Abstract
Purpose of Review
This article aims to shed light on the various value-added opportunities arising from the valorization of digestate nutrients. As opposed to the conventional applications of digestate for land/soil applications, this review discusses the key bioconversion routes to microalgae, biofuels, biochemicals, and enzymes production in which digestate is utilized as a feedstock for microorganisms to produce high-value bio-based products.
Recent Findings
A comprehensive analysis of key digestate valorization schemes in this review showed that microalgal cultivation and biopesticide production results obtained using digestate provide new directions to optimally utilize this resource. Pilot scale and long-term performance, and environmental assessment of these digestate-based productions will govern their success within the bioeconomy scheme in the near future.
Summary
Recovery of nutrients from digestate of anaerobic digestion (AD) and its use as a feedstock in biotechnological processes is an environmentally benign and socially responsible method to treat the waste digestate appearing from digestion plants. The replacement of cost-intensive pure nutrients by digestate could improve the economic feasibility of bioprocesses. This fits in the advanced biorefinery model within the circular bioeconomy scheme by providing new and alternative markets for digestate while increasing the economic incentives for AD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic digestion (AD) is an established technology which involves the degradation of organic matter by action of microorganisms in the absence of oxygen to produce methane-rich biogas. It is an effective and environment-friendly process that produces renewable bioenergy (methane) and helps in waste stabilization [1]. Its simplicity and capacity to process a diverse range of substrates from high solid feedstocks, i.e., municipal solid waste (MSW), food waste, animal manure, and lignocellulosic biomass to municipal and industrial wastewaters make it a preferred waste treatment option [2]. This approach of conversion of (bio)waste for energy production by biotechnological means also fulfills the sustainable development goals of energy and material efficiency and reduced waste generation. However, while producing clean and renewable energy from waste resources on one hand, huge quantities of nutrient-rich digestate are left behind by AD.
Digestate consists of a suspended solid fraction and a liquid fraction containing soluble nutrients and is the material that remains at the end of the AD process. It is estimated that between 90 and 95% of the feed to the digester remains behind as digestate [3]. Digestate has an organic matter content of 68–72%, thus indicating that it is a rich nutrient source [4•]. Consequently, the major outlet for digestate has been agricultural applications, e.g., fertilizer or soil improver. However, the use of digestate for land applications has raised serious concerns [5]. First, it requires to be stored at AD plants until further use for land application and this can lead to the escape of gases including CH4, CO2, NH3, and N2O, thereby contributing to atmospheric pollution [6]. Furthermore, the use of digestate is governed by strict regulations, for example, in the UK, only if the digestate meets the standards defined in the Quality Protocol PAS110, it is not considered a waste and is marketed for beneficial use [7].
On the other hand, large quantities of digestate are being generated. For an instance, an AD plant with a 500 kW power production generates more than 10,000 tons of digestate per year. In EU, 80 million tons of digestate was produced from 117 AD plants processing food waste, farm waste, manure, and crops [8••]. With limited land application and increasing availability of digestate, there is a strong and compelling need to develop alternative markets for digestate to ensure that this resource is used optimally and it does not become a “new waste” in the nearest future. Valorization of digestate into high-value products is important to (i) provide increased economic incentives to the AD plants and increase their sustainability, and (ii) generate increased opportunity for reuse of biodegradable waste and development of advanced biorefineries [9•]. Implications of increased digestate generation and strategies for its sustainable management have raised the concern among researchers and governments. Recent initiatives include The Waste and Resources Action Programme (WRAP, UK) and EU Vision 2020 with increased focus on digestate bioresource management and bio-based products in the circular economy scheme. Therefore, the focus of the present review is to discuss the key valorization and/or bioconversion schemes which utilize digestate nutrients in the form of feedstock or cultivation medium for fermenting microorganisms to produce value-added products. A comprehensive analysis of properties of digestate suitable for specific bioconversion, the bioprocess scheme and production efficiencies achieved using digestate, has been performed.
Digestate as a Feedstock for Bioprocesses
The obtained digestate from AD plant can be utilized directly without any further processing and/or modification or it can be subjected to various separation and processing technologies. The ability to use whole digestate would be ideal to ensure optimal use of both solid and liquid fractions and no further remaining waste from digestate itself. However, usually the digestate is separated into solid and liquid fractions and only one fraction is utilized in most bioprocesses. Dewatering of digestate is done by specialized processes and/or technologies including decanter centrifuges and screw press separators. This represents a substantial investment and high operational costs [8••]. On the other hand, it also dramatically reduces the transportation costs. The final consideration for digestate processing also involves finding a suitable outlet for remaining (left-over) fraction disposal and/or management lest it might become a cost-intensive operation.
A standard procedure in bioprocesses is to sterilize the nutrient medium. The ability to sterilize the digestate and/or separate or concentrate its nutrients by membrane technology is receiving considerable attention. Use of membrane technology including microfiltration and ultrafiltration has been performed to prepare the digestate as a liquid medium for microalgae cultivation and polyhydroxyalkanoate (PHA) bioplastic production. Microfiltration (0.2 μm) helps to remove solids from digestate, avoids the inclusion of pathogens, and helps to adjust the nitrogen/phosphate (N/P) value of digestate based on molecular weight cut-off of the membrane, as per the needs of the microorganism [10••]. In addition to the above merits of filtration method, the advances in membrane technology in the recent years have allowed the setting up of cost-effective large-scale filtration units for various biotechnological applications (e.g., for wastewater treatment) [11]. This further makes this technology amenable for the preparation of digestate as the fermentation medium.
In some other bioprocesses, the solid fraction of digestate has been used as a substrate for fermentation, i.e., in solid-state fermentation (SSF) [12••]. Previous to its use as a feedstock, the solid digestate is hygienized by maintaining the digestate for 1 h at 70 °C and then cooling it down to room temperature. Such a digestate processing method is a part of EU Regulation 14/2011 according to which the digestate must be hygienized prior to its valorization. This has been used for SSF of biopesticides, biosurfactants, and enzymes (the “Bioconversions of Digestate Nutrients to High-Value Products” section). Another digestate processing technique is autoclaving in which the digestate is heated at 121 °C for 15 min and is similar to the autoclaving which is generally used for sterilization of refined nutrients of synthetic medium in bioprocesses. Autoclaving of digestate was employed for fungal fermentation to produce chitin [13]. The use of thermal treatment such as in hygienization and autoclaving leads to the removal of pathogens that are usually present in the digestate [14]. Although both being energy- and cost-intensive techniques, autoclaving offers an added advantage of increased solubilization of digestate solids which contain most of the nutrients of digestate. This increases the availability of nutrients to the fermenting microorganism when autoclaved digestate is used in a bioprocess, thereby also positively affecting the process performance.
Bioconversions of Digestate Nutrients to High-Value Products
Microalgae Cultivation for Various End Applications
Microalgae are miniature, plantlike oxygen-releasing photosynthetic microorganisms. They naturally grow in numerous water environments, including fresh and marine water, and a variety of wastewaters, e.g., municipal, agricultural, domestic, industrial, and other types of wastewaters [15, 16]. Research efforts are now being focused on using digestate streams as potential feedstock which can significantly reduce the water and nutrient demands for microalgal culture [17]. Exploration of algal biomass produced on digestates has the advantage of direct integration of microalgal culture with (AD) technologies that are already present and widespread. Therefore, this obviates the need for a separate infrastructure which might be a requirement for microalgal processes utilizing other biomass-based feedstocks.
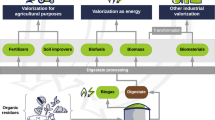
Majority of reports on digestate cultivation of microalgae have been focused on freshwater microalgae such as Chlorella and Scenedesmus and some marine microalgae such as Nannochloris. Mostly, the cultures have been carried out at a laboratory scale, with only a few outdoor pilot scale tests. Culture systems range from flasks, bags, photobioreactors to raceways and in batch and continuous mode [18]. These studies help in understanding various factors that influence the nutrient utilization, growth kinetics, and applicability of these digestate-grown microalgal cultures in an industrial setting. Agro-zootechnical digestates derived from AD of liquid cow manure, cheese whey, dairy products, pig manure, and chicken manure as well as biosludge digestates from wastewater treatment plants and food industry (dairies, slaughterhouses) and leachate water (biodegradable waste from households and composting plants) can act as suitable feedstocks for microalgae [19] (Fig. 1).
While producing microalgae as high-value commodities and providing an efficient means to remediate digestate, a suitable system is required to prepare an optimal digestate medium which will be suitable for microalgal growth. For this, usually the digestate derived from AD is kept in a settling tank for a hydraulic retention time (HRT) of >8 h [10••]. This is necessary since the digestate typically obtained from a mesophilic AD plant has a temperature between 27 and 42 °C and an alkaline pH of 7.4–8.2. These values are higher than the optimal values required for microalgal culture, thereby making the adjustment of digestate necessary before its utilization as a feedstock. This makes digestate usage different from the conventional microalgal feedstocks, e.g., defined nutrient media. Appropriate dilution and dosing of digestate for a proper nutrient balance is one of the key parameters for using liquid digestate as microalgal growth medium [19]. Pretreatment methods are often implemented to decrease the suspended particle population in the medium and prevent potential toxic effects of highly concentrated inorganic matters. Suspended solid removal can be carried out through dilution, centrifugation, hydrogen peroxide treatment, filtration, and/or supernatant extraction. Diluting the digestate and allowing the solids to settle over time for supernatant extraction has been the most effective and least expensive method for efficient microalgal growth [20]. Ultrafiltration provides an advantage of reducing microbial contamination and preventing damage to the quality of the final biomass product [11]. In order to maximize the growth of autotrophic and mixotrophic cultivation of microalgae optimum, illumination is required for photosynthesis. Furthermore, darker shades of digestates often negatively impact the microalgal growth and light wavelength and intensity adjustments are required [21]. Additionally, the nutritional characteristics of digestate have a direct influence on microalgae biomass. The availability of carbon, nitrogen, and phosphorous is the most important requirement. Quantities of nitrogen in culture medium directly alter the cell growth. Decline in nitrogen concentration reduces the biomass productivity; however, this triggers a greater formation of carbohydrate and lipids by redirection of cell metabolism into the biosynthesis of these reserve materials. Carbon levels too influence the culture dynamics of microalgae and are often described in digestate chemical oxygen demand (COD) and/or dissolved organic carbon (DOC). Digestates with a COD of 3–5 g COD/L and a DOC of 0.15–0.54 g/L have been proved to be highly optimum for microalgal growth [22, 23]. Total carbon and carbon/nitrogen (C/N) ratio are also found to be a function of pH in Nannochloris sp. dominant cultures. It is important that a feedstock such as digestate should allow microalgae production with high productivity and high lipid content for further applications in food and/or biodiesel production. Microalgae while removing the organic carbon and volatile fatty acids in digestates simultaneously upgrade the biomass profile in terms of carbohydrates and lipids. Biomass yield and fatty acid contents of the digestate-produced microalgae have varied between 570–1117 mg/L and 3.9–24.5%, respectively. Fatty acid profiling of microalgal extracts showed that oleic, palmitic, and linolenic acids were the most abundant fatty acids, which was similar to the results on synthetic media [24].
Biofuels Production Using Digestate
Next-generation biofuels which are based on non-agricultural feedstock answer the problems of competition with food and environmental complications. Production of liquid biofuels such as bioethanol and biodiesel has been reported using digestate as feedstock.
Bioethanol production has been investigated on the solid fraction of digestate, i.e., the undigested solids in digestate (Fig. 1). For such a utilization, a treatment step is often required on the digestate fibers prior to enzymatic hydrolysis and fermentation. This facilitates the solubilization of lignin from lignocellulosic matrix and elevates the cellulose content of the remaining fraction [4•]. A dilute alkaline pretreatment of manure fibers using 2% sodium hydroxide was performed at 130 °C for 2 h followed by enzymatic hydrolysis to produce 51 g/L glucose [25]. Use of this hydrolysate for ethanol fermentation with Saccharomyces cerevisiae generated a 72% ethanol yield. In another report, the replacement of freshwater and synthetic nutrients with anaerobic digestate effluent increased the ethanol concentration yields to up to 81 g/L in comparison with 59.7 g/L obtained using synthetic nutrients [26]. Recovery of carbon from recalcitrant solid digestate through ozone combined with aqueous ammonia followed by a thermophilic process produced 24.3% more ethanol as compared with that achieved on solid digestate after mesophilic process. Optimum net energy balance for this process was calculated as 6416 kJ/kg [27].
Biodiesel is another type of renewable source of energy and has gained attention worldwide. Conventionally, biodiesel is sourced from lipid feedstock, animal fats, and waste cooking oil by transesterification reaction. Among the type and availability of feedstock (i.e., raw material), production method, additives, and operational costs, raw materials contribute to the major portion of biodiesel production cost. Removal of feedstock-derived, catalytic residues and other undesired products (triglycerides, free glycerine) also plagues the current biodiesel production methods. To overcome these challenges, digestate can be used as a suitable source for biodiesel through an oleaginous microbe-based cascade biorefinery scheme [4•].
Digestate utilization was initiated by alkali processing of feed mixture of solid and liquid digestates, monosugar release by enzymatic hydrolysis, and detoxification through overliming [28] (Fig. 1). Subsequently, a lipid accumulation of 3.16 g/L was achieved through repeated batch fungal fermentations. The mass and energy balance analysis successfully showed the establishment of a self-sustaining, freshwater-free, and energy-efficient process of lipid production as a biodiesel precursor. Few studies have also established the superiority of mixed cultures over monocultures of oleaginous yeasts with other oleaginous microbes such as microalgae. Mixed cultures on liquid digestate of dairy wastewater with added glycerol as a carbon source proved the conjunction of mixed culture-based digestate utilization and biodiesel generation. These cultures generated a higher yield of biomass (1.62 g/L), lipid (0.31 g/L), and protein (0.51 g/L) and had a higher heating value (34.06 kJ/L) as compared with monocultures. The biological utilization of nitrogen and phosphorus was also elevated in mixed cultures [29]. Similar results were achieved using liquid digestate of yeast industry as a seed feed for mixed batch cultures of microalgae Chlorella vulgaris and yeast Yarrowia lipolytica. It was shown that the biomass concentration (1.39–1.56 g/L), lipids yields (0.073–0.154 g/L), and heating value (20.06–29.76 kJ/L) were higher as compared with monocultures [30]. The approach of mixed cultures with digestates for biodiesel production can be further studied for selection and engineering of suitable strain, underlying synergistic mechanisms, and mutual nutrient recycling benefits.
Biochemicals and Other Bioproducts Derived From Digestate Bioconversion
The digestate nutrients can also be utilized by various microorganisms to produce useful bio-based chemicals and products such as biosurfactants, biopesticides, bioplastics, and enzymes which have numerous commercial applications. Biosurfactant is an amphiphilic, detergent-like compound which is produced biologically through the fermentation process. Currently, two of the most commonly investigated biosurfactants are sophorolipids and rhamnolipids. In our recent investigation, rhamnolipids have been successfully produced at multigram scale using digestate from sludge and food waste (unpublished results). A recent report investigated the production of sophorolipids from mixture of hygienized biowaste digestate, 1% glucose and 10% oleic acid through SSF by Starmerella bombicola ATCC22214 in 0.45 L reactor (Fig. 1) [31•]. The production yield was lower at 0.020 g sophorolipids/g dry matter which was probably due to the lower carbon nutrients in their digestate than is usually required for sophorolipids production [32].
Another high-value product from digestate bioconversion which has been extensively studied is biopesticides. The production of biopesticides from hygienized biowaste digestate was performed through SSF of Bacillus thuringiensis var. kurstaky in 0.45 L reactor [31•]. Biopesticides were successfully produced and reached a maximum spore count of 2.85 × 107 colony-forming unit (CFU)/g dry matter during sporulation of B. thuringiensis. Spore count is an important parameter in biopesticide production since δ-endotoxin is simultaneously produced during sporulation when the nutrient is limited. In another report, the production of biopesticide by SSF of digestate using B. thuringiensis was attempted at pilot scale using different configurations of reactors. Successful pesticide production was achieved under non-insulated stirrer reactor at 100 L scale which resulted in an increment of spore counts by 3.8 with respect to initial inoculation values [11].
Among the bioplastics, polyhydroxyalkanoate (PHA) has been one of the most investigated categories. PHA could be used to form bio-thermoplastics such as poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (poly(3HB-co-3HHx)) which are biodegradable under anaerobic sludge digestion and aerobic seawater environments [33]. Food waste–based digestate from wheat fed digesters was used and supplemented with 1% v/v acetic acid for PHA fermentation by Cupriavidus necator DSMZ 545 in a 5-L fed-batch fermenter [34]. A PHA yield of 12.3 g/L and 90% PHA content was successfully produced in this digestate-based fermentation, which was comparable with the PHA yield obtained on refined media and other waste materials [35]. A more recent study demonstrated that PHA could be produced from a mixture of waste sunflower oil and micro-filtered digestate liquor of chicken manure [36]. The fermentation process produced 4.6 g/L PHA with a 75% cell dry weight content in 96 h.
Enzymes are another important bioproducts which can be derived from digestate-based bioconversions. A large proportion of available carbon in the digestate is stored in the form of solid structural molecules such as lignin and cellulose. This forms an interesting value-added chain in which the residual carbon in the digestate solids can be accessed by enzyme-producing microbes. The available functional molecules and nutrients in the digestate induce the enzyme biosynthesis and the produced enzyme acts simultaneously to hydrolyze lignin and cellulose in digestate. Thus, a second advantage of this process is the consequent degradability of the digestate residue which can then be used back in AD to increase the biogas yield. This makes the utilization of digestate for enzyme production technologically and economically more attractive than using conventional substrates [37]. Some of the important enzymes which have been produced on digestate include manganese peroxidase and laccase (as ligninolytic enzymes), and β-glucosidase and endo-β-1,4-glucanase (as cellulases). These enzymes have a high market volume and their potential production using inexpensive and readily available feedstock is particularly attractive.
Most of the research on digestate to enzyme production has utilized mainly two types of digestates, i.e., the digestate derived from agricultural wastes and the digestate from distillery spent wash. Santi et al. [38] reported the expression of endoglucanase (2300 U/g protein), cellobiohydrolase (700 U/g protein), and xylanase (3000 U/g protein) in 20 days during SSF by Pleurotus ostreatus using corn silage digestate as substrate. More recently, Fang et al. [39] successfully produced ligninolytic enzyme from digestate of crop residues (wasted residues of fruits and vegetables) under anaerobic condition with no pH control and temperature of 30 °C. Manganese peroxidase activity of 103.1 U/g volatile solids was observed at 5 weeks in anaerobic SSF by Pleurotus sajor-caju strain MES 03464 and a laccase activity of 284.9 U/g volatile solids was observed at 3 weeks in anaerobic SSF of Trametes versicolor strain MES 1191. Cerda et al. [31•] also used SSF to investigate the production of cellulases and proteases from autochthonous microbe using digestate as feedstock and SSF was carried out in packed-bed reactors for 48 h. A low cellulase production ranging at 0.5–1.5 FPU per gram dry matter was observed while a higher protease production of ~ 65 U per gram dry matter was obtained.
Submerged fermentation (SMF) has also been investigated for enzyme production on digestate (Fig. 1). Musatti et al. [37] investigated the first submerged fermentation of solid agricultural waste digestate from cow manure, cheese whey, poultry manure, olive pomace, and corn sugar using different microorganisms. At optimum condition of 150 rpm, pH 6, 25 °C temperature, and a cultivation time of 7 days, cellulase, xylanase, and laccase were successfully produced. Cellulase was produced by Irpex lateus DSM1183 with endoglucanase activity of 236 IU/g total solids and β-glucosidase activity of 52 IU/g total solids. Maximum xylanase activity of 494 IU/g total solids and a laccase activity of 124 IU/g total solids were expressed by Schizophyllum commune CBS30132 and Pleurotus ostreatus ATCC96997, respectively.
Distillery spent wash (DSW) digestate is commonly diluted with water to a 5–60% (v/v) concentration and subsequently used as a medium to produce lignocellulosic enzymes and xylanase [40]. Xylanase was successfully produced with an activity of 5200–5600 U/g using Burkholderia sp. DMAX in SSF of 37 h [41]. DSW digestate was used at 10% concentration and supplemented with wheat bran. Similar investigation was performed by Chapla et al. [42] who used 10.5% DSW digestate supplemented with wheat bran through SSF in shake flask using Aspergillus foetidus MTCC 4898. Xylanase activity of 8450 U/g was observed at 80 h when optimum conditions of pH 5.0 and temperature 30 °C were provided.
In addition to xylanase, cellulase has also been produced by utilizing 40% DSW digestate mixed with wheat straw by SSF of Aspergillus ellipticus. At pH 3, β-glucosidase activity peaked at 26.95 U/g-substrate, while highest endo-β-1,4-glucanase activity of 130.92 U/g-substrate was observed at pH 5 [43]. Lastly, ligninolytic enzymes have also been produced using DSW digestate. Yadav and Chandra [44] reported that a manganese peroxidase activity of 1.93 U/mL and a laccase activity of 0.84 U/mL was observed during decolorization of DSW digestate supplemented with 1% glucose and 0.1% peptone. A bacteria consortium (4:3:1:1 ratio) of Proteus mirabilis, Bacillus sp., Raoultella planticola, and Enterobacter sakazakii was used in this study.
Conclusion
Examples of bio-valorization routes for digestate provided in this review explicitly demonstrate the advancement towards realization of their applications. The environment footprint and cost are the major drivers for development of bioprocesses based on digestate. To this end, the demonstration of scalability of such processes is a key requirement to reap real benefits. The studies on digestate-based bioconversions to produce high-value products have been largely performed at laboratory scale. However, there have been few reports on pilot scale investigations for microalgae cultivation and biopesticide production. This is already impressive and provides confidence in the effective utilization of an unconventional waste such as digestate for high-value product formation.
Following the pilot scale and long-term data, the next essential step in the digestate-to-high value product chain will be the life cycle assessment of the proposed valorization schemes. The inputs of infrastructure (photobioreactors, microbial fermenters), energy, and water for processes including cultivation, filtration/centrifugation, and product extraction and processing to final (finished) product need to be carefully evaluated. The key question to be answered in the future is whether the above burdens are outweighed by the environmental credits associated with waste-based and biotechnologically produced high-value products. Thus, both technological and environmental efficiency of the digestate valorization schemes will govern their success in the future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kaur G, Luo L, Chen G, Wong JWC. Integrated food waste and sewage treatment - a better approach than conventional food waste-sludge co-digestion for higher energy recovery via anaerobic digestion. Bioresour Technol. 2019;289:121698. https://doi.org/10.1016/j.biortech.2019.121698.
Obulisamy PK, Chakraborty D, Selvam A, Wong JW. Anaerobic co-digestion of food waste and chemically enhanced primary-treated sludge under mesophilic and thermophilic conditions. Environ Technol. 2016;37(24):3200–7. https://doi.org/10.1080/09593330.2016.1181112.
Tampio E, Marttinen S, Rintala J. Liquid fertilizer products from anaerobic digestion of food waste: mass, nutrient and energy balance of four digestate liquid treatment systems. J Clean Prod. 2016;125:22–32. https://doi.org/10.1016/j.jclepro.2016.03.127.
• Monlau F, Sambusiti C, Ficara E, Aboulkas A, Barakat A, Carrère H. New opportunities for agricultural digestate valorization: current situation and perspectives. Energy Environ Sci. 2015;8(9):2600–21. https://doi.org/10.1039/c5ee01633aA comprehensive review of state-of-the-art approaches in conversion of agricultural-derived digestate. This review focusses on thermal processes e.g. combustion, hydrothermal carbonization and pyrolysis for production of energy and char and/or activated carbons.
Gioelli F, Dinuccio E, Balsari P. Residual biogas potential from the storage tanks of non-separated digestate and digested liquid fraction. Bioresour Technol. 2011;102(22):10248–51. https://doi.org/10.1016/j.biortech.2011.08.076.
Sambusiti C, Ficara E, Malpei F, Steyer JP, Carrere H. Benefit of sodium hydroxide pretreatment of ensiled sorghum forage on the anaerobic reactor stability and methane production. Bioresour Technol. 2013;144:149–55. https://doi.org/10.1016/j.biortech.2013.06.095.
WRAP. Enhancement and treatment of digestates from anaerobic digestion. 2012.
•• Pérez-Camacho MN, Curry R. Regional assessment of bioeconomy options using the anaerobic biorefinery concept. Proc Inst Civ Eng Waste Resour Manag. 2018;171(4):104–13. https://doi.org/10.1680/jwarm.17.00015This paper provides an assessment of potential significance of anaerobic digestion biorefineries. It shows how the evaluation of biogas and digestate management pathways in an integrated way within an anaerobic digestion biorefinery can contribute to the development of a road map for a regional bioeconomy.
• Kaur G, Uisan K, Ong KL, Ki Lin CS. Recent trends in green and sustainable chemistry & waste valorisation: rethinking plastics in a circular economy. Curr Opin Green Sustain Chem. 2018;9:30–9. https://doi.org/10.1016/j.cogsc.2017.11.003This critical review article provides perspective on how the biorefinery approach can contribute to the advancement and development of a circular (bio)economy.
•• WAV S, Styles D, Chapman SP, Esteves S, Bywater A, Melville L, et al. Using microalgae in the circular economy to valorise anaerobic digestate: challenges and opportunities. Bioresour Technol. 2018;267:732–42. https://doi.org/10.1016/j.biortech.2018.07.100This paper discusses the prospects and challenges in digestate utilization for microalgae cultivation. It further demonstrates how the regional digestate excesses can be effectively managed by this route to produce livestock feed and fuel and to enhance sustainability.
Veronesiv D, D’Imporzano G, Salati S, Adani F. Pre-treated digestate as culture media for producing algal biomass. Ecol Eng. 2017;105:335–40. https://doi.org/10.1016/j.ecoleng.2017.05.007.
•• Rodríguez P, Cerda A, Font X, Sánchez A, Artola A. Valorisation of biowaste digestate through solid state fermentation to produce biopesticides from Bacillus thuringiensis. Waste Manag. 2019;93:63–71. https://doi.org/10.1016/j.wasman.2019.05.026This paper demonstrates the pilot scale production of biopesticides using digestate as the feedstock for producer microrganism.
Liu Z, Liao W, Liu Y. A sustainable biorefinery to convert agricultural residues into value-added chemicals. Biotechnol Biofuels. 2016;9:197. https://doi.org/10.1186/s13068-016-0609-8.
Mininni G, Blanch AR, Lucena F, Berselli S. EU policy on sewage sludge utilization and perspectives on new approaches of sludge management. Environ Sci Pollut Res. 2015;22:7361–74. https://doi.org/10.1007/s11356-014-3132-0.
Koyande AK, Chew KW, Rambabu K, Tao Y, Chu D-T, Show P-L. Microalgae: a potential alternative to health supplementation for humans. Food Sci Human Wellness. 2019;8(1):16–24. https://doi.org/10.1016/j.fshw.2019.03.001.
Li K, Liu Q, Fang F, Luo R, Lu Q, Zhou W et al. Microalgae-based wastewater treatment for nutrients recovery: a review. Bioresour Technol 2019:121934. doi:https://doi.org/10.1016/j.biortech.2019.121934.
Li R, Duan N, Zhang Y, Liu Z, Li B, Zhang D, et al. Co-digestion of chicken manure and microalgae Chlorella 1067 grown in the recycled digestate: nutrients reuse and biogas enhancement. Waste Manag. 2017;70:247–54. https://doi.org/10.1016/j.wasman.2017.09.016.
Pizzera A, Scaglione D, Bellucci M, Marazzi F, Mezzanotte V, Parati K, et al. Digestate treatment with algae-bacteria consortia: a field pilot-scale experimentation in a sub-optimal climate area. Bioresour Technol. 2019;274:232–43. https://doi.org/10.1016/j.biortech.2018.11.067.
Massa M, Buono S, Langellotti AL, Castaldo L, Martello A, Paduano A, et al. Evaluation of anaerobic digestates from different feedstocks as growth media for Tetradesmus obliquus, Botryococcus braunii, Phaeodactylum tricornutum and Arthrospira maxima. New Biotechnol. 2017;36:8–16. https://doi.org/10.1016/j.nbt.2016.12.007.
Abu Hajar HA, Guy Riefler R, Stuart BJ. Anaerobic digestate as a nutrient medium for the growth of the green microalga Neochloris oleoabundans. Environ Eng Res. 2016;21:265–75. https://doi.org/10.4491/eer.2016.005.
Lavrič L, Cerar A, Fanedl L, Lazar B, Žitnik M, Marinšek LR. Thermal pretreatment and bioaugmentation improve methane yield of microalgal mix produced in thermophilic anaerobic digestate. Anaerobe. 2017;46:162–9. https://doi.org/10.1016/j.anaerobe.2017.02.001.
Thi Nguyen M-L, Lin C-Y, Lay C-H. Microalgae cultivation using biogas and digestate carbon sources. Biomass Bioenergy. 2019;122:426–32. https://doi.org/10.1016/j.biombioe.2019.01.050.
Tao R, Lakaniemi A-M, Rintala JA. Cultivation of Scenedesmus acuminatus in different liquid digestates from anaerobic digestion of pulp and paper industry biosludge. Bioresour Technol. 2017;245:706–13. https://doi.org/10.1016/j.biortech.2017.08.218.
Koutra E, Grammatikopoulos G, Kornaros M. Selection of microalgae intended for valorization of digestate from agro-waste mixtures. Waste Manag. 2018;73:123–9. https://doi.org/10.1016/j.wasman.2017.12.030.
Yue Z-B, Teater C, Liu Y, Maclellan J, Liao W. A sustainable pathway of cellulosic ethanol production integrating anaerobic digestion with biorefining. Biotechnol Bioeng. 2010;105:1031–9. https://doi.org/10.1002/bit.22627.
Gao T, Li X. Using thermophilic anaerobic digestate effluent to replace freshwater for bioethanol production. Bioresour Technol. 2011;102(2):2126–9. https://doi.org/10.1016/j.biortech.2010.08.088.
Wang D, Xi J, Ai P, Yu L, Zhai H, Yan S, et al. Enhancing ethanol production from thermophilic and mesophilic solid digestate using ozone combined with aqueous ammonia pretreatment. Bioresour Technol. 2016;207:52–8. https://doi.org/10.1016/j.biortech.2016.01.119.
Zhong Y, Liu Z, Isaguirre C, Liu Y, Liao W. Fungal fermentation on anaerobic digestate for lipid-based biofuel production. Biotechnol Biofuels. 2016;9:253. https://doi.org/10.1186/s13068-016-0654-3.
Qin L, Liu L, Wang Z, Chen W, Wei D. Efficient resource recycling from liquid digestate by microalgae-yeast mixed culture and the assessment of key gene transcription related to nitrogen assimilation in microalgae. Bioresour Technol. 2018;264:90–7. https://doi.org/10.1016/j.biortech.2018.05.061.
Qin L, Wei D, Wang Z, Alam MA. Advantage assessment of mixed culture of Chlorella vulgaris and Yarrowia lipolytica for treatment of liquid Digestate of yeast industry and cogeneration of biofuel feedstock. Appl Biochem Biotechnol. 2019;187(3):856–69. https://doi.org/10.1007/s12010-018-2854-8.
• Cerda A, Mejias L, Rodríguez P, Rodríguez A, Artola A, Font X, et al. Valorisation of digestate from biowaste through solid-state fermentation to obtain value added bioproducts: a first approach. Bioresour Technol. 2019;271:409–16. https://doi.org/10.1016/j.biortech.2018.09.131This article demonstrates the utilization of solid digestate in bio-based processes for multiple bio-products formation.
Kaur G, Wang H, To MH, Roelants SLKW, Soetaert W, Lin CSK. Efficient sophorolipids production using food waste. J Clean Prod. 2019;232:1–11. https://doi.org/10.1016/j.jclepro.2019.05.326.
Wang S, Lydon KA, White EM, Grubbs JB, Lipp EK, Locklin J, et al. Biodegradation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) plastic under anaerobic sludge and aerobic seawater conditions: gas evolution and microbial diversity. Environ Sci Technol. 2018;52(10):5700–9. https://doi.org/10.1021/acs.est.7b06688.
Passanha P, Esteves SR, Kedia G, Dinsdale RM, Guwy AJ. Increasing polyhydroxyalkanoate (PHA) yields from Cupriavidus necator by using filtered digestate liquors. Bioresour Technol. 2013;147:345–52. https://doi.org/10.1016/j.biortech.2013.08.050.
Berezina N, Martelli SM. Polyhydroxyalkanoates: structure, properties and sources. In: Ipsita R, Visakh, P.M., editor. Polyhydroxyalkanoate (PHA) based blends, composites and nanocomposites. Royal Society of Chemistry; 2015.
Altun M. Polyhydroxyalkanoate production using waste vegetable oil and filtered digestate liquor of chicken manure. Prep Biochem Biotechnol. 2019. https://doi.org/10.1080/10826068.2019.1587626.
Musatti A, Ficara E, Mapelli C, Sambusiti C, Rollini M. Use of solid digestate for lignocellulolytic enzymes production through submerged fungal fermentation. J Environ Manag. 2017;199:1–6. https://doi.org/10.1016/j.jenvman.2017.05.022.
Santi G, Muzzini VG, Galli E, Proietti S, Moscatello S, Battistelli A. Mycelial growth and enzymatic activities of white-rot fungi on anaerobic digestates from industrial biogas plants. Environ Eng Manag J. 2015;14(7):1713–9. https://doi.org/10.30638/eemj.2015.182.
Fang W, Zhang P, Zhang X, Zhu X, van Lier JB, Spanjers H. White rot fungi pretreatment to advance volatile fatty acid production from solid-state fermentation of solid digestate: efficiency and mechanisms. Energy. 2018;162:534–41. https://doi.org/10.1016/j.energy.2018.08.082.
Singh A, Bajar S, Bishnoi NR, Singh N. Laccase production by Aspergillus heteromorphus using distillery spent wash and lignocellulosic biomass. J Hazard Mater. 2010;176:1079–82.
Mohana S, Shah A, Divecha J, Madamwar D. Xylanase production by Burkholderia sp. DMAX strain under solid state fermentation using distillery spent wash. Bioresour Technol. 2008;99(16):7533–64. https://doi.org/10.1016/j.biortech.2008.02.009.
Chapla D, Divecha J, Madamwar D, Shah A. Utilization of agro-industrial waste for xylanase production by Aspergillus foetidus MTCC 4898 under solid state fermentation and its application in saccharification. Biochem Eng J. 2010;49(3):361–9. https://doi.org/10.1016/j.bej.2010.01.012.
Acharya BK, Mohana S, Jog R, Divecha J, Madamwar D. Utilization of anaerobically treated distillery spent wash for production of cellulases under solid-state fermentation. J Environ Manag. 2010;91(10):2019–27. https://doi.org/10.1016/j.jenvman.2010.05.001.
Yadav S, Chandra R. Detection of persistent organic compounds from biomethanated distillery spent wash (BMDS) and their degradation by manganese peroxidase and laccase producing bacterial strains. J Environ Biol 2012.
Funding
This work is supported by the Innovation and Technology Commission, Hong Kong, under Innovation and Technology Fund (Grant No. ITS/176/18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Bioconversion
Rights and permissions
About this article
Cite this article
Kaur, G., Wong, J.W.C., Kumar, R. et al. Value Addition of Anaerobic Digestate From Biowaste: Thinking Beyond Agriculture. Curr Sustainable Renewable Energy Rep 7, 48–55 (2020). https://doi.org/10.1007/s40518-020-00148-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40518-020-00148-2