Abstract
Many factors contribute to the decline of skeletal muscle that occurs as we age. This is a reality that we may combat, but not prevent because it is written into our genome. The series of records from World Master Athletes reveals that skeletal muscle power begins to decline at the age of 30 years and continues, almost linearly, to zero at the age of 110 years. Here we discuss evidence that denervation contributes to the atrophy and slowness of aged muscle. We compared muscle from lifelong active seniors to that of sedentary elderly people and found that the sportsmen have more muscle bulk and slow fiber type groupings, providing evidence that physical activity maintains slow motoneurons which reinnervate muscle fibers. Further, accelerated muscle atrophy/degeneration occurs with irreversible Conus and Cauda Equina syndrome, a spinal cord injury in which the human leg muscles may be permanently disconnected from the nervous system with complete loss of muscle fibers within 5–8 years. We used histological morphometry and Muscle Color Computed Tomography to evaluate muscle from these peculiar persons and reveal that contraction produced by home-based Functional Electrical Stimulation (h-bFES) recovers muscle size and function which is reversed if h-bFES is discontinued. FES also reverses muscle atrophy in sedentary seniors and modulates mitochondria in horse muscles. All together these observations indicate that FES modifies muscle fibers by increasing contractions per day. Thus, FES should be considered in critical care units, rehabilitation centers and nursing facilities when patients are unable or reluctant to exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The scientific literature on the aging nervous system is immense, in particular the works on the decay of cognition and mobility. However, the impact of muscle denervation on aging skeletal muscle fibers is a relatively an orphan topic. This is related in part to the difficulties in determining by molecular approaches if motoneurons release chemical neurotrophic agents to the muscle fibers of the motor units [1]. It is well known that such mechanisms contribute to neuromuscular junction development and maintenance; however, if and what chemical trophic factors influence the synchronized expression of the hundreds of nuclei belonging to a single muscle fiber remains a subject of hypotheses, while the synchronized spread of muscle action potential seems to be a more rational mechanism [1]. The conclusions of two recent reviews [2, 3] that provide a summary of what is known or hypothesized about this subject will be discussed in the following chapters. The aim of our effort is to contribute the experience of muscle anatomists, biochemists, physiologists and skeletal muscle rehabilitation specialists concerning the impact of the denervation/reinnervation processes on shaping aging muscle fibers. We will focus on our own approaches and results which are poorly recognized in the aging community. We hope to provide rational explanations for contradictions existing in the literature on the nature and effects of the aging-related decrease in muscle bulk and on the increasing “slowness” of the aging muscle.
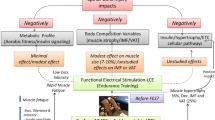
It is well established that the muscle deterioration that occurs with aging may be combatted by coordinated nutritional approaches and physical exercise. We will thus start with the main contradiction to this statement: the world record series of Master Athletes shows an almost linear decay in muscle power that starts at age 30 years and declines to the null value at around 110 years [4]. It is worth stressing that this occurs despite the fact that these record-holding men are the best that the human species can provide in terms of genetics and capacity to train under the best possible advice. Herein, we discuss a compilation of our investigations, involving Master Athletes, active or sedentary seniors, Spinal Cord Injured (SCI) patients, and horses. Altogether, these studies highlight the idea that volitional exercise and home-based Functional Electrical Stimulation (h-bFES) are crucial for maintaining both motor neurons and muscle fibers [5].
Lessons from Master Athletes
A 1925 paper by Hill [6] stated that valuable information concerning mobility may be found in the records of sport competitions. Accordingly, we studied the performance of world record holding Master Athletes and deduced relevant hints about the pattern of changes in muscle. Our results produce trend lines which show that power decline commences at 30 years and decreases toward zero at the age of 110 years (Fig. 1). Muscle power declines in a linear fashion, and this loss of power is a rather consistent 25 % every 20 years [4]. Each world champion is the best in his area, representing just one out of billions of people today. Even these exceptional athletes (conceivably, with optimal genetic backgrounds, focused attitudes toward training and performing, and access to the best trainers) lose power drastically as they age. Thus, it appears that something in our genome dictates this decline and nothing (as of yet) has been found to prevent it. The size and power of our muscles can go up and down several times in our lifetime in accordance with our nutritional and activity statuses; however, no matter how well we may fight aging, in the end, we will lose. First conclusion: Do not expect too much from training and rehabilitation!
Lessons from persons with or without a lifelong history of high-level activity
To address the situation for non-exceptionally athletic persons, we collected data and compared the muscle of young (not world class) sportsmen with that of two groups of senior people, either sedentary persons or those with a lifelong history of a high activity level. Age-related decay is strongly influenced by lifestyle, in particular activity level. [2, 7, 8]. Electrophysiological and histological measurements of skeletal muscle in older adults have detected reductions in the number of motor units and the presence of fiber type groupings [9–12] that are suggestive of denervation [13–16].
In our group of sedentary seniors, the majority of muscle fibers appear to co-express both slow and fast Myosin Heavy Chain (MHC), and some of these fibers are peculiarly small and angulated in appearance—likely a result of being denervated [8]. In contrast, muscle fibers are larger in the senior amateur sportsmen, and there is a larger number of “slow fiber type groupings” in these muscles [8]. The “type grouping” is strong evidence that the muscle fibers were “denervated” and then “reinnervated” by different motoneuron axons [8, 17]. Figure 2 shows that the denervation/reinnervation process starts with a checkerboard cluster of muscle fibers. In Fig. 2a, green fluorescence indicates fast MHC type fibers while in Fig. 2b the red indicates slow MHC type fibers. Fibers that co-express both fast and slow MHC (Fig. 2c) are orange and either of normal size or atrophic (white stars). It is likely that these are fibers which have been denervated by axonal damage and then reinnervated by a single regenerated slow motor axon. Incremental summation of denervation/reinnvervation processes may occur to such a degree that whole muscle biopsies might exhibit very large, almost complete reinnervation [7]. More importantly muscle fibers co-expressing both slow and fast MHC are normal in size in the senior sportsmen and often fill in the gaps that occur between clusters of slow myofibers [8].
Denervation/reinnervation process starts with a checkerboard cluster of muscle fibers. Green fluorescence in panel a identifies fast MHC type fibers while the red in panel b slow MHC type fibers. Fibers that co-express both fast and slow MHC (circled in c) are either normal-sized or atrophic (white star). It is likely that these are fibers which have been denervated by axonal damage and then reinnervated by a single regenerated motor axon
Consonant findings are reported in a recent study of older obese adults, showing that 5 months of resistance training enhanced skeletal muscle innervation [18]. Indirect evidence of the occurrence of background denervation with aging was reported from the electrostimulation of leg muscles in old rats [19]. Contractile activity generated by electrical stimulation eliminated age-related losses in muscle mass and reduced the deficit in force by 50 %, providing support for the hypothesis that during aging decreased contractile activity in fibers contributes to muscle weakness and even exacerbates atrophy.
Why muscle fibers are preferentially reinnervated by axons sprouting from slow motoneurons with age is a critical question. Our opinion is that this is related to the fact that slow motoneurons are activated much more often per day than fast motoneurons [20] and that their frequent firing preferentially spares them [1]. The idea that denervation occurs naturally with aging is based on evidence of reinnervation, and we may say this because in the normal muscle of young sportsmen, there are few to no type groupings [21]. In a recent review, Hepple and Rice [3] state that “ageing motor units manifest … a reduction in motor unit number secondary to motor neuron loss, fiber type grouping due to repeating cycles of denervation-reinnervation,… Regular muscle activation through habitual physical activity … can attenuate some of these changes…”. We may underscore these statements: Our conclusion is that senior sportsmen have greater myofiber diameters, a lower percentage of denervated myofibers and a higher number of slow type groupings because they were more physically active for a greater portion of their lives. It confirms that exercise has beneficial effects on age-related muscle degradation because it promotes also muscle fiber reinnervation, preferentially rescuing slow-type motoneurons. Indeed, the opposite life-style (i.e., the low level of daily activity of sedentary persons and the even lower activity of persons with mobility impairments), produces progressive muscle weakness with muscle atrophy [2, 7, 8, 17].
Thus, high-level daily activity not only maintains trophism of muscle fibers, but also trophism of slow motoneurons and/or increases their axon sprouting potentials [2, 3, 8, 18].
Lessons from sedentary elderly persons: effects and mechanisms of muscle Functional Electrical Stimulation (FES)
In addition to a progressive loss of muscle mass, aging skeletal muscle also presents with a conspicuous reduction in myofiber plasticity [2, 17, 22] and alterations in muscle-specific transcriptional mechanisms. During the aging process, protein synthetic rates decrease and an increase in protein degradation follows [23, 24], affecting characteristics of muscle fibers [25]. It is generally accepted that the failure to repair damage is a contributory cause of functional impairment with aging [26–29] and promotes the detrimental replacement of functional contractile muscle with fibrous tissue [25].
Volitional physical exercise can reverse these damaging processes [2, 3]. Interestingly, it has been shown that both acute and prolonged resistance exercise stimulate the proliferation of satellite cells in healthy sedentary elderly subjects [30–34], though blunted in elderly people. This fact may be explained by the increased levels of myostatin [35], a negative regulator of muscle mass [35, 36]. An increase in autophagy in the muscle of athletic people has been reported [37–39], suggesting that exercise may activate an important system that detoxifies muscle cells. Another major factor that is associated with physical exercise is Insulin-like Growth factor 1 (IGF-1) [40]. IGF-1 production by muscle increases after 5–10 min of moderate- to high-intensity exercise [41–43]. The evidence suggests that training and regular exercise, by modulating functional autophagy, myokines (IL-6) and IGF-1 expression, can increase muscle strength and attenuate sarcopenia [21, 37, 44]. There is strong evidence that IGF-1 secretion by muscle fibers also influences synaptogenesis in the brain [45] and at neuromuscular junctions [46, 47]. Further, agrin (along with other proteins such as MuSK, rapsyn, ChAT, Hb9, munc18, etc.) is important to denervation/reinnervation processes [48].
Unfortunately, elderly people may be unable or reluctant to adequately participate in physical exercise. We suggest that an alternative approach is functional electrical stimulation. A stimulator for neuromuscular electrical stimulation (ES) was designed, which especially suits the requirements of elderly people [49]. As detailed in Kern et al. [23], subjects were exposed to regular neuromuscular ES training for a period of 9 weeks. The outcome was an increase in muscle strength, associated with an increase of fast fibers, which are the first to respond to ES and are related to the power of skeletal muscle [23]. Because IGF-1 is one of the factors that is activated during physical exercise, we asked if ES would induce an increase in expression of IGF-1 and other components of downstream pathways. Indeed, we discovered that ES increases expression of IGF1 and of markers of both satellite cell proliferation and extracellular matrix remodeling and that it downregulates expression of proteases [23]. We also explored collagen expression and reported that there is remodeling during both volitional physical exercise and ES. Three different forms of collagen are also upregulated in electrically stimulated muscle [23], but the increase in collagen likely does not stimulate fibrosis as is shown by both morphological evidence and at the level of important fibrosis modulators, namely the increase in expression of miR29 [50].
Several longitudinal studies have shown that regular exercise is beneficial to aged population [2, 3, 12, 40, 51]. Thus, we wanted to compare the effects of regular exercise and ES on senior people. The Interreg IVa project recruited sedentary seniors with a normal lifestyle who were trained for 9 weeks with either leg press (LP) exercise [52] or ES [22, 23, 49]. Functional tests of trained subjects showed that LP and ES induced improvements in leg muscle force and of mobility [53, 54]. ES significantly increased the size of fast-type muscle fibers, together with a significant increase in the number of Pax7 and NCAM positive satellite cells. Further, no signs of muscle damage and/or inflammation were observed in muscle biopsies. Altogether the results demonstrate that physical exercise, either voluntary (LP) or induced by ES, improves the functional performance of aging muscles. However, neither training can stop the aging process as we have learned from the Master Athletes. The data show that ES is a safe home-based method able to counteract atrophy of fast-type muscle fibers, a biomarker of skeletal muscle aging in sedentary seniors [17, 22].
Lessons from persons affected by spinal cord injury
Premature aging may occur as a result of many chronic diseases, including those which involve loss of muscle innervation. One extreme case is irreversible Conus and Cauda Equina syndrome, a spinal cord injury (SCI) sequela. In this syndrome, the leg muscles may be completely and permanently disconnected from the nervous system and their fibers, thus, undergo severe atrophy and finally fibro-fatty degeneration. [55–63]. In the case of SCI with only upper motor neuron lesions, likewise those with complete transverse lesion at the thoracic level, muscle fibers below the lesion remain innervated but disused; nonetheless, their disuse induces, at most, a 50 % decrease in fiber size which remains stable for at least 20 years post-SCI [64]. Such patients exhibit a stable histochemical ATPase checkerboard pattern, and the slow-type fibers may stain more lightly [64].
Otherwise, looking at the effects of long-term denervation on human muscle in irreversible Conus and Cauda Equina syndrome (Fig. 3), four phases have been identified: (1) ultrastructural disorganization and loss of contractile response to ES (within a few months); (2) muscle atrophy (up to 2 years after SCI); (3) muscle degeneration with severe muscle atrophy (3–6 years after SCI); and (4) loss of myofibers (more than 3 years after SCI) [55–63, 65].
On the other hand, we have shown that home-based Functional Electrical Stimulation (h-bFES) of permanently denervated muscles stops and reverses the degeneration of skeletal muscle tissue [55–63]. Indeed, 2D Muscle Color Computer Tomography scans of thigh muscles in transverse section (Fig. 4) demonstrate the effect of h-bFES on long-term permanently denervated leg muscles. After two additional years of permanent denervation, but with FES treatment (Fig. 4g–j), the muscles began to resemble normal tissue. Indeed, the colors represent different tissues, computed on the basis of their radiodensity. These results are quite astonishing, highlighting the utility of h-bFES to greatly improve even badly degenerated denervated muscle tissue [55–63, 65]. As further evidence of the efficacy of h-bFES, Fig. 5 shows that 5 years after discontinuation of FES the recovered leg muscle deteriorates again [66–68]. It is evident, by our data, that h-bFES training (five times per week at 3 h per day) reverses atrophy and maintains microscopic and macroscopic features of the muscle fibers [56, 58, 60–63, 65]. If FES training is started within the first year following SCI, the retention of muscle is almost complete and may even rescue the ability to stand up and perform “walking in place” exercises during the second year of h-bFES [61]. If one induces many contractions daily in those muscle fibers that are otherwise destined to die, these fibers will survive and contract for up to dozens of years, but only if one stimulates them with very long, high amplitude impulses delivered by large surface electrodes designed for stimulating denervated muscles, i.e., using the patterns of stimulation developed in Vienna by Prof. Helmut Kern, Eng. Christian Hofer, Prof. Winfried Mayr and collaborators [55–65]. Commercial devices designed for electrical stimulation of long-term permanently denervated muscles that are capable of producing the needed stimulation patterns are currently available. Therefore, this excellent therapy should become accessible to more people for the preservation of skeletal muscle.
2D Color CT evidence of recovery from permanent denervation (i.e., premature muscle aging) by home-based Functional Electrical Stimulation (h-bFES). Color scans of thigh muscles before (b–e) and after 2 years (g–j) of h-bFES. Each panel Cross-sectional area and the quality of quadriceps muscles in patients starting h-bFES at different time points after denervation (b 1.2; c 1.7; d 3.2; e 5.4 years) increased after 2 years of home training (g–j, respectively). Moreover, the interstitial tissues that increased with post-denervation time (compare yellow, green and blue areas in b–e) decreased in the respective patient after 2 years of h-bFES (g–j, respectively)
3D Color Muscle CT reconstruction of the rectus femoris: reversibility of h-bFES-induced muscle recovery in a non-compliant patient. 3D reconstructions of rectus femoris from the SCI patient at three time points. a Four years after SCI, b after 4 years of h-bFES, and c 5 years after discontinuing h-bFES training. Five years after discontinuation of FES the recovered leg muscle deteriorates again
Research conducted over the past 50 years has demonstrated that muscle activity, not neurotrophic substances, is the most important factor in the regulation of size and of specific physiological and biochemical properties of muscle fibers. Application of this knowledge has led to considerable experimentation with chronic electrical stimulation as a possible clinical tool for the treatment of denervated muscles. Evidence accumulated from animal studies has indicated that direct electrical stimulation of denervated muscles can, to a significant extent, directly substitute reinnervation and preserve or restore the normal properties of these muscles. Appropriate stimulation parameters were critical for successful intervention, and the best results were obtained when the stimulation pattern resembled the differential firing pattern of motoneurons [1, 69, 70]. Nonetheless, even now, the vast majority of neurologists and physiatrists, who are not yet aware of those results, profess that denervated muscle cannot be maintained and certainly not regenerated [56, 63, 71]. Furthermore, based mainly on animal studies during the stage of removal of polyneural innervation in developing muscle fibers [72], there is a common sentiment that FES may hamper reinnervation despite the mounting evidence of the role of exercise in maintaining the integrity of the neuromuscular junction [73].
Contrastingly, many pioneering and recent experiments are showing the contrary. Denervated muscles, stimulated electrically for 4 days prior to reinnervation, show preserved endplate structure as well as accelerated recovery of normal function in reinnervated muscle fibers after 11 days of denervation [74]. Furthermore, electrical stimulation improves muscle reinnervation both functionally and structurally [5, 75–78].
Apparently, additional evidence is necessary to fully convince skeptics that FES is effective. A by-product of our previous studies, clinical imaging by 2D and 3D Muscle Color Computed Tomography (Fig. 6a, b) may be useful in collecting final evidence of the effectiveness of FES. In particular, this 3D methodology allows for evaluation of the effects of FES on an entire muscle [66–68]. Widely used in cardiac imaging [79], the false color approach is basically ignored in clinical imaging of skeletal muscle tissue. Our aim is to convince readers that the advantages inherent to this imaging technique offset the low risks of irradiation, in particular during follow-up of supervised trials.
2D and 3D Muscle Color CT scan. a The distribution analysis of the ranges of Hounsfield Unit in the histograms (left panels) allows for much more detailed quantitation of the changes occurring within the soft tissues of the leg (i.e., fat in yellow and muscles in red or orange, according to their density). Two months of conventional physiotherapy and electrical stimulation increased the content of low density muscle in the right leg by 2 % at the expense of the intramuscular fat and fibrous-dense connective tissue. The histograms in a refer to the muscle density on the tibialis anterior that is highlighted with black contours in all cross sections. b Density distribution and segmented areas on specific cross sections
Readers interested in neuromuscular electrical stimulation (NMES) by surface or implantable devices for other more frequent syndromes due to SCI or stroke may find valuable information in other literature sources [80–82]. Furthermore, the debated topic of electrical stimulation and/or exercise to promote regeneration of damaged peripheral nerves is reviewed in detail in Gordon T, English AW 2016 [5].
Lessons from horse models
Finally, we would like to discuss a recent example of the effectiveness of FES for treatment of equine epaxial muscle spasm. In this example, the different types of mitochondria present in skeletal muscle fibers, either subsarcolemmal or intermyofibrillar and their differential responses to needs and activation loads of muscle fibers were explored [83–85]. Preliminary histopathologic analyses had suggested that stimulated muscles were more damaged after treatment than they were before (Sheila Schils, personal communication). More careful morphometric analyses of the same samples excluded this possibility. Indeed, only one horse presented with obvious evidence of post-FES muscle damage, whereas the other five horses, which had the same type and amount of electrical stimulation, displayed scarce or none evidence of muscle atrophy/damage in both pre- and post-stimulation biopsies [84, 85]. If one does morphometry properly, the correlation between FES and muscle damage often disappears.
Regardless, in the post-FES horse biopsies, NADH-TR staining of mitochondria showed an increase of mitochondria that are localized near the sarcolemma. It is interesting to note that in recent observations comparing young and very old (30 months) rat muscles (EDL, soleus and diaphragm) subjected to neurectomy [86], we found that the abundance of subsarcolemmal mitochondria decreases with age—even more so after 1 week of denervation, whatever the animal’s age (manuscript in preparation). Feliciano Protasi and others have shown very interesting data concerning the structural relationships of mitochondria with triads in the intermyofibrillar spaces [56, 58, 61]. They conclude that physical interactions are part of the control mechanisms that provide the needed amount of ATP to the contractile machinery [87, 88]. In muscle fibers, there are different types of mitochondria, and they respond differently to various muscle fiber needs. Mitochondrial distributions and densities are significantly changed in horse post-FES muscle biopsies. This indicates that the clinical improvements observed in these horses are related to increased muscle perfusion induced by FES stimulation [83–85]. Thus, as in human cases [55–65], FES provides clinically relevant results in the treatments of equine epaxial muscle spasm. Interesting results were also recently published on the effects of FES by an implantable device on denervated laryngeal muscle in horses. Eight-week daily electrical stimulation produced a 30 % increase in fiber diameter in comparison with an unstimulated control group, as well as a trend toward a decrease in the proportion of type 1 (slow) fibers. The data confirm that ES by an implanted device, in accordance with the use of a relatively conservative set of stimulation parameters, can reverse the muscle fiber atrophy produced by complete denervation [89].
Conclusion and perspectives
In summary, it appears that the age-related decline in muscle power is partially attributable to a loss of innervation and that this loss can be deferred by lifelong high-level activity [7, 8]. Diseases involving permanent denervation show similar, but premature, aging and much more severe muscle deterioration. Despite doubts and criticisms of related literature [80, 82], we have shown that, with appropriate protocols, h-bFES can inhibit degeneration of denervated muscle and even reverse it [61, 65]. Thus, it should be possible to defer age-related muscle decline in aging people and in others who have become unable to participate in daily physical activities. Its use will be hopefully extended to muscle reinnervation after peripheral nerve lesions. In truth, the data indicate that FES should be considered for use in critical care units, rehabilitation centers, nursing facilities and at home when patients are unable or reluctant to perform volitional physical exercise even for short periods of time.
References
Lømo T (2014) The response of denervated muscle to long-term stimulation (1985, revisited here in 2014). Eur J Transl Myol Basic Appl Myol 24:13–19
Mitchell WK, Williams J, Atherton P et al (2012) Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3:260. doi:10.3389/fphys.2012.00260
Hepple RT, Rice CL (2015) Innervation and neuromuscular control in ageing skeletal muscle. J Physiol. doi:10.1113/JP270561
Gava P, Kern H, Carraro U (2015) Age-associated power decline from running, jumping, and throwing male masters world records. Exp Aging Res 41:115–135. doi:10.1080/0361073X.2015.1001648
Gordon T, English AW (2016) Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci 43:336–350. doi:10.1111/ejn.13005
Hill AV (1925) The physiological basis of athletic records. Sci Month 2:409–428
Mosole S, Rossini K, Kern H et al (2013) Significant increase of vastus lateralis reinnervation in 70-year sportsmen with a lifelong history of high-level exercise. Eur J Transl Myol Basic Appl Myol 23:117–122
Mosole S, Carraro U, Kern H et al (2014) Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol 73:284–294. doi:10.1097/NEN.0000000000000032
Stålberg E, Fawcett PR (1982) Macro EMG in healthy subjects of different ages. J Neurol Neurosurg Psychiatry 45:870–878
Doherty TJ (2003) Invited review: aging and sarcopenia. J Appl Physiol 95:1717–1727. doi:10.1152/japplphysiol.00347.2003
Lexell J, Downham DY (1991) The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81:377–381
Ling SM, Conwit RA, Ferrucci L et al (2009) Age-associated changes in motor unit physiology: observations from the Baltimore Longitudinal Study of Aging. Arch Phys Med Rehabil 90:1237–1240
Lexell J, Taylor CC, Sjostrom M (1988) What is the cause of ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84:275–294
Doherty TJ, Vandervoort AA, Taylor AW et al (1985) Effects of motor unit losses on strength in older men and women. J Appl Physiol 1993:868–874
Payne AM, Delbono O (2004) Neurogenesis of excitation-contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev 32:36–40
Delbono O (2003) Neural control of aging skeletal muscle. Aging Cell 2:21–29
Zampieri S, Pietrangelo L, Loefler S et al (2015) Lifelong physical exercise delays age-associated skeletal muscle decline. J Gerontol A Biol Sci Med Sci 70:163–173. doi:10.1093/gerona/glu006
Messi ML, Li T, Wang ZM et al (2015) Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J Gerontol A Biol Sci Med Sci. pii:glv176
Dow DE, Dennis RG, Faulkner JA (2005) Electrical stimulation attenuates denervation and age-related atrophy in extensor digitorum longus muscles of old rats. J Gerontol A Biol Sci Med Sci 60:416–424
Hennig R, Lømo T (1985) Firing patterns of motor units in normal rats. Nature 314:164–166
Kern H, Pelosi L, Coletto L et al (2011) Atrophy/hypertrophy cell signaling in muscles of young athletes trained with vibrational-proprioceptive stimulation. Neurol Res 33:998–1009
Zampieri S, Mosole S, Löfler S et al (2015) Physical exercise in Aging: nine weeks of leg press or electrical stimulation training in 70 years old sedentary elderly people. Eur J Transl Myol Basic Appl Myol 25:237–242
Kern H, Barberi L, Löfler S et al (2014) Electrical stimulation counteracts muscle decline in seniors. Front Aging Neurosci 6:189
Carnio S, LoVerso F, Baraibar MA et al (2014) Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep 8:1509–1521. doi:10.1016/j.celrep.2014.07.061
Barberi L, Scicchitano BM, Musaro A (2015) Molecular and cellular mechanisms of muscle aging and sarcopenia and effects of electrical stimulation in seniors. Eur J Transl Myol Basic Appl Myol 25:231–236
Scicchitano BM, Rizzuto E, Musarò A (2009) Counteracting muscle wasting in aging and neuromuscular diseases: the critical role of IGF-1. Aging (Albany NY) 1(5):451–457
Vinciguerra M, Musaro A, Rosenthal N (2010) Regulation of muscle atrophy in aging and disease. Adv Exp Med Biol 694:211–233
Carosio S, Berardinelli MG, Aucello M et al (2011) Impact of ageing on muscle cell regeneration. Ageing Res Rev 10:35–42
Barberi L, Scicchitano BM, De Rossi M et al (2013) Age-dependent alteration in muscle regeneration: the critical role of tissue niche. Biogerontology 14:273–292
Musarò A (2014) The basis of muscle regeneration. Adv Biol 2014:1–16
Snijders T, Verdijk LB, van Loon LJ (2009) The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res Rev 8:328–338
Mikkelsen UR, Langberg H, Helmark IC et al (2009) Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol 107:1600–1611
Farup J, Rahbek SK, Riis S et al (2014) Influence of exercise contraction mode and protein supplementation on human skeletal muscle satellite cell content and muscle fiber growth. J Appl Physiol 117:898–909
Farup J, Rahbek SK, Knudsen IS et al (2014) Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids 46:2503–2516
McKay BR, Ogborn DI, Bellamy LM et al (2012) Myostatin is associated with age related human muscle stem cell dysfunction. FASEB J 26:2509–2521
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Sandri M, Barberi L, Bijlsma AY et al (2013) Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 14:303–323
Kadi F, Schjerling P, Andersen LL et al (2004) The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. Physiol J 558:1005–1012
Mackey AL, Holm L, Reitelseder S et al (2010) Myogenic response of human skeletal muscle to 12 weeks of resistance training at light loading intensity. Scand J Med Sci Sports 21:773–782
Adamo ML, Farrar RP (2006) Resistance training, and IGF involvement in the maintenance of muscle mass during the aging process. Ageing Res Rev 5:310–331
Wallace JD, Cuneo RC, Baxter R et al (1999) Responses of the growth hormone (GH) and insulin-like growth factor axis to exercise, GH administration, and GH withdrawal in trained adult males: a potential test for GH abuse in sport. J Clin Endocrinol Metab 84:3591–3601
Kostka T, Patricot MC, Mathian B et al (2003) Anabolic and catabolic hormonal responses to experimental two-set low-volume resistance exercise in sedentary and active elderly people. Aging Clin Exp Res 15:123–130
Berg U, Bang P (2004) Exercise and circulating insulin-like growth factor I. Horm Res 62:50–58
Pelosi L, Berardinelli MG, De Pasquale L (2015) Functional and morphological improvement of dystrophic muscle by interleukin 6 receptor blockade. EBioMedicine 2:274–275
Nieto-Estévez V, Defterali Ç, Vicario-Abejón C (2016) IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci 10:52. doi:10.3389/fnins.2016.00052
Apel PJ, Ma J, Callahan M et al (2010) Effect of locally delivered IGF-1 on nerve regeneration during aging: an experimental study in rats. Muscle Nerve 41:335–341. doi:10.1002/mus.21485
Wang J, Jokerst JV (2016) Stem cell imaging: tools to improve cell delivery and viability. Stem Cells Int 2016:9240652. doi:10.1155/2016/9240652
Tezuka T, Inoue A, Hoshi T et al (2014) The MuSK activator agrin has a separate role essential for postnatal maintenance of neuromuscular synapses. Proc Natl Acad Sci USA 111:16556–16561. doi:10.1073/pnas.1408409111
Krenn M, Haller M, Bijak M et al (2011) Safe neuromuscular electrical stimulator designed for the elderly. Artif Organs 35:253–256
He Y, Huang C, Lin X et al (2013) MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie 95:1355–1359
Carraro U, Franceschi C (1997) Apoptosis of skeletal and cardiac muscles and physical exercise. Aging 9(1–2):19–34 (Review)
Hamar D (2015) Universal linear motor driven Leg Press Dynamometer and concept of Serial Stretch Loading. Eur J Transl Myol Basic Appl Myol 25:215–219
Cvecka J, Tirpakova V, Sedliak M et al (2015) Physical activity in elderly. Eur J Transl Myol Basic Appl Myol 25:249–252
Sarabon N, Löfler S, Hosszu G et al (2015) Mobility test protocols for the elderly: a methodological note. Eur J Transl Myol Basic Appl Myol 25:253–256
Kern H (1995) Funktionelle Elektrostimulation Paraplegischer Patienten. Österreichi sche Zeitschrift für Physikalische Medizin: ÖZPM 5:1–75. ISSN 1021-4348
Kern H, Boncompagni S, Rossini K et al (2004) Long-term denervation in humans causes degeneration of both contractile and excitation contraction coupling apparatus, which is reversible by functional electrical stimulation (FES). A role for myofiber regeneration? J Neuropathol Exp Neurol 63:919–931
Kern H, Rossini K, Carraro U et al (2005) Muscle biopsies show that FES of denervated muscles reverses human muscle degeneration from permanent spinal motoneuron lesion. J Rehabil Res Dev 42:43–53
Boncompagni S, Kern H, Rossini K et al (2007) Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc Natl Acad Sci USA 104:19339–19344
Kern H, Hofer C, Mayr W (2008) Protocols for clinical work package of the European project RISE. Eur J Transl Myol Basic Appl Myol 18:39–44
Kern H, Carraro U, Adami N et al (2010) One year of home-based Functional Electrical Stimulation (FES) in complete lower motor neuron paraplegia: recovery of tetanic contractility drives the structural improvements of denervated muscle. Neurol Res 32:5–12. doi:10.1189/184313209X385644
Kern H, Carraro U, Adami N et al (2010) Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabil Neural Repair 24:709–721. doi:10.1177/1545968310366129
Rossini K, Zanin ME, Carraro U (2002) To stage and quantify regenerative myogenesis in human long-term permanent denervated muscle. Basic Appl Myol 12:277–287
Carraro U, Rossini K, Mayr W et al (2005) Muscle fiber regeneration in human permanent lower motoneuron denervation: relevance to safety and effectiveness of FES-training, which induces muscle recovery in SCI subjects. Artif Organs 29:187–191
Kern H, Hofer C, Mödlin M et al (2008) Stable muscle atrophy in long-term paraplegics with complete upper motor neuron lesion from 3- to 20-year SCI. Spinal Cord 46:293–304
Kern H, Carraro U (2014) Home-based Functional Electrical Stimulation (h-b FES) for long-term denervated human muscle: history, basics, results and perspectives of the Vienna Rehabilitation Strategy. Eur J Transl Myol Basic Appl Myol 24:27–40
Gargiulo P, Helgason T, Reynisson PJ et al (2011) Monitoring of muscle and bone recovery in spinal cord injury patients treated with electrical stimulation using three-dimensional imaging and segmentation techniques: methodological assessment. Artif Organs 35:275–281. doi:10.1111/j.1525-1594.2011.01214.x
Gargiulo P, Reynisson PJ, Helgason B et al (2011) Muscle, tendons, and bone: structural changes during denervation and FES treatment. Neurol Res 33:750–758. doi:10.1179/1743132811Y.0000000007
Carraro U, Edmunds KJ, Gargiulo P (2015) 3D false color computed tomography for diagnosis and follow-up of permanent denervated human muscles submitted to home-based Functional Electrical Stimulation. Eur J Transl Myol Basic Appl Myol 25:129–140
Eberstein A, Eberstein S (1996) Electrical stimulation of denervated muscle: is it worthwhile? Med Sci Sports Exerc 28:1463–1469
Salmons S, Ashley Z, Sutherland H et al (2005) Functional electrical stimulation of denervated muscles: basic issues. Artif Organs 29:199–202
Carraro U, Boncompagni S, Gobbo V et al (2015) Persistent muscle fiber regeneration in long term denervation. Past, present, future. Eur J Transl Myol Basic Appl Myol 25:77–92
Brown MC, Holland RL (1979) A central role for denervated tissues in causing nerve sprouting. Nature 282(5740):724–726
Nishimune H, Stanford JA, Mori Y (2014) Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve 49:315–324. doi:10.1002/mus.24095
Eberstein A, Pachter BR (1986) The effect of electrical stimulation on reinnervation of rat muscle: contractile properties and endplate morphometry. Brain Res 384:304–310
Willand MP, Chiang CD, Zhang JJ et al (2015) Daily electrical muscle stimulation enhances functional recovery following nerve transection and repair in rats. Neurorehabil Neural Repair 29:690–700. doi:10.1177/1545968314562117
Willand MP, Nguyen MA, Borschel GH et al (2015) Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil Neural Repair (Epub ahead of print) (Review)
Willand MP, Nguyen MA, Borschel GH, Gordon T (2016) Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil Neural Repair 30(5):490–496. doi:10.1177/1545968315604399
Chan KM, Curran M, Gordon T (2016) The use of brief post-surgical low frequency electrical stimulation to enhance nerve regeneration in clinical practice. J Physiol. doi:10.1113/JP270892
Wang R, Meinel FG, Schoepf UJ et al (2015) Performance of automated software in the assessment of segmental left ventricular function in cardiac CT: comparison with cardiac magnetic resonance. Eur Radiol (Epub ahead of print)
Bersch I, Tesini S, Bersch U et al (2015) Functional electrical stimulation in spinal cord injury: clinical evidence versus daily practice. Artif Organs 39:849–854. doi:10.1111/aor.12618
Donovan-Hall MK, Burridge J, Dibb B et al (2011) The views of people with spinal cord injury about the use of functional electrical stimulation. Artif Organs 35:204–211. doi:10.1111/j.1525-1594.2011.01211.x
Hughes AM, Burridge JH, Demain SH et al (2014) Translation of evidence-based assistive technologies into stroke rehabilitation: users’ perceptions of the barriers and opportunities. BMC Health Serv Res 14:124. doi:10.1186/1472-6963-14-124
Schils SJ, Turner TA (2014) Functional Electrical Stimulation for equine epaxial muscle spasms: retrospective study of 241 clinical cases. Comp Exerc Physiol 10:89–97
Ravara B, Gobbo V, Carraro U et al (2015) Functional electrical stimulation as a safe and effective treatment for equine epaxial muscle spasms: clinical evaluations and histochemical morphometry of mitochondria in muscle biopsies. Eur J Transl Myol Basic Appl Myol 25:109–120
Schils S, Carraro U, Turner T et al (2015) Functional electrical stimulation for equine muscle hypertonicity: histological changes in mitochondrial density and distribution. J Equine Vet Sci 35:907–916
Mosole S, Zampieri S, Germinario E et al (2015) Structural and functional characteristics of denervated muscles from oldest-old rats: a relevant animal model for FES of denervated myofibers of the diaphragm in ALS? Eur J Transl Myol Basic Appl Myol 25:151
Mammucari C, Gherardi G, Zamparo I et al (2015) The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep 2015(10):1269–1279. doi:10.1016/j.celrep.01.056
Franzini-Armstrong C (2015) Electron microscopy: from 2D to 3D images with special reference to muscle. Eur J Transl Myol Basic Appl Myol 25(1):5–13
Cheetham J, Perkins JD, Jarvis JC et al (2015) Effects of functional electrical stimulation on denervated laryngeal muscle in a large animal model. Artif Organs 39(10):876–885. doi:10.1111/aor.12624
Acknowledgments
This work was supported by the European Regional Development Fund—Cross Border Cooperation Programme Slovakia—Austria 2007–2013 (Interreg-IVa), project Mobilität im Alter, MOBIL, N_00033 (Partners: Ludwig Boltzmann Institute of Electrical Stimulation and Physical Rehabilitation, Austria, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Austria, and Faculty of Physical Education and Sports, Comenius University in Bratislava, Slovakia); Austrian national co-financing of the Austrian Federal Ministry of Science and Research; Ludwig Boltzmann Society (Vienna, Austria) and supported by EU Commission Shared Cost Project RISE (Contract No. QLG5-CT-2001-02191) co-financed by the Austrian Ministry of Science. Some of the research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number NIH NIAMS 1R03AR053706-01A2 to ALP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Ugo Carraro thanks the IRCCS Fondazione Ospedale San Camillo, Venice, Italy for hospitality and scientific support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
All participants in the senior sportsmen studies were healthy and declared not to have any specific physical/disease issues (for detailed inclusion and exclusion criteria, see ClinicalTrials.gov: NCT01679977). All of the senior sportsmen declared to have a lifelong (30 years) history of high-level training. We certify that all applicable rules concerning the ethical use of human volunteers were followed during the course of this research (approval of ethical committee, Vienna, Austria: EK-02-068-0702).
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual partecipants included in the study.
Rights and permissions
About this article
Cite this article
Carraro, U., Kern, H., Gava, P. et al. Recovery from muscle weakness by exercise and FES: lessons from Masters, active or sedentary seniors and SCI patients. Aging Clin Exp Res 29, 579–590 (2017). https://doi.org/10.1007/s40520-016-0619-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-016-0619-1