA-deficient L. monocytogenes are 1000-fold attenuated compared with wild-type bacteria [29], and importantly, the LADD strain induces similar antigen-specific T cell responses to wild-type L. monocytogenes [28]. Advaxis takes a different approach to attenuate L. monocytogenes. The Advaxis vaccine platform, termed LmddA, is deleted at actA, and the dal and dat loci which are required for the synthesis of d-alanine, effectively making the bacteria auxotrophic and replication deficient in vivo [30]. By comparison, the LADD strain is replication competent, which may enhance the absolute abundance of antigens produced within an infected cell.
In addition to differences in mechanisms of attenuation, the Aduro Biotech and Advaxis platforms utilize different approaches for tumor antigen expression. Aduro Biotech’s vaccine platform utilizes expression of TAA’s fused to the actA N-terminal 100 amino acids under the control of the ActA promoter, stably integrated into the L. monocytogenes genome [31]. Advaxis on the other hand episomally expresses TAAs fused to LLO under the control of the hly promoter [30]. Creating in-frame fusions with endogenous L. monocytogenes proteins enhances vaccine immunogenicity [32], although the mechanisms underlying the enhanced immunogenicity of the fusion-based vaccines are not completely understood. Fusion of TAAs to ActA enhances vaccine immunogenicity and antitumor activity [33], as does fusion of TAAs to LLO [32]. However, the enhanced immunogenicity of ActA-based fusions may not rely entirely on enhanced bacterial secretion. Injected protein-based vaccines consisting of TAAs either fused to or administered in conjunction with ActA are more effective than TAAs alone, suggesting that in addition to enhancing antigen presentation, L. monocytogenes ActA may also act as an adjuvant to enhance immune responses [33]. Similar adjuvant-like effects have been observed for LLO [34].
The efficacy of ActA- and LLO-TAA fusion vaccines at generating CD8+ T cell responses has been directly compared. In mice with human papillomavirus (HPV)-16 E7-expressing tumors, vaccination with L. monocytogenes expressing LLO fused with E7 resulted in a greater number of E7-specific CD8+ T cells compared with mice immunized an ActA-E7 fusion vaccine. Interestingly, both vaccine strategies conferred similar tumor control [35]. In an HPV-16-driven model of autochthonous thyroid cancer, the frequency of E7-specific CD8+ T cells was no different in the spleens of immunized mice, while in tumors, the LLO vaccine produced a threefold increase in E7-specific T cells [36]. Tumor control was similar for both vaccines. Given that both ActA and LLO have ostensible adjuvant-like properties, why differences in T cell number did not correlate with differences in tumor control is unclear. Perhaps LLO and ActA differ in their adjuvant-like properties resulting in different changes in the tumor microenvironment. Alternatively, TAA expression or secretion levels may have differed between the two vaccines, a consideration that was not directly addressed.
While fusion of TAAs to secreted L. monocytogenes virulence factors increases immunogenicity, it remains unclear if there is an optimal antigen fusion partner. Although LLO-TAA vaccines appear to induce greater numbers of antigen-specific T cells than ActA-TAA vaccines in some experimental settings, it is difficult to argue in favor of either as both seem to control tumors to the same extent. Future studies are needed to understand why LLO fusions induce more antigen-specific T cells, and why this does not translate to enhanced tumor control over ActA-based vaccines. Although LLO- and ActA-TAA fusions are the most extensively studied in the context of cancer immunotherapy, L. monocytogenes secretes a plethora of proteins inside host cells [37], any of which may be even better fusion partners for TAAs in L. monocytogenes vaccines. Finally, it is unclear if the replication-competent LADD strain or replication-deficient LmddA strain is superior at generating antitumor immune responses. A direct comparison would prove valuable to inform future vaccine design strategies.
L. monocytogenes Effects on Immune Cells in the Tumor Microenvironment
L. monocytogenes–based vaccines generate robust antigen-specific CD8+ T cell responses; however, tumor protection likely involves additional mechanisms. Mounting evidence suggests L. monocytogenes immunization dramatically alters the tumor microenvironment (TME). This may include alterations in the frequency and/or function of both pro- or antitumor immune cells. Suppressive cell types found in the TME include regulatory T cells (T-regs) on the adaptive side [38], and myeloid-derived suppressor cells (MDSCs) on the innate side [39], which both dampen immune responses through various mechanisms.
Deng et al. demonstrated that L. monocytogenes vaccines control tumor growth in two different tumor models in different mouse backgrounds. Importantly, this study attributed the effectiveness of L. monocytogenes vaccination to modulation of the TME, most notably decreases in tumor-infiltrating FOXP3+ T-reg frequency, increases in pro-inflammatory cytokine production, and a shift in tumor-associated macrophage (TAM) phenotype from M2 to M1. Similarly, PD-1 expression was decreased on tumor-infiltrating CD8+ T cells relative to unvaccinated animals. Notably, some of these results (decreased PD-1 expression and FOXP3+ T-reg frequency) were also observed in mice vaccinated with empty L. monocytogenes suggesting that the bacterium alone affects the TME immune milieu in addition to contributing to T cell priming [40••]. Additional groups have also noted decreases in T-reg frequency [41, 42] and PD-1 expression associated with L. monocytogenes vaccination [43]. Consistent with observations from Deng et al., in a 4T1 breast cancer model, LADD treatment repolarized TAMs from M2 to M1. Interestingly however, in this model, macrophage polarization was dependent on LADD accumulation in the TME suggesting that in addition to priming T cells in the periphery, L. monocytogenes vaccines can act locally at the tumor site if access is granted [44]. Others have demonstrated that L. monocytogenes vaccines are particularly effective due to alterations in the frequency of MDSCs. L. monocytogenes vaccination reduces the number of MDSC-like cells both in the peripheral blood and in the TME of tumor-bearing animals. Notably, in the remaining MDSC-like cells, expression of the pro-inflammatory cytokine IL-12 was increased, reflecting an overall phenotypic shift from immunosuppressive to pro-inflammatory [45, 46].
Taken together, the data indicate that L. monocytogenes vaccination is particularly effective due in part to its effects in the TME in addition to contributing to T cell priming. While the importance of L. monocytogenes’ ability to generate antigen-specific T cells cannot be understated, L. monocytogenes vaccination also induces a profound reduction in the frequency of immunosuppressive cell types including FOXP3+ T-regs and MDSCs. Additionally, tumor-infiltrating immune cells display a markedly more immunogenic phenotype in response to L. monocytogenes vaccination. CD8+ T cells have reduced PD-1 expression, while TAMs display increased iNOS and reduced expression of M2 markers. Given the effects of L. monocytogenes vaccination, tumors heavily infiltrated with immunosuppressive cell types or where PD-1 blocking antibody has failed despite high PD-1 expression would make rational targets. Additionally, L. monocytogenes vaccination might be particularly well suited to tumors with high CD8+ T cell infiltration but low effector function due to its ability to induce a pro-inflammatory state.
Combination Approaches for Enhanced L. monocytogenes Vaccines
Although in many cases L. monocytogenes is administered in combination with standard of care chemotherapies, significant interest exists in exploring L. monocytogenes in combination with other immunotherapies and radiation therapy. Combining immune checkpoint inhibitors with therapeutic L. monocytogenes vaccination is a logical next step in enhancing vaccine efficacy. TAA-expressing L. monocytogenes vaccination in combination with α-PD-1 antibody was shown to induce complete tumor regression in 20% of mice bearing HPV-positive TC-1 tumors, whereas no mice were cured by either monotherapy [42], while a similar strategy eradicated all tumors in a model of breast cancer [40••]. Another recent study explored combining TAA-expressing L. monocytogenes vaccination with α-PD-1 antibody in a cancer with low mutational burden, pancreatic ductal adenocarcinoma (PDAC) [47]. The vaccine targeted Annexin-A2, which is frequently overexpressed in metastatic lesions of pancreatic ductal adenocarcinoma but is rarely mutated. Astonishingly, despite targeting a self-antigen, the vaccine resulted in tumor control which was enhanced by α-PD-1 antibody and induction of Annexin-A2-specific T cells [48]. Others have similarly demonstrated the capacity of L. monocytogenes–based vaccines to break central tolerance against several proteins [35, 49, 50]. Critical questions include how L. monocytogenes breaks central tolerance, and which self-antigens are suitable targets for L. monocytogenes–based vaccines. While a discussion of epitope discovery is beyond the scope of this review, it is important to mention that many preclinical studies are using epitopes specific to mice which may not apply to human disease. Future studies will need to employ humanized mouse models to test immunogenic epitopes in relevant human cancer antigens. Indeed, a recent study utilizing human prostate cancer antigens in HLA-A2- and HLA-DRI-expressing mice demonstrated the increased efficacy of combining DNA tumor vaccines with L. monocytogenes tumor vaccines in specific prime-boost regimens [51].
Finally, studies on the use of combination therapy with L. monocytogenes are not limited to checkpoint inhibitors and DNA vaccines as two recent studies investigated the combined effect of TAA-expressing LADD and radiation therapy. Treatment with TAA-expressing LADD alone resulted in tumor control, and this was enhanced by combination with radiation treatment. This correlated with a massive influx of TAA-specific T cells with enhanced effector activity [52, 53]. Ultimately, to rationally combine L. monocytogenes–based immunotherapies with other treatment modalities, we need a better mechanistic understanding of why some tumors respond better to combined L. monocytogenes vaccination and immune checkpoint blockade as well as how the kinetics of dosing influence efficacy. Likewise, although there have been no preclinical studies combining α-CTLA-4 antibody with L. monocytogenes vaccination, L. monocytogenes vaccination was shown to induce CTLA-4 expression on CD4+ T cells in the spleens and livers of mice bearing metastatic colon cancer. While slowed tumor growth was observed in vaccinated mice, no cures were achieved [43] suggesting that CTLA-4 blockade might be a rational choice in this model. Other immune checkpoints such as VISTA, Lag-3, Tim-3, and TIGIT (as well as others) have largely been unexplored as monotherapies or in combination with therapeutic anti-cancer vaccines [54]. Therefore, future studies are needed to address if these checkpoints are relevant during therapeutic L. monocytogenes vaccine schemes and if so, which tumor types are most likely to respond to which combination therapies.
Clinical Trials
Current Clinical Trials
A comprehensive review of the state of L. monocytogenes vaccine trials was recently published [27] and as such we will focus our discussion here on new avenues of clinical application, namely combination therapy and precision medicine approaches. Aduro Biotech’s CRS-207 (LADD platform expressing the TAA mesothelin) is currently in clinical trials for patients with pancreatic cancer and mesothelioma in various combinations with pembrolizumab, ipilimumab, nivolumab, chemotherapeutics, and IDO inhibitors (NCT02243371, NCT03190265, NCT03006302, and NCT01675765). Aduro Biotech’s other platform currently in clinical trials, pLADD, is based on an all-together different approach to engineering anti-cancer vaccines and is one mirrored in the ADXS-NEO platform described below. In the era of personized medicine, treatment options are increasingly focusing on driving T cell responses toward patient-specific mutations termed neoantigens. Indeed, neoantigens generate an enhanced repertoire of strongly immunogenic epitopes which are often presented in the context of MHC-I and recognized as foreign antigens [55]. By sequencing colorectal tumors and formulating LADD-based vaccines using predicted neoantigen epitopes, the pLADD approach is taking personalized medicine to the next level (NCT03189030). While the active trials will be completed, Aduro Biotech announced that it will not be initiating any further clinical trials based on its LADD platform leaving open the question of the future of pLADD and the LADD platform more generally.
Advaxis has also developed a patient-specific vaccine termed ADXS-NEO that similarly uses patient-specific neoantigens expressed by L. monocytogenes to personalize tumor immunotherapy (NCT03265080). Here patients with multiple tumor types are included and combination with pembrolizumab is being explored. In addition to ADXS-NEO, Advaxis has three other L. monocytogenes vaccines in active clinical trials. ADXS11-001 expressing HPV E7 is in active trials for patients with HPV-positive oropharyngeal cancer, cervical cancer, and anorectal cancer as a monotherapy (NCT02002182, NCT01266460, NCT02853604, and NCT02399813). ADXS31-142 targets the prostate cancer antigen PSA and is being tested in combination with pembrolizumab in prostate cancer (NCT02325557). Finally, ADXS-503 is a vaccine formulated to express epitopes from ten frequently mutated genes in a variety of tumor types and is being used in combination with pembrolizumab (NCT03847519). This is intended to be an off the shelf treatment for multiple tumor types.
Safety of L. monocytogenes–Based Clinical Trials
Overall, the published clinical data suggest L. monocytogenes anti-cancer vaccines are well tolerated. Adverse clinical events associated with vaccination, most often pyrexia and chills, are frequently reported but are well managed [56,57,58,59]. However, one patient that received an Advaxis L. monocytogenes vaccine in 2013 died in 2015, with trace amounts of the bacteria detected in her blood. The FDA put a hold on L. monocytogenes vaccine trials, which was lifted shortly after. Then again, briefly, in 2016, the FDA put a halt on an Aduro Biotech trial due to the detection of disseminated bacteria in the blood of a cervical cancer patient treated with CRS-207 [60]. The trials were reinstated after Aduro Biotech and Advaxis reevaluated their patient monitoring and management practices and will exclude patients that receive immunosuppressive drugs or have certain prosthetics including indwelling ports.
New platforms are being developed for use in immunocompromised patients. Recently, a strain of L. monocytogenes that dies upon entry into host cells was shown to be cleared rapidly in immunocompromised mice [61]. Importantly, this strain retains comparable immunogenicity to LADD-based vaccines. Therefore, therapeutic L. monocytogenes vaccines are incredibly safe and well tolerated, especially in comparison with chemotherapy and other cytotoxic agents.
Engineering More Efficacious Anti-cancer Vaccines: Modulating Innate Immune Pathways Activated by L. monocytogenes
L. monocytogenes infection activates many innate immune signaling pathways [62]. Access to the cytosol is essential for L. monocytogenes activation of protective CD8+ T cell responses [22] leading to the hypothesis that innate immune pathways triggered specifically by cytosolic bacteria are essential for driving robust CD8+ T cell priming. Consistent with this hypothesis, Toll-like receptor (TLR) signaling, which is triggered at the cell surface or in endosomes, is robustly triggered by L. monocytogenes infection but is dispensable for T cell priming [63,64,65,66, 67•]. Surprisingly however, many cytosolic innate immune signaling pathways are not only dispensable for optimal T cell priming; in some cases, they appear to be actively detrimental.
STING/cGAS
One of the earliest known innate responses specific to cytosolic L. monocytogenes was activation of type I interferons [68]. Considerable effort was focused on elucidating the bacterial PAMP(s) and host signaling pathway(s) leading to IFNβ production with the expectation that it would be a key signal for T cell priming due to the canonical role of IFNβ in T cell expansion and activation [69]. These efforts ultimately demonstrated that L. monocytogenes activates the Stimulator of interferon genes (STING) pathway directly through the secretion of cyclic-di-AMP [70, 71] as well as through cytosolic DNA recognition by the cyclic GMP-AMP synthase (cGAS) [72]. cGAS binds cytosolic DNA and catalyzes the formation of cyclic-di-nucleotides (CDNs) which are recognized by STING, initiating a signaling cascade through interferon regulatory factor 3 (IRF3) and resulting in the production of the type I interferon (IFN) IFNβ. Surprisingly however, STING-deficient mice develop enhanced T cell responses and better protective immunity relative to wild-type mice [67•]. This strongly suggests that during L. monocytogenes immunization, limiting systemic STING-induced inflammation produces an ideal environment for T cell priming. Deficits in cellular immunity are mediated by type I interferons, as IFNαβR-deficient mice generate comparable T cell responses to STING-deficient mice [67•].
In contrast, evidence from human tumors suggests that STING activation at the tumor site can be beneficial to the generation of T cell responses. Analysis of gene signatures from human cancer metastases demonstrated that CD8+ T cell infiltration correlates with IFNβ expression [73] and STING expression in tumors can predict prognosis [74]. Work from multiple groups utilizing various tumor models has demonstrated that STING activation at the tumor site has potent antitumor effects [45, 75, 76]. Why systemic STING activation during L. monocytogenes infection is detrimental to the generation of adaptive immunity, but beneficial in the TME in some models, is just beginning to be unraveled.
In a 4T1 mouse breast cancer model, TAA-expressing L. monocytogenes vaccination and intratumoral CDN injection result in enhanced tumor control compared with the vaccine or CDNs alone [45]. However, antigen-specific CD8+ T cells were decreased by CDN treatment compared with the L. monocytogenes vaccine alone. Rather, tumor control in CDN-treated mice was attributed to Caspase-3-dependent apoptosis in the tumor cells. Finally, reducing the amount of CDNs 10,000-fold led to an expansion of antigen-specific T cells in mice that received the combination treatment compared with vaccine alone [45] hinting that modest STING activation may be beneficial to generating T cell responses. Excessive IFNβ may inhibit the induction of robust cellular immunity through numerous mechanisms [77, 78]. Consistent with this observation, Sivick et al. found that high doses of STING agonist delivered to directly to tumor beds kill tumor cells through Caspase-3-dependent apoptosis, but limits systemic T cell immunity, whereas low doses at the tumor site activate innate immune cells to prime CD8+ T cell responses resulting in greater systemic immunity [79•]. Therefore, it appears that the magnitude of STING signaling is critical for shaping the adaptive immune response.
Collectively, although systemic activation of the STING/cGAS pathway appears to inhibit cellular immunity during L. monocytogenes infection, the extent of STING activation in acute infection models may exceed a threshold for enhancing CD8+ T cell responses. Experiments utilizing L. monocytogenes strains that limit secretion of CDNs or are less susceptible to cytosolic bacteriolysis (limiting L. monocytogenes genomic DNA in the cytosol) may lead to the generation of vaccine platforms that induce increased T cell responses. Furthermore, the combined approach utilizing low-dose intratumoral CDN injection to optimize T cell priming at the tumor bed and IV-delivered L. monocytogenes to optimize priming in the periphery should synergize to yield a profoundly more effective immunotherapeutic approach.
Inflammasomes
In addition to STING/cGAS activation, activation of the inflammasome is an innate response specific to L. monocytogenes in the cytosol [80,81,82,83,84,85]. Canonical inflammasomes are cytosolic multi-protein complexes comprised of a receptor, the adaptor apoptosis-associated Spec-like protein containing a Caspase recruitment domain (ASC), and Caspase-1 [86]. Inflammasome activation leads to the secretion of the inflammatory cytokines IL-1β and IL-18 [87], secretion of inflammatory lipid signaling molecules known as eicosanoids [88], and an inflammatory type of cell death known as pyroptosis [89]. The AIM2 inflammasome is the predominant receptor activated during L. monocytogenes infection due to bacteriolysis in the host cytosol [84].
Surprisingly, similar to STING activation, inflammasome activation limits the generation of adaptive immunity during L. monocytogenes infection [90, 91••, 92]. Although the obvious hypothesis is that inflammasome-mediated APC death via pyroptosis could impair adaptive immunity by destroying the cells responsible for priming CD8+ T cells, Theisen et al. demonstrated instead that the inflammation associated with inflammasome activation is responsible for inhibiting optimal T cell priming [91••]. Although the mechanisms underlying inflammasome-mediated inhibition of T cell priming remain unknown (inhibition is independent of IL-1R/IL18R (data not shown)), limiting activation of this pathway may produce a more efficacious vaccine due to its effects on both immune cells and tumor cells.
Multiple studies have documented a role for inflammasome activation in cancer development and progression in both humans and mice [93,94,95,96]. Patient data demonstrate a positive correlation between IL-1β and IL-18 and pro-tumor cytokine expression [97], whereas lower IL-1β and IL-18 expression confers better prognosis [97,98,99]. Inflammasome activation also inhibits NK cell responses and enhances tumor growth in a model of melanoma. Depleting inflammasome activity specifically in MDSC-like cells completely abrogated these defects, strongly suggesting that inflammasome activation in immune cells is detrimental to antitumor immunity [100]. Inflammasome activation also promotes an M2 phenotype in TAMs [101] and blocking IL-1R signaling reduces the accumulation of MDSCs in the TME [102]. As with many innate immune responses, however, magnitude and location are likely important as there is also conflicting data that suggests inflammasome activation can be protective against cancer in some contexts [103,104,105,106,107].
Although it is unclear why inflammasome activation has both pro- and antitumorigenic functions during cancer development, in the context of L. monocytogenes vaccination, it appears that limiting inflammasome activation should result in enhanced vaccine efficacy. Several inflammasome inhibitors are being tested in preclinical models showing antitumor benefits [108] and could be paired with L. monocytogenes vaccines for a combination approach. Additionally, strains of L. monocytogenes that are less susceptible to cytosolic bacteriolysis may prove to be better at priming T cells by limiting AIM2 activation.
Eicosanoids
Finally, eicosanoid production is also associated with L. monocytogenes access to the cytosol [109, 110]. Eicosanoids are produced following the liberation of arachidonic acid from the plasma membrane by the cytosolic phospholipase A2 at which point arachidonic acid can be further metabolized by cyclooxygenases or lipoxygenases [111]. Among the best studied eicosanoids, prostaglandin E2 (PGE2) is produced from arachidonic acid first through the activity of cyclooxygenase-1 or cyclooxygenase-2 (COX-1 and COX-2) to produce PGH2, and then subsequently into PGE2 by a prostaglandin E synthase [112]. Inhibition of both COX enzymes by indomethacin (an ibuprofen analog) impairs adaptive immunity to L. monocytogenes infection. However, specific knockout of COX-1 enhances adaptive immunity, while COX-2 inhibition by celecoxib impairs adaptive immunity, suggesting contrasting roles for the two enzymes [113•]. PGE2 is the key eicosanoid downstream of COX-2 necessary for optimal T cell priming as add back of PGE2 alone to celecoxib-treated mice restored both T cell priming and protective immunity. Together, these data suggest that eicosanoid signaling plays a key role in L. monocytogenes–stimulated immunity and that care should be taken in the choosing of analgesics following administration of L. monocytogenes vaccines.
In contrast to its essential role in L. monocytogenes T cell priming, COX-2 inhibition enhances the therapeutic efficacy of non–L. monocytogenes anti-cancer vaccines. In 4T1 tumor-bearing mice, expression of COX-2 by tumor cells impairs T cell ingress into tumors and inhibition by celecoxib in the context of dendritic cell vaccines improves tumor control [114]. Similarly celecoxib treatment in the context of adenovirus vaccination improves tumor control due to increased T cell influx into tumors [115]. Indeed, tumor-derived PGE2 has been shown to inhibit immune cell infiltration [116], indicating that local, tumor-derived PGE2 can impair vaccine efficacy by inhibiting immune cell infiltration in a tumor cell–autonomous manner. Given the contrasting roles of COX-2 systemically and in the TME, COX enzyme inhibitors could be withheld immediately after vaccination allowing for development of optimal T cell responses and administered later to enhance immune cell infiltration into tumors.
The function of PGE2 during vaccination is poorly understood as PGE2 has both pro- and anti-T cell functions in various vaccine contexts and has historically been associated with impaired immune function [117,118,119,120,121,122]. More specifically, there are four PGE2 receptors, EP1, EP2, EP3, and EP4 [123], each ascribed varying functions [119, 121, 124,125,126]. Which PGE2 receptors drive pro–T cell priming functions vs those that shut down inflammation and T cell function remains to be fully elucidated. How L. monocytogenes infection drives PGE2 production and whether or not strains that modulate PGE2 production may be ideal vaccine platforms need to be addressed. Furthermore, pharmacologic inhibitors and/or agonists of specific PGE2 receptors could be combined with therapeutic L. monocytogenes vaccines to enhance the development of adaptive immunity. Ultimately the role of PGE2, the COX enzymes, and eicosanoids more broadly in anti-cancer vaccines and specifically L. monocytogenes vaccines remains to be fully elucidated.
Conclusion
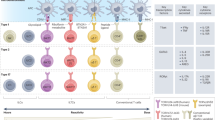
L. monocytogenes is poised to become a major player in the therapeutic armament against cancer. The infectious cycle that results in secretion of antigens directly into the cytosol while driving robust and specific innate immune responses uniquely positions L. monocytogenes as a powerful platform to generate adaptive immune responses toward ectopically expressed antigens. Nevertheless, many questions remain (Fig. 1):
-
1.
What is the optimal attenuated vaccine platform (LADD, LmddA, other?) for driving both antigen-specific T cell responses and beneficial modulation of the TME?
-
2.
What is the ideal TAA fusion partner (LLO, ActA, other?) and how do these partners augment immunogenicity?
-
3.
What combination therapies (checkpoint inhibitors, radiation therapy, and others) will be most effective when paired with a L. monocytogenes vaccine and which indications will require such therapeutic augmentations?
-
4.
How does L. monocytogenes vaccination modulate the TME and can this be optimized?
-
5.
Why are systemic STING and inflammasome activation detrimental and can these pathways be modulated with either pharmacological agents or with modified L. monocytogenes strains to enhance vaccine efficacy?
-
6.
How does L. monocytogenes activate COX-1 and what are the downstream products of COX-1 that inhibit T cell immunity?
-
7.
Which cell types make and respond to PGE2, what are the relevant PGE2 receptors and can PGE2 signaling be modulated in a rational way to enhance vaccine efficacy?
-
8.
And finally, what other innate immune pathways are activated by L. monocytogenes that can be capitalized upon to enhance vaccine efficacy?
To fully harness the potential of L. monocytogenes vaccines, these and many other questions must be answered. Nevertheless, as evidenced by the approval of a L. monocytogenes–based osteosarcoma immunotherapy [127] for use in dogs last year which more than doubled median survival, future of L. monocytogenes cancer vaccines is now.
(A) Schematic representation of L. monocytogenes anti-cancer vaccine administration, and (B) the effects on innate immune cells (left) and adaptive immune cells (right) in the TME, and (C) innate immune pathways triggered by L. monocytogenes that are detrimental (left) and beneficial to adaptive immunity (right). Numbered question marks reference the future directions discussed in the “Conclusion” section
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51.
Spitzer MH, Carmi Y, Reticker-Flynn NE, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168:487–502.
Davis RJ, Van Waes C, Allen CT. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016;58:59–70.
Carretero-González A, Lora D, Ghanem I, Zugazagoitia J, Castellano D, Sepúlveda JM, et al. Analysis of response rate with anti-PD1/PDL1 antibodies in advanced solid tumors: a meta-analysis of randomized clinical trials (RCT). J Clin Oncol. 2018;35:8706–15.
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23.
Fousek K, Ahmed N. The evolution of T-cell therapies for solid malignancies. Clin Cancer Res. 2015;21:3384–92.
Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2) - specific chimeric antigen receptor - modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33:1688–96.
Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H, et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci. 2016;59:468–79.
Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;10:487–511.
Herr HW, Morales A. History of Bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol. 2008;179:53–6.
National Institute of Health. Home - ClinicalTrials.gov. US Natl Libr Med. 2018. https://doi.org/10.1242/jeb.083188.
Ferreira V, Wiedmann M, Teixeira P, Stasiewicz MJ. Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J Food Prot. 2014;77:150–70.
Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–32.
Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000;103:501–10.
Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–7.
Hamon MA, Ribet D, Stavru F, Cossart P. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol. 2012;20:360–8.
Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P, et al. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–31.
de Chastellier C, Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994;62:543–53.
Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect. 2007;9:1208–15.
Brunt LM, Portnoy DA, Unanue ER. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–6.
Ikonomidis G, Paterson Y, Kos FJ, Portnoy DA. Delivery of a viral antigen to the class i processing and presentation pathway by listeria monocytogenes. J Exp Med. 1994;180:2209–18.
Bahjat KS, Liu W, Lemmens EE, Schoenberger SP, Portnoy DA, Dubensky TW, et al. Cytosolic entry controls CD8+-T-cell potency during bacterial infection. Infect Immun. 2006;74:6387–97.
Bahjat KS, Meyer-Morse N, Lemmens EE, Shugart JA, Dubensky TW, Brockstedt DG, et al. Suppression of cell-mediated immunity following recognition of phagosome-confined bacteria. PLoS Pathog. 2009;5:e1000568.
Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–45.
Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–8.
Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16:32–46.
Flickinger J, Rodeck U, Snook A. Listeria monocytogenes as a vector for cancer immunotherapy: current understanding and progress. Vaccines. 2018;6:48.
Brocksted DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–7.
Starks H, Bruhn KW, Shen H, et al. Listeria monocytogenes as a vaccine vector: virulence attenuation or existing antivector immunity does not diminish therapeutic efficacy. J Immunol. 2004;173:420–7.
Wallecha A, Maciag PC, Rivera S, Paterson Y, Shahabi V. Construction and characterization of an attenuated Listeria monocytogenes strain for clinical use in cancer immunotherapy. Clin Vaccine Immunol. 2009;16:96–103.
Lauer P, Chow MYN, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184:4177–86.
Gunn GR, Zubair A, Peters C, Pan Z-KZ-K WT-CT-C, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortal. J Immunol. 2001;167:6471–9.
Wood LM, Pan ZK, Shahabi V, Paterson Y. Listeria-derived ActA is an effective adjuvant for primary and metastatic tumor immunotherapy. Cancer Immunol Immunother. 2010;59:1049–58.
Jin MP, Ng VH, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200:1647–55.
Souders NC, Sewell DA, Pan ZK, Hussain SF, Rodriguez A, Wallecha A, et al. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun. 2007;7:2.
Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008;26:5315–20.
Port GC, Freitag NE. Identification of novel Listeria monocytogenes secreted virulence factors following mutational activation of the central virulence regulator, PrfA. Infect Immun. 2007;75:5886–97.
Zhao H, Liao X, Kang Y. Tregs: where we are and what comes next? Front Immunol. 2017;8:1578.
Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res. 2017;5:3–8.
•• Deng W, Lira V, Hudson TE, et al. Recombinant Listeria promotes tumor rejection by CD8 + T cell-dependent remodeling of the tumor microenvironment. Proc Natl Acad Sci U S A. 2018;115:8179–84 This study describes mechanisms of howL. monocytogenesvaccines modify the tumor microenvironment in both antigen-specific and non-specific ways. Importantly, the group shows thatL. monocytogenesvaccines are particularly effective in comparison with other therapeutic modalities in part, due to the effects on the TME.
Shrimali R, Ahmad S, Berrong Z, Okoev G, Matevosyan A, Razavi GSE, et al. Agonist anti-GITR antibody significantly enhances the therapeutic efficacy of Listeria monocytogenes-based immunotherapy. J Immunother Cancer. 2017;5:1–9. https://doi.org/10.1186/s40425-017-0266-x.
Mkrtichyan M, Chong N, Eid RA, Wallecha A, Singh R, Rothman J, et al. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer. 2013;1:15.
Olino K, Wada S, Edil BH, Pan X, Meckel K, Weber W, et al. Tumor-associated antigen expressing listeria monocytogenes induces effective primary and memory T-cell responses against hepatic colorectal cancer metastases. Ann Surg Oncol. 2012;19:597–607. https://doi.org/10.1245/s10434-011-2037-0.
Lizotte PH, Baird JR, Stevens CA, Lauer P, Green WR, Brockstedt DG, et al. Attenuated Listeria monocytogenes reprograms M2-polarized tumor-associated macrophages in ovarian cancer leading to iNOS-mediated tumor cell lysis. Oncoimmunology. 2014;3:e28926.
Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, et al. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res. 2014;2:901–10.
Keenan BP, Saenger Y, Kafrouni MI, et al. A listeria vaccine and depletion of t-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology. 2014;146:1784–94.
Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016;30:355–85.
Kim VM, Blair AB, Lauer P, et al. Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies. J Immunother Cancer. 2019;7:132.
Singh R, Paterson Y. In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/neu induced by Listeria monocytogenes-based vaccines. Cancer Immunol Immunother. 2007;56:927–38.
Bruhn KW, Craft N, Nguyen BD, Yip J, Miller JF. Characterization of anti-self CD8 T-cell responses stimulated by recombinant Listeria monocytogenes expressing the melanoma antigen TRP-2. Vaccine. 2005;23:4263–72.
Johnson LE, Brockstedt D, Leong M, Lauer P, Theisen E, Sauer JD, et al. Heterologous vaccination targeting prostatic acid phosphatase (PAP) using DNA and Listeria vaccines elicits superior anti-tumor immunity dependent on CD4+ T cells elicited by DNA priming. Oncoimmunology. 2018;7:e1456603.
Lim JY, Brockstedt DG, Lord EM, Gerber SA. Radiation therapy combined with Listeria monocytogenes-based cancer vaccine synergize to enhance tumor control in the B16 melanoma model. Oncoimmunology. 2014;3:e29028.
Hannan R, Zhang H, Wallecha A, Singh R, Liu L, Cohen P, et al. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer Immunol Immunother. 2012;61:2227–38.
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11:39.
Yarchoan M, Johnson BA, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:209–22.
Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–83.
Le DT, Ko AH, Wainberg ZA, et al. Results from a phase 2b, randomized, multicenter study of GVAX pancreas and CRS-207 compared to chemotherapy in adults with previously-treated metastatic pancreatic adenocarcinoma (ECLIPSE Study). J Clin Oncol. 2017;35:345–5.
Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated listeria vaccine (ANZ-100) and a live-attenuated listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–68.
Basu P, Mehta A, Jain M, Gupta S, Nagarkar RV, John S, et al. A randomized phase 2 study of ADXS11-001 Listeria monocytogenes-listeriolysin O immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int J Gynecol Cancer. 2018;28:764–72.
Denham JD, Lee DH, Castro M, Pandya S, Aslam S, Nanjappa S, et al. Two cases of disseminated infection following live organism anti-cancer vaccine administration in cancer patients. Int J Infect Dis. 2018;72:1–2.
Hanson WG, Benanti EL, Lemmens EE, et al. A potent and effective suicidal Listeria vaccine platform. Infect Immun. 2019;87:e00144–19.
Witte CE, Archer KA, Rae CS, Sauer JD, Woodward JJ, Portnoy DA. Innate immune pathways triggered by listeria monocytogenes and their role in the induction of cell-mediated immunity. Adv Immunol. 2012:135–56.
Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–900.
Torres D, Barrier M, Bihl F, Quesniaux VJF, Maillet I, Akira S, et al. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect Immun. 2004;72:2131–9.
Way SS, Kollmann TR, Hajjar AM, Wilson CB. Cutting edge: protective cell-mediated immunity to Listeria monocytogenes in the absence of myeloid differentiation factor 88. J Immunol. 2003;171:533–7.
Tam MA, Wick MJ. MyD88 and interferon-α/β are differentially required for dendritic cell maturation but dispensable for development of protective memory against Listeria. Immunology. 2009;128:429–38.
• Archer KA, Durack J, Portnoy DA. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog. 2014;10:1003861 This study examines how STING-dependent type I interferon negatively impacts the generation of antigen-specific T cell responses toL. monocytogenesinfection.
O’Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci U S A. 2002;99:13861–6.
Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012;8:e1002352.
Woodward JJ, Lavarone AT, Portnoy DA. C-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–5.
Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun. 2011;79:688–94.
Hansen K, Prabakaran T, Laustsen A, et al. Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 2014;33:1654–66.
Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8 + T-CeII recruitment. Cancer Res. 2009;69:3077–85.
Song S, Peng P, Tang Z, et al. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci Rep. 2017;7:39858.
Fu J, Kanne DB, Leong M, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7:283ra52.
Corrales L, Gajewski TF. Endogenous and pharmacologic targeting of the STING pathway in cancer immunotherapy. Cytokine. 2016;77:245–7.
Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–40.
Dikopoulos N, Bertoletti A, Kröger A, Hauser H, Schirmbeck R, Reimann J. Type I IFN negatively regulates CD8 + T cell responses through IL-10-producing CD4 + T regulatory 1 cells. J Immunol. 2005;174:99–109.
• Sivick KE, Desbien AL, Glickman LH, et al. Magnitude of therapeutic STING activation determines CD8 + T cell-mediated anti-tumor immunity. Cell Rep. 2018;25:3074–85 This study demonstrates that the magnitude and location (i.e., systemic vs local) of STING-activation are critical for shaping T cell responses in tumors.
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32.
Kanneganti TD, Özören N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–6.
Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol. 2010;30:693–702.
Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, et al. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010;40:1545–51.
Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7:412–9.
Theisen E, Sauer JD. Listeria monocytogenes and the inflammasome: from cytosolic bacteriolysis to tumor immunotherapy. Curr Top Microbiol Immunol. 2016;397:133–60.
McDougal C, Sauer JD. Listeria monocytogenes: the impact of cell death on infection and immunity. Pathogens. 2018;7:8.
Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, et al. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8.
von Moltke J, Trinidad NJ, Moayeri M, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–11.
von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE. Recognition of bacteria by inflammasomes. Annu Rev Immunol. 2013;31:73–106.
Sauer JD, Pereyre S, Archer KA, Burke TP, Hanson B, Lauer P, Portnoy DA (2011) Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are. poor inducers of protective immunity. Proc Natl Acad Sci U S A 108:12419–24.
•• Theisen E, Sauer JD. Listeria monocytogenes-induced cell death inhibits the generation of cell-mediated immunity. Infect Immunol. 2017;85:e00733–16 This study examines the mechanism of how inflammasome activation impairs the generation of antigen-specific T cell responses duringL. monocytogenesinfection. Importantly, the authors demonstrate that rather than pyroptotic death of APCs, it is the inflammation associated with inflammasome activation that impairs adaptive immunity.
Williams CR, Dustin ML, Sauer JD. Inflammasome-mediated inhibition of listeria monocytogenes-stimulated immunity is independent of myelomonocytic function. PLoS One. 2013;8:83191.
Kolb R, Phan L, Borcherding N, et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat Commun. 2016;7:13007.
Drexler SK, Bonsignore L, Masin M, Tardivel A, Jackstadt R, Hermeking H, et al. Tissue-specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis. Proc Natl Acad Sci U S A. 2012;109:18384–9.
Wang H, Luo Q, Feng X, Zhang R, Li J, Chen F. NLRP3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. BMC Cancer. 2018;18:500.
Huang CF, Chen L, Li YC, Wu L, Yu GT, Zhang WF, et al. NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36:116.
Kim JW, Koh Y, Kim DW, et al. Clinical implications of VEGF, TGF-β1, and IL-1β in patients with advanced non-small cell lung cancer. Cancer Res Treat. 2013;45:325–33.
Tas F, Tilgen Yasasever C, Karabulut S, Tastekin D, Duranyildiz D. Clinical significance of serum interleukin-18 (IL-18) levels in patients with gastric cancer. Biomed Pharmacother. 2015;70:19–23.
Xue Y, Du H-D, Tang D, et al. Correlation between the NLRP3 inflammasome and the prognosis of patients with LSCC. Front Oncol. 2019;9:588.
Chow MT, Sceneay J, Paget C, Wong CSF, Duret H, Tschopp J, et al. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012;72:5721–32.
Daley D, Mani VR, Mohan N, Akkad N, Pandian GSDB, Savadkar S, et al. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med. 2017;214:1711–24.
Guo B, Fu S, Zhang J, Liu B, Li Z. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci Rep. 2016;6:36107.
Chen IF, Ou-Yang F, Hung JY, Liu JC, Wang H, Wang SC, et al. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol Cancer Ther. 2006;5:1–7.
Liu ZY, Yi J, Liu FE. The molecular mechanism of breast cancer cell apoptosis induction by absent in melanoma (AIM2). Int J Clin Exp Med. 2015;8:14750–8.
Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med. 2015;21:906–13.
Liu R, Truax AD, Chen L, Hu P, Li Z, Chen J, et al. Expression profile of innate immune receptors, NLRs and AIM2, in human colorectal cancer: correlation with cancer stages and inflammasome components. Oncotarget. 2015;6:33456–69.
Dihlmann S, Tao S, Echterdiek F, Herpel E, Jansen L, Chang-Claude J, et al. Lack of absent in melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer. 2014;135:2387–96.
Karan D. Inflammasomes: emerging central players in cancer immunology and immunotherapy. Front Immunol. 2018;9:3028.
Sibelius U, Rose F, Chakraborty T, Darji A, Wehland J, Weiss S, et al. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect Immun. 1996;64:674–6.
Noor S, Goldfine H, Tucker DE, Suram S, Lenz LL, Akira S, et al. Activation of cytosolic phospholipase A2α in resident peritoneal macrophages by Listeria monocytogenes involves listeriolysin O and TLR2. J Biol Chem. 2008;283:4744–55.
Lone AM, Taskén K. Proinflammatory and immunoregulatory roles of eicosanoids in T cells. Front Immunol. 2013;4:130.
Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119:229–40.
• Theisen E, McDougal CE, Nakanishi M, Stevenson DM, Amador-Noguez D, Rosenberg DW, et al. Cyclooxygenase-1 and -2 play contrasting roles in Listeria-stimulated immunity. J Immunol. 2018;200:3729–38 This study demonstrates that eicosanoids are produced duringL. monocytogenesinfection and influence T cell priming. Importantly, the authors show that the critical eicosanoid mediating the generation of antigen-specific T cell responses is PGE2.
Hahn T, Alvarez I, Kobie JJ, Ramanathapuram L, Dial S, Fulton A, et al. Short-term dietary administration of celecoxib enhances the efficacy of tumor lysate-pulsed dendritic cell vaccines in treating murine breast cancer. Int J Cancer. 2006;118:2220–31.
Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, et al. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 2006;12:214–22.
Zelenay S, Van Der Veen AG, Böttcher JP, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162:1257–70.
Walker W, Rotondo D. Prostaglandin E2 is a potent regulator of interleukin-12- and interleukin-18-induced natural killer cell interferon-γ synthesis. Immunology. 2004;111:298–305.
Joshi PC, Zhou X, Cuchens M, Jones Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common γ-chain. J Immunol. 2001;166:885–91.
Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–65.
Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721–6.
Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13.
Kaliński P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35.
Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2 -induced inflammation: relevance of prostaglandin e receptors. Biochim Biophys Acta Mol Cell Biol Lipids. 2015;1851:414–21.
Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–8.
Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93.
Luft T, Jefford M, Luetjens P, Toy T, Hochrein H, Masterman KA, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E2 regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72.
Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, et al. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res. 2016;22:4380–90.
Funding
This study was supported by grant R01 CA188034 from the National Institutes of Health (JDS). In addition, this material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program (Z.T.M) under Grant No. DGE-1747503. Support was also provided by the Graduate School and the Office of the Vice Chancellor for Research and Graduate Education at the University of Wisconsin-Madison with funding from the Wisconsin Alumni Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Microbial Anti-cancer Therapy and Prevention
Rights and permissions
About this article
Cite this article
Morrow, Z.T., Powers, Z.M. & Sauer, JD. Listeria monocytogenes Cancer Vaccines: Bridging Innate and Adaptive Immunity. Curr Clin Micro Rpt 6, 213–224 (2019). https://doi.org/10.1007/s40588-019-00133-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-019-00133-4