Abstract
Purpose of Review
Human immunodeficiency virus (HIV) has infected over 36 million individuals worldwide and presents a tremendous public health concern, yet much remains unknown about the effect of immune responses on infection. In this review, we discuss the current status of mathematical modeling of HIV-immune system dynamics and how advances in modeling approaches have contributed to our understanding of the role of immune responses in virus infection.
Recent Findings
Recent advances provide important quantitative findings about CD8+ T cell and antibody responses. Specifically, these models explain important dynamical features such as the intracellular eclipse phase, and they estimate immune escape rates, the timing of MHC downregulation, and the proportion of virus in antibody-viral complexes.
Summary
Models of HIV-immune system dynamics, validated with experimental data, advance our quantitative understanding of infection and can generate hypotheses for further experiments. Greater insight on immune responses in HIV infection dynamics can lead to the development of vaccines and ultimately a cure for this infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of virus dynamics using mathematical models has had a substantial impact on understanding within-host dynamics of human immunodeficiency virus type 1 (HIV-1) and other viral infections [1]. This understanding has led to advances in treatment strategies. However, it is well known that immune responses can significantly alter viral dynamics and treatment effectiveness. Despite being an important component of virus-host interactions and highly relevant for vaccination strategies, the role of immune responses on HIV infection is yet to be fully illuminated. Immune response modeling in viral dynamics is one of the rapidly growing subfields of within-host infection. Here, we briefly review the current status of modeling HIV-immune system dynamics, and in particular, how contributions over the past 5 years have advanced our knowledge of the role of immune responses in virus infection.
Virus Dynamics Models
The standard model of virus infection dynamics considers three populations: free-virus, uninfected (or target) cells, and infected cells. Despite its remarkable simplicity, this model can reasonably explain early viral load data in individuals infected with HIV. This model, along with some of its variants, has been reviewed extensively [1–6]. The standard model does not explicitly take the immune response into consideration. However, evidence from experimental studies have shown that CD8+ T cell depletion in simian immunodeficiency virus (SIV)-infected macaques results in an increase in viral load [7–10], implying that the immune response has an effect on the overall viral dynamics not explained by the standard model. Mathematical modeling also indicates a role for immune control [11, 12].
Additional studies have suggested that CD8+ T cells play a limited role in HIV control. A vaccine that increases CD8+ T cells before SIV infection does not change the viral growth or post-peak decay rate observed during primary infection of non-immunized SIV-infected macaques [13]. However, specific cellular and humoral immune responses to HIV are detected throughout clinical infection and are taken into consideration in escape studies. Furthermore, data from other studies indicate a role for CD8+ T cells, especially in elite controllers [14–16]. A study in which CD8+ T cells were depleted in early SIV infection resulted in the viral load persisting at high levels instead of exhibiting the typical peak and drop in virus dynamics [10]. Moreover, CD8+ T cell escape mutants begin accumulating around the time of peak viremia, apparently emerging in response to inhibition by CD8+ T cells.
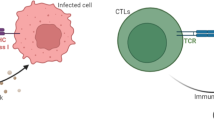
The standard model of virus dynamics has been modified in recent years by a number of researchers to include immune responses (Fig. 1). Figure 1 shows a schematic diagram of a virus dynamics model with immune responses; most useful models contain a subset of these elements in an effort to keep the model and its analysis tractable. Several models contain multiple subsets of infected cells, such as infected non-virus-producing cells and productively infected cells. Some models also include multiple populations of viral strains, with their corresponding infected cells and the effector responses (cells and antibodies) levied against them. Several of the recent studies on modeling HIV-immune system dynamics focus on immune escape, or the process of viral evolution to evade the established immune responses. This review provides an update on recent progress in the past 5 years specifically on models of HIV virus dynamics that include immune responses.
Schematic diagram showing the modeling of HIV infection dynamics with adaptive immune responses. Populations include uninfected cells, infected cells in the intracellular eclipse phase before the production of virus begins, productively infected cells, free virus, CD8+ effector cells, and antibodies. The potential effects of CD8+ T cells and antibodies that have been considered by mathematical models are depicted
Modeling CD8+ T Cell Responses
Thus far, the majority of work on modeling HIV dynamics with immune responses has focused on CD8+ T cell responses. In this section, we review recent work that incorporates the CD8+ response into the standard model of HIV and related infections.
Intracellular eclipse phase models make for a better fit to HIV data, improve escape rate estimates, estimate the timing of MHC downregulation, and resolve the mechanism of CD8+ activity.
A number of recent modeling efforts, each pursued to investigate different questions, subdivided the infected pool into cells in the intracellular eclipse phase and productively infected cells.
A recent study by Althaus and de Boer [17], modeling the eclipse phase and productively infected cells separately, considers that HIV is able to partially evade killing by CD8+ T cells through downregulation of MHC-I molecules on infected cells. They estimated that this downregulation occurs as early as 12 h post infection, suggesting that cytotoxic T lymphocyte (CTL) killing during the eclipse phase can be more efficient than at later viral life stages because the downregulation increases as time progresses. In a further study by the same authors, an expanded model is presented that considers not only an intracellular delay phase but also multiple infections of cells [18]. Other CD8+ T cell-based models that expand the standard model by subdividing the infected pool into cells in the eclipse phase and productively infected cells also take into account that most escape events occur early, and the escape rate slows considerably over time [19]. Investigations by these models concluded that HIV evolves only a small number of escape mutants when the CD8+ T cell response is sufficiently broad, and so escaping one of the many CD8+ T cell responses provides little advantage to the virus after the viral set point (where the viral load stabilizes and reaches a steady state) has been approached. Pawelek et al. alternatively modeled the eclipse phase by incorporating into the standard model a time delay for the production of virus after infection [20]. This model also included the population of effector cells that kill infected cells as well as a second time delay for the emergence of killing ability. They found an improved fit of their model to viral load data from 10 patients during primary HIV-1 infection, although this alteration was not a statistically significant improvement compared to the model without time delays. Using their model, they found that the time between infection and virion production was less than 1.5 days, and the time between infection and the emergence of killing ability was between 19 and 32 days.
The mechanism of CD8+ T cell activity has been a topic of active research in the last several years. In particular, researchers questioned whether CD8+ T cells exert their influence on virus infection by killing infected cells, or if their effect is mediated through a secreted soluble factor that has an inhibitory effect on virus production or cell infection [16, 21–26]. CD8+ non-cytolytic factors include the CD8+ cell antiviral factor (CAF) [27, 28], prothymosin-alpha [29], or other soluble factors secreted by HIV-specific CD8+ T cells with antiviral activity [30]. In a counterargument, others contend that modeling supports the direct killing mechanism of CTLs [31] and conclude that a CTL killing mechanism describes the viral load data well when the model includes the eclipse phase before virus production [32••]. This debate has been reviewed extensively elsewhere; see Gadhamsetty et al. [33]. Perhaps future models can delineate the extent of activity of both mechanisms, or can help elucidate the precise role and timing of each effect. If nothing else, this controversy points towards a need for clarification on this issue regarding CD8+ T cell function, and it can shed light on which experiments can resolve the discrepancy.
Other models that include CD8+ T cell responses delineate controllers from viral rebounders or establish thresholds between control and AIDS.
In work on the latent reservoir, Conway and Perelson [34••] focused their study on why only a small fraction of patients on antiretroviral therapy exhibit post-treatment control, i.e., viral load maintained at RNA <50 copies per milliliter after cessation of therapy. They hypothesized that antiretroviral therapy, initiated early during primary infection, permits post-treatment control by limiting the size of the latent reservoir, which, if small enough at treatment termination, may allow the adaptive immune response to prevent viral rebound and control infection. Using their model, they provided the range of the CTL killing strength and the latent reservoir size at treatment cessation for viral rebound, post-treatment control, or elite controllers. They conclude that the size of the latent reservoir and killing rate delineate these outcomes.
In a model by Huang et al. [35] that included a population of CTLs in the standard model, they established thresholds that characterize HIV persistence and the progression to AIDS. One threshold delineates persistence of infection, and another allows sufficient CTL killing of infected cells for the individual to progress to AIDS. In viral persistence, the CTLs are not stimulated enough to clear the virus; in AIDS, the CTL activity is so high that all the CD4+ T cells are killed, resulting in immunodeficiency.
Recent modeling efforts improve estimates of the timing and the rate of escape, for single and multiple escape events.
Models that calculate the rate of emergence of escape variants provide kinetics that give an estimate of the strength of the immune response and the pressure it exerts on the virus. The basic model considers populations of two viral strains, representing the wild-type virus and a mutant that is capable of escaping the CD8+ T cell response [1]. This simplified model does not explicitly include the dynamics of infected cell populations, based on the quasi steady state assumption that the infected cell population densities are proportional to the densities of the respective virus populations throughout infection [1, 36]. A key idea incorporated in this model is that the escape variant avoids death from CD8+ T cell responses, since these viral strains are resistant to the CD8+ T cell response against the wild type. The model solution yields the frequencies of the wild-type and mutant populations, from which the escape rate is calculated. The escape rate increases as the rate of killing of the wild type increases, and it decreases as the fitness cost of the escape mutation increases. Therefore, the fastest escapes happen when CD8+ T cell pressure is high and the fitness cost of escape is low [37].
Some recent work includes alternate methods to calculate escape rates. Wick and Yang [38••] introduced an “escape formula” based on branching processes to calculate the escape rate that takes into account the relative escape advantage, fitness loss, mutation rate, and effective population size of productively infected cells. Using the two-strain mathematical model and longitudinal data from three HIV-infected patients, Ganusov et al. quantified the CD8+ T cell escape rate for single and multiple epitopes [39]. Their calculations, based on the relative frequencies of wild-type and mutant virus and the initial frequency of the mutant, showed that the escape rate decreases over time between infection and the viral set point. The study suggested that the low escape rates observed in chronic/late infection are due to a high fitness cost for escape mutations and an increase in the diversity of CD8+ T cell responses with time. In another study, Ganusov et al. concluded that through mutation, the virus population escapes from around half of the CD8+ T cell responses encountered in the first year [36]. However, estimated escape rates can vary depending on the mathematical model and method used.
Using a similar two-strain model, Martyushev et al. investigated patterns of epitope-specific CD8+ T cell escape in 25 SIV-infected macaques in which multiple escape mutants emerge simultaneously [40•]. It was observed that immune escape occurs on average 18 days after the epitope-specific CD8+ T cells reach 0.5 % of total CD8+ T cells. Upon escape, two patterns emerge: monomorphic and variable escape. They concluded that the timing of escape is largely determined by the kinetics of epitope-specific CD8+ T cells and is independent of the variability of the epitope. Applying an approach that considers the entire HIV genotype rather than single-escape epitopes that are treated independently, Kessinger et al. used sequential data from three HIV-infected patients to calculate escape rates [41•]. They predicted escape rates that were substantially higher than previously estimated rates: 0.3–0.4 escapes per day for the first 4–6 escape mutations. Furthermore, they showed that the timing of escape events depends on epitope entropy and immunodominance. Leviyang and Ganusov [42•] posit that current methods of estimating CD8+ T cell escape rates that assume independent escapes can be biased when escape proceeds from multiple CTL responses concurrently. They also contend that other models considering escape across multiple epitopes [36, 41•, 42•] are computationally complex and highly parameterized. To overcome these difficulties, they proposed an alternate method that applies multi-epitope logistic models to HIV patient data. Implementing their method, they estimated that concurrent escape at multiple epitopes occurs at rates of 0.03 to 0.4 escapes per day, in line with the 0.1 to 0.2 escapes per day observed in patient data.
Several recent studies investigate the triggers, characteristics, and mechanisms of escape.
In this area, modeling has contributed to our understanding of conditions that permit virus persistence, clearance, control, and escape. Liu et al. investigated early immune escape in primary infection, expanding on previous models of CD8+ T cell responses at a single epitope [43]. They showed that CD8+ T cells exert selective pressure that favors rapid escape, so an increase in the magnitude of T cell responses significantly increases the risk of escape. However, slow escape does not indicate inadequate immune pressure, as increased epitope entropy was also associated with faster escape. Entropy, however, was not a good predictor of resulting virus fitness. Surprisingly, they found that rapid virus escape was not more common in epitopes restricted by protective HLA allotypes. In an effort to understand the impact of CTL escape on immunopathology, Johnson et al. developed a mathematical model to explore the conditions under which CD8+ T cells lead to protection from infection or immunopathology [44]. Their investigation showed that production of escape mutants has an insignificant effect on pathology, even though escape can change the dynamics of the infection. By developing an innovative “escape clock” approach that measures the replacement of wild-type virus by CTL escape mutant strains, Reece et al. [45] estimated the turnover of viral DNA in SIV-infected macaques. They concluded that treatment should be given early or during active virus replication, to reduce the formation of stable viral DNA in the host. Konrad et al. constructed a two-strain model in which therapeutic vaccines that stimulate the CTL response can, under certain conditions, theoretically eliminate both wild-type and mutant strains. They also evaluated the conditions for escape mutants to emerge, resulting in vaccine failure. Within their framework, they were able to demonstrate how imperfect adherence to the vaccine regimen may result in persistence of a mutant strain at low levels, allowing the mutant to outcompete and escape [46].
Computational simulations explore the conditions and consequences of CTL escape.
A number of studies explored escape via constructing computational simulations. Kadolsky and Asquith quantified the impact of CD8+ T cell escape on viral load in chronic HIV infection [47]. Their study found that CTLs result in a modest increase in viral load. They also noted a significant positive association between viral load and escape events involving mutations in the pol gene and a negative association with the gag gene. A stochastic agent-based model of CTL escape developed by Schwartz et al. examines the conditions under which escape mutants occur in both acute and chronic HIV infections [48]. Their simulations reproduced CTL escape seen in clinical data, namely, early escape, late escape, and the rapid escape events occurring in late infection. Similarly, using computational simulations, Gadhamsetty et al. observed that CTL killing efficiency is determined by the relative densities of target cells and CTLs, which depend on if a CTL can kill multiple cells concurrently and if a target cell can be killed by multiple CTLs together [49]. Simulations of HIV and CD8+ T cell immune responses are also performed by Castiglione and Celeda, using the immune system computational simulation model called C-IMMSIM [50].
Modeling Antibody Responses
Recently, more clinical and experimental data have become available for the study of antibodies in HIV infection, expanding the potential of modeling to understand the mechanisms by which antibodies aid in control of infection. Models have investigated the roles of both neutralizing and non-neutralizing antibodies in HIV and related infections.
Modeling studies predict how neutralizing antibodies may determine virus control or persistence.
A study by a combined group of experimentalists and modelers showed that the composition of virus-antibody immune complexes is dynamic over the course of HIV-1 infection [51]. Their study determined the relative concentration of immune complexes during acute and chronic infection and found significantly fewer HIV-antibody immune complexes in acute infections than in chronic infections. Additionally, they estimated that the mean number of neutralizing antibodies bound to each virion in acute infections is approximately one antibody per virion. A theoretical study investigating strain-specific and cross-reactive (or poly-specific) HIV antibodies compared the efficacy of antibodies that can bind multiple strains of virus to those that only bind one strain [52]. Results of this model indicate that when only poly-specific antibodies are present, they are capable of controlling multiple strains and can lead to viral clearance. However, a mixed antibody response is dominated by strain-specific antibodies and results in viral persistence. Another study by Ciupe [53] examined the neutralizing capability of antibodies and their impact on persistent infection. By modeling antibody-binding kinetics, Ciupe showed that in the early stages of infection, HIV virions are largely free, with very few forming antibody-virion complexes, while at the 1-year mark, only about 1 % of the virus remains free, with the remaining proportion in the form of antibody-virion complexes, thereby drastically reducing the infectious virus pool. This reduction in infectivity over time is consistent with the previous SIV infection model with time-decaying infectivity that fits the SIV viral load better than the standard model [54]. In another study, Wikramaratna et al. combined CD8+ T cells with antibody responses (both specific and cross-reactive) to explore how virus control breaks down, leading to AIDS [55••]. Their novel model takes into account CD8+ T cell escape as well as that specific antibody populations are long-lived, while CD8+ T cell activity is short-lived. Intriguingly, their results posit that CD8+ T cell escape, as well as the loss of virus control, can be prevented by strong antibody responses.
Models estimate antibody escape rates.
Studies showing antibody escape events in HIV-infected individuals have permitted the beginning of estimation of calculations of antibody escape rates. These studies show that low titers of broadly neutralizing antibodies appear to select for escape mutants due to a relatively low concentration of the antibody capable of neutralizing a variety of sub-strains [56]. This supports an early need for a high titer of broadly neutralizing antibodies to control infection.
Experimental infection of horses with equine infectious anemia virus (EIAV), a lentivirus similar to HIV, evidenced a single antibody escape variant that arose several weeks after infusion of SCID horses with EIAV-specific antibodies [57, 58]. Using these data from EIAV-infected horses to study antibody escape, Schwartz et al. developed an improved mathematical model to quantify the antibody escape kinetics of EIAV [59•]. This study provided the rates of viral escape due to antibody pressure for two different antibody dosages and two different antibody specificities. In addition, they calculated antibody blocking rates of wild-type virus, fitness costs of mutant virus, and growth rates of both viruses. By determining how rapidly the virus escaped from the antibody response, this study estimated the strength of antibody pressure in these EIAV infections. Similarly, Ciupe and Schwartz examined antibody protection and escape in EIAV infection, including an analysis of the role of antibody neutralization on competition and escape in the presence of two viral strains [60]. Expanding on this analysis, Schwartz and Smith? [61] modeled these data from EIAV-infected immunodeficient horses presuming three viral strains and identified the correlates of antibody escape and protection. In a study on cell-to-cell transmission with antibody responses, Allen and Schwartz [62] developed models to evaluate the role of the transmission mode within a single host (i.e., cell-to-cell vs. free-virus transmission) on EIAV infection and predict the potential impact of interventions that block only one mode of transmission. In a more general approach, Schwartz et al. constructed a model that contains both antibodies and CTLs in order to project the long-term behaviors observed in EIAV infection [63].
Models predict the roles for antibodies in vaccine strategies.
One of the major hurdles in the development of an effective vaccine for HIV is the capacity of a vaccine to produce broadly neutralizing antibodies. A study by Luo and Perelson [64] suggests that current vaccine studies do not utilize a broad range of viral subtypes in their vaccine design, and thus do not elicit broadly neutralizing antibodies as quickly as a more diverse vaccine could. A more diverse vaccine could help counteract the virus-antibody coevolution that occurs in the early stages of infection. This study also explains that broadly neutralizing antibodies arise late in infection partly due to competition in the earlier stages of infection. In contrast, an in silico computational model [65] suggested that generating cross-reactive HIV antibodies is uncommon because the affinity maturation process gets “frustrated” by different variants of the virus. These results predict that, for a vaccine to produce a cross-reactive immune response, one should immunize with antigenic variants sequentially instead of delivering several different antigens at once.
More generally, Ali Tabei et al. [66] investigated the minimum threshold of broadly neutralizing antibody needed to effectively decrease viral load. Using the standard model expanded to include broadly neutralizing antibodies and virion-antibody complexes, they showed that the antibody response needed to control infection can be achieved through the immune response and is therefore feasible by therapeutic vaccination. Future modeling efforts will likely continue to contribute to understanding of how antibodies can help control HIV infection.
Conclusion
Besides improving our grasp of viral infection mechanisms, mathematical models can also generate new hypotheses that can be evaluated experimentally. Future modeling studies should continue to investigate the mechanisms of CD8+ T cell and antibody activity on HIV. Whether CD8+ T cells exert their effects predominantly through killing infected cells, secreting soluble factors, or both (perhaps to different extents at different stages) needs additional clarification through both experimental and mathematical studies.
Continued efforts are also essential to construct mathematical models that can help us understand antibody activity against HIV. The mechanisms through which antibodies (neutralizing and non-neutralizing) help control HIV infection are largely unknown. In this respect, additional clarity is needed on clearing virus, blocking infection of cells, or involvement in other mechanisms. Models may be useful to investigate whether antibodies clear infection by direct binding to virus, or if their effects are mediated by indirect mechanisms such as ADCC (antibody dependent cell cytotoxicity), ADCVI (antibody dependent cell-mediated virus inhibition), or tagging infected cells for destruction by phagocytes. The timing (i.e., during which phases of infection) and degree of these responses will advance our understanding of virus control by antibodies. Ultimately, a successful vaccine will elicit broadly neutralizing antibodies as the virus evolves in response to antibody (and presumably other immune) pressure, and modeling studies can provide insight into this effort.
Furthermore, the kinetics of effective immune responses in HIV infection are still largely unknown. Control strategies will benefit from knowledge of which responses are active in different stages of infection, as well as to what extent. Additional areas of future investigation include the role of innate immune responses in HIV infection and the role that immune responses exert against cell-to-cell infection. Most importantly, models should investigate how immune responses can be harnessed to develop effective vaccine strategies. Future models should consider both CD8+ T cell and antibody-stimulating strategies, so that vaccines can be developed with improved efficacy from both responses working together.
For further reading, we refer the reader to the many in-depth reviews written on other aspects of within-host viral dynamics modeling that are beyond the scope of the current review and not covered here (Table 1).
References
Recently published papers of particular interest have been highlighted as: • Of importance •• Of major importance
Perelson AS, Ribeiro RM. Modeling the within-host dynamics of HIV infection. BMC Biol. 2013;11:96.
Alizon S, Magnus C. Modelling the course of an HIV infection: insights from ecology and evolution. Viruses. 2012;4:1984–2013.
De Boer RJ, Perelson AS. Target cell limited and immune control models of HIV infection: a comparison. J Theor Biol. 1998;190:201–14.
Padmanabhan P, Dixit NM. Models of viral population dynamics. Curr Top Microbiol Immunol. 2016;392:277–302.
Perelson AS. Modelling viral and immune system dynamics. Nat Rev Immunol. 2002;2:28–36.
Wodarz D. Modeling T cell responses to antigenic challenge. J Pharmacokinet Pharmacodyn. 2014;41:415–29.
Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–8.
Matano T, Shibata R, Siemon C, Connors M, Lane HC, et al. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–9.
Schmitz JE, Johnson RP, McClure HM, Manson KH, Wyand MS, et al. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques. J Virol. 2005;79:8131–41.
Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60.
Regoes RR, Antia R, Garber DA, Silvestri G, Feinberg MB, et al. Roles of target cells and virus-specific cellular immunity in primary simian immunodeficiency virus infection. J Virol. 2004;78:4866–75.
Kouyos RD, Gordon SN, Staprans SI, Silvestri G, Regoes RR. Similar impact of CD8+ T cell responses on early virus dynamics during SIV infections of rhesus macaques and sooty mangabeys. PLoS Comput Biol. 2010;6(8): e1000901. doi:10.1371/journal.pcbi.1000901.
Davenport MP, Ribeiro RM, Perelson AS. Kinetics of virus-specific CD8+ T cells and the control of human immunodeficiency virus infection. J Virol. 2004;78:10096–103.
Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9.
Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–16.
Elemans M, Thiebaut R, Kaur A, Asquith B. Quantification of the relative importance of CTL, B cell, NK cell, and target cell limitation in the control of primary SIV-infection. PLoS Comput Biol. 2011;7:e1001103.
Althaus CL, De Boer RJ. Implications of CTL-mediated killing of HIV-infected cells during the non-productive stage of infection. PLoS One. 2011;6:e16468.
Althaus CL, De Boer RJ. Impaired immune evasion in HIV through intracellular delays and multiple infection of cells. Proc Biol Sci. 2012;279:3003–10.
van Deutekom HW, Wijnker G, de Boer RJ. The rate of immune escape vanishes when multiple immune responses control an HIV infection. J Immunol. 2013;191:3277–86.
Pawelek KA, Liu S, Pahlevani F, Rong L. A model of HIV-1 infection with two time delays: mathematical analysis and comparison with patient data. Math Biosci. 2012;235:98–109.
Balamurali M, Petravic J, Loh L, Alcantara S, Kent SJ, et al. Does cytolysis by CD8+ T cells drive immune escape in HIV infection? J Immunol. 2010;185:5093–101.
Elemans M, Florins A, Willems L, Asquith B. Rates of CTL killing in persistent viral infection in vivo. PLoS Comput Biol. 2014;10:e1003534.
Elemans M, Seich Al Basatena NK, Klatt NR, Gkekas C, Silvestri G, et al. Why don’t CD8+ T cells reduce the lifespan of SIV-infected cells in vivo? PLoS Comput Biol. 2011;7:e1002200.
Klatt NR, Shudo E, Ortiz AM, Engram JC, Paiardini M, et al. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 2010;6:e1000747.
Seich Al Basatena NK, Chatzimichalis K, Graw F, Frost SD, Regoes RR, et al. Can non-lytic CD8+ T cells drive HIV-1 escape? PLoS Pathog. 2013;9:e1003656.
Wong JK, Strain MC, Porrata R, Reay E, Sankaran-Walters S, et al. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 2010;6:e1000748.
Shridhar V, Chen Y, Gupta P. The CD8 antiviral factor (CAF) can suppress HIV-1 transcription from the long terminal repeat (LTR) promoter in the absence of elements upstream of the CATATAA box. Virol J. 2014;11:130.
Bonneau KR, Ng S, Foster H, Choi KB, Berkhout B, et al. Derivation of infectious HIV-1 molecular clones with LTR mutations: sensitivity to the CD8+ cell noncytotoxic anti-HIV response. Virology. 2008;373:30–8.
Mosoian A, Teixeira A, Burns CS, Sander LE, Gusella GL, et al. Prothymosin-alpha inhibits HIV-1 via toll-like receptor 4-mediated type I interferon induction. Proc Natl Acad Sci U S A. 2010;107:10178–83.
DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol. 2004;2:401–13.
Wick WD, Yang OO. Biologically-directed modeling reflects cytolytic clearance of SIV-infected cells in vivo in macaques. PLoS One. 2012;7:e44778.
Gadhamsetty S, Coorens T, de Boer RJ. Notwithstanding circumstantial alibis, cytotoxic T cells can be major killers of HIV-1-infected cells. J Virol. 2016;90:7066–83. The authors showed that mathematical modeling supports the direct killing mechanism of CTLs, and conclude that a CTL killing mechanism fits well when the model includes the eclipse phase before virus production and when killing is fast and varies over the life cycle of infected cells.
Gadhamsetty S, Beltman JB, de Boer RJ. What do mathematical models tell us about killing rates during HIV-1 infection? Immunol Lett. 2015;168:1–6.
Conway JM, Perelson AS. Post-treatment control of HIV infection. Proc Natl Acad Sci U S A. 2015;112:5467–72. This study develops a mathematical model to describe the potential mechanism by which only a small fraction of patients exhibit post-treatment virus control. The study further gives a quantitative description of the roles of the CD8+ T cell response and latent reservoir size on patients showing viral rebound or the elite controller phenotype.
Huang G, Takeuchi Y, Korobeinikov A. HIV evolution and progression of the infection to AIDS. J Theor Biol. 2012;307:149–59.
Ganusov VV, Neher RA, Perelson AS. Mathematical modeling of escape of HIV from cytotoxic T lymphocyte responses. J Stat Mech. 2013;2013:P01010.
Kent SJ, Fernandez CS, Dale CJ, Davenport MP. Reversion of immune escape HIV variants upon transmission: insights into effective viral immunity. Trends Microbiol. 2005;13:243–6.
Wick WD, Yang OO. War in the body : the evolutionary arms race between HIV and the human immune system and the implications for vaccines. New York: Springer; 2013. 298 pages. The authors derived a formula to calculate the escape rate of HIV to predict the expected number of times per generation that a new HIV lineage with escape mutations emerge and evade extinction.
Ganusov VV, Goonetilleke N, Liu MK, Ferrari G, Shaw GM, et al. Fitness costs and diversity of the cytotoxic T lymphocyte (CTL) response determine the rate of CTL escape during acute and chronic phases of HIV infection. J Virol. 2011;85:10518–28.
Martyushev AP, Petravic J, Grimm AJ, Alinejad-Rokny H, Gooneratne SL, et al. Epitope-specific CD8+ T cell kinetics rather than viral variability determine the timing of immune escape in simian immunodeficiency virus infection. J Immunol. 2015;194:4112–21. The authors analyzed epitope-specific CD8+ T cells in pigtail macaques infected with SIV to look for patterns of escape mutations. They found that timing of escape is largely determined by the kinetics of epitope-specific T cells and not by the conservation or variability of an epitope.
Kessinger TA, Perelson AS, Neher RA. Inferring HIV escape rates from multi-locus genotype data. Front Immunol. 2013;4:252. The authors compare escape rates for modeled infections with serially sampled sequence data from three HIV-infected individuals. They predicted escape rates that were higher than previously estimated.
Leviyang S, Ganusov VV. Broad CTL response in early HIV infection drives multiple concurrent CTL escapes. PLoS Comput Biol. 2015;11:e1004492. This paper uses a robust method of CD8+ T cell escape rate calculation that corrects for inherent biases against multiple escapes at the same epitope. Their results are able to bring estimates to the 0.1-0.2 escape events per day predicted by clinical data.
Liu MK, Hawkins N, Ritchie AJ, Ganusov VV, Whale V, et al. Vertical T cell immunodominance and epitope entropy determine HIV-1 escape. J Clin Invest. 2013;123:380–93.
Johnson PL, Kochin BF, McAfee MS, Stromnes IM, Regoes RR, et al. Vaccination alters the balance between protective immunity, exhaustion, escape, and death in chronic infections. J Virol. 2011;85:5565–70.
Reece J, Petravic J, Balamurali M, Loh L, Gooneratne S, et al. An “escape clock” for estimating the turnover of SIV DNA in resting CD4(+) T cells. PLoS Pathog. 2012;8:e1002615.
Konrad BP, Vaidya NK, Smith RJ. Modelling mutation to a cytotoxic T-lymphocyte HIV vaccine. Math Popul Stud. 2011;18:122–49.
Kadolsky UD, Asquith B. Quantifying the impact of human immunodeficiency virus-1 escape from cytotoxic T-lymphocytes. PLoS Comput Biol. 2010;6:e1000981.
Schwartz EJ, Yang OO, Cumberland WC, de Pillis LG. Computational model of HIV-1 escape from the cytotoxic T lymphocyte response. Can Appl Math Q. 2013;21:261–79.
Gadhamsetty S, Maree AF, Beltman JB, de Boer RJ. A general functional response of cytotoxic T lymphocyte-mediated killing of target cells. Biophys J. 2014;106:1780–91.
Castiglione F, Celada F. Immune system modelling and simulation. Boca Raton: CRC; 2015. p. 286.
Liu P, Overman RG, Yates NL, Alam SM, Vandergrift N, et al. Dynamic antibody specificities and virion concentrations in circulating immune complexes in acute to chronic HIV-1 infection. J Virol. 2011;85:11196–207.
Ciupe SM, De Leenheer P, Kepler TB. Paradoxical suppression of poly-specific broadly neutralizing antibodies in the presence of strain-specific neutralizing antibodies following HIV infection. J Theor Biol. 2011;277:55–66.
Ciupe SM. Mathematical model of multivalent virus-antibody complex formation in humans following acute and chronic HIV infections. J Math Biol. 2015;71:513–32.
Vaidya NK, Ribeiro RM, Miller CJ, Perelson AS. Viral dynamics during primary simian immunodeficiency virus infection: effect of time-dependent virus infectivity. J Virol. 2010;84:4302–10.
Wikramaratna PS, Lourenco J, Klenerman P, Pybus OG, Gupta S. Effects of neutralizing antibodies on escape from CD8+ T-cell responses in HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2015. doi:10.1098/rstb.2014.0290. This model includes both CD8+ T cells and the antibody response and suggests a role for antibodies that explains the breakdown of virus control as well as CD8+ T cell escape.
Bar KJ, Tsao CY, Iyer SS, Decker JM, Yang Y, et al. Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog. 2012;8:e1002721.
Taylor SD, Leib SR, Carpenter S, Mealey RH. Selection of a rare neutralization-resistant variant following passive transfer of convalescent immune plasma in equine infectious anemia virus-challenged SCID horses. J Virol. 2010;84:6536–48.
Taylor SD, Leib SR, Wu W, Nelson R, Carpenter S, et al. Protective effects of broadly neutralizing immunoglobulin against homologous and heterologous equine infectious anemia virus infection in horses with severe combined immunodeficiency. J Virol. 2011;85:6814–8.
Schwartz EJ, Nanda S, Mealey RH. Antibody escape kinetics of equine infectious anemia virus infection of horses. J Virol. 2015;89:6945–51. This study calculates antibody escape kinetics of equine infectious anemia virus, pioneering the estimation of antibody blocking rates of wild-type virus, fitness costs of mutant virus, and growth rates of both viruses. Such quantitative kinetic estimates may help develop antibody-eliciting vaccine strategies.
Ciupe SM, Schwartz EJ. Understanding virus-host dynamics following EIAV infection in SCID horses. J Theor Biol. 2014;343:1–8.
Schwartz EJ, Smith? RJ. Identifying the conditions under which antibodies protect against infection by equine infectious anemia virus. Vaccines. 2014;2:397–421.
Allen LJS, Schwartz EJ. Free-virus and cell-to-cell transmission in models of equine infectious anemia virus infection. Math Biosci. 2015;270:237–48.
Schwartz EJ, Pawelek KA, Harrington K, Cangelosi R, Madrid S. Immune control of equine infectious anemia virus infection by cell-mediated and humoral responses. Appl Math. 2013;4:171–7.
Luo S, Perelson AS. Competitive exclusion by autologous antibodies can prevent broad HIV-1 antibodies from arising. Proc Natl Acad Sci U S A. 2015;112:11654–9.
Wang S, Mata-Fink J, Kriegsman B, Hanson M, Irvine DJ, et al. Manipulating the selection forces during affinity maturation to generate cross-reactive HIV antibodies. Cell. 2015;160:785–97.
Ali Tabei SM, Li Y, Weigert M, Dinner AR. Model for competition from self during passive immunization, with application to broadly neutralizing antibodies for HIV. Vaccine. 2012;30:607–13.
De Boer RJ, Perelson AS. Quantifying T lymphocyte turnover. J Theor Biol. 2013;327:45–87.
Elemans M, Seich Al Basatena NK, Asquith B. The efficiency of the human CD8+ T cell response: how should we quantify it, what determines it, and does it matter? PLoS Comput Biol. 2012;8:e1002381.
Luo S, Perelson AS. The challenges of modelling antibody repertoire dynamics in HIV infection. Philos Trans R Soc Lond B Biol Sci. 2015. doi:10.1098/rstb.2014.0247.
Acknowledgments
The authors thank A. T. Dawes, R. Tyson, and C. A. Cobbold for careful reading of the manuscript and helpful comments. This work is supported by NSF grant DMS-1616299 (to NKV), a grant from the Association for Women in Mathematics (to EJS), and an international research award from Washington State University (to EJS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Virology
Rights and permissions
About this article
Cite this article
Schwartz, E.J., Biggs, K.R.H., Bailes, C. et al. HIV Dynamics With Immune Responses: Perspectives From Mathematical Modeling. Curr Clin Micro Rpt 3, 216–224 (2016). https://doi.org/10.1007/s40588-016-0049-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-016-0049-z